Abstract
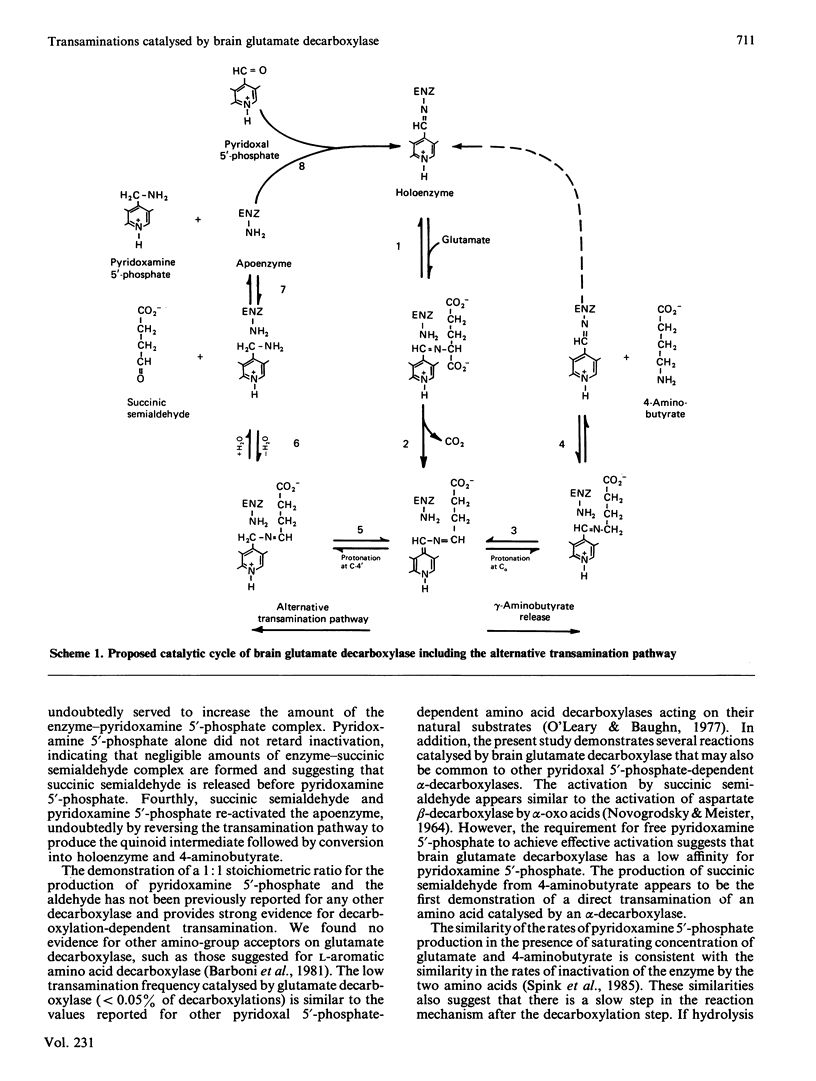
In addition to normal decarboxylation of glutamate to 4-aminobutyrate, glutamate decarboxylase from pig brain was shown to catalyse decarboxylation-dependent transamination of L-glutamate and direct transamination of 4-aminobutyrate with pyridoxal 5'-phosphate to yield succinic semialdehyde and pyridoxamine 5'-phosphate in a 1:1 stoichiometric ratio. Both reactions result in conversion of holoenzyme into apoenzyme. With glutamate as substrate the rates of transamination differed markedly among the three forms of the enzyme (0.008, 0.012 and 0.029% of the rate of 4-aminobutyrate production by the alpha-, beta- and gamma-forms at pH 7.2) and accounted for the differences among the forms in rates of inactivation by glutamate and 4-aminobutyrate. Rates of transamination were maximal at about pH 8 and varied in parallel with the rate constants for inactivation from pH 6.5 to 8.0. Rates of transamination of glutamate and 4-aminobutyrate were similar, suggesting that the decarboxylation step is not entirely rate-limiting in the normal mechanism. The transamination was reversible, and apoenzyme could be reconstituted to holoenzyme by reverse transamination with succinic semialdehyde and pyridoxamine 5'-phosphate. As a major route of apoenzyme formation, the transamination reaction appears to be physiologically significant and could account for the high proportion of apoenzyme in brain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barboni E., Voltattorni C. B., D'Erme M., Fiori A., Minelli A., Rosei M. A., Turano C. An abnormal reaction occurring in the presence of L-aromatic aminoacid decarboxylase. Biochem Biophys Res Commun. 1981 Mar 31;99(2):576–583. doi: 10.1016/0006-291x(81)91784-8. [DOI] [PubMed] [Google Scholar]
- Cash C., Ciesielski L., Maitre M., Mandel P. Purification and properties of rat brain succinic semialdehyde dehydrogenase. Biochimie. 1977;59(3):257–268. doi: 10.1016/s0300-9084(77)80142-9. [DOI] [PubMed] [Google Scholar]
- Hill D. W., Walters F. H., Wilson T. D., Stuart J. D. High performance liquid chromatographic determination of amino acids in the picomole range. Anal Chem. 1979 Jul;51(8):1338–1341. doi: 10.1021/ac50044a055. [DOI] [PubMed] [Google Scholar]
- Meeley M. P., Martin D. L. Inactivation of brain glutamate decarboxylase and the effects of adenosine 5'-triphosphate and inorganic phosphate. Cell Mol Neurobiol. 1983 Mar;3(1):39–54. doi: 10.1007/BF00734997. [DOI] [PubMed] [Google Scholar]
- Miller L. P., Martin D. L., Mazumder A., Walters J. R. Studies on the regulation of GABA synthesis: substrate-promoted dissociation of pyridoxal-5'-phosphate from GAD. J Neurochem. 1978 Feb;30(2):361–369. doi: 10.1111/j.1471-4159.1978.tb06538.x. [DOI] [PubMed] [Google Scholar]
- NOVOGRODSKY A., MEISTER A. CONTROL OF ASPARTATE BETA-DECARBOXYLASE ACTIVITY BY TRANSAMINATION. J Biol Chem. 1964 Mar;239:879–888. [PubMed] [Google Scholar]
- O'Leary M. H., Baughn R. L. Decarboxylation-dependent transamination catalyzed by mammalian 3,4-dihydroxyphenylalanine decarboxylase. J Biol Chem. 1977 Oct 25;252(20):7168–7173. [PubMed] [Google Scholar]
- O'Leary M. H., Herreid R. M. Mechanism of inactivation of ornithine decarboxylase by alpha-methylornithine. Biochemistry. 1978 Mar 21;17(6):1010–1014. doi: 10.1021/bi00599a011. [DOI] [PubMed] [Google Scholar]
- O'Leary M. H., Piazza G. J. Specificity in enzymatic decarboxylation. J Am Chem Soc. 1978 Jan 18;100(2):632–633. doi: 10.1021/ja00470a049. [DOI] [PubMed] [Google Scholar]
- Pitts F. N., Jr, Quick C. Brain succinate semialdehyde dehydrogenase. I. Assay and distribution. J Neurochem. 1965 Sep-Oct;12(9):893–900. doi: 10.1111/j.1471-4159.1965.tb10275.x. [DOI] [PubMed] [Google Scholar]
- Porter T. G., Martin D. L. Evidence for feedback regulation of glutamate decarboxylase by gamma-aminobutyric acid. J Neurochem. 1984 Nov;43(5):1464–1467. doi: 10.1111/j.1471-4159.1984.tb05409.x. [DOI] [PubMed] [Google Scholar]
- Spink D. C., Porter T. G., Wu S. J., Martin D. L. Characterization of three kinetically distinct forms of glutamate decarboxylase from pig brain. Biochem J. 1985 Nov 1;231(3):695–703. doi: 10.1042/bj2310695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voltattorni C. B., Minelli A., Turano C. Spectral properties of the coenzyme bound to DOPA decarboxylase from pig kidney. FEBS Lett. 1971 Oct 1;17(2):231–235. doi: 10.1016/0014-5793(71)80153-9. [DOI] [PubMed] [Google Scholar]
- Yamada H., O'Leary M. H. A solvent isotope effect probe for enzyme-mediated proton transfers. J Am Chem Soc. 1977 Mar 2;99(5):1660–1661. doi: 10.1021/ja00447a071. [DOI] [PubMed] [Google Scholar]


