Abstract
The present study was designed to assess the possible effects of platelet rich plasma (PRP) when used individually and in combination with nitazoxanide (NTZ) on experimental Cryptosporidium parvum (C. parvum) infection. It was conducted on 100 male albino mice, laboratory bred in Theodore Bilharz Research Institute. Starting from the 7th day post infection (p.i), therapeutics were given to immunosuppressed infected mice, which were divided as follows; oral NTZ treated group (0.2 mg/g/day for 6 consecutive days), six-PRP-treated groups (0.5 μl/g/week) to be administered intravenously (IV) in 1st, 2nd, 3rd week as PRP alone in (3 groups) and combined with oral NTZ (0.2 mg/g/day for 6 consecutive days) in (3 groups). Parasitological, histopathological and immunohistochemical assessments of therapeutics under study were done. Fecal pellets collected from groups at different intervals were stained using modified Ziehl–Neelsen and examined under microscope. Among PRP-treated groups, the highest significant percentage of oocyst reduction (89.96%) was observed in the group received 3 doses of PRP in combination with NTZ on the 35th day post infection. Likewise, the histopathological examination of small intestinal tissue sections showed improvement in villous architecture with mild to moderate stunting and moderate inflammatory infiltrates in lamina propria. Immunohistochemical staining of small intestinal tissue sections showed moderate increase in the expression of TGF-β1. Therefore, PRP can be a novel strategy in the treatment of cryptosporidiosis particularly when combined with NTZ.
Keywords: Cryptosporidium parvum, Platelet rich plasma, Nitazoxanide, Transforming growth factor-β1, Murine infection
Introduction
Cryptosporidium species are opportunistic intracellular parasites which are responsible for a disease called cryptosporidiosis with a worldwide distribution (Liu et al. 2020). The epithelium of the gastrointestinal tract is the normal habitat for Cryptosporidium spp. in humans and other vertebrate hosts. Infection with Cryptosporidium spp. damages the intestinal epithelium, interrupts absorption of different nutrients and affects barrier function of the intestine, resulting in mild-to-severe diarrhea (Bouzid et al. 2013; Khalil et al. 2018).
Acquiring infection with Cryptosporidium spp. and the severity of the disease depend on the host immune status. Both children with immature immune system and individuals with suppressed immunity experience severe life-threatening cryptosporidiosis (Bouzid et al. 2013).
Food and Drug Administration (FDA) has approved nitazoxanide as the only treatment for cryptosporidiosis. Unfortunately, it has weaker effect in malnourished children, minimal or no effect in patients with acquired immuno-deficiency syndrome (AIDs) and cannot be used in patients below one year old, which makes finding an effective and safe treatment for those types of patients an urgent need (Atia et al. 2016; Zhang et al. 2022).
Problems facing scientists in developing effective anti-Cryptosporidium treatment include: lack of dependable in vitro culture system and protocols, incomplete knowledge about the parasite biology and the high expense of developing a de novo drug making the use of ready-approved drugs a more convenient option (Pinto et al. 2022).
Platelet Rich Plasma is a volume of plasma containing high platelet concentration of three to five folds more than the baseline prepared by blood centrifugation. It shows high concentration of several growth factors including platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), transforming growth factor beta (TGF-β) and epithelial growth factor (EGF) (Choi et al. 2012).
PRP possesses many properties as hemostatic functions, ability to fight inflammation, modulating the immune system as well as promoting cell proliferation. Recently, it is used in various medical fields such as dermatology, gynecology, ophthalmology, urology, oncology, musculoskeletal field and different surgical properties (Montero et al. 2015; Luzo et al. 2020). Many recent studies proposed that PRP has antimicrobial properties, therefore it can be used as biological therapy along with the conventional therapy (Zhang et al. 2019; Bayoumi et al. 2023).
The aim of this study is to assess and compare the results of using PRP as a potential anti-Cryptosporidium therapeutic in immunocompromised mice infected with C. parvum versus the commercially anti-C. parvum nitazoxanide.
Material and methods
Experimental animals
About 100 laboratory bred male Swiss albino mice of C57BL/6 strain were provided by and housed in the animal house of Theodor Bilharz Research Institute (TBRI). The mice aged 6–8 weeks and weighed about 20–30 gm.
During the course of the study, animals were fed on a standard diet containing 24% protein, 4% fat, 4–5% fiber and water as needed. Animals were kept in appropriate plastic cages at a humidity level of 50–60% and a temperature of 21 ± 2 ºC. As regards PRP collection & preparation, Rattus norvegicus albino male rats weighing (200-300gm) were used according to Van Herck et al (2000).
The experiment was validated by the institutional animal care and use committee (IACUC) of Cairo University (Number: CU III F 42 21), the ethical committee of the Kasralainy School of Medicine, and the ethical committee of TBRI. Animals were manipulated in line with the recommendations of the "Guide for The Care and Use of Laboratory Animals."
Immunosuppression
90 mice out of 100 received immunosuppression using dexazone (Al Kahira Pharmaceuticals (CPCI), Cairo, Egypt) using a dose of 0.25μg/g/day orally via esophageal tube. Immunosuppression started 14 days before infecting mice and continued during the course of the experiment (Rehg et al. 1988).
Infection of animals
Cryptosporidium parvum oocysts, preserved in potassium dichromate 2.5% solution at 4 ºC, were supplied from TBRI after molecular identification (Current et al. 1983; Khalifa et al. 2001). Oocysts were then washed in distilled water to remove the potassium dichromate, followed by 10 min centrifugation at 1500xg (Arrowood 1996). Using an esophageal tube, 80 mice were orally infected with Cryptosporidium parvum oocysts at a dose of around 103 oocysts/mouse (Abdelhamed et al. 2019).
Drug and PRP preparation and administration
All therapeutics started to be used on the 7th day post infection (p.i) according to protocol of administration of each one.
- NTZ (Nanazoxid) (ATCO pharma, Quesna, Egypt) was administered orally using a dose of 0.2 mg/g daily for 6 successive days (Moawad et al. 2021).
- PRP preparation started with blood sample collection by inserting the tip of standard non-heparinized micro-hematocrit capillary tube into medial canthus of the albino rat eyes. When an amount of blood equaled to 2.0 ml were collected, the tube was withdrawn and slight pressure with a piece of sterile gauze on the eyeball was done for 85–140 s to prevent further bleeding (Van Herck et al. 2000).
Blood samples were obtained in acid citrate dextrose tubes then centrifuged at 1000 revolutions per minute (rpm) speed for 15 min (1st spin). Platelet-containing supernatant plasma was transferred into another sterile centrifuge tube. A platelet concentrate is obtained after a higher speed of centrifugation (3000 rpm) for 10 min (2nd spin). The lower 3rd of the tube contained PRP with platelet pellets at the bottom of the tube (Dhurat and Sukesh 2014). PRP concentrate was then dissolved in phosphate buffered saline (PBS) PH: 7.2 (1:1) (Lucarelli et al. 2003; Hesami et al. 2014). When platelets were counted, they ranged in number from 800,000/μl to 1,700,000/μl using Medonic hematology analyzer device (model: DD II C) (Shoeib et al. 2018).
- A dose of 0.5 μl/g of freshly prepared PRP was injected using insulin syringe intravenously (IV) into lateral tail vein of mice once per week (Hesami et al. 2014).
Experimental design
100 male albino mice were involved in this experiment which were divided as the following:
Group I: Non infected non treated immunocompetent naïve group (10 mice).
Group II: Non infected non treated immunosuppressed group (10 mice).
Group III (C): Infected non treated immunosuppressed group (10mice) (infection control group).
Group IV: Infected treated immunosuppressed (70 mice) received treatment as follows:
Group IVa (N):
10 infected immunosuppressed animals treated with 0.2mg/g NTZ daily for 6 consecutive days started on 7th day p.i.
Group IVb:
60 infected immunosuppressed animals treated with IV injection of 0.5 μl/g PRP as follows:
Group IVb1(PRP1): 10 infected immunosuppressed animals treated with single dose of PRP once on the 7th day p.i.
Group IVb2 (PRP2): 10 infected immunosuppressed animals treated with 2 doses of PRP once/week started on the 7th day p.i for 2 consecutive weeks.
Group IVb3 (PRP3): 10 infected immunosuppressed animals treated with 3 doses of PRP once/week started on the 7th day p.i for 3 consecutive weeks.
Group IVb4 (PRP1+N): 10 infected immunosuppressed animals treated with single dose of PRP once on the 7th day p.i combined with 0.2 mg/g NTZ once daily for 6 consecutive days.
Group IVb5 (PRP2+N): 10 infected immunosuppressed animals treated with 2 doses of PRP once/week started on the 7th day p.i for 2 consecutive weeks combined with 0.2mg/g NTZ once daily for 6 consecutive days.
Group IVb6 (PRP3+N): 10 infected immunosuppressed animals treated with 3 doses of PRP once/week started on the 7th day p.i for 3 consecutive weeks combined with 0.2 mg/g NTZ once daily for 6 consecutive days.
Mice were sacrificed 2 weeks after stoppage of treatment: intraperitoneal (IP) anesthetic overdose was given in the form of thiopental 0.5 mg/g body weight/ mouse then chest was opened & heart was inspected to confirm death (Liang et al. 1987).
After dissection, the small intestine' isolated segments were labelled according to their groups and subgroups and prepared for histopathological and immunohistochemical studies.
Parasitological assessment
Fecal pieces from infected mice were collected on 7th, 14th, 21st, 28th, 35th days p.i. After measuring their weight, each fecal sample was dissolved in 1ml of formalin 10%. A volume of 50 µl was obtained from each mixture, stained with MZN, and the number of C. parvum oocysts was counted per gram of feces (Garcia 2007; Benamrouz et al. 2012).
Three microscopic slides were prepared for each group, stained, examined and oocysts were counted. The mean number of oocysts was calculated to estimate the change occurred in the number of oocysts/gm feces (Khalifa 2016).
Stained C. parvum oocysts appeared as pink round bodies against blue background using oil immersion lens (× 1000). They were measured 4–6 μm using eyepiece micrometer and some of the four sporozoites were visible in the C. parvum oocysts (Garcia 2007).
Histopathological and immunohistochemical assessment
-
A.
Histopathological assessment: According to Drury and Wallington 1980, the excised segments were fixed and stained with Hematoxylin & Eosin stain (Hx&E). Histopathological examination was done at the National research institute for the detection of histopathological changes.
-
-
The degree of tissue response was identified by the increase in inflammatory cell density mainly lympho-plasma cells. Classification was according to the intensity of the cellular infiltration (mild, moderate and severe).
-
-
Villous stunting (decreased villous height in relation to crypt length) was described subjectively into mild, moderate and severe discrepancy in villous crypt ratio in small intestinal tissue sections.
-
B.
Immunohistochemical staining of small intestinal tissue sections was done to detect TGF-β1using Polyclonal anti-TGF-β antibody IHC Kit (TGFB1 Rabbit pAb, Catalog No: A16640, USA) after examining by light microscope.
-
-
Slides were examined in semi-quantitative pattern subjectively and classified into mild, moderate and severe according to degree of staining using OLYMPUS SX 41 microscope, microphotography was captured using OLYMPUS SC camera and analyzed using analSIS getlT software.
Statistical analysis of data
Entering and coding data was followed by calculating the mean and standard deviation. Statistical analysis of variance (ANOVA) with multiple comparisons post hoc test was used to compare the groups. P values less than 0.05 were regarded as statistically significant. All analyses were done using the statistical software for the social sciences (SPSS) version 25 (IBM Corp., Armonk, NY, USA) (Chan 2003).
Results
Effects of immunosuppression
mice began to exhibit immunosuppressive symptoms, such as hair loss, skin ulceration and abscess formation, after receiving dexamethasone through an esophageal tube for one week.
Parasitological assessment
Oocysts shedding in the 7th day p.i: 7th day p.i was the first day of therapeutic administration in treated groups, and therefore the passage of C. parvum oocysts were nearly similar between those groups and (C) group (Infection immunosuppressed control group) in stool.
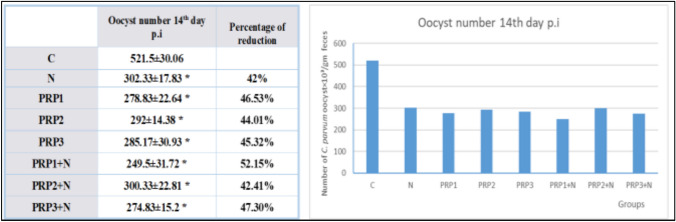
Oocysts shedding in the 14th day p.i (As shown in Fig. 1): Groups treated with PRP alone (PRP1, PRP2, PRP3) showed statistically significant decrease in oocyst shedding in comparison to group (C) (Infection control group) (P-value < 0.05). Maximum reduction occurred in the group that received single dose of PRP (PRP1) with 46.53% reduction in oocyst shedding compared to group (C) and least reduction was observed with the group received 2 doses of PRP (PRP2) with 44.01% reduction in oocyst shedding compared to group (C) but there was no statistically significant difference between the 3 groups. Treated groups with PRP combined with NTZ showed statistically significant reduction in oocyst shedding compared to group (C) (P-value < 0.05). Maximum reduction was in group (PRP1 + N) (Infected treated with combination of single dose of PRP & NTZ) with 52.15% reduction in oocyst shedding compared to group (C) and minimum reduction was observed with group (PRP2 + N) (Infected treated with combination of 2 PRP doses & NTZ) with 42.41% reduction in oocyst shedding compared to group (C). However, there was no statistical difference between the 3 groups treated with PRP combined with NTZ. In group (N) (Infected treated with NTZ) oocyst shedding reduced by 42% in comparison to group (C) (P-value < 0.05).
Fig. 1.
Demonstration of the effect of PRP used individually and in combination with NTZ in different groups on oocyst shedding on the 14th day p.i in comparison to control group and NTZ treated group
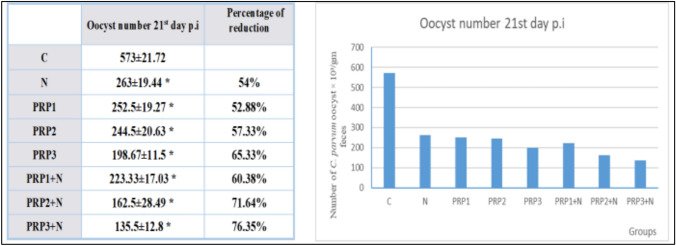
Oocysts shedding in the 21st day p.i (As shown in Fig. 2): Groups treated with PRP alone (PRP1, PRP2, PRP3) showed statistically significant reduction in oocyst shedding in comparison to group (C) (P-value < 0.05). Maximum reduction occurred in group PRP3 (Infected treated with 3 doses of PRP doses) with 65.33% reduction in oocyst shedding compared to group (C) This result was statistically significant from results of the other 2 groups (PRP1, PRP2). Least reduction was observed with the group received 1 dose of PRP (PRP1) with 52.88% reduction in oocyst shedding compared to group (C). Treated groups with PRP combined with NTZ showed statistically significant reduction in oocyst shedding compared to group (C) (P-value < 0.05). Maximum reduction was in group (PRP3 + N) (Infected treated with combination of 3 doses of PRP & NTZ) with 76.35% reduction in oocyst shedding compared to group (C). This result was statistically significant from the results of the other 2 groups (PRP1 + N, PRP2 + N). Minimum reduction was observed with group (PRP1 + N) (Infected treated with combination of single dose of PRP & NTZ) with 60.38% reduction in oocyst shedding compared to group (C). In Group (N) (Infected treated with NTZ) oocyst shedding reduced by 54% in comparison to group (C) (P-value < 0.05).
Fig. 2.
Demonstration of the effect of PRP used individually and in combination with NTZ in different groups on oocyst shedding on the 21st day p.i in comparison to control group and NTZ treated group
Oocysts shedding in the 28th day p.i (As shown in Fig. 3): Oocyst shedding reduction in the 2 remained groups receiving PRP alone (PRP2, PRP3) was statistically significant compared to group (C) (P-value < 0.05). There was statistically significant difference between the 2 groups with better results in PRP3 group (Infected treated with 3 doses of PRP doses) with 82.94% reduction in oocyst shedding compared to group (C) while group PRP 2 (Infected treated with 2 doses of PRP doses) showed 69.81% reduction in oocyst shedding compared to group (C). Treated groups with PRP combined with NTZ showed statistically significant reduction in oocyst shedding compared to group (C) (P-value < 0.05). Furthermore, those groups had better results than groups treated with PRP alone based on statistical analysis. Best result was in group (PRP3 + N) (Infected treated with combination of 3 doses of PRP & NTZ) with 89.89% reduction in oocyst shedding compared to group (C). This group showed statistically significant difference compared to the other 2 groups (PRP1 + N, PRP2 + N) regarding oocyst shedding. Minimum reduction was observed with group (PRP1 + N) (Infected treated with combination of single dose of PRP & NTZ) with 73.40% reduction in oocyst shedding compared to group (C). In group (N) (Infected treated with NTZ) oocyst shedding reduced by 69% in comparison to group (C) (P-value < 0.05).
Fig. 3.
Demonstration of the effect of PRP used individually and in combination with NTZ in different groups on oocyst shedding on the 28th day p.i in comparison to control group and NTZ treated group
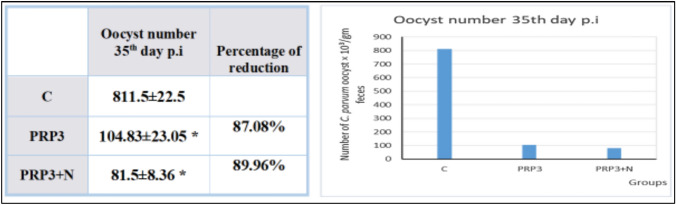
Oocysts shedding in the 35th day p.i (As shown in Fig. 4): PRP3 group (Infected treated with 3 does of PRP) was the only remaining of PRP alone treated groups and revealed statistically significant reduction in oocyst shedding 87.08% compared to group (C) (P-value < 0.05). From groups treated with PRP combined with NTZ, group PRP3 + N (Infected treated with combination of 3 doses of PRP & NTZ) was the only remaining group and showed statistically significant reduction in oocyst shedding 89.96% compared to group (C) (P-value < 0.05).
Fig. 4.
Demonstration of the effect of PRP used individually and in combination with NTZ in different groups on oocyst shedding on the 35th day p.i in comparison to control group and NTZ treated group
The effect of PRP used individually and in combination with NTZ in different groups on oocyst shedding throughout different days p.i in comparison to control group and NTZ treated group was summarized in Fig. 5.
Fig. 5.
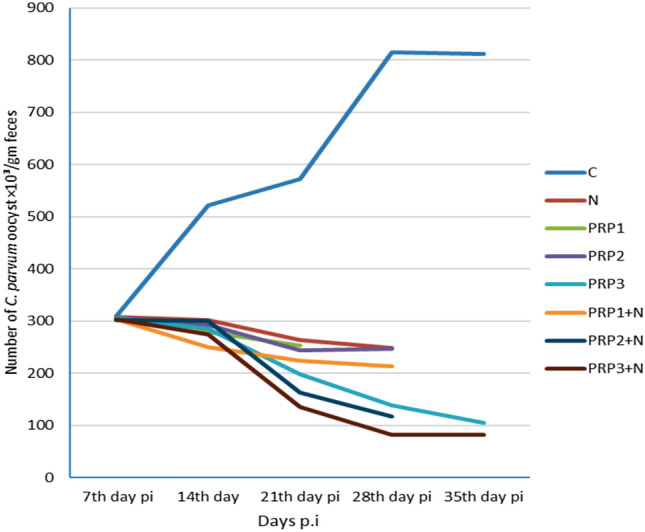
Demonstration of the effect of PRP used individually and in combination with NTZ in different groups on oocyst shedding throughout different days p.i in comparison to control group and NTZ treated group
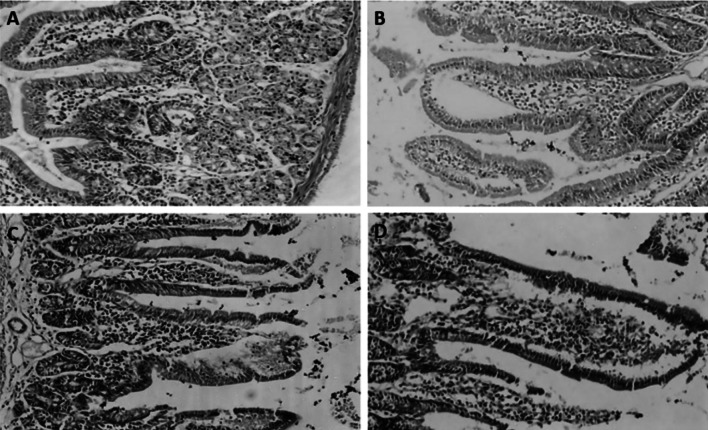
Histopathological assessment (as shown in Figs. 6 and 7)
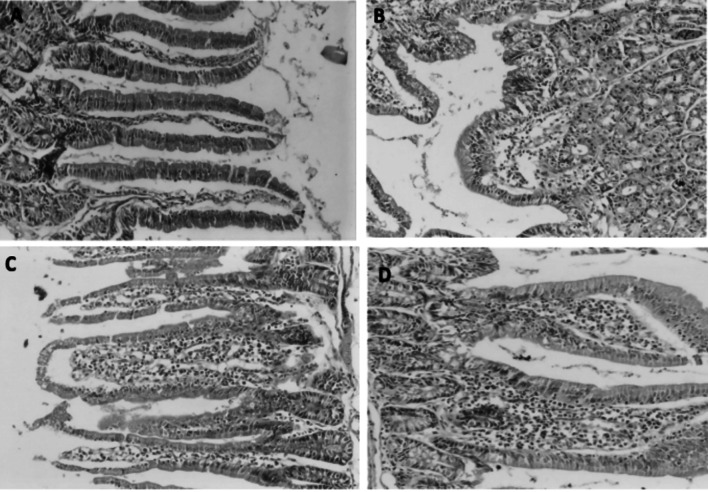
Fig. 6.
A Normal small intestinal section showing normal villous architecture with normal epithelium, goblet cells, crypts and normal lympho-plasma cells in lamina propria stained with H &E (magnification power: × 100). B, D Small intestinal tissue sections obtained from infection control group showing severe villous stunting and severe inflammatory tissue response. C Small intestinal tissue section obtained from NTZ treated group (N) showing moderate villous stunting and moderate inflammatory tissue response
Fig. 7.
A Small intestinal tissue section showing moderate villous stunting and mild inflammatory infiltrate in lamina propria in immunocompromised mice treated with single dose of PRP. B Small intestinal tissue section showing moderate villous stunting (Decreased villous height in relation to crypt length) and moderate inflammatory tissue response in groups of immunocompromised mice treated with 2 doses of PRP. C Small intestinal tissue section showing mild villous stunting and moderate inflammatory tissue response in groups of immunocompromised mice treated with 3 doses of PRP. D Small intestinal tissue section showing mild villous stunting and moderate inflammatory tissue response in groups of immunocompromised mice treated with PRP combined with NTZ
Infection control group (C) tissue sections showed severe villous stunting with reduced ratio of villous to crypt length along with severe infiltration with inflammatory cells mainly lympho-plasma cells. While, sections from nitazoxanide treated group (N) revealed moderate villous stunting and moderate lamina propria infiltration with inflammatory lympho-plasma cells.
Groups treated with PRP alone showed moderate villous stunting and mild inflammatory infiltrate in lamina propria in case of receiving single dose, while the group treated with 2 doses of PRP showed moderate villous stunting but with moderate infiltration of lamina propria with lympho-plasma cells and the group treated with 3 doses of PRP showed mild villous stunting together with moderate infiltrate of lamina propria with lympho-plasma cell. Whereas groups treated with PRP combined with NTZ showed better results than groups treated with PRP alone regarding villous stunting, only mild stunting was present accompanied by moderate lympho-plasma cellular infiltration of lamina propria.
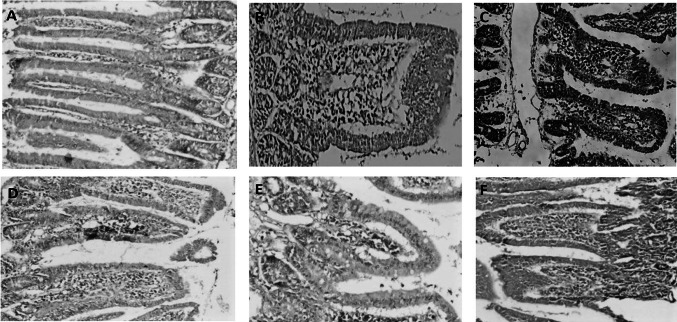
Immunohistochemical assessment (as shown in Fig. 8)
Fig. 8.
A Immunohistochemical staining of normal small intestinal tissue section for TGF-β1 showing minimal expression of TGF-β1 (magnification power: × 100). B, C Immunohistochemical staining of small intestinal tissue sections for TGF-β1 in infection control group showing severe expression of TGF-β1. D Immunohistochemical staining of small intestinal tissue sections for TGF-β1 in infected immunosuppressed animals treated with NTZ showing moderate expression of TGF-β1. E Immunohistochemical staining of small intestinal tissue sections for TGF-β1 in infected immunosuppressed animals treated with single dose of PRP showing mild expression of TGF-β1. F Immunohistochemical staining of small intestinal tissue sections for TGF-β1 in infected immunosuppressed animals treated with 2 doses of PRP showing moderate expression of TGF-β1
Evaluation of immunohistochemistry was done subjectively according to the number of lymphocytes expressing TGF-β1 and classified into mild, moderate and severe increase in TGF-β1 expression.
TGF-β1 is normally expressed by villous or crypt epithelial cells which are considered as positive internal control that ensure correct staining process and expression is detected as brown cytoplasmic and nuclear staining within the inflammatory cells.
Severe increase in TGF-β1 expression was noticed in infection control group (C) group.
Moderate increase of TGF-β1 expression was detected in nitazoxanide treated group (N) group.
Mild increase in TGF-β1 expression was observed in the group treated with single dose of PRP while all other groups treated with PRP alone or in combination with NTZ showed moderate increase in the expression of TGF-β1.
Discussion
In our study, regarding infected immunocompromised mice that were treated with IV injection of PRP alone as single, double and triple doses, all groups showed statistically significant reduction of C. parvum oocyst shedding (P-value < 0.05) compared to infection immunosuppressed control group. Highest percentage of reduction of oocyst shedding was 87.08% in the group that had received 3 doses of PRP at a dose of 0.5 µl/g/week started on the 7th day p.i for 3 consecutive weeks. The least percentage of reduction of oocyst shedding was 52.88% in the group that had received a single dose of PRP (0.5 µl/g) on the 7th day p.i.
These results can be referred to confirmed antimicrobial properties of PRP and its ability to fight many pathogens (Fabbro et al. 2016). It is postulated that PRP releases immunomodulatory mediators that attract and activate immune cells in addition to mediators with direct effect on microbes and parasites as peptides, chemokines and peroxides (Li et al. 2019).
El-Kholy et al. 2021 found also that IP administration of PRP twice weekly for 4 weeks in immunosuppressed rats infected with C. parvum decreased parasite passage in stool by 59.17% on 35th day p.i when used alone.
Treated groups with IV injection of PRP in consecutive doses combined with oral NTZ had better results than each of them individually with statistically significant reduction in oocyst shedding compared to infection immunosuppressed control group (P-value < 0.05). Highest percentage of reduction was 89.96% in the group received 3 doses of PRP combined with NTZ and lowest percentage of reduction was 57.33% in the group received only a single dose of PRP combined with NTZ.
Various trials have been conducted to evaluate the potential impact of PRP as an adjuvant therapy in combination with the conventional medications used to treat various parasites (El-Refai et al. 2018; El-Kholy et al. 2021; Eissa et al. 2022; Ibrahim et al. 2022).
Closely related results were published by El-Kholy et al. (2021) who concluded that combination of IP injection of PRP twice weekly and oral NTZ (65 mg/day) for 4 weeks starting one-week p.i showed higher percentage of reduction than when each of them used alone. Percentage of reduction of oocyst shedding was 71.32% in immunosuppressed rats infected with C. parvum on 35th day p.i.
Ibrahim et al. (2022) tested IP injection of PRP on another apicomplexan parasite (Toxoplasma gondii) in Swiss albino mice infected with ME49 strain. PRP treated groups as twice/week for 4 weeks showed statistically significant reduction of brain tissue cysts. Much better results were achieved in the group treated with spiramycin and PRP combination causing 80.3% reduction compared to only 29.6% reduction when PRP used alone and 66% reduction when spiramycin used alone.
In concordance with the present study, El-Refai et al. (2018) research on experimental Schistosomiasis mansoni stated statistically significant reduction in worm burden (86.5%), intestinal eggs (87.8%) and intrahepatic eggs (86%) in group of mice infected and treated with PRP (twice/week for 4 weeks IP) combined with praziquantel (500 mg/kg for 2 days). In the same study, results of the group treated with PRP alone disagreed with our results. It showed statistically insignificant reduction in worm burden obtained from liver and mesenteric vessels, intestinal and intrahepatic eggs (P-value > 0.05).
Additional studies have been performed by Eissa et al. (2022) in the same concern who found that intramuscular PRP injection (0.5 mL/kg) combined with oral albendazole (50 mg/kg/day for 5 days) treatment in rats experimentally infected with Trichinella spiralis has better results rather than usage of PRP alone. The average percentage of reduction in encysted larvae in muscles was 95.2% in group treated with 3 doses of PRP combined with albendazole and about 86.08% in group treated with PRP alone.
In our study, groups treated with PRP alone showed mild to moderate villous stunting and mild to moderate invasion of lamina propria with lympho-plasma cells. On the other hand, groups treated with PRP combined with NTZ showed mild stunting of villi in attempt to restore normal architecture while moderate inflammatory infiltrate of lamina propria was still present.
Such results may be attributable to the ability of PRP to modulate the immune response by various mechanisms that end in inhibiting inflammatory process and promoting tissue repair (Dos Santos et al. 2021).
Similar results were reported by El-Kholy et al. (2021) who demonstrated moderate villous stunting with moderate villous stunting and moderate inflammatory infiltrates in group treated with PRP alone and normal appearance of intestinal villi with mild inflammatory infiltrates in group treated with PRP combined with NTZ.
Anti-inflammatory properties of PRP were reported in the same context by Ibrahim et al. (2022) who found marked reduction in inflammatory cells in brain tissue of Toxoplasma gondii infected mice treated with PRP alone and nearly restoration of normal brain tissue in group treated with RRP combined with spiramycin.
El-Refai et al. (2018) observed a more cellular granuloma with lower fibrous area in Schistosoma mansoni infected mice, receiving PRP and praziquantel combination, in comparison to infected nontreated mice..
Eissa et al. (2022) noticed mild infiltration of muscle tissue sections with inflammatory cells in Trichinella spiralis infected rats when treated with PRP alone. In the same study groups treated with PRP and albendazole combination showed better results with no infiltrates in muscle tissue but with minimal fibrosis.
Regarding immunohistochemical staining of small intestinal tissue sections, mild increase in TGF-β1 expression was observed in the group treated with single dose of PRP, while all other groups treated with PRP alone or in combination with NTZ showed moderate increase in the expression of TGF-β.
These results may be due to the immunomodulatory effects of PRP and its capacity to promote healing process by increasing the expression and secretion of TGF-β1 as mentioned by Li et al. (2019), which may explain the mild expression in PRP single dose treatment.
Conclusion
It was concluded that PRP can give promising results in the treatment of infectious agents such as cryptosporidiosis. As shown in the previous results, PRP gave superior results in the treatment of C. parvum in immunosuppressed host than the routinely used NTZ. It is also obvious that PRP has an adjuvant effect when used in combination with NTZ in the treatment of C. parvum infection in immunosuppressed host. Additionally, using PRP in multiple doses for the treatment of cryptosporidiosis has better effect than when used in only single dose either individually or in combination with NTZ. Employment of different microbial models and therapeutic approaches is essential to elaborate clearer picture of PRP effectiveness in those diseases.
Author contributions
All authors contributed in all the process of study including designing, methodology preparation, data collection and analysis, and final manuscript proofreading.
Funding
Authors did not receive any form of financial support at any stage of this manuscript.
Declarations
Ethics approval
This study was validated by The Institutional Animal Care & Use Committee (IACUC) of Cairo University (Number: CU III F 42 21).
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdelhamed EF, Fawzy EM, Ahmed SM, Zalat RS, Rashed HE (2019) Effect of nitazoxanide, artesunate loaded polymeric nano fiber and their combination on experimental cryptosporidiosis. Iran J Parasitol 14(2):240 [PMC free article] [PubMed] [Google Scholar]
- Arrowood MJ (1996) Improved purification methods for calf-derived C parvum oocysts using discontinuous sucrose and cesium chloride gradients. J Eukaryot Microbiol 43:89 [DOI] [PubMed] [Google Scholar]
- Atia MM, Abdul Fattah MM, Abdel Rahman HA, Mohammed FA, Al Ghandour AM (2016) Assessing the efficacy of nitazoxanide in treatment of cryptosporidiosis using PCR examination. J Egypt Soc Parasitol 46(3):683–692 [PubMed] [Google Scholar]
- Bayoumi AM, Ismail MA, Mahmoud SS, Soliman AS, Mousa AM, Yousof HAS (2023) The potential curative and hepatoprotective effects of platelet rich plasma on liver fibrosis in Schistosoma mansoni experimentally infected mice. J Parasit Dis 47(2):349–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benamrouz S, Guyot K, Gazzola S, Mouray A, Chassat T, Delaire B et al (2012) Cryptosporidium parvum infection in SCID mice infected with only one oocyst: qPCR assessment of parasite replication in tissues and development of digestive cancer. PLoS ONE 7(12):512–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzid M, Hunter PR, Chalmers RM, Tyler KM (2013) Cryptosporidium pathogenicity and virulence. Clin Microbiol Rev 26(1):115–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YH (2003) Biostatistics102: quantitative data—parametric & non-parametric tests. Singap Med J 44(8):391–396 [PubMed] [Google Scholar]
- Choi J, Minn KW, Chang H (2012) The efficacy and safety of platelet-rich plasma and adipose-derived stem cells: an update. Arch Plast Surg 39(06):585–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Current WL, Reese NC, Ernst JV, Batley WS, Heyman MB, Weinsten WM (1983) Human cryptosporidiosis in immunocompetent and immunodeficient persons. Studies of an outbreak and experimental transmission. N Engl J Med 308:1252–1267 [DOI] [PubMed] [Google Scholar]
- Dhurat R, Sukesh MS (2014) Principles and methods of preparation of platelet-rich plasma: a review and author’s perspective. J Cutan Aesthet Surg 7(4):189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos RG, Santos GS, Alkass N, Chiesa TL, Azzini GO, da Fonseca LF et al (2021) The regenerative mechanisms of platelet-rich plasma: a review. Cytokine 144:155560 [DOI] [PubMed] [Google Scholar]
- Drury RAB, Wallington EA (1980) Carleton’s histological technique, 5th edn. Oxford University Press, Oxford, New York, Toronto [Google Scholar]
- Eissa FM, Eassa AH, Zalat RS, Negm MS, Elmallawany MA (2022) Potential therapeutic effect of platelet-rich plasma and albendazole on the muscular phase of experimental Trichinella spiralis infection. Food Waterborne Parasitol 28:e00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kholy W, Elgohary S, El Kholy A, El-Ashkar A (2021) The efficacy of platelet rich plasma as adjuvant therapy in the treatment of cryptosporidiosis in experimentally infected immunosuppressed rats. Parasitol United J 14(2):162–170 [Google Scholar]
- El-Refai SA, El-aswad BEDW, Mohamed AH, Shereen FM (2018) Human platelet rich plasma alleviates liver fibrosis in murine Schistosomiasis Mansoni. Med J Cairo Univ 86(December):3807–3823 [Google Scholar]
- Fabbro MD, Bortolin M, Taschieri S, Ceci C, Weinstein RL (2016) Antimicrobial properties of platelet-rich preparations. A systematic review of the current pre-clinical evidence. Platelets 27(4):276–285 [DOI] [PubMed] [Google Scholar]
- Garcia LS (2007) Macroscopic and microscopic examination of fecal specimens. In: Washington DC (ed) Diagnostic medical parasitology, 5th edn. ASM Press, pp 782–830 [Google Scholar]
- Hesami Z, Jamshidzadeh A, Ayatollahi M, Geramizadeh B, Farshad O, Vahdati A (2014) Effect of platelet-rich plasma on CCl4-induced chronic liver injury in male rats. Int J Hepatol [DOI] [PMC free article] [PubMed]
- Ibrahim SM, Mohamed S, Foaad HH, Ahmad HK, Al-Ghandour AM (2022) Efficacy of murine platelet rich-plasma versus spiramycin in treatment of chronic toxoplasmosis infected mice. J Egypt Soc Parasitol 52(2):267–278 [Google Scholar]
- Khalifa EA (2016) Probiotics as a promising treatment of experimental cryptosporidiosis in an immuno suppressed mouse model. Int J Curr Microbiol App Sci 5(3):97–106 [Google Scholar]
- Khalifa AM, El Temsahy MM, Abou El Naga IF (2001) Effect of ozone on the viability of some protozoa in drinking water. J Egypt Soc Parasitol 31:603–616 [PubMed] [Google Scholar]
- Khalil IA, Troeger C, Rao PC, Blacker BF, Brown A, Brewer TG et al (2018) Morbidity, mortality, and long-term consequences associated with diarrhoea from Cryptosporidium infection in children younger than 5 years: a meta-analyses study. Lancet Glob Health 6(7):e758–e768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Ma Y, Wang M, Wang T, Wei J, Ren R et al (2019) Platelet-rich plasma plays an antibacterial, anti-inflammatory and cell proliferation-promoting role in an in vitro model for diabetic infected wounds. Infect Drug Resist 12:297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YS, Bruce JI, Boyd DA (1987) Proceeding of the First Sino-American Symposium 1:34–48 [Google Scholar]
- Liu A, Gong B, Liu X, Shen Y, Wu Y, Zhang W et al (2020) A retrospective epidemiological analysis of human Cryptosporidium infection in China during the past three decades (1987–2018). PLoS Negl Trop Dis 14(3):e0008146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucarelli E, Beccheroni A, Donati D, Sangiorgi L, Cenacchi A, el Del Vento AM, al. (2003) Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials 24(18):3095–3100 [DOI] [PubMed] [Google Scholar]
- Luzo AC, Fávaro WJ, Seabra AB, Durán N (2020) What is the potential use of platelet-rich-plasma (PRP) in cancer treatment? A mini review. Heliyon 6(3):e03660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moawad HSF, Hegab MHAEH, Badawey MSR, Ashoush SE, Ibrahim SM, Ali AAELS (2021) Assessment of chitosan nanoparticles in improving the efficacy of nitazoxanide on cryptosporidiosis in immunosuppressed and immunocompetent murine models. J Parasit Dis 45(3):606–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero EC, Santos MF, Fernández RS (2015) Platelet-rich plasma: applications in dermatology. Actas Dermo-Sifiliográficas (english Edition) 106(2):104–111 [DOI] [PubMed] [Google Scholar]
- Pinto P, Ribeiro CA, Kváč M, Tsaousis AD (2022) Cryptosporidium. In: de Souza W (eds) Lifecycles of pathogenic protists in humans. Microbiology monographs, vol 35. Springer, Cham, pp 331–389
- Rehg JE, Hancock ML, Woodmansee DB (1988) Characterization of a dexamethasone treated rat model of cryptosporidial infection. Jpn J Infect Dis 158:1406–1407 [DOI] [PubMed] [Google Scholar]
- Shoeib HM, Keshk WA, Foda AM, Abo El Noeman SEDAE (2018) A study on the regenerative effect of platelet-rich plasma on experimentally induced hepatic damage in albino rats. Can J Physiol Pharmacol 96(6):630–636 [DOI] [PubMed] [Google Scholar]
- Van Herck H, Baumans V, Boere HAG, Hesp APM, Van Lith HA, Beynen AC (2000) Orbital sinus blood sampling in rats: effects upon selected behavioural variables. Lab Anim 34(1):10–19 [DOI] [PubMed] [Google Scholar]
- Zhang W, Guo Y, Kuss M, Shi W, Aldrich AL, Untrauer J et al (2019) Platelet-rich plasma for the treatment of tissue infection: preparation and clinical evaluation. Tissue Eng Part B Rev 25(3):225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CX, Love MS, McNamara CW, Chi V, Woods AK, Joseph S et al (2022) Pharmacokinetics and pharmacodynamics of clofazimine for treatment of cryptosporidiosis. Antimicrob Agents Chemother 66(1):e01560-e1621 [DOI] [PMC free article] [PubMed] [Google Scholar]