Abstract
Four-membered carbocycles are among the most sought-after backbones which are commonly found in biologically active molecules. However, difficulties on their producing are existing due to its highly strained ring system. On the other hand, cyclobutanols can be straightforwardly prepared and can serves as precursors for synthesizing cyclobutane derivatives. Here we report an example of regioselective aminocarbonylation of cyclobutanols in which the cyclobutane core remained intact. The method exhibits good functional group compatibility, as well as high regio- and stereoselectivity, offering new pathways for synthesizing several pharmaceuticals. Furthermore, this strategy enables the rapid installation of cyclobutane as a conformational restricted skeleton, greatly facilitating direct access to valuable drug molecules that require conformational restriction.
Subject terms: Synthetic chemistry methodology, Homogeneous catalysis
Functionalized cyclobutanes are prized motifs in pharmaceutically active molecules, but due to the ring strain involved, their syntheses have been associated with lots of challenges. Here the authors present a methodology to transform cyclobutanols, which are relatively easily prepared, to cyclobutanecarboxamides via palladium catalysis.
Introduction
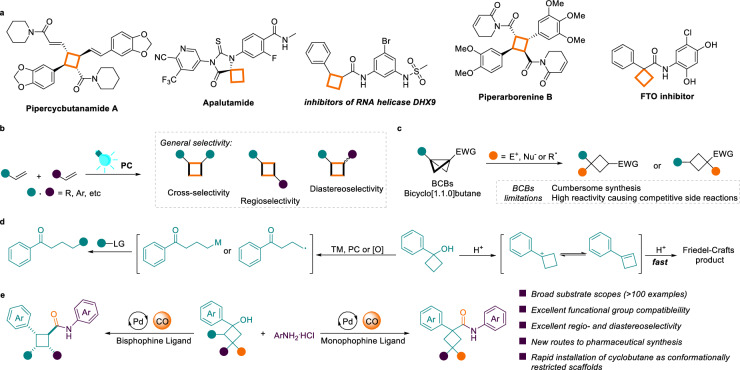
Cyclobutanecarboxamides exist in a variety of biologically active molecules, which are common motifs found in both natural products and pharmaceutical molecules (Fig. 1a)1–7. This can be attributed to distinctive nonplanarity, sp3-enrichment and relatively rigid three-dimensional structure of cyclobutanes8,9, which enable them to perform as conformationally restricted scaffolds10–13, as well as being among the most favored backbones in the concept of “escape from flatland”14–16. Photocatalytic [2 + 2] cycloaddition of alkenes is one of the most straightforward strategies for the synthesis of cyclobutanes, with numerous elegant [2 + 2] approaches having been developed (Fig. 1b)17–23. Regrettably, the vast majority of [2 + 2] photocycloadditions depend on alkenes with activating groups and controlling the selectivity of intermolecular cycloaddition reactions proves challenging24–27. In this context, implementing direct functionalization of pre-incorporated cyclobutanes emerges as a fascinating strategy. Bicyclo[1.1.0]butanes (BCBs) are a category of molecules with high strain energy that are currently heavily favored for their cleavage and strain release reactions (Fig. 1c)28–34. However, several limitations remain, such as cumbersome synthesis of BCBs and increased complexity of the reaction due to its high reactivity35–38.
Fig. 1. Reaction design and developments.
a Representative biologically active cyclobutanecarboxamides. b Photochemical [2 + 2] cycloaddition. c Direct functionalization of bicyclo[1.1.0]butanes (BCBs). d Conventional reactions of cyclobutanols. e This work: ligand-controlled regioselectivity aminocarbonylation of cyclobutanols.
Cyclobutanols are easily prepared and crucial synthetic building blocks39,40. However, the high ring strain of cyclobutanols renders them susceptible to ring-opening reactions in the presence of transition metals, photocatalysts, or radical initiators (Fig. 1d)41–48. In addition, intermolecular Friedel-Crafts reactions occur rapidly under acid-catalyzed conditions49. Therefore, direct functionalization of cyclobutanols while retaining the cyclobutane core is still highly challenging.
Transition metal-catalyzed carbonylation reactions are among the most efficient strategies for the installation of carbonyl and associated functional groups50–52. Besides synthesizing carbonylated compounds, achieving regioselectivity control to access different products from the same substrate is even more fascinating53–56. In recent years, various ligand-controlled regiodivergent carbonylations of alkenes57–65, alkynes66–68, allenes69, and imines70,71 have been developed. Despite these achievements, the direct access functionalized cyclobutanes through regiodivergent carbonylation of cyclobutanol has never been realized. With these considerations in mind, we envisioned the modulation of the ligand to modify the structural and electronic properties of the metallic complex, aiming for regioselective aminocarbonylation of cyclobutanols. Among the significances and challenges, the development of a suitable catalytic system to avoid ring breakage as well as to inhibit carbocation intermediate formation is the foundation for the success of this research. In this work, we present our study on palladium-catalyzed highly regioselective aminocarbonylation of cyclobutanols to afford 1,1- and 1,2-substituted cyclobutanecarboxamides (Fig. 1e). The regioselectivity of this aminocarbonylation of cyclobutanols can be tuned by choosing different ligands with good functional group compatibility.
Results
Reaction development
Our initial studies focused on the aminocarbonylation between cyclobutanol 1a and aniline hydrochloride 2a with palladium as the catalyst (Fig. 2). After screening various bisphosphine ligands, we were surprised to discover that variations in the bite angle of the ligands had a significant effect on the reaction. Carbonylation products 3a or 4a were not observed when the bite angle of the ligand was less than 104° (Fig. 2, entries 1–5). The first observation of amide 3a in 44% yield as well as trace amount of 4a when using Xantphos as the ligand with 111° bite angle (Fig. 2, entry 6). As the bite angle of the bisphosphine ligand increased, the amide 3a and 4a yields both increased significantly (Fig. 2, entries 7–8). In which 3a was obtained in 77% yield with 8:1 rr with Nixantphos as the ligand. Fortunately reducing the pressure was effective in decreasing the formation of 4a, and when the CO pressure was reduced to 6 bar and prolongation of time afforded 1,2-substituted cyclobutanecarboxamide 3a in 95% yield (90% isolated yield) with 43:1 rr and trans/cis > 20:1 (Fig. 2, entry 12, condition A). When the bite angle of the bisphosphine ligand reached 138°, a large amount of amide 4a was obtained (Fig. 2, entry 9). We speculated that this may be attributed to the larger bite angle of DBFphos and the ligand’s tendency to lean toward monophosphine ligands, which prevents the formation of the catalysis-active species P–Pd–P complex A (mechanism)72–76. Considering this, we examined a range of monophosphine ligands to increase the yield of amide 4a. The results indicated that L10 containing the electron-withdrawing trifluoromethyl group was the best ligand for this transformation and afforded the desired amide 4a in 91% yield with 18:1 rr. In addition, replacing Pd(TFA)2 with Pd(acac)2 could further increase 1,1-substituted cyclobutanecarboxamide 4a to 93% yield (92% isolated yield) with 23:1 rr (Fig. 2, entry 11, condition B). It’s worth to mention that several byproducts could be detected during the optimization process, including N-(1-phenylcyclobutyl)aniline, (1-chlorocyclobutyl)benzene, buta-1,3-dien-2-ylbenzene, and 2a’-phenyl-1’,2’,2a’,7a’-tetrahydrospiro[cyclobutane-1,7’-cyclobuta[a]indene].
Fig. 2. Optimization of the catalyst systems for aminocarbonylation.
Reaction conditions: 1a (0.3 mmol), 2a (0.2 mmol), Pd(TFA)2 (1 mol%), bisphosphine ligand (1.2 mol%) or monophosphine ligand (2.4 mol%), CO (20 bar), DCE (2.0 mL) at 110 °C. Entries 1–9: 12 h. Entries 10–12: 24 h. The yield of products and regioisomeric ratios (rr) were determined by GC with hexadecane as internal standard. [a] Condition B: 1a (0.3 mmol), 2a (0.2 mmol), Pd(acac)2 (1 mol%), (4-CF3C6H4)3P (2.4 mol%), CO (20 bar), DCE (2.0 mL), 24 h at 110 °C, isolated yield of 4a: 92%, 23:1 rr. [b] Condition A: 1a (0.3 mmol), 2a (0.2 mmol), Pd(TFA)2 (1 mol%), NIXantphos (1.2 mol%), CO (6 bar), DCE (2.0 mL), 24 h at 110 °C, isolated yield of 3a: 90%, 43:1 rr, trans/cis > 20:1.
Substrate scope
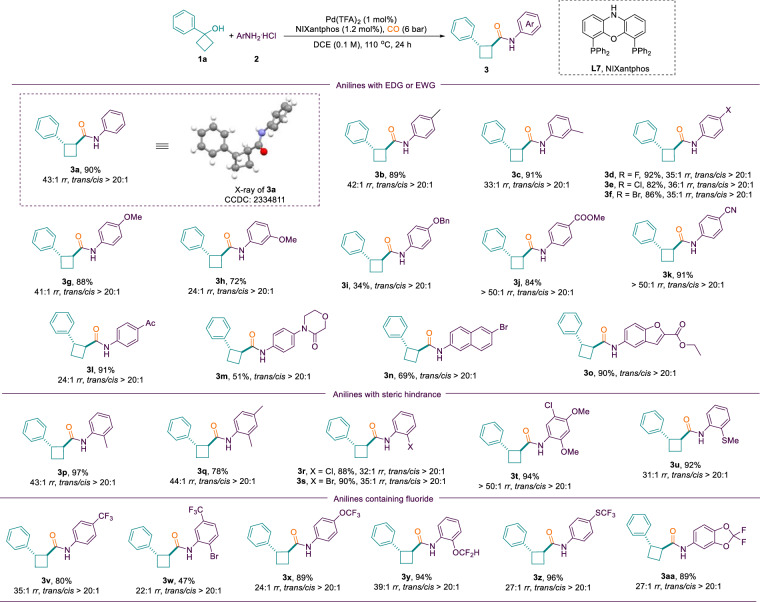
With the optimized reaction conditions in hand, the scope of this aminocarbonylation of cyclobutanols was explored using this protocol to afford 1,2-substituted cyclobutanecarboxamides 3. As shown in Fig. 3, the present method has excellent regioselectivity and diastereoselectivity, with both regioisomeric and diastereoisomeric ratios reaching >20:1. A number of anilines bearing electron withdrawing groups-including halogens (F, Cl, Br, 3d-3f, 3r, 3s), ester (3j), cyano (3k), acyl (3l), trifluoromethyl (3v), as well as electron donating groups such as alkoxy (3g-3l), thiomethyl (3u) can be successfully applied to the reaction and provided the corresponding 1,2-substituted cyclobutanecarboxamides in good to excellent yields (Fig. 3, 3a–3aa). In addition, anilines bearing amide (3m), naphthyl (3n) and benzofuranyl (3o) were well tolerated and afforded the corresponding compounds in moderate to high yields. Subsequently, to explore the effects of steric hindrance on the reaction, some ortho-substituted anilines were tested. To our delight, there were no detrimental effects observed in the presence of ortho-steric hindrance groups (3p-3u). Notably, the aniline with multiple functional groups, also obtained the desired product 3t in 94% yield with >50:1 rr and trans/cis > 20:1. Because of the increasing applications of fluorine-containing organic compounds in pharmaceuticals, agrochemicals, and advanced materials77,78, it was necessary to examine the stability of fluorine-containing molecules in this method. Fortunately, fluorine-containing functional groups such as trifluoromethyl (3v, 3w), trifluoromethoxy (3x), difluoromethyl (3y), trifluoromethylthio (3z), and fluorinated heterocycle (3aa) survived in the reaction conditions and afforded the corresponding amides in excellent yields with outstanding regioselectivities and diastereoselectivities. Despite the success of the anilines, subjecting aliphatic amine to identical reaction conditions yielded only trace amount of the desired product. We attribute that to the strong basicity of the aliphatic amines (pKb < 5), which inhibited the production of the key intermediate palladium hydride species57,79. Furthermore, an X-ray crystal structure of 3a (CCDC 2334811) confirmed the trans relationship between the Ar and amide groups.
Fig. 3. Anilines scope of aminocarbonylation toward 1,2-substituted cyclobutanecarboxamides.
Reaction conditions: 1a (0.3 mmol), 2a (0.2 mmol), Pd(TFA)2 (1 mol%), NIXantphos (1.2 mol%), CO (6 bar), DCE (2.0 mL), 24 h at 110 °C. Regioisomeric ratios (rr) were determined by GC analysis of the crude products. The trans/cis ratios were determined by 1H NMR.
Next, we studied the effect of the substituents in cyclobutanols on the syntheses of 1,2-substituted cyclobutanecarboxamides (Fig. 4). In terms of the employed substrates, the ortho-, meta-, and para-substitution pattern on the aromatic ring of the cyclobutanols were all effective, provided the corresponding products in good to excellent yields (3ab-3am). However, para-methoxy substituted cyclobutanol was observed to yield only 36% of product 3ai, as well as a significant amount of the self-coupling product from the Friedel-Crafts reaction49. This was due to the para-methoxy increasing the electron density of the benzene ring, resulting in the production of more electrophilic substitution product. The electron-withdrawing group CF3-substituted cyclobutanol 1al required the extra addition of TsOH to promote dehydration. In addition, several heterocycles such as benzofuran (3an), dibenzofuran (3ao), benzothiophene (3ap), dibenzothiophene (3aq), naphthalene (3ar), and fluorinated heterocycle (3as) were all tolerated in our protocol. However, as an example of alkyl substituted cyclobutanol, no desired product could be detected when 1-(3-phenylpropyl)cyclobutan-1-ol was tested under our standard conditions. Finally, the reactions with 1-(furan-2-yl)cyclobutan-1-ol, 1-(thiophen-2-yl)cyclobutan-1-ol, 1-phenylcyclopropan-1-ol, and 1-phenylcyclopentan-1-ol all failed with aniline.
Fig. 4. Cyclobutanols scope of aminocarbonylation toward 1,2-substituted cyclobutanecarboxamides.
Reaction conditions: 1a (0.3 mmol), 2a (0.2 mmol), Pd(TFA)2 (1 mol%), NIXantphos (1.2 mol%), CO (6 bar), DCE (2.0 mL), 24 h at 110 °C. Condition C: 1a (0.3 mmol), 2a (0.2 mmol), Pd(TFA)2 (3 mol%), NIXantphos (3.6 mol%), CO (6 bar), DCE (2.0 mL), 24 h at 110 °C. [a] 5 mol% TsOH. Regioisomeric ratios (rr) were determined by GC analysis of the crude products. The trans/cis ratios were determined by 1H NMR.
It’s worth mentioning that only small variations in the reaction conditions (condition C) enabled substrate 1at with trans-cis ratio of 2.9:1 to afford the desired product 3at in 95% yield with >20:1 dr. Encouraged by this result, we tested multi-substituted cyclobutanols with cis-trans isomerism. The length of the alkyl chain (3at-3aw) and the chlorine attached to the alkyl group (3aw) had no significant effect on the reaction outcome. Using 1,1,2-substituted cyclobutanol as substrate, the product 3ax was obtained with 2.8:1 dr. Interestingly, the substrates containing bridged hydrocarbons were also applicable to the method, and the corresponding products were obtained in 64–71% yields (3ay-3ba). The benzoyl group can also be introduced into the product from the corresponding starting material, albeit in relatively low yield (3bb).
Our next goal is to evaluate the scope of 1,1-substituted cyclobutanecarboxamides 4. As shown in Fig. 5, cyclobutanol 1a was used to test the scope of anilines. We were pleased to find that anilines with different functional groups such as halogens (4e-4g), methoxy (4h), cyano (4i), thiomethyl (4k), acyl (4l), ester (4m), naphthyl (4n), benzofuranyl (4o) and some fluorine-containing molecules (4p-4s) all worked well to afford the desired products in moderate to excellent yields with excellent regioisomeric ratios. The excellent functional group compatibility demonstrated the applicability of the protocol. Subsequently, a variety of cyclobutanols were explored under the condition B and were transformed into the corresponding products in good yields with excellent regioselectivity (4t-4ai). The ortho-substituted cyclobutanols were restricted, for instance the product 4v was obtained only in 19% yield with 2:1 rr. We speculate that the result was mainly attributed to the steric hindrance effect of the substrates. Furthermore, 3,3-substituted cyclobutanol was proven to be suitable partner in the reaction and the product 4ai was afforded in 73% yield with 2.5:1 cis/trans.
Fig. 5. Scope of aminocarbonylation toward 1,1-substituted cyclobutanecarboxamides.
Reaction conditions: 1a (0.3 mmol), 2a (0.2 mmol), Pd(acac)2 (1 mol%), (4-CF3C6H4)3P (2.4 mol%), CO (20 bar), DCE (2.0 mL), 24 h at 110 °C. Regioisomeric ratios (rr) were determined by GC analysis of the crude products.
Synthetic applications
To further demonstrate the robustness of the approach, 1a and 2c were available for reactions in the larger scale (3 mmol) under the condition A and condition B, afforded the desired products 3c and 4c without loss of efficiency and selectivity (Fig. 6a). Subsequently, to emphasize the applicability of this palladium-catalyzed aminocarbonylation, we performed late-stage functionalization of biorelevant molecules, such as ibuprofen, ioxoprofen, gemfibrozil, L-menthol, and vitamin E. These molecules were examined to afford the corresponding 1,2-substituted cyclobutanecarboxamides (5–9) or 1,1-substituted cyclobutanecarboxamides (10–14) in high yields (average 88% yield) and excellent selectivity (Fig. 6b). Next, we proceeded to achieve the total synthesis of pharmaceutically active molecules to demonstrate the practical value of the methodology (Fig. 6c). As shown in Fig. 6c–i, 1,1-substituted cyclobutanecarboxamide 16 (N-CDPCB) is recognized as an inhibitor of the fat mass and obesity associated protein (FTO)80. In comparison to the reported synthetic route, the new protocol required merely two steps of aminocarbonylation and methoxy deprotection to gain the product 16 (N-CDPCB), with an overall yield of 71%. The pharmaceutically active molecule 18, manufactured by accent therapeutics, is an inhibitor of the RNA helicase DHX9 and could be used in the treatment of cancer81. It is pleasing to observe that the 1,2-substituted cyclobutanecarboxamide 18 was obtained in 81% yield in only one step under condition C (Fig. 6c–ii). In addition to cyclobutanecarboxamides, this protocol also allows access to several molecules containing cyclobutane units. For instance, cannabinoid derivative 23 used for neuroprotection, treating inflammation, reducing pain, and addressing other central nervous system (CNS) disorders, can be obtained through subsequent transformations82. It is should be noted that through this synthetic route only a single trans-isomer was obtained, which has not been reported previously. On the other hand, conformational restriction is among the important strategies in pharmaceutical development. There are numerous instances where the introduction of a cyclobutane-restricted conformation has been shown to enhance pharmaceutical activity effectively83–87. As shown in Fig. 6d, a number of pharmaceutically active molecules such as GPR132-A-888 (G protein-coupled receptor 132, modulation of immune function), DSC-79-BIS89 (enzyme monoacylglycerol lipase-MAGL inhibitors, inhibiting cell viability of tumor cell lines) and MAZ188790 (inhibitors of glycosylphosphatidylinositol GPI-anchor biosynthesis, inhibiting fungal growth and treating fungal infections) could be readily introduced into cyclobutane using this method, yielding 1,2-substituted cyclobutanecarboxamides with a single stabilized trans-conformation and excellent yields. This protocol offers significant convenience for directly accessing valuable pharmaceutical molecules that require conformational restriction.
Fig. 6. Synthetic applications of the aminocarbonylation.
a Large-scale synthesis. b Applications in bioactive molecules. c Total synthesis of pharmacologically active molecules. d Applications of cyclobutane as conformationally restricted scaffolds.
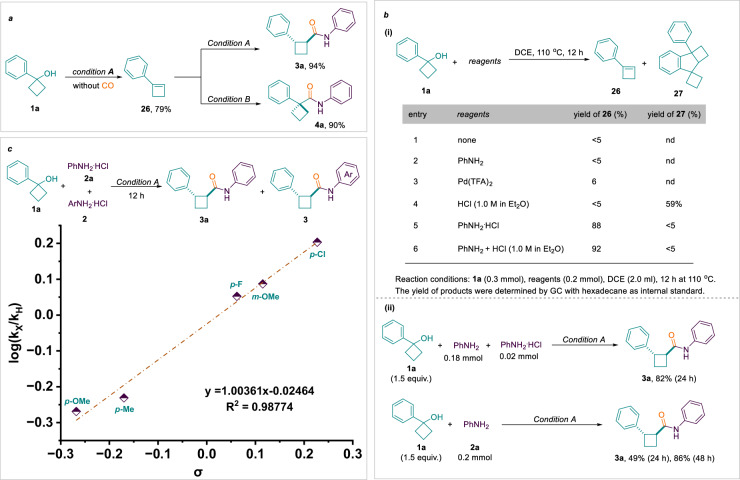
To gain a greater understanding of the mechanism of this regiodivergent carbonylation, a series of control experiments were conducted. We obtained cyclobutene 26 in 79% isolated yield without CO (Fig. 7a). Afterwards the cyclobutene 26 provides the corresponding amides 3a or 4a under conditions A or B, respectively, which confirms that cyclobutene 26 is the critical intermediate for this reaction. Controlled experiments indicate the significance of HCl in the dehydration of cyclobutanol (Fig. 7b–i, entries 1–3). However, the presence of significant amounts of H+ in the system contributes to the formation of the by-product 27 from the Friedel-Crafts reaction (Fig. 7b–i, entry 4). Fortunately, the self-coupling product 27 from the Friedel-Crafts reaction almost disappeared after the addition of aniline as well as cyclobutene 26 was preserved abundantly (Fig. 7b–i, entries 5 and 6). We speculate that aniline plays a slow-release role for HCl in which it reduces the concentration of H+ in the reaction and Friedel-Crafts reaction occurs with difficulty. In addition, when a catalytic amount of HCl was added, the amide 3 was still provided in 82% yield (Fig. 7b–ii). Surprisingly, the replacement of aniline hydrochloride with aniline also afforded amides, albeit in low yields, which continued to increase with prolonged reaction times. In addition, investigating the effect of aniline substituents on the reaction rate, the plotted Hammett showed a positive linear correlation, suggesting that ammonolysis is probably the rate-determining step of route A.
Fig. 7. Mechanistic investigations.
a Intermediate verification experiment. b Control experiments for the dehydration of cyclobutanol. c Rate-determining step verification experiment - Hammett plot.
Based on the above results and previous mechanistic studies of other carbonylation reactions59,64,91, a possible reaction mechanism has been suggested (Fig. 8). Initially, the active Pd–H complex A and D is formed from palladium salt with acid or H2O. Then, anti-Markovnikov or Markovnikov addition of the Pd–H complex to the cyclobutene formed after acid or high-temperature dehydration produces the corresponding 1,2- or 1,1-substituted alkyl-palladium intermediate B or E. Intermediate B or E could be converted to acyl-palladium complex C or F through coordination and insertion of CO. Finally, the aniline attacks the acyl-palladium complex to provide the desired 1,1- and 1,2-substituted cyclobutanecarboxamide and regenerate the Pd–H complex for the next catalytic cycle.
Fig. 8. Proposed mechanism.
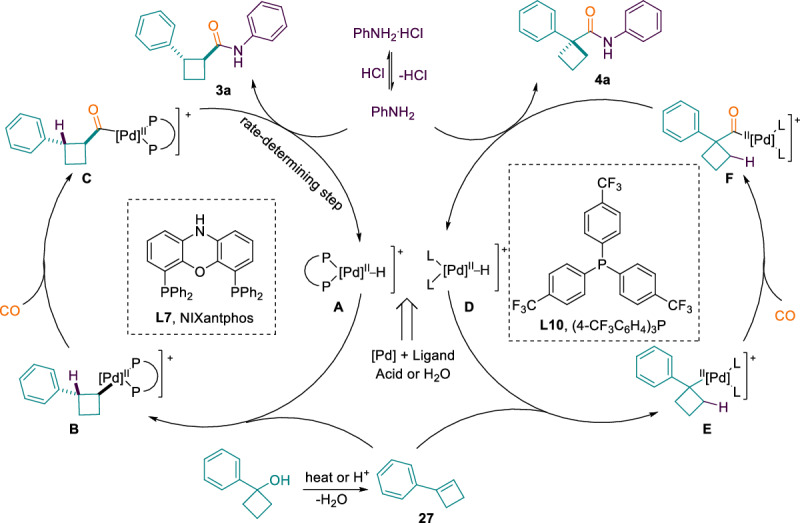
Reaction pathways for the two types of products.
Discussion
In summary, we have developed a ligand-controlled regiodivergent aminocarbonylation of cyclobutanols. Ligand modulation allows for the straightforward production of a variety of desired 1,1- or 1,2-substituted cyclobutanecarboxamides with excellent regioselectivity. The protocol demonstrates wide substrate scope, high regio- and stereoselectivity, as well as outstanding performance in scale-up reactions. In addition, the rapid installation of cyclobutane offers significant advantages for pharmaceutical production and modifications. Further investigation of this procedure for preparing cyclobutene-related medicals and natural products are proceeding in our group.
Methods
General procedure I: regioselective amino-carbonylation of cyclobutanols toward 1,2-substituted cyclobutanecarboxamides
Condition A
A 4 mL screw-cap vial was charged with Pd(TFA)2 (0.7 mg, 1 mol%), NiXantphos (1.4 mg, 1.2 mol%), ArNH2.HCl (0.2 mmol, 1.0 equiv.), cyclobutanols (0.3 mmol, 1.5 equiv.), and an oven-dried stirring bar. The vial was closed with a Teflon septum and cap and connected to the atmosphere via a needle. After DCE (1.0 mL) was added with a syringe under argon atmosphere, the vial was moved to an alloy plate and put into a Parr 4560 series autoclave (300 mL) under argon atmosphere. At room temperature, the autoclave was flushed with CO three times and charged with 6 atm CO. The autoclave was placed on a heating plate equipped with a magnetic stirrer and an aluminum block. The reaction mixture was heated to 110 °C for 24 h. After reaction, cooling to room temperature. The crude product was purified by silica gel chromatography (pentane/EA) to afford the corresponding product.
Condition C
A 4 mL screw-cap vial was charged with Pd(TFA)2 (2.1 mg, 3 mol%), NiXantphos (4.2 mg, 3.6 mol%), ArNH2.HCl (0.2 mmol, 1.0 equiv.), cyclobutanols (0.3 mmol, 1.5 equiv.), and an oven-dried stirring bar. The vial was closed with a Teflon septum and cap and connected to the atmosphere via a needle. After DCE (1.0 mL) was added with a syringe under argon atmosphere, the vial was moved to an alloy plate and put into a Parr 4560 series autoclave (300 mL) under argon atmosphere. At room temperature, the autoclave was flushed with CO three times and charged with 6 atm CO. The autoclave was placed on a heating plate equipped with a magnetic stirrer and an aluminum block. The reaction mixture was heated to 110 °C for 24 h. After reaction, cooling to room temperature. The crude product was purified by silica gel chromatography (pentane/EA) to afford the corresponding product.
General procedure II: regioselective amino-carbonylation of cyclobutanols toward 1,1-substituted cyclobutanecarboxamides
Condition B
A 4 mL screw-cap vial was charged with Pd(acac)2 (1.2 mg, 1 mol%), (4-CF3C6H4)3P (2.3 mg, 1.2 mol%), ArNH2.HCl (0.2 mmol, 1.0 equiv.), cyclobutanols (0.3 mmol, 1.5 equiv.), and an oven-dried stirring bar. The vial was closed with a Teflon septum and cap and connected to the atmosphere via a needle. After DCE (1.0 mL) was added with a syringe under argon atmosphere, the vial was moved to an alloy plate and put into a Parr 4560 series autoclave (300 mL) under argon atmosphere. At room temperature, the autoclave was flushed with CO three times and charged with 20 atm CO. The autoclave was placed on a heating plate equipped with a magnetic stirrer and an aluminum block. The reaction mixture was heated to 110 °C for 24 h. After reaction, cooling to room temperature. The crude product was purified by silica gel chromatography (pentane/EA) to afford the corresponding product.
Supplementary information
Acknowledgements
We appreciate the financial support provided by the National Key R&D Program of China (2023YFA1507500), the International Partnership Program of the Chinese Academy of Sciences (Grant No. 028GJHZ2023045FN).
Author contributions
X.F.W. conceived and directed the project. X.W.G. and Y.H.Z. performed experiments and prepared the supplementary information. X.F.W. and X.W.G. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Peer review
Peer review information
Nature Communications thanks Hequan Yao, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data supporting the findings of this study are available within this article and its Supplementary Information, which contains experimental details, characterization data, copies of NMR spectra for all products.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xing-Wei Gu, Yan-Hua Zhao.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-53571-0.
References
- 1.Dembitsky, V. M. Bioactive cyclobutane-containing alkaloids. J. Nat. Med.62, 1–33 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Fan, Y.-Y., Gao, X.-H. & Yue, J.-M. Attractive natural products with strained cyclopropane and/or cyclobutane ring systems. Sci. China. Chem.59, 1126–1141 (2016). [Google Scholar]
- 3.Hui, C., Wang, Z., Xie, Y. & Liu, J. Contemporary synthesis of bioactive cyclobutane natural products. Green Synth. Catal.4, 1–6 (2023). [Google Scholar]
- 4.Taylor, R. D., MacCoss, M. & Lawson, A. D. Rings in drugs. J. Med. Chem.57, 5845–5859 (2014). [DOI] [PubMed] [Google Scholar]
- 5.van der Kolk, M. R., Janssen, M., Rutjes, F. & Blanco-Ania, D. Cyclobutanes in small-molecule drug candidates. ChemMedChem17, e202200020 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang, M. & Lu, P. Catalytic approaches to assemble cyclobutane motifs in natural product synthesis. Org. Chem. Front.5, 254–259 (2018). [Google Scholar]
- 7.Yang, P., Jia, Q., Song, S. & Huang, X. [2 + 2]-Cycloaddition-derived cyclobutane natural products: structural diversity, sources, bioactivities, and biomimetic syntheses. Nat. Prod. Rep.40, 1094–1129 (2023). [DOI] [PubMed] [Google Scholar]
- 8.Bauer, M. R. et al. Put a ring on it: application of small aliphatic rings in medicinal chemistry. RSC Med. Chem.12, 448–471 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui, C., Liu, Y., Jiang, M. & Wu, P. Cyclobutane-containing scaffolds in bioactive small molecules. Trends Chem4, 677–681 (2022). [Google Scholar]
- 10.Komiskey, H. L. et al. Inhibition of synaptosomal uptake of norepinephrine and dopamine by conformationally restricted sympathomimetic amines. Eur. J. Pharmacol.52, 37–45 (1978). [DOI] [PubMed] [Google Scholar]
- 11.Martın-Vila, M. et al. Enantioselective synthetic approaches to cyclopropane and cyclobutane β-amino acids: synthesis and structural study of a conformationally constrained β-dipeptide. Tetrahedron: Asymmetry11, 3569–3584 (2000). [Google Scholar]
- 12.Wrobleski, M. L. et al. Cyclobutane derivatives as potent NK1 selective antagonists. Bioorg. Med. Chem. Lett.16, 3859–3863 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Chen, B. et al. Cyclobutane-bearing restricted anchoring residues enabled geometry-specific hydrocarbon peptide stapling. Chem. Sci.14, 11499–11506 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem.52, 6752–6756 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Marson, C. M. New and unusual scaffolds in medicinal chemistry. Chem. Soc. Rev.40, 5514–5533 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Cox, B., Booker-Milburn, K. I., Elliott, L. D., Robertson-Ralph, M. & Zdorichenko, V. Escaping from flatland: [2+2] photocycloaddition; conformationally constrained sp3-rich scaffolds for lead generation. ACS Med. Chem. Lett.10, 1512–1517 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poplata, S., Troster, A., Zou, Y. Q. & Bach, T. Recent advances in the synthesis of cyclobutanes by olefin [2+2] photocycloaddition reactions. Chem. Rev.116, 9748–9815 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarkar, D., Bera, N. & Ghosh, S. [2+2] Photochemical cycloaddition in organic synthesis. Eur. J. Org. Chem.2020, 1310–1326 (2019). [Google Scholar]
- 19.Sicignano, M., Rodriguez, R. I. & Aleman, J. Recent visible light and metal free strategies in [2+2] and [4+2] photocycloadditions. Eur. J. Org. Chem.2021, 3303–3321 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ischay, M. A., Ament, M. S. & Yoon, T. P. Crossed Intermolecular [2+2] cycloaddition of styrenes by visible light photocatalysis. Chem. Sci.3, 2807–2811 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleinmans, R. et al. Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer. Nature605, 477–482 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Liu, Y., Ni, D. & Brown, M. K. Boronic ester enabled [2+2]-cycloadditions by temporary coordination: synthesis of artochamin J and piperarborenine B. J. Am. Chem. Soc.144, 18790–18796 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansson, C. M. F. & Burns, N. Z. Aqueous amine-tolerant [2+2] photocycloadditions of unactivated olefins. J. Am. Chem. Soc.144, 19689–19694 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Li, R. et al. Photocatalytic regioselective and stereoselective [2+2] cycloaddition of styrene derivatives using a heterogeneous organic photocatalyst. ACS Catal7, 3097–3101 (2017). [Google Scholar]
- 25.Jiang, Y., Wang, C., Rogers, C. R., Kodaimati, M. S. & Weiss, E. A. Regio- and diastereoselective intermolecular [2+2] cycloadditions photocatalysed by quantum dots. Nat. Chem.11, 1034–1040 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray, P. R. D. et al. Intermolecular crossed [2+2] cycloaddition promoted by visible-light triplet photosensitization: expedient access to polysubstituted 2-oxaspiro[3.3]heptanes. J. Am. Chem. Soc.143, 4055–4063 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Guo, J. et al. Visible light-mediated intermolecular crossed [2+2] cycloadditions using a MOF-supported copper triplet photosensitizer. Nat. Catal.7, 307–320 (2024). [Google Scholar]
- 28.Pramanik, M. M. D., Qian, H., Xiao, W.-J. & Chen, J.-R. Photoinduced strategies towards strained molecules. Org. Chem. Front.7, 2531–2537 (2020). [Google Scholar]
- 29.Turkowska, J., Durka, J. & Gryko, D. Strain release—an old tool for new transformations. Chem. Commun.56, 5718–5734 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Golfmann, M. & Walker, J. C. L. Bicyclobutanes as unusual building blocks for complexity generation in organic synthesis. Commun. Chem.6, 9 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silvi, M. & Aggarwal, V. K. Radical addition to strained σ-bonds enables the stereocontrolled synthesis of cyclobutyl boronic esters. J. Am. Chem. Soc.141, 9511–9515 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Ociepa, M., Wierzba, A. J., Turkowska, J. & Gryko, D. Polarity-reversal strategy for the functionalization of electrophilic strained molecules via light-driven cobalt catalysis. J. Am. Chem. Soc.142, 5355–5361 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Ernouf, G., Chirkin, E., Rhyman, L., Ramasami, P. & Cintrat, J. C. Photochemical strain-release-driven cyclobutylation of C(sp3)-centered radicals. Angew. Chem. Int. Ed.59, 2618–2622 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Dutta, S. et al. Double strain-release [2π+2σ]-photocycloaddition. J. Am. Chem. Soc.146, 5232–5241 (2024). [DOI] [PubMed] [Google Scholar]
- 35.Kelly, C. B., Milligan, J. A., Tilley, L. J. & Sodano, T. M. Bicyclobutanes: from curiosities to versatile reagents and covalent warheads. Chem. Sci.13, 11721–11737 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo, L., Noble, A. & Aggarwal, V. K. α-Selective ring-opening reactions of bicyclo[1.1.0]butyl boronic ester with nucleophiles. Angew. Chem. Int. Ed.60, 212–216 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Xiao, Y. et al. Photochemical α-selective radical ring-opening reactions of 1,3-disubstituted acyl bicyclobutanes with alkyl halides: modular access to functionalized cyclobutenes. Chem. Sci.14, 13060–13066 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dasgupta, A. et al. Stereoselective Alder-Ene reactions of bicyclo[1.1.0]butanes: facile synthesis of cyclopropyl- and aryl-substituted cyclobutenes. J. Am. Chem. Soc.146, 1196–1203 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu, X. & Zhu, C. Recent advances in alkoxy radical-promoted C-C and C-H bond functionalization starting from free alcohols. Chem. Commun.55, 9747–9756 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Han, W. J., Zhan, J. L., Yang, F. L. & Liu, L. Ring-opening functionalization/cyclization reactions of cycloalkanols under transition-metal-free conditions. Eur. J. Org. Chem. 27, e202301215 (2024).
- 41.Chang, L., An, Q., Duan, L., Feng, K. & Zuo, Z. Alkoxy radicals see the light: new paradigms of photochemical synthesis. Chem. Rev.122, 2429–2486 (2022). [DOI] [PubMed] [Google Scholar]
- 42.Murakami, M. & Ishida, N. Cleavage of carbon-carbon σ‑bonds of four-membered rings. Chem. Rev.121, 264–299 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Jha, N., Mishra, P. & Kapur, M. Strained cycloalkanols in C–C bond formation reactions: a boon in disguise! Org. Chem. Front.10, 4941–4971 (2023). [Google Scholar]
- 44.Nishimura, T. & Uemura, S. Palladium-catalyzed arylation of tert-cyclobutanols with aryl bromide via C-C bond cleavage: new approach for the γ-arylated ketones. J. Am. Chem. Soc.121, 11010–11011 (1999). [Google Scholar]
- 45.Ren, R., Wu, Z., Xu, Y. & Zhu, C. C-C bond-forming strategy by manganese-catalyzed oxidative ring-opening cyanation and ethynylation of cyclobutanol derivatives. Angew. Chem. Int. Ed.55, 2866–2869 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Seiser, T. & Cramer, N. Rhodium-catalyzed C-C bond cleavage: construction of acyclic methyl substituted quaternary stereogenic centers. J. Am. Chem. Soc.132, 5340–5341 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Yang, Z. et al. Electrophotochemical Ce-catalyzed ring-opening functionalization of cycloalkanols under redox-neutral conditions: scope and mechanism. J. Am. Chem. Soc.144, 13895–13902 (2022). [DOI] [PubMed] [Google Scholar]
- 48.Zhao, H., Fan, X., Yu, J. & Zhu, C. Silver-catalyzed ring-opening strategy for the synthesis of β- and γ‑fluorinated ketones. J. Am. Chem. Soc.137, 3490–3493 (2015). [DOI] [PubMed] [Google Scholar]
- 49.An, Z., Liu, Y., Sun, Y. & Yan, R. TFA-catalyzed [3+2] spiroannulation of cyclobutanols: a route to spiro[cyclobuta[a]indene-7,1’-cyclobutane] skeletons. Chem. Asian J.15, 3812–3815 (2020). [DOI] [PubMed] [Google Scholar]
- 50.Matsubara, H., Kawamoto, T., Fukuyama, T. & Ryu, I. Applications of radical carbonylation and amine addition chemistry: 1,4-hydrogen transfer of 1-hydroxylallyl radicals. Acc. Chem. Res.51, 2023–2035 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Peng, J. B., Wu, F. P. & Wu, X. F. First-row transition-metal-catalyzed carbonylative transformations of carbon electrophiles. Chem. Rev.119, 2090–2127 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Wu, X. F., Neumann, H. & Beller, M. Synthesis of heterocycles via palladium-catalyzed carbonylations. Chem. Rev.113, 1–35 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Boogaerts, I. I., White, D. F. & Cole-Hamilton, D. J. High chemo and regioselective formation of alcohols from the hydrocarbonylation of alkenes using cooperative ligand effects. Chem. Commun.46, 2194–2196 (2010). [DOI] [PubMed] [Google Scholar]
- 54.Peng, J. B. & Wu, X. F. Ligand- and solvent-controlled regio- and chemodivergent carbonylative reactions. Angew. Chem. Int. Ed.57, 1152–1160 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Yang, J. et al. Direct synthesis of adipic acid esters via palladiumcatalyzed carbonylation of 1,3-dienes. Science366, 1514–1517 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Zhang, Y., Torker, S., Sigrist, M., Bregovic, N. & Dydio, P. Binuclear Pd(I)-Pd(I) catalysis assisted by iodide ligands for selective hydroformylation of alkenes and alkynes. J. Am. Chem. Soc.142, 18251–18265 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Zhang, G., Gao, B. & Huang, H. Palladium-catalyzed hydroaminocarbonylation of alkenes with amines: a strategy to overcome the basicity barrier imparted by aliphatic amines. Angew. Chem. Int. Ed.54, 7657–7661 (2015). [DOI] [PubMed] [Google Scholar]
- 58.Williams, D. B., Shaw, M. L., Green, M. J. & Holzapfel, C. W. Aluminum triflate as a highly active and efficient nonprotic cocatalyst in the palladium-catalyzed methoxycarbonylation reaction. Angew. Chem. Int. Ed.47, 560–563 (2008). [DOI] [PubMed] [Google Scholar]
- 59.Xu, T., Sha, F. & Alper, H. Highly ligand-controlled regioselective Pd-catalyzed aminocarbonylation of styrenes with aminophenols. J. Am. Chem. Soc.138, 6629–6635 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Gao, B., Zhang, G., Zhou, X. & Huang, H. Palladium-catalyzed regiodivergent hydroaminocarbonylation of alkenes to primary amides with ammonium chloride. Chem. Sci.9, 380–386 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dong, K. et al. Palladium-catalyzed carbonylation of sec- and tert-alcohols. Angew. Chem. Int. Ed.56, 6203–6207 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Wu, F. et al. Palladium-catalyzed regiodivergent hydrochlorocarbonylation of alkenes for formation of acid chlorides. Nat. Commun.14, 3167 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao, Y. H. et al. Asymmetric Markovnikov hydroaminocarbonylation of alkenes enabled by palladium-monodentate phosphoramidite catalysis. J. Am. Chem. Soc.143, 85–91 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Yang, H. Y., Yao, Y. H., Chen, M., Ren, Z. H. & Guan, Z. H. Palladium-catalyzed Markovnikov hydroaminocarbonylation of 1,1-disubstituted and 1,1,2-trisubstituted alkenes for formation of amides with quaternary carbon. J. Am. Chem. Soc.143, 7298–7305 (2021). [DOI] [PubMed] [Google Scholar]
- 65.Yang, H.-Y., Lin, L.-Q., Li, N.-Q., Ren, Z.-H. & Guan, Z.-H. Palladium-catalyzed 3,4-hydroaminocarbonylation of conjugated dienes for formation of β,γ-unsaturated amides. Sci. China Chem.66, 1474–1481 (2023). [Google Scholar]
- 66.Sha, F. & Alper, H. Ligand- and additive-controlled Pd-catalyzed aminocarbonylation of alkynes with aminophenols: highly chemo- and regioselective synthesis of α,β-unsaturated amides. ACS Catal7, 2220–2229 (2017). [Google Scholar]
- 67.Liu, J. et al. Tuning the selectivity of palladium catalysts for hydroformylation and semihydrogenation of alkynes: experimental and mechanistic studies. ACS Catal10, 12167–12181 (2020). [Google Scholar]
- 68.Ai, H. J., Lu, W. & Wu, X. F. Ligand-controlled regiodivergent thiocarbonylation of alkynes toward linear and branched α,β-unsaturated thioesters. Angew. Chem. Int. Ed.60, 17178–17184 (2021). [DOI] [PubMed] [Google Scholar]
- 69.Liu, J., Liu, Q., Franke, R., Jackstell, R. & Beller, M. Ligand-controlled palladium-catalyzed alkoxycarbonylation of allenes: regioselective synthesis of α,β- and β,γ-unsaturated esters. J. Am. Chem. Soc.137, 8556–8563 (2015). [DOI] [PubMed] [Google Scholar]
- 70.Hoshimoto, Y., Ohata, T., Sasaoka, Y., Ohashi, M. & Ogoshi, S. Nickel(0)-catalyzed [2+2 +1] carbonylative cycloaddition of imines and alkynes or norbornene leading to γ-lactams. J. Am. Chem. Soc.136, 15877–15880 (2014). [DOI] [PubMed] [Google Scholar]
- 71.Wu, F. P. & Wu, X. F. Ligand-controlled copper-catalyzed regiodivergent carbonylative synthesis of α-amino ketones and alpha-boryl amides from imines and alkyl iodides. Angew. Chem. Int. Ed.60, 695–700 (2021). [DOI] [PubMed] [Google Scholar]
- 72.Kranenburg, M., Kamer, P. C. J. & van Leeuwen, P. W. N. M. The effect of the bite angle of diphosphane ligands on activity and selectivity in palladium-catalyzed cross-coupling. Eur. J. Inorg. Chem.1998, 155–157 (1998). [Google Scholar]
- 73.Leeuwen, P., Kamer, P. & Reek, J. The bite angle makes the catalyst. Pure Appl. Chem.71, 1443–1452 (1999). [Google Scholar]
- 74.Kamer, P., Leeuwen, P. & Reek, J. Wide bite angle diphosphines: xantphos ligands in transition metal complexes and catalysis. Acc. Chem. Res.34, 895–904 (2001). [DOI] [PubMed] [Google Scholar]
- 75.Birkholz, M. N., Freixa, Z. & van Leeuwen, P. W. Bite angle effects of diphosphines in C-C and C-X bond forming cross coupling reactions. Chem. Soc. Rev.38, 1099–1118 (2009). [DOI] [PubMed] [Google Scholar]
- 76.van Leeuwen, P. W. N. M. & Kamer, P. C. J. Featuring xantphos. Catal. Sci. Technol.8, 26–113 (2018). [Google Scholar]
- 77.Purser, S., Moore, P. R., Swallow, S. & Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev.37, 320–330 (2008). [DOI] [PubMed] [Google Scholar]
- 78.Berger, R., Resnati, G., Metrangolo, P., Weber, E. & Hulliger, J. Organic fluorine compounds: a great opportunity for enhanced materials properties. Chem. Soc. Rev.40, 3496–3508 (2011). [DOI] [PubMed] [Google Scholar]
- 79.Liu, J. et al. Selective palladium-catalyzed aminocarbonylation of olefins to branched amides. Angew. Chem. Int. Ed.55, 13544–13548 (2016). [DOI] [PubMed] [Google Scholar]
- 80.He, W. et al. Identification of a novel small-molecule binding site of the fat mass and obesity associated protein (FTO). J. Med. Chem.58, 7341–7348 (2015). [DOI] [PubMed] [Google Scholar]
- 81.Daniels, M. H. et al. Inhibitors of RNA Helicase DHX9 and Uses Thereof. WO2023/154519A1 (2023).
- 82.Jagtap, P., Shoken, D., Avidanshlomovich, S. & Salzman, A. L. Cannabinoid Derivatives and Conjugates and Uses Thereof. WO2019/159168A1 (2019).
- 83.Allan, R. D. et al. Cyclobutane analogs of GABA. Neurochemical Res.5, 393–400 (1980). [DOI] [PubMed] [Google Scholar]
- 84.Reddy, V. K. et al. Conformationally restricted analogues of 1N,12N-bisethylspermine: synthesis and growth inhibitory effects on human tumor cell lines. J. Med. Chem.41, 4723–4732 (1998). [DOI] [PubMed] [Google Scholar]
- 85.Fang, Z., Song, Y., Zhan, P., Zhang, Q. & Liu, X. Conformational restriction: an effective tactic in ‘follow-on’-based drug discovery. Future Med. Chem.6, 885–901 (2014). [DOI] [PubMed] [Google Scholar]
- 86.Awada, H. et al. Practical syntheses of both enantiomers of the conformationally restricted GABA analogue cis-(2-aminocyclobutyl)acetic acid. Eur. J. Org. Chem.2014, 7148–7155 (2014). [Google Scholar]
- 87.Alibes, R. et al. Synthesis and conformational analysis of new cyclobutane-fused nucleosides. Org. Lett.8, 491–494 (2006). [DOI] [PubMed] [Google Scholar]
- 88.Jiao, N. et al. Preparation of Carboxylic Acid Or Carboxylate Compounds as GPCR Regulators. CN116903562A (2023).
- 89.Granchi, C. et al. Preparation of Fluorinated Hydroxybiphenyl Amides as Monoacylglycerol Lipase (MAGL) Inhibitors for the Treatment and Prevention of Diseases. EP3889132A1 (2021).
- 90.Mclellan, C., Mazitschek, R., Whitesell, L. & Lindquist, S. L. Compounds for Treating Infectious Diseases. WO2013/192517A2 (2013).
- 91.Ge, Y. et al. Synthesis of non-equivalent diamides and amido-esters via Pd-catalysed carbonylation. Nat. Synth.3, 202–213 (2023). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within this article and its Supplementary Information, which contains experimental details, characterization data, copies of NMR spectra for all products.