Abstract
Purpose
Tinnitus, the perception of sound without any external sound source, is a prevalent hearing health concern. Mounting evidence suggests that a confluence of genetic, environmental, and lifestyle factors can influence the pathogenesis of tinnitus. We hypothesized that alteration in DNA methylation, an epigenetic modification that occurs at cytosines of cytosine-phosphate-guanine (CpG) dinucleotide sites, where a methyl group from S-adenyl methionine gets transferred to the fifth carbon of the cytosine, could contribute to tinnitus. DNA methylation patterns are tissue-specific, but the tissues involved in tinnitus are not easily accessible in humans. This pilot study used saliva as a surrogate tissue to identify differentially methylated CpG regions (DMRs) associated with tinnitus. The study was conducted on healthy young adults reporting bilateral continuous chronic tinnitus to limit the influence of age-related confounding factors and health-related comorbidities.
Methods
The present study evaluated the genome-wide methylation levels from saliva-derived DNA samples from 24 healthy young adults with bilateral continuous chronic tinnitus (> 1 year) and 24 age, sex, and ethnicity-matched controls with no tinnitus. Genome-wide DNA methylation was evaluated for > 850,000 CpG sites using the Infinium Human Methylation EPIC BeadChip. The association analysis used the Bumphunter algorithm on 23 cases and 20 controls meeting the quality control standards. The methylation level was expressed as the area under the curve of CpG sites within DMRs.The FDR-adjusted p-value threshold of 0.05 was used to identify statistically significant DMRs associated with tinnitus.
Results
We obtained 25 differentially methylated regions (DMRs) associated with tinnitus. Genes within or in the proximity of the hypermethylated DMRs related to tinnitus included LCLAT1, RUNX1, RUFY1, NUDT12, TTC23, SLC43A2, C4orf27 (STPG2), and EFCAB4B. Genes within or in the proximity of hypomethylated DMRs associated with tinnitus included HLA-DPB2, PM20D1, TMEM18, SNTG2, MUC4, MIR886, MIR596, TXNRD1, EID3, SDHAP3, HLA-DPB2, LASS3 (CERS3), C10orf11 (LRMDA), HLA-DQB1, NADK, SZRD1, MFAP2, NUP210L, TPM3, INTS9, and SLC2A14. The burden of genetic variation could explain the differences in the methylation levels for DMRs involving HLA-DPB2, HLA-DQB1, and MUC4, indicating the need for replication in large independent cohorts.
Conclusion
Consistent with the literature on comorbidities associated with tinnitus, we identified genes within or close to DMRs involved in auditory functions, chemical dependency, cardiovascular diseases, psychiatric conditions, immune disorders, and metabolic syndromes. These results indicate that epigenetic mechanisms could influence tinnitus, and saliva can be a good surrogate for identifying the epigenetic underpinnings of tinnitus in humans. Further research with a larger sample size is needed to identify epigenetic biomarkers and investigate their influence on the phenotypic expression of tinnitus.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10162-024-00961-2.
Keywords: Tinnitus, Tinnitus in young adults, Noise, Noise exposure background, Hyperacusis, Epigenetics, Genomics, Epigenomics, Differentially methylated regions, Differentially methylated positions, EbGSEA
Introduction
Tinnitus, the phantom perception of sound without an external sound source, is an etiologically heterogeneous trait. Almost 20 million US adults struggle with bothersome tinnitus, and 2 million have a severe form of debilitating tinnitus [1]. Tinnitus is the most prevalent service-connected health concern among military veterans [2]. Tinnitus is a common hearing health concern among individuals exposed to traumatic sound levels for occupational reasons. About 15% of workers exposed to occupational noise experience chronic bothersome tinnitus [3]. Almost 50% of individuals fail to inform their employers or co-workers about their tinnitus because they feel others would be incapable of understanding their condition [3, 4]. Tinnitus is associated with several otological diseases and clinical conditions [5]. Aging, smoking, ototoxicity, and ear infections are the major risk factors for tinnitus [6–8]. However, the molecular mechanisms underlying the association between these risk factors and tinnitus remain elusive.
Recent twin studies estimated tinnitus heritability (h2) at 0.56, which rises to 0.68 for bilateral tinnitus in men, indicating a high genetic contribution to bilateral tinnitus [9–12]. Candidate gene studies identified some genetic variants associated with tinnitus, but they were not highly successful at interrogating the genetic architecture of tinnitus [13]. A recent genome-wide association study (GWAS), using population-based health databases (such as UK Biobank and the Million Veteran Program), identified single nucleotide polymorphism (SNPs), explaining about 6% of heritability and highlighting a polygenic architecture of tinnitus [14]. Other genome-wide studies using the UK Biobank identified SNPs near RCOR1 and GPM6A associated with tinnitus [15, 16]. A recent GWAS identified 39 genomic loci showing significant association with tinnitus [17]. Using the whole-exome sequencing from the UK Biobank, a recent GWAS identified 17 suggestive SNPs in 13 genes in two sex-separated cohorts [18]. Rare variants in ANK2, AKAP3, and TSC2 were associated with extreme clinical manifestation of tinnitus (i.e., tinnitus with a high level of psychological distress in daily living) [19]. Recent genetic studies showed a polygenic architecture of tinnitus and highlighted the need to identify novel biomarkers. Aside from SNPs, additional disease mechanisms, such as epigenetic modulation of genes, have not been investigated well in humans.
DNA methylation, an epigenetic modification influencing gene expression, can impact pathogenesis [20]. DNA methylation is a post-replication DNA modification that often occurs at cytosines of cytosine-phosphate-guanine (CpG) dinucleotide sites, where a methyl group from S-adenyl methionine gets transferred to the fifth carbon of the cytosine [21]. CpG dinucleotides comprise about 1% of the human genome [22, 23]. Methylation at a single CpG site on a DNA strand is a binary phenomenon, but it is measured as a quantitative trait in the experimental samples. DNA extracted from the samples contains a population of DNA strands with some methylated and unmethylated at any specific CpG site, collectively giving rise to a continuous level of methylation [24]. A genome-wide bisulfite DNA sequencing study revealed that about 30% of CpG sites are unmethylated [25]. CpG islands, genomic regions of > 200 bp to 2 kb containing at least 50% of CpG sites, play a central role in gene regulation through their methylation. The CpG sites are found abundantly in the promoters of genes [26, 27]. The human genome contains more than 30,000 CpG islands, which are critical in embryological development, cell differentiation, genetic imprinting, and X-inactivation [28].
DNA methylation patterns are tissue-specific [29]. Past studies investigated the association between adult-onset health conditions and DNA methylation using invasive approaches for obtaining relevant disease tissues [30, 31]. Some studies investigated post-mortem tissues to identify the association between health conditions and DNA methylation [32]. There is an increasing level of concern regarding the stability of DNA methylation in the post-mortem tissues [33]. Access to biologically relevant tissues from patients with tinnitus could be challenging, limiting the direct epigenetic interrogation of tinnitus. Recent studies showed high cross-tissue correlations between DNA methylation levels between peripheral tissues (e.g., saliva, blood, buccal mucosa) and the live human brain tissues [34, 35], suggesting that the peripheral tissues could be used to investigate the epigenetic basis of complex health conditions [e.g., 36].
Peripheral blood-derived DNA was used to investigate the epigenetic basis of tinnitus in past studies [37–39]. An unbiased genome-wide DNA methylation analysis of 59 veterans exposed to impulse noise (29 reporting tinnitus) revealed 11 DMRs associated with tinnitus. The DMRs associated with tinnitus involve genes important for auditory functions, such as KCNN3, SOD3, MUC4, GALR1, and WDR45B [38]. DNA methylation levels in candidate CpG sites of brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor (GDNF), and glucocorticoid receptor (NR3C1) were associated with tinnitus [37, 39]. Smoking, a known risk factor for tinnitus, has been observed to change DNA methylation levels [40, 41]. Recent studies identified DMRs associated with cigarette smoking, both using DNA derived directly from lung tissues and saliva as a surrogate [42–45]. Noise exposure, another significant risk factor for tinnitus, influences DNA methylation [46]. The prevalence of tinnitus increases with age, and aging is highly correlated with DNA methylation [47, 48]. These studies collectively suggested that epigenetic modification to gene expression could influence tinnitus. However, DMRs involved in tinnitus remain elusive.
The present study evaluated genome-wide DNA methylation markers in healthy young adults with bilateral continuous chronic tinnitus. The pilot study aimed to compare the DNA methylation markers between cases and controls to identify DMRs associated with tinnitus. We evaluated healthy young adults with tinnitus because older adults with tinnitus exhibit age-related confounding factors (e.g., age-related hearing loss and systemic diseases), which could obscure the identification of the epigenetic biomarkers. Tinnitus in older adults (including military veterans) is likely influenced by lifetime exposure to environmental stressors, such as exposure to ototoxic drugs and occupational and recreational noise/music exposure. Young adults exhibit relatively lower environmental exposure to these risk factors than older adults, facilitating the identification of the most susceptible individuals. We utilized saliva-derived DNA to identify DMRs associated with tinnitus, which is supported by the current literature indicating high correlations in DNA methylation levels between saliva and live human brain tissues [35].
Methods
Participants
The Institutional Review Board of the University of Iowa approved the protocol for the present study (IRB#202,010,165). The informed consent was obtained from all participants. A screening questionnaire was distributed via sending campus-wide emails and conducting an in-class questionnaire. The questionnaire inquired about demographic details (e.g., age, sex, ethnicity), tinnitus, and general health-related information. Tinnitus questions were adopted from the National Health and Nutrition Examination Survey (NHANES). The questionnaire assessed tinnitus by inquiring, “Q1. In the past 12 months, have you been bothered by ringing, roaring, buzzing, or any other type of sounds in ears or head that lasts from 5 min or more?”. If the participants answered “yes,” it further inquired, “Q2. How long have you been bothered by things ringing, roaring, buzzing, or any other types of sounds in your ears or head?”. The options included “less than three months,” “three months to a year,” “1 to 4 years,” “5 to 9 years,” and “10 or more years.” The participants were inquired about their health status and were asked to list health conditions they have or had in the past. Participants with chronic bothersome tinnitus perception (“yes” to Q1 and > 1 year of tinnitus perception) were invited to participate in the present study. We created a registry of 5128 adults aged 18–40 years (520 with chronic tinnitus). The registry was used to send email invites to potential participants. Individuals reporting systemic diseases and health conditions were excluded. Individuals reporting good health status, absence of active health conditions, and absence of systemic diseases were included. These criteria were used to control for the effects of health-related confounding factors. A case–control cohort included 24 individuals with chronic tinnitus and 24 without tinnitus. Age (± 1 year, ranging from 18 to 35 years) and biological sex were used to match cases and controls. All participants included in the present study reported European ethnicity (self and both parents). The study sample included 20 males (10 case–control pairs) and 28 females (14 case–control pairs). The average age of cases was 22 years (SD = 3.7 years), and the average age of controls was 21.88 years (SD = 3.65 years). The age difference between the cases and controls was not statistically significant (t(46) = 0.15, mean difference = 0.16 years, p = 0.86). The case–control cohort was invited to participate in the study. The inclusion and exclusion criteria helped to identify young adults reporting chronic tinnitus who were otherwise healthy. Though the exact cause(s) of chronic tinnitus in the study sample remains unknown, occupational noise, recreational noise/music, smoking, and history of reoccurring ear infections are known risk factors for tinnitus in youth [49], which could have influenced tinnitus pathogenesis in the study cohort.
Tinnitus Evaluation
All participants completed a tinnitus questionnaire adopted from the NHANES [49]. A certified and state-licensed clinical audiologist with > 10 years of experience in tinnitus evaluation and management verified their responses by offering clarification on the question text, asking follow-up questions, and rephrasing the questions to assure the reliability of the responses. Individuals reporting continuous bilateral bothersome tinnitus perception for > 1 year were asked to spend 5 min in a double-walled sound-treated booth meeting ANSI standards for maximum permission ambient noise in audiometric rooms. After 5 min, they were asked, “Did you experience ringing, roaring, buzzing, or perceiving any other types of sounds in your ears or head in the last 5 min?”. Further questions were asked to evaluate tinnitus laterality. The present study included cases with bilateral continuous chronic tinnitus (i.e., bothersome tinnitus > 1 year) with continuous tinnitus perception during 5 min in the sound-treated booth, and their age, sex, and ethnicity-matched controls with no tinnitus and no tinnitus perception in the sound-treated booth.
Tinnitus-related distress in daily living was evaluated using two questionnaires widely used in clinics—the Tinnitus Handicap Inventory (THI) and the Tinnitus Functional Index (TFI). THI evaluates three domains: functional, emotional, and catastrophic. THI investigates tinnitus-related distress using 25 questions. It elicits responses with a forced choice method (yes/sometimes/no). Participants receive a score of 4 for “yes,” 2 for “sometimes,” and 0 for “no.” The overall THI score is obtained by calculating the sum of question scores. A high THI score indicates high perceived tinnitus-related distress in daily living [50]. TFI evaluates tinnitus-related distress on eight subscales: intrusive, sense of control, cognitive, sleep, auditory, relaxation, quality of life, and emotional. Participants rated their agreement with a question on a 0–10 scale. The overall TFI scores were obtained by adding question-specific scores, dividing by the number of questions with valid answers, and multiplying by 10. The score was expressed on a 0–100 scale, with high scores indicating more distress [51].
Audiometric tests
The case–control cohort went through an otoscopic examination. Individuals with normal otoscopic findings were tested further with immittance audiometry using Titan IMP440 (Interacoustics, Middelfart, Denmark). All audiometric procedures were conducted in a double-walled sound-treated room meeting ANSI standards (ANSI S3.1–1999). Participants with normal tympanometric findings (e.g., static compliance: 0.3–1.7, middle ear pressure: + 25 to − 100 daPa) were tested with puretone audiometry. Behavioral puretone audiometric thresholds were obtained with the modified Hughson-Westlake procedure (AVANT Stealth Clinical Audiometer, MedRx Inc., Largo, FL). We used IP30 insert receivers (RadioEar, Middelfart, Denmark) to obtain hearing thresholds at 250, 500, 1000, 2000, 3000, 4000, 6000, and 8000 Hz. The extended-high frequency audiometry was performed with a circumaural headset DD450 (RadioEar, Middelfart, Denmark) at 9000, 10,000, 11,200, 12,500, 14,000, and 16,000 Hz.
Saliva Samples and DNA Extraction
The saliva samples were collected using Oragene DISCOVER™ (DNA Genotek Inc., OGR-500). The saliva samples were collected following an hour of data collection session. Participants were requested to swish their mouths at the beginning of the session and avoid eating, drinking, and smoking. Following the audiometric tests and questionnaire responses, participants were requested to provide a 2 ml saliva sample. The stabilized saliva samples were stored at room temperature for around 90 days before extracting DNA. The DNA extraction was conducted with a prepIT-L2P reagent kit (DNA Genotek Inc., PT-L2P-5). The extracted DNA samples were evaluated with NanoDrop spectrometry (ThermoFisher Scientific Inc., NanoDrop). DNA concentration was quantified with the Qubit™ 1X dsDNA Broad Range Assay Kit (ThermoFisher Scientific Inc., Q32850).
Genome-Wide DNA Methylation Assay
Qubit-derived DNA concentration was calculated, and 500 ng of DNA was bisulfite converted using the EZ DNA Methylation kit (Zymo Research, D5002). Infinium Human MethylationEPIC BeadChip kit (Illumina, WG-317) was used to evaluate genome-wide DNA methylation. The first batch included 32 samples (16 cases and 16 matched controls) (Illumina, WG-317–1002), and the second batch included 16 samples (8 cases and 8 matched controls) (Illumina, WG-317–1001). The samples were placed in the 96-well plates, and 8 samples were hybridized on the same array. Cases and controls were placed on the same array (4 cases and 4 matched controls) to minimize the effect of the hybridization. The arrays were scanned with the Illumina iScan system (Illumina, iScan system) at the University of Iowa Genomic Core.
Preprocessing and Quality Control
Methylation data processing and analysis steps were implemented using parameters and practices recommended in the end-to-end pipeline ChAMP [52]. The input data consisted of a sample specification sheet representing the population metadata and raw IDAT files representing two different color channels prior to normalization and measurements on 411 control probes. The raw signals were extracted from the IDAT files and converted into beta values. The beta values were calculated as the ratio of methylated CpG probe to total probe intensities. It ranges between 0 (unmethylated) and 1 (methylated) [53]. Samples filtered with a failed CpG fraction threshold of 0.10 were removed, excluding 5 samples from the association analysis (4 controls and 1 case). Next, probes with a detection p-value above 0.01 were removed (5721 probes removed), and probes with a bead count < 3 were filtered out (10,045 probes removed). Non-CG probes were ignored as well (2963 non-CG probes removed). Multi-hit probes, which align to multiple locations, and probes on X and Y chromosomes were removed (8483 multi-hit probes and 18,692 X/Y probes removed). After all quality control steps, a total of 820,014 probes remained. We applied additional quality control checks such as multidimensional scaling plots to visualize the similarity of samples, and density plots to verify the beta distributions for each sample and find outlier samples. Any options or parameters not listed here were set as CHAMP pipeline’s defaults [54].
Normalization and Correction
After initial preprocessing and quality control, we applied a beta-mixture quantile normalization method for correcting probe design bias (BMIQ algorithm) and singular value decomposition (SVD) to detect correlations with biological factors of interest and technical sources of variation (batch effect detection) [54]. Any detected batch effects were then corrected using an empirical Bayes algorithm (ComBat) [55]. The normalized and batch-corrected data was then processed through differential methylation analysis.
Genome-Wide DNA Methylation Analysis for Identifying DMR Involved in Tinnitus
We focused on identifying DMRs through an unbiased genome-wide approach. DMR identification involves fewer hypothesis tests than testing individual CpG sites across the genome, making it suitable for the pilot study. To identify DMRs between cases and controls, we applied a standard Bumphunter algorithm that identifies candidate regions (bumps) as clusters of consecutive CpG probes that are differentially methylated when comparing cases versus controls [56]. The methylation level was expressed as the area under the curve of individual CpG sites within the region of interest. DMR effect sizes were calculated as log (fold change) = log10 (Methylation LevelTinnitus /Methylation LevelNo tinnitus). To identify DMRs associated with tinnitus, we set an FDR-adjusted p-value cutoff of 0.05. To adjust for the DMR size bias (i.e., larger DMRs are more likely to show significant results by random chance), we calculated p-values representing the proportion of random differentially methylated regions that show higher total methylation levels than the target DMR. The adjusted p-value is corrected by bootstrapping for the DMR size bias [for details, 52, 56]. The genes within the associated DMRs were reported. If no genes were identified within the associated DMRs, we searched for the nearest gene(s) by setting a maximum distance threshold of 300 Kbp upstream and downstream of the target DMRs.
Enrichment Analysis
We identified differentially methylated genes through the Empirical Bayesian Gene Set Enrichment Analysis (ebGSEA) algorithm, which ranks genes according to the overall level of differential methylation of its CpGs/probes, allowing unbiased and sensitive detection of enriched pathways [57]. The method uses global tests to directly detect the significance of genes from DNA methylation data sets instead of simply selecting genes mapped by DMRs. After the global test, the Empirical Bayes method uses the Wilcox test to enrich genes to pathways. The threshold of Benjamini–Hochberg false discovery rate (FDR) adjusted p-value < 0.05 was used for determining statistically significant pathways.
Results
Demographic Details
Table 1 presents the descriptive statistics of the study sample. The sample included 24 healthy adults aged 18–35 with bilateral continuous chronic tinnitus. The control group included age (difference between cases and controls < 1 year) and sex-matched healthy young adults. The case–control cohort included all individuals reporting European ethnicity.
Table 1.
Descriptive statistics of the study sample (N = 48)
| Pair | Group | Age | Sex | Self-reported ethnicity | Reoccurring ear infection | Ever smoked tobacco? |
|---|---|---|---|---|---|---|
| 1 | Tinnitus | 20 | Male | European | No | No |
| No tinnitus | 20 | Male | European | No | No | |
| 2 | Tinnitus | 19 | Male | European | No | No |
| No tinnitus | 19 | Male | European | Yes | No | |
| 3 | Tinnitus | 21 | Male | European | No | No |
| No tinnitus | 21 | Male | European | No | No | |
| 4 | Tinnitus | 21 | Male | European | No | No |
| No tinnitus | 21 | Male | European | No | No | |
| 5 | Tinnitus | 35 | Male | European | No | Yes |
| No tinnitus | 35 | Male | European | No | No | |
| 6 | Tinnitus | 20 | Male | European | No | No |
| No tinnitus | 20 | Male | European | No | No | |
| 7 | Tinnitus | 24 | Male | European | Yes | No |
| No tinnitus | 25 | Male | European | No | No | |
| 8 | Tinnitus | 22 | Male | European | No | No |
| No tinnitus | 23 | Male | European | No | No | |
| 9 | Tinnitus | 20 | Male | European | No | No |
| No tinnitus | 21 | Male | European | No | No | |
| 10 | Tinnitus | 21 | Male | European | Yes | No |
| No tinnitus | 21 | Male | European | No | No | |
| 11 | Tinnitus | 18 | Female | European | Yes | No |
| No tinnitus | 18 | Female | European | No | No | |
| 12 | Tinnitus | 18 | Female | European | Yes | No |
| No tinnitus | 19 | Female | European | No | No | |
| 13 | Tinnitus | 19 | Female | European | Yes | No |
| No tinnitus | 19 | Female | European | No | No | |
| 14 | Tinnitus | 20 | Female | European | Yes | No |
| No tinnitus | 20 | Female | European | No | No | |
| 15 | Tinnitus | 20 | Female | European | Yes | No |
| No tinnitus | 20 | Female | European | Yes | No | |
| 16 | Tinnitus | 20 | Female | European | Yes | No |
| No tinnitus | 20 | Female | European | No | No | |
| 17 | Tinnitus | 21 | Female | European | No | No |
| No tinnitus | 21 | Female | European | No | No | |
| 18 | Tinnitus | 21 | Female | European | Yes | No |
| No tinnitus | 21 | Female | European | Yes | No | |
| 19 | Tinnitus | 22 | Female | European | No | No |
| No tinnitus | 21 | Female | European | Yes | No | |
| 20 | Tinnitus | 22 | Female | European | Yes | No |
| No tinnitus | 22 | Female | European | Yes | No | |
| 21 | Tinnitus | 23 | Female | European | No | No |
| No tinnitus | 23 | Female | European | No | No | |
| 22 | Tinnitus | 24 | Female | European | Yes | No |
| No tinnitus | 24 | Female | European | No | No | |
| 23 | Tinnitus | 27 | Female | European | No | No |
| No tinnitus | 27 | Female | European | No | No | |
| 24 | Tinnitus | 27 | Female | European | Yes | No |
| No tinnitus | 28 | Female | European | No | No |
Audiometric Findings
Table 2 presents tinnitus-related phenotypic details. Tinnitus perception was reported as “ringing” in all cases. THI scores ranged from slight to severe (4 to 62), which were highly correlated with TFI scores (r(48) = 0.90, p < 10−16). Figure 1 presents audiometric thresholds between the case–control cohort. The tinnitus group showed slightly elevated average hearing thresholds across the frequency range than their matched controls. The independent sample t-test showed no significant group difference between hearing thresholds across the frequency range (false discovery rate (FDR) p < 0.05).
Table 2.
Tinnitus-related phenotypic details
| Pair | Tinnitus | THI score | TFI score | Duration (in years) | Laterality | Continuous tinnitus | Self-reported loudness | Self-reported pitch |
|---|---|---|---|---|---|---|---|---|
| 1 | Ringing, hissing | 20 | 18.8 | > 10 years | Both ears/head | Yes | 75 | 80 |
| 2 | Ringing, roaring | 10 | 4.8 | 5–9 years | Both ears/head | Yes | 50 | 75 |
| 3 | Ringing | 4 | 6 | 1–4 years | Both ears/head | Yes | 50 | 80 |
| 4 | Ringing | 26 | 32 | 5–9 years | Both ears/head | Yes | 50 | 70 |
| 5 | Ringing | 14 | 11.2 | > 10 years | Both ears/head | Yes | 25 | 85 |
| 6 | Ringing | 20 | 26.4 | > 10 years | Both ears/head | Yes | 70 | 75 |
| 7 | Ringing | 8 | 13.6 | 1–4 years | Both ears/head—more in the left ear | Yes | 20 | 80 |
| 8 | Ringing | 6 | 3.6 | > 10 years | Both ears/head | Yes | 30 | 70 |
| 9 | Ringing, roaring | 14 | 14.4 | 1–4 years | Both ears/head | Yes | 40 | 80 |
| 10 | Ringing, pulsating | 14 | 8.4 | 5–9 years | Both ears/head | Yes | 37.5 | 85 |
| 11 | Ringing, pulsating | 62 | 55.2 | 1–4 years | Both ears/head | Yes | 70 | 90 |
| 12 | Ringing, buzzing, pulsating | 50 | 19.6 | 1–4 years | Both ears/head | Yes | 20 | 88 |
| 13 | Ringing, buzzing, pulsating, rushing water | 40 | 45.6 | 1–4 years | Both ears/head | Yes | 80 | 70 |
| 14 | Hissing, roaring | 22 | 15.6 | 1–4 years | Both ears/head | Yes | 7 | 50 |
| 15 | Ringing, buzzing | 16 | 19.6 | > 10 years | Both ears/head | Yes | 70 | 90 |
| 16 | Ringing, hissing, roaring | 4 | 5.6 | 1–4 years | Both ears/head | Yes | 30 | 60 |
| 17 | Ringing | 22 | 17.2 | 1–4 years | Both ears/head | Yes | 15 | 60 |
| 18 | Ringing, buzzing, hissing | 48 | 54 | > 10 years | Both ears/head | Yes | 19 | 65 |
| 19 | Ringing, buzzing, pulsating | 26 | 39.2 | 1–4 years | Both ears/head | Yes | 40 | 95 |
| 20 | Ringing, pulsating | 26 | 29.6 | 1–4 years | Both ears/head | Yes | 15 | 75 |
| 21 | Ringing | 4 | 6.4 | > 10 years | Both ears/head | Yes | 30 | 70 |
| 22 | Ringing, buzzing | 6 | 18.8 | > 10 years | Both ears/head | Yes | 25 | 60 |
| 23 | Ringing, buzzing, pulsating | 26 | 19.2 | > 10 years | Both ears/head | Yes | 30 | 60 |
| 24 | Ringing | 8 | 17.2 | > 10 years | Both ears/head | Yes | 20 | 70 |
Loudness scale: 0 = very soft, 50 = sound level of normal conversation, 100 = painfully loud
Pitch scale: 0 = very low (example: foghorn), 100 = very high (example: whistle)
THI categorization: 0–16 = slight, 18–36 = mild, 38–56 = moderate, 58–76 = severe, and 78–100 = catastrophic
Fig. 1.
Hearing thresholds as a function of audiometric frequency between individuals with chronic tinnitus and their age, sex, and ethnicity-matched controls with no tinnitus. The error bar presents a 95% confidence interval. The spaghetti charts present hearing thresholds between cases and their respective controls
DMRs Associated with Tinnitus
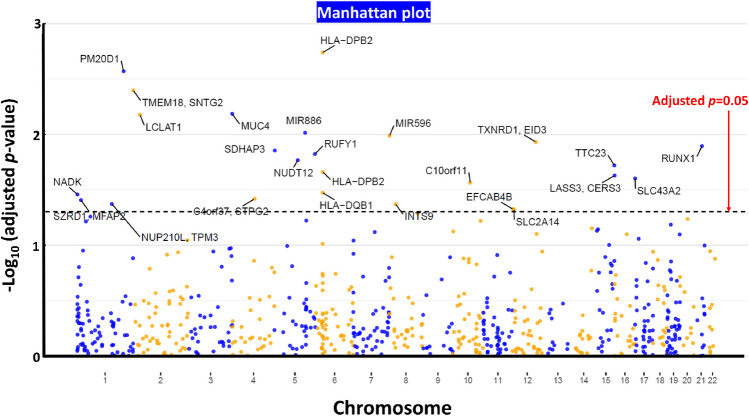
We obtained 25 DMRs showing significant associations with tinnitus (adjusted p-value < 0.05) (Supplementary File S1). Table 3 presents the top DMR results. Genes within or in the proximity of hypermethylated (i.e., gain in average methylation levels) DMRs associated with tinnitus included LCLAT1, RUNX1, RUFY1, NUDT12, TTC23, SLC43A2, C4orf27 (STPG2), and EFCAB4B. Genes within or in the proximity of hypomethylated (i.e. loss in average methylation levels) DMRs associated with tinnitus included HLA-DPB2, PM20D1, TMEM18, SNTG2, MUC4, MIR886, MIR596, TXNRD1, EID3, SDHAP3, HLA-DPB2, LASS3 (CERS3), C10orf11 (LRMDA), HLA-DQB1, NADK, SZRD1, MFAP2, NUP210L, TPM3, INTS9, and SLC2A14. Figure 2 presents the Manhattan plot showing the significant DMR results.
Table 3.
The results of the differential methylation region analysis
| Chr | MTinnitus | MNoTinnitus | Change (%) | Gain/loss | Adj p (FDR) | Genes | Gene distance |
|---|---|---|---|---|---|---|---|
| chr6 | 2.08 | 2.58 | 19.4 | Loss | 0.0018 | HLA-DPB2 | Within DMR* |
| chr1 | − 0.43 | 0.15 | 373.8 | Loss | 0.0026 | PM20D1 | Within DMR |
| chr2 | 1.31 | 2.03 | 35.4 | Loss | 0.0040 | TMEM18, SNTG2 | 238,432, 3734 |
| chr3 | 0.76 | 1.37 | 44.0 | Loss | 0.0065 | MUC4 | Within DMR* |
| chr2 | − 3.69 | − 4.19 | 11.9 | Gain | 0.0066 | LCLAT1 | Within DMR |
| chr5 | − 1.41 | − 1.10 | 28.3 | Loss | 0.0096 | miR886 | Within DMR |
| chr8 | − 4.15 | − 3.76 | 10.3 | Loss | 0.0102 | miR596 | Within DMR |
| chr12 | − 3.49 | − 3.13 | 11.4 | Loss | 0.0117 | TXNRD1, EID3 | Within DMR |
| chr21 | − 2.04 | − 2.43 | 15.9 | Gain | 0.0127 | RUNX1 | Within DMR |
| chr5 | − 3.65 | − 3.14 | 16 | Loss | 0.0139 | SDHAP3 | Within DMR |
| chr5 | − 3.17 | − 3.49 | 9.3 | Gain | 0.0150 | RUFY1 | Within DMR |
| chr5 | − 3.61 | − 4.05 | 10.9 | Gain | 0.0170 | NUDT12 | Within DMR |
| chr15 | − 4.12 | − 4.49 | 8.2 | Gain | 0.0190 | TTC23 | Within DMR |
| chr6 | 1.57 | 1.88 | 16.6 | Loss | 0.0217 | HLA-DPB2 | Within DMR* |
| chr15 | 1.10 | 1.51 | 27.0 | Loss | 0.0235 | LASS3 (CERS3) | Within DMR |
| chr17 | − 0.40 | − 0.84 | 52.3 | Gain | 0.0249 | SLC43A2 | Within DMR |
| chr10 | 1.40 | 1.91 | 26.9 | Loss | 0.0273 | C10orf11 (LRMDA) | Within DMR |
| chr6 | − 3.57 | − 3.29 | 8.7 | Loss | 0.0335 | HLA-DQB1 | Within DMR* |
| chr1 | 3.27 | 3.83 | 14.5 | Loss | 0.0348 | NADK | Within DMR |
| chr4 | − 4.78 | − 5.09 | 6.1 | Gain | 0.0381 | C4orf37 (STPG2) | Within DMR |
| chr1 | − 2.73 | − 2.23 | 22.4 | Loss | 0.0391 | SZRD1, MFAP2 | 6045, 12 |
| chr1 | 1.84 | 2.31 | 20.5 | Loss | 0.0424 | NUP210L, TPM3 | Within DMR |
| chr8 | − 5.66 | − 5.25 | 7.8 | Loss | 0.0425 | INTS9 | Within DMR |
| chr12 | − 5.04 | − 5.60 | 10.0 | Gain | 0.0474 | EFCAB4B | Within DMR |
| chr12 | − 3.84 | − 3.49 | 9.7 | Loss | 0.0474 | SLC2A14 | Within DMR |
*DMR within the region typically not included in the genome-wide association studies. These associations are at a high risk of being false-positive hits (see Supplementary File S1)
MTinnitus and MNoTinnitus present average M-values for CpG probes within DMRs for tinnitus and no tinnitus groups, respectively. Gain (or hypermethylation) was defined as an average M-value higher (more positive) for the tinnitus group than no tinnitus group. Loss (or hypomethylation) was defined as an average M-value lower (less positive) for the tinnitus group than no tinnitus group
Fig. 2.
A Manhattan plot presenting the significance levels across the genome-wide CpG islands showing the differentially methylation regions (DMR) associated with chronic tinnitus
DMRs Involving Regions Typically Excluded in Genetic Association Studies
DMRs involving HLA-DPB2, HLA-DQB1, and MUC4 were within the major histocompatibility complex (HLA) regions (Table 3). The methylation levels of the CpG probes within these regions could be driven by nearby genetic variants. The present study included 45 participants for whom we had access to low-pass whole genome sequencing data [for methodological details, review 58]. We extracted single nucleotide polymorphisms (SNPs) within or near the associated DMRs (Supplementary File S1). The relationship between the candidate SNPs and CpG probes within the associated DMRs was investigated. Two SNPs (rs2550262 and rs113387976) within DMR involving MUC4 revealed significant associations with methylation levels of the CpG probes, but they revealed no association with tinnitus (Supplementary File S1). The main effect of tinnitus was not significant after controlling for the effect of SNPs using the linear mixed model. These results indicate that the relationship between tinnitus and methylation levels in the region involving MUC4 could be influenced by common SNPs within the region. For DMRs involving HLA-DPB2 and HLA-DQB1, we identified common SNPs showing significant associations with the methylation levels of CpG probes. The main effect of tinnitus was not significant after controlling for the effect of SNPs using the linear mixed model. These observations indicate that the burden of genetic variation could influence differences in methylation levels within the regions. It is imperative to exercise caution while interpreting the relationships between tinnitus and DMRs involving HLA-DPB2, HLA-DQB1, and MUC4 (Table 3). The replication analysis should be conducted on large independent cohorts for accurate identification of DMRs involving the HLA region.
ebGSEA Enrichment Analysis
Reactome olfactory signaling pathway (AUC = 0.58, p = 3*10−7, adjusted p = 0.002) and Kegg olfactory signaling pathway (AUC = 0.56, p = 5*10−6, adj p = 0.02) revealed significant association with tinnitus. No other gene set revealed a significant association with tinnitus.
Cluster Analysis
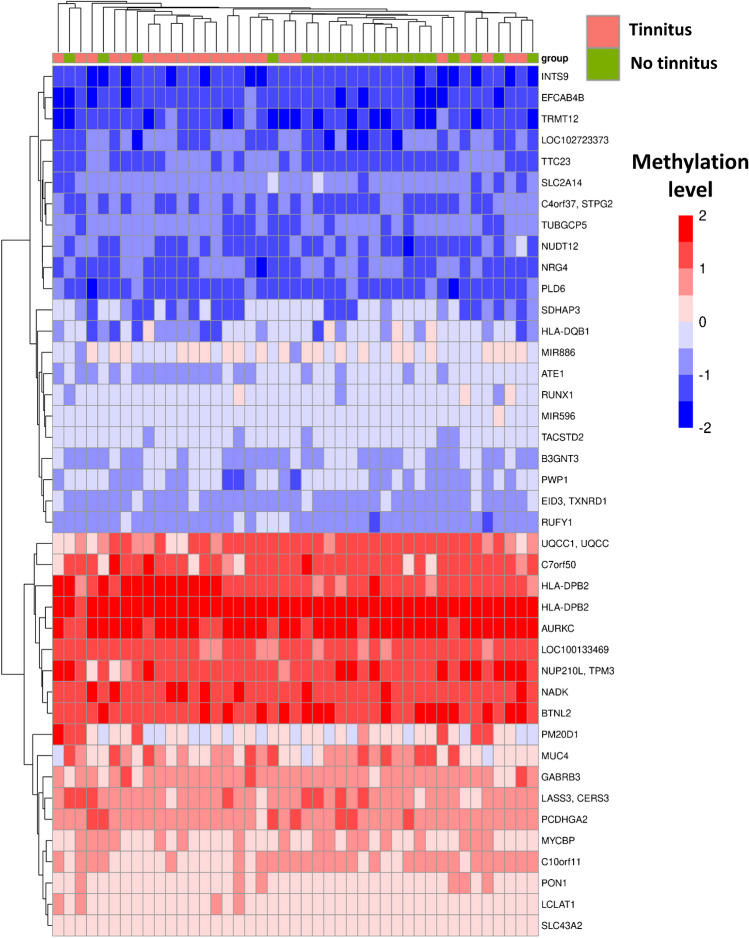
We performed a cluster analysis using the average methylation levels for DMRs associated with tinnitus. The analysis included 25 DMRs achieving adjusted p-value < 0.1 (Supplementary File S1). Figure 3 presents the heatmap showing the cluster analysis results using K-mean clustering. The figure clusters participants on the X-axis and methylation levels of the associated DMRs (genes) on the Y-axis. We found that the average methylation levels at the associated DMRs could efficiently separate individuals with tinnitus from those without tinnitus, with 20 cases (out of 24) clustered on the left-hand side.
Fig. 3.
A heatmap showing the results of the cluster analysis. The average methylation levels are shown for the DMRs associated with chronic tinnitus (for adjusted p-value < 0.1). The figure clusters participants on the X-axis and methylation levels of the associated DMRs (genes) on the Y-axis. The figure suggests that average methylation levels at the associated DMRs collectively could cluster individuals with tinnitus (20/24 on the left-hand side) and no tinnitus (20/24 on the right-hand side)
Discussion
This pilot study examined DNA methylation markers using saliva-derived DNA to identify molecular mechanisms involved in tinnitus. The study was conducted on a cohort of healthy young adults. The cases and controls were matched for age, sex, and ethnicity. We obtained 25 DMRs associated with continuous bilateral chronic tinnitus in young adults. Individuals with tinnitus revealed a significantly higher prevalence of a history of reoccurring ear infections, consistent with past studies [8, 49]. Consistent with the prior knowledge of comorbid conditions associated with tinnitus, the present study identified genes within or close to the DMRs involved in auditory functions, chemical dependency, cardiovascular diseases, immune, metabolic, reproductive, aging, cancer, and neurologic conditions (Table 4). Our pilot study highlighted the need for a large-scale epigenetic investigation of tinnitus to accurately identify DMRs involved in tinnitus. Table 4 presents the top significant DMR results associated with chronic tinnitus, genes found within or near DMRs, and diseases related to these genes. We used the Harmonize search engine to conduct the literature search [58].
Table 4.
Genes within or close to the DMRs associated with tinnitus, and their associations with traits and diseases (obtained with a Harmonizome search engine: https://maayanlab.cloud/Harmonizome/)
| DMR# | Genes within DMR | Gene name | Gene-disease associations (obtained with GAD and CDT databases) | High-level gene-disease associations |
|---|---|---|---|---|
| DMRs 2 and 5 | HLA-DPB2 | Major histocompatibility complex, class II, DP beta 2 (pseudogene) | Abortion—spontaneous, creatinine, electrocardiography, glomerulonephritis, myocardial infarction, tunica media, systemic sclerosis, | Cardiovascular, immune, metabolic, renal, reproduction |
| DMR 3 | PM20D1 | Peptidase M20 domain containing 1 | Human immunodeficiency virus disease, Parkinson’s disease | Infection, neurological |
| DMR 4 | TMEM18 | Transmembrane protein 18 | Body mass index, body weight, obesity, celiac disease, type 2 diabetes, anorexia nervosa, asthma | Immune, metabolic, psychological |
| SNTG2 | Syntrophin gamma 2 | Eosinophils, child development disorders, autistic disorder, drug-induced liver injury, hyperplasia, necrosis | Hematological, metabolic | |
| DMR 6 | MUC4 | Mucin 4, cell surface associated | Asthma, bronchiolitis, respiratory syncytial virus infections, atopy | Immune, infection, reproduction |
| DMR 9 | LCLAT1 | Lysocardiolipin acyltransferase 1 | Alcoholism, hyperplasia, inflammation | Cardiovascular, chemical dependency |
| DMR 10 | miR886 | A non-coding RNA | Not reported (miRNA) | Not reported |
| DMR 13 | miR596 | A non-coding RNA | Not reported (miRNA) | Not reported |
| DMR 17 | TXNRD1 | Thioredoxin reductase 1 | Acquired immunodeficiency syndrome, adenoma, colorectal neoplasms, aging—telomere length, amyotrophic lateral sclerosis, glucose, arsnic exposure, breast cancer, cognitive traits | Aging, cancer, infection, metabolic, neurological, pharmacogenomic |
| EID3 | EP300 Interacting Inhibitor of Differentiation 3 | Inflammation, drug-induced liver injury, kindness diseases, necrosis | Metabolic | |
| DMR 21 | RUNX1 | RUNX family transcription factor 1 | Arthritis, rheumatoid, bilirubin, bone mineral density, colitis, Crohn’s disease, esophageal neoplasms, hepatopulmonary syndrome, liver cirrhosis, leukemia, myeloid, myelodysplastic syndromes, stroke, tobacco use disorder, type 2 diabetes, edema rosiglitazone, Vitamin D, asthma, chronic myelomonocytic leukemia, diabetes, type 1, leukemia, lupus erythematosus, prostate cancer, psoriasis, rheumatoid arthritis, schizophrenia | Cancer, chemical dependency, hematological, immune, metabolic, neurological, other, pharmacogenomic |
| DMR 22 | SDHAP3 | SDHA pseudogene 3 | Blood pressure, cholesterol, echocardiography, erythrocytes, hemoglobin | Cardiovascular, hematological, metabolic |
| DMR 23 | RUFY1 | RUN and FYVE domain containing 1 | Amyotrophic lateral sclerosis | Neurological |
| DMR 24 | NUDT12 | Nudix hydrolase 12 | Alanine transaminase, hearing loss, arteries, blood proteins, body height, body weights and measures, platelet count, tobacco use disorder, waist circumference, | Cardiovascular, chemical dependency, developmental, hematological, metabolic, other |
| DMR 27 | TTC23 | Tetratricopeptide Repeat Domain 23 | Erythrocytes, stroke, drug-induced liver injury | Hematological, cardiovascular |
| DMR 32 | CERS3 (LASS3) | Ceramide Synthase 3 | Albumins, triglycerides | Metabolic |
| DMR 28 | SLC43A2 | Solute carrier family 43 member 2 | Tobacco use disorder, type 2 diabetes, edema, rosiglitazone | Chemical dependency, pharmacogenomic |
| DMR 14 | C10orf11 (LRMDA) | Leucine Rich Melanocyte Differentiation Associated | Blood flow velocity, calcium, coronary artery disease, erythrocyte count, respiratory function tests, tamoxifen, tobacco use disorder, uric acid | Not reported |
| DMR 33 | HLA-DQB1 | Major histocompatibility complex, class II, DQ beta 1(HLA-DQB1) | Abortion, acquired immunodeficiency syndrome, tuberculosis, Addison disease, allergies, arthritis, asthma, diabetes type 1 and type 2, autoimmune diseases, rheumatic fever, Whipple disease, and more | Immune, infection, cancer, metabolic, normal variation, cardiovascular, reproduction, neurological, hematological, psychiatric, renal, vision, pharmacogenomic |
| DMR 1 | NADK | NAD kinase(NADK) | Tobacco use disorder | Chemical dependency |
| DMR 34 | C4orf37 (STPG2) | Sperm Tail PG-Rich Repeat Containing 2 | Tobacco use disorder | Chemical dependency |
| DMR 15 | SZRD1 | SUZ RNA Binding Domain Containing 1 | Hypertrophy, liver neoplasms, kidney diseases, inflammation, weight gain, and more | Not reported |
| MFAP2 | Microfibril Associated Protein 2 | Body height, exfoliation syndrome, glaucoma, respiratory function tests | Developmental, vision, cardiovascular | |
| DMR 18 | NUP210L | Nucleoporin 210 kDa-Like | ||
| TPM3 | Tropomyosin 3 | Nemaline myopathy | Other | |
| DMR 35 | INTS9 | Integrator Complex Subunit 9 | ||
| DMR 36 | EFCAB4B | EF-hand calcium binding domain 4B | Not reported | Not reported |
| DMR 37 | SLC2A14 | Solute Carrier Family 2 Member 14 | Alcoholism | Chemical dependency |
| DMR 38 | TRMT12 | TRNA Methyltransferase 12 Homolog | Infertility (male), drug-induced liver injury, necrosis, inflammation, cardiovascular abnormalities | Not reported |
DMR Involving MUC4 Associated with Tinnitus in Military Veterans
DMR involving MUC4 revealed a significant association with tinnitus. Two nearby SNPs revealed significant associations with methylation levels of the CpG probes within the region, but both SNPs showed no association with tinnitus. The results indicate weak evidence of the association between DMR involving MUC4 and tinnitus, and a high risk of a false positive association. DMR involving MUC4 revealed a significant association with tinnitus in a past genome-wide DNA methylation study with 59 veterans exposed to impulse noise, 29 reporting tinnitus [38]. MUC4 is a member of the mucin gene family that forms glycoproteins in mucus, essential for the protection, renewal, and differentiation of the epithelial cells [59]. MUC4 is expressed in human middle ear epithelial tissues and is associated with middle ear effusion [60, 61]. Epidemiological studies showed a significant association between tinnitus and reoccurring ear infections in youth [8, 49]. In addition to the expression in the epithelial tissue of the middle ear, MUC4 is associated with metastatic cancers and is vital for orchestrating the immune response [62, 63]. Future research is necessary to investigate the burden of genetic variants on the methylation levels and influence of epigenetic modification of MUC4 on tinnitus.
DMRs Associated with Tinnitus in Young Adults
A loss of methylation levels in DMR involving Peptidase M20 domain containing 1 (PM20D1) was associated with tinnitus in the present study. PM20D1 encodes a biosynthesized enzyme that is important for adaptive thermogenesis by catalyzing fatty acids and amino acids into bioactive N-acyl amino acids without engaging uncoupling protein 1 [64]. PM20D1 is associated with metabolic diseases, such as obesity, insulin resistance, and type II diabetes [65]. Tinnitus is associated with obesity, body mass index, type II diabetes, and other metabolic phenotypes [66, 67]. Epigenetic modification of PM20D1 is associated with Alzheimer's disease [68]. Tinnitus is associated with a higher risk for Alzheimer's disease [69]. The epigenetic modification of PM20D1 might lie at the crossroads of tinnitus, metabolic diseases, and neuropsychiatric conditions.
Genes, such as SLC43A2, NADK, NUDT12, and C4orf37 (STPG2), involved in chemical dependency that are also essential for metabolic processes, showed association with tinnitus. Active and passive smoking are known risk factors for tinnitus in children and young adults. Tinnitus is associated with toxic chemical exposure [70]. The long-term effects of environmental exposure (including toxic chemicals, smoking, and noise) might be reflected in DNA methylation [71]. In this study, only one participant with tinnitus reported exposure to smoking. Self-reported smoking status might not be accurate as young adults frequently underreport smoking behavior [72]. Besides, the exposure to passive smoking in early childhood might not be efficiently quantified using self-reported measures. The epigenetic modifications of the genes involved in chemical dependency essential for regulating metabolic processes might reflect a subclinical influence of toxic compounds on tinnitus.
SLC43A2 is a solute carrier (SLC) family member that encodes LAT4 amino acid transporter. DMR involving SLC43A2 was associated with tinnitus (Table 3). Epigenetic modification of SLC43A2 is associated with smoking and social anxiety disorder [42, 73]. Exposure to cognitive behavioral therapy, frequently prescribed to individuals with anxiety disorder (and tinnitus), could modify methylation levels in SLC43A2 [74]. Another gene from the SLC family, SLC2A14, implemented in glucose and vitamin C transport [75], showed an association with tinnitus. Individuals with tinnitus revealed significantly lower methylation levels at the CpG region involving SLC2A14.
NADK encodes a protein that catalyzes nicotinamide adenine dinucleotide phosphate (NADP). It is essential for energy metabolism and signal transduction [76]. Hypomethylation in NADK is associated with cigarette smoking, maternal exposure to heavy metals during pregnancy, and obesity [42, 77, 78]. Nicotinamide adenine dinucleotide (NAD) + metabolism is associated with acquired hearing loss [79]. Another gene involved in NAD + metabolism, NUDT12, an enzyme that facilitates de-capping RNAs with NAD caps [80], is associated with tinnitus in the present study. The epigenetic modification of NUDT12 is related to genomic imprinting [81].
Cardiovascular diseases are known risk factors for tinnitus [82]. DMR involving genes related to cardiovascular diseases, such as SDHAP3 and TTC23, were associated with tinnitus. Epigenetic modification of DMR involving SDHAP3 is associated with atherosclerosis, autism, and endemic fluorosis [83–85]. A DMR involving another protein-coding gene, TTC23, involved in the hedgehog signaling pathway essential for cochlear development and homeostasis [86, 87], showed significant association with tinnitus. Genetic mutations in TTC23 are associated with ciliopathies and Ring chromosome 15 syndrome [88, 89]. It is important to note that the present study included healthy young adults with no significant medical history. The changes in the methylation status might reflect subclinical changes in the physiological status that often precede clinical diagnosis.
DMR involving RUNX1 showed a significant association with chronic tinnitus. RUNX1 encodes a master transcription factor that interacts with core binding factor beta to form a core binding complex, which is essential for hematopoiesis. Somatic mutation in RUNX1 is associated with various hematological traits [90]. RUNX1 is implemented in DNR repair processes and is necessary for maintaining genomic integrity following environmental stresses [91]. Emerging evidence suggests that RUNX1 interacts with the epigenome machinery to regulate DNA methylation [92]. The functional significance of RUNX1 in hearing loss and tinnitus remains elusive. Further research is needed to investigate the role of epigenetic modulation of RUNX1 in tinnitus.
DMR involving SNTG2 and TMEM18 revealed a significant association with tinnitus. SNTG2 is a protein-coding gene and a member of the syntrophin family, which encodes sytrophins. Syntrophins are found in neuromuscular junctions and the central nervous system, regulating mechanosensitive sodium channels [93]. Syntrophins function as scaffolding proteins localized exclusively in inhibitory post-synaptic junctions [94], where the epigenetic modification of SNTG2 might impair inhibitory processes involved in tinnitus. Within the DMR involving SNTG2, we identified another gene, TMEM18, a transmembrane protein coding gene putatively involved in cell migration. TMEM18 is expressed in the central nervous system. It is involved in regulating appetite and body weight [95]. Dysregulated TMEM18 activities are associated with obesity-related phenotypes [96, 97].
Non-coding RNAs are essential for gene expression and regulation. A growing body of literature highlights their functional significance in multisensory organisms [98]. In the present study, DMRs involving non-coding RNAs, such as miR886 and miR596, were significantly associated with tinnitus. miR886 is associated with Friedreich ataxia, which often accompanies sensory loss and peripheral neural dysfunction [99]. miR596 is an epigenetically modifiable tumor suppressor associated with cancer and idiopathic pulmonary artery hypertension [100, 101].
Enrichment Analysis with ebGSEA
ebGSEA is a method for conducting enrichment analysis using genome-wide DNA methylation data [56]. It performs a global test using all CpG probes mapped to a specific gene and ranks their overall methylation differences to run GSEA; thereby, it avoids a classical bias of favoring genes containing more CpG probes in the enrichment analysis. Intriguingly, the olfactory signaling pathway revealed a significant association with tinnitus. About 28 olfactory genes, including OR1A2, OR2A25, and OR4N4, were shared between a gene set associated with tinnitus in the present study and the olfactory signaling pathway (Supplementary File S1). Olfactory dysfunction is typically not evaluated in tinnitus patients in audiology clinics. Recent evidence from a large-scale epidemiological study showed that individuals with tinnitus exhibit a higher prevalence of olfactory dysfunction, with the severity of olfactory dysfunction showing a significant correlation with tinnitus severity [102]. The neurobiological substrate of the association remains elusive. The studies investigating auditory-olfactory integration showed that about 19% of olfactory tubercle single units could respond to sound and showed phase synchrony between auditory and olfactory cortices [103]. Further research is needed to investigate the influence of epigenetic modification of genes involved in tinnitus and olfactory signaling pathways.
Limitations of the Study
The present study investigated the associations between tinnitus and DMRs in a small case–control cohort of European ethnicity. The results of the pilot study must be replicated using large biobank-scale cohorts including individuals with diverse ethnic backgrounds. Our study utilized Infinium Human Methylation EPIC BeadChip, a cost-effective method for quantifying methylation levels at a predefined set of CpG sites. However, the EPIC BeadChip covers only a fraction of the total CpG sites in the genome. Additionally, probe-specific biases and cross-reactivity could confound the DNA methylation levels estimated with Infinium methylation arrays [104]. Whole genome bisulfite sequencing is more accurate in quantifying DNA methylation across the genome [105]. Future studies should utilize whole genome bisulfite sequencing to investigate the epigenetic landscape of tinnitus.
Conclusions
Our pilot genome-wide methylation analysis utilizing saliva-derived DNA samples identified 25 DMRs involved in tinnitus. Consistent with the literature on comorbidities associated with tinnitus, we identified genes within or close to DMRs involved in auditory functions, chemical dependency, cardiovascular diseases, psychiatric conditions, immune disorders, and metabolic syndromes. Further research with a larger sample size is required to identify novel epigenetic biomarkers of tinnitus and replicate our findings. Further mechanistic studies are necessary to understand the molecular mechanisms underlying the epidemiological associations.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- DMR
Differentially methylated regions
- GWAS
Genome-wide association study
- SNPs
Single nucleotide polymorphism
- CpG
Cytosine-phosphate-guanine
- NADP
Nicotinamide adenine dinucleotide phosphate
- NAD
Nicotinamide adenine dinucleotide
- ebGSEA
Empirical Bayesian Gene Set Enrichment Analysis
Author Contribution
The authors are responsible for the content and writing of the paper. IB performed data collection and analysis and prepared the first draft of the manuscript. JARG prepared Supplementary file S1. RD conducted the statistical analyses involving methylation data. AT supervised the statistical analysis and helped with the study design.
Funding
The study utilized a case–control sample collected as part of a project funded by the National Institute on Deafness and Other Communication Disorders, R21DC016704-01A1.
Data Availability
The deidentified data will be shared on dbGaP after the completion of the project (R21DC016704-01A1).
Code Availability
The codes used for data processing and statistical analysis are available at https://github.com/Bhatt-lab-bioinfo-tools/ewas_tinnitus.
Declarations
Ethics Approval
The Institutional Review Board of the University of Iowa approved the protocol for the present study (IRB#202010165).
Completing Interests
The author reports no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Tinnitus Association (2023) Why are my ears ringing? https://www.ata.org/about-tinnitus/why-are-my-ears-ringing/. Accessed 18 Aug 2023
- 2.Theodoroff SM, Lewis MS, Folmer RL, Henry JA, Carlson KF (2015) Hearing impairment and tinnitus: prevalence, risk factors, and outcomes in US service members and veterans deployed to the Iraq and Afghanistan wars. Epidemiol Rev 37(1):71–85 [DOI] [PubMed] [Google Scholar]
- 3.Masterson EA, Themann CL, Luckhaupt SE, Li J, Calvert GM (2016) Hearing difficulty and tinnitus among US workers and non-workers in 2007. Am J Ind Med 59(4):290–300 [DOI] [PubMed] [Google Scholar]
- 4.Langguth B, Kreuzer PM, Kleinjung T, De Ridder D (2013) Tinnitus: causes and clinical management. The Lancet Neurology 12(9):920–930 [DOI] [PubMed] [Google Scholar]
- 5.Henry JA, Roberts LE, Caspary DM, Theodoroff SM, Salvi RJ (2014) Underlying mechanisms of tinnitus: review and clinical implications. J Am Acad Audiol 25(01):005–022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kostev K, Alymova S, Kössl M, Jacob L (2019) Risk factors for tinnitus in 37,692 patients followed in general practices in Germany. Otol Neurotol 40(4):436–440 [DOI] [PubMed] [Google Scholar]
- 7.Bhatt JM, Lin HW, Bhattacharyya N (2016) Prevalence, severity, exposures, and treatment patterns of tinnitus in the United States. JAMA Otolaryngol-Head Neck Surg 142(10):959–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahboubi H, Oliaei S, Kiumehr S, Dwabe S, Djalilian HR (2013) The prevalence and characteristics of tinnitus in the youth population of the United States. Laryngoscope 123(8):2001–2008 [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Escamez JA, Amanat S (2020) Heritability and genetics contribution to tinnitus. Otolaryngol Clin North Am 53(4):501–513 [DOI] [PubMed] [Google Scholar]
- 10.Cederroth CR, PirouziFard M, Trpchevska N, Idrizbegovic E, Canlon B, Sundquist J, Sundquist K, Zöller B (2019) Association of genetic vs environmental factors in Swedish adoptees with clinically significant tinnitus. JAMA Otolaryngol-Head Neck Surg 145(3):222–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogo R, Farah A, Karlsson KK, Pedersen NL, Svartengren M, Skjönsberg Å (2017) Prevalence, incidence proportion, and heritability for tinnitus: a longitudinal twin study. Ear Hear 38(3):292–300 [DOI] [PubMed] [Google Scholar]
- 12.Maas IL, Brüggemann P, Requena T, Bulla J, Edvall NK, Hjelmborg JVB, Szczepek AJ et al (2017) Genetic susceptibility to bilateral tinnitus in a Swedish twin cohort. Genetics in Medicine 19(9):1007–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vona B, Nanda I, Shehata-Dieler W, Haaf T (2017) Genetics of tinnitus: still in its infancy. Front Neurosci 11:236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clifford RE, Maihofer AX, Stein MB, Ryan AF, Nievergelt CM (2020) Novel risk loci in tinnitus and causal inference with neuropsychiatric disorders among adults of European ancestry. JAMA Otolaryngol-Head Neck Surg 146(11):1015–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatt IS, Wilson N, Dias R, Torkamani A (2022) A genome-wide association study of tinnitus reveals shared genetic links to neuropsychiatric disorders. Sci Rep 12(1):22511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells HR, Abidin FNZ, Freidin MB, Williams FM, Dawson SJ (2021) Genome-wide association study suggests that variation at the RCOR1 locus is associated with tinnitus in UK Biobank. Sci Rep 11(1):6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clifford RE, Maihofer AX, Chatzinakos C, Coleman JR, Daskalakis NP, Gasperi M et al (2024) Genetic architecture distinguishes tinnitus from hearing loss. Nat Commun 15(1):614 [DOI] [PMC free article] [PubMed]
- 18.Urbanek ME, Zuo J (2021) Genetic predisposition to tinnitus in the UK Biobank population. Sci Rep 11(1):18150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amanat S, Gallego-Martinez A, Sollini J, Perez-Carpena P, Espinosa-Sanchez JM, Aran I et al (2021) Burden of rare variants in synaptic genes in patients with severe tinnitus: An exome based extreme phenotype study. EBioMedicine 66 [DOI] [PMC free article] [PubMed]
- 20.Jin Z, Liu Y (2018) DNA methylation in human diseases. Genes & diseases 5(1):1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore LD, Le T, Fan G (2013) DNA methylation and its basic function. Neuropsychopharmacology 38(1):23–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han L, Su B, Li WH, Zhao Z (2008) CpG island density and its correlations with genomic features in mammalian genomes. Genome Biol 9(5):1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Human Genome Sequencing Consortium (2001) Initial sequencing and analysis of the human genome. Nature 409(6822):860–921 [DOI] [PubMed]
- 24.Bibikova M, Barnes B, Tsan C, Ho V, Klotzle B, Le JM et al (2011) High density DNA methylation array with single CpG site resolution. Genomics 98(4):288–295 [DOI] [PubMed]
- 25.Eckhardt F, Lewin J, Cortese R, Rakyan VK, Attwood J, Burger M et al (2006) DNA methylation profiling of human chromosomes 6, 20 and 22. Nat Gen 38(12):1378–1385 [DOI] [PMC free article] [PubMed]
- 26.Medvedeva YA, Fridman MV, Oparina NJ, Malko DB, Ermakova EO, Kulakovskiy IV, Heinzel A, Makeev VJ (2010) Intergenic, gene terminal, and intragenic CpG islands in the human genome. BMC Genomics 11(1):1–16 [DOI] [PMC free article] [PubMed]
- 27.Gardiner-Garden M, Frommer M (1987) CpG islands in vertebrate genomes. J Mol Biol 196(2):261–282 [DOI] [PubMed] [Google Scholar]
- 28.Antequera F, Bird A (1993) Number of CpG islands and genes in human and mouse. Proc Natl Acad Sci 90(24):11995–11999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varley KE, Gertz J, Bowling KM, Parker SL, Reddy TE, Pauli-Behn F et al (2013) Dynamic DNA methylation across diverse human cell lines and tissues. Genome Res 23(3):555–567 [DOI] [PMC free article] [PubMed]
- 30.Montano C, Taub MA, Jaffe A, Briem E, Feinberg JI, Trygvadottir R et al (2016) Association of DNA methylation differences with schizophrenia in an epigenome-wide association study. JAMA Psychiatry 73(5):506–514 [DOI] [PMC free article] [PubMed]
- 31.Sabunciyan S, Aryee MJ, Irizarry RA, Rongione M, Webster MJ et al (2012) Genome-wide DNA methylation scan in major depressive disorder. PLoS One 7(4):e34451 [DOI] [PMC free article] [PubMed]
- 32.Pidsley R, Mill J (2011) Epigenetic studies of psychosis: current findings, methodological approaches, and implications for postmortem research. Biol Psychiat 69(2):146–156 [DOI] [PubMed] [Google Scholar]
- 33.Sjöholm LK, Ransome Y, Ekström TJ, Karlsson O (2018) Evaluation of post-mortem effects on global brain DNA methylation and hydroxymethylation. Basic Clin Pharmacol Toxicol 122(2):208–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishitani S, Isozaki M, Yao A, Higashino Y, Yamauchi T, Kidoguchi M et al (2023) Cross-tissue correlations of genome-wide DNA methylation in Japanese live human brain and blood, saliva, and buccal epithelial tissues. Transl Psychiatry 13(1):72 [DOI] [PMC free article] [PubMed]
- 35.Braun PR, Han S, Hing B, Nagahama Y, Gaul LN, Heinzman JT et al (2019) Genome-wide DNA methylation comparison between live human brain and peripheral tissues within individuals. Transl Psychiatry 9(1):47 [DOI] [PMC free article] [PubMed]
- 36.Langie SA, Moisse M, Declerck K, Koppen G, Godderis L, Vanden Berghe W et al (2017) Salivary DNA methylation profiling: aspects to consider for biomarker identification. Basic Clin Pharmacol Toxicol 121:93–101 [DOI] [PMC free article] [PubMed]
- 37.Fransen E, Cassiers LL, Chubar V, Gilles A, Van Rompaey V, van der Werf I et al (2023) Differential effect of panic on the DNA methylation of the glucocorticoid receptor gene exon 1F in chronic subjective tinnitus with distress. Psychiatric Gen 33(4):134–144 [DOI] [PMC free article] [PubMed]
- 38.Wang Z, Wilson CM, Ge Y, Nemes J, LaValle C, Boutté A et al (2020) DNA methylation patterns of chronic explosive breaching in U.S. Military warfighters. Front Neurol 11:1010 [DOI] [PMC free article] [PubMed]
- 39.Orenay-Boyacioglu S, Caliskan M, Boyacioglu O, Coskunoglu A, Bozkurt G, Cam FS (2019) Chronic tinnitus and BDNF/GDNF CpG promoter methylations: a case–control study. Mol Biol Rep 46(4):3929–3936 [DOI] [PubMed] [Google Scholar]
- 40.de Vries M, van der Plaat DA, Nedeljkovic I, Verkaik-Schakel RN, Kooistra W, Amin N et al (2018) From blood to lung tissue: effect of cigarette smoke on DNA methylation and lung function. Respiratory Res 19(1):1–9 [DOI] [PMC free article] [PubMed]
- 41.Lee KW, Pausova Z (2013) Cigarette smoking and DNA methylation. Front Genet 4:132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joehanes R, Just AC, Marioni RE, Pilling LC, Reynolds LM, Mandaviya PR et al (2016) Epigenetic signatures of cigarette smoking. Circ Cardiovasc Genet 9(5):436–447 [DOI] [PMC free article] [PubMed]
- 43.Christensen CH, Chang JT, Rostron BL, Hammad HT, van Bemmel DM, Del Valle-Pinero AY et al (2021) Biomarkers of inflammation and oxidative stress among adult former smoker, current e-cigarette users—results from wave 1 PATH study. Cancer Epidemiol Biomarkers 30(10):1947–1955 [DOI] [PMC free article] [PubMed]
- 44.Ringh MV, Hagemann-Jensen M, Needhamsen M, Kular L, Breeze CE, Sjöholm LK et al (2019) Tobacco smoking induces changes in true DNA methylation, hydroxymethylation and gene expression in bronchoalveolar lavage cells. EBioMedicine 46:290–304 [DOI] [PMC free article] [PubMed]
- 45.Barcelona V, Huang Y, Brown K, Liu J, Zhao W, Yu M et al (2019) Novel DNA methylation sites associated with cigarette smoking among African Americans. Epigenetics 14(4):l383–391 [DOI] [PMC free article] [PubMed]
- 46.Eze IC, Jeong A, Schaffner E, Rezwan FI, Ghantous A, Foraster M et al (2020) Genome-wide DNA methylation in peripheral blood and long-term exposure to source-specific transportation noise and air pollution: the SAPALDIA study. Environ Health Perspect 128(6):67003. [DOI] [PMC free article] [PubMed]
- 47.Horvath S (2013) DNA methylation age of human tissues and cell types. Genome Biol 14(10):1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horvath S, Zhang Y, Langfelder P, Kahn RS, Boks MP, van Eijk K, van den Berg LH, Ophoff RA (2012) Aging effects on DNA methylation modules in human brain and blood tissue. Genome Biol 13(10):1–18 [DOI] [PMC free article] [PubMed]
- 49.Bhatt IS (2018) Prevalence of and risk factors for tinnitus and tinnitus-related handicap in a college-aged population. Ear Hear 39(3):517–526 [DOI] [PubMed] [Google Scholar]
- 50.Newman CW, Jacobson GP, Spitzer JB (1996) Development of the tinnitus handicap inventory. Arch Otolaryngol-Head Neck Surg 122(2):143–148 [DOI] [PubMed] [Google Scholar]
- 51.Meikle MB, Henry JA, Griest SE, Stewart BJ, Abrams HB, McArdle R et al (2012) The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear 33(2):153–176 [DOI] [PubMed]
- 52.Morris TJ, Butcher LM, Feber A, Teschendorff AE, Chakravarthy AR, Wojdacz TK, Beck S (2014) ChAMP: 450k chip analysis methylation pipeline. Bioinformatics 30(3):428–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aryee MJ, Jaffe AE, Corrada-Bravo H, Ladd-Acosta C, Feinberg AP, Hansen KD, Irizarry RA (2014) Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 30(10):1363–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fortin JP, Triche TJ Jr, Hansen KD (2017) Preprocessing, normalization and integration of the Illumina HumanMethylationEPIC array with minfi. Bioinformatics 33(4):558–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson WE, Li C, Rabinovic A (2007) Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8(1):118–127 [DOI] [PubMed] [Google Scholar]
- 56.Jaffe AE, Murakami P, Lee H, Leek JT, Fallin MD, Feinberg AP, Irizarry RA (2012) Bump hunting to identify differentially methylated regions in epigenetic epidemiology studies. Int J Epidemiol 41(1):200–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong D, Tian Y, Zheng SC, Teschendorff AE (2019) ebGSEA: an improved gene set enrichment analysis method for epigenome-wide-association studies. Bioinformatics 35(18):3514–3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rouillard AD, Gundersen GW, Fernandez NF, Wang Z, Monteiro CD, McDermott MG, Ma’ayan A (2016) The harmonizome: a collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database 2016:baw100. 10.1093/database/baw100 [DOI] [PMC free article] [PubMed]
- 59.Carraway KL, Theodoropoulos G, Kozloski GA, Carothers Carraway CA (2009) Muc4/MUC4 functions and regulation in cancer. Future Oncol 5(10):1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Val S, Poley M, Anna K, Nino G, Brown K, Pérez-Losada M, Gordish-Dressman H, Preciado D (2018). Characterization of mucoid and serous middle ear effusions from patients with chronic otitis media: implication of different biological mechanisms?. Pediatric Res 84(2):296–305 [DOI] [PMC free article] [PubMed]
- 61.Kerschner JE, Khampang P, Erbe CB, Kolker A, Cioffi JA (2009) Mucin gene 19 (MUC19) expression and response to inflammatory cytokines in middle ear epithelium. Glycoconj J 26(9):1275–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao XP, Dong JJ, Xie T, Guan X (2021). Integrative analysis of MUC4 to prognosis and immune infiltration in pan-cancer: friend or foe? Front Cell Dev Biol 9. 10.3389/fcell.2021.695544 [DOI] [PMC free article] [PubMed]
- 63.Sagar S, Leiphrakpam PD, Thomas D, McAndrews KL, Caffrey TC, Swanson BJ et al (2021) MUC4 enhances gemcitabine resistance and malignant behaviour in pancreatic cancer cells expressing cancer-associated short O-glycans. Cancer Lett 503:91–102 [DOI] [PMC free article] [PubMed]
- 64.Long JZ, Svensson KJ, Bateman LA, Lin H, Kamenecka T, Lokurkar IA et al (2016) The secreted enzyme PM20D1 regulates lipidated amino acid uncouplers of mitochondria. Cell 166(2):424–435 [DOI] [PMC free article] [PubMed]
- 65.Yang R, Hu Y, Lee CH, Liu Y, Diaz-Canestro C, Fong CHY et al (2021) PM20D1 is a circulating biomarker closely associated with obesity, insulin resistance and metabolic syndrome. Eur J Endocrinol 186(2):151–161 [DOI] [PubMed]
- 66.Haro-Hernandez E, Perez-Carpena P, Unnikrishnan V, Spiliopoulou M, Lopez-Escamez JA (2022) Standardized Clinical Profiling in Spanish Patients with Chronic Tinnitus. J Clin Med 11(4):978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spankovich C, Gonzalez VB, Su D, Bishop CE (2018) Self reported hearing difficulty, tinnitus, and normal audiometric thresholds, the National Health and Nutrition Examination Survey 1999–2002. Hear Res 358:30–36 [DOI] [PubMed] [Google Scholar]
- 68.Sanchez-Mut JV, Heyn H, Silva BA, Dixsaut L, Garcia-Esparcia P, Vidal E et al (2018) PM20D1 is a quantitative trait locus associated with Alzheimer's disease. Nat Med 24(5):598–603 [DOI] [PubMed]
- 69.Chu HT, Liang CS, Yeh TC, Hu LY, Yang AC, Tsai SJ, Shen CC (2020) Tinnitus and risk of Alzheimer’s and Parkinson’s disease: a retrospective nationwide population-based cohort study. Sci Rep 10(1):1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kirk KM, McGuire A, Nielsen L, Cosgrove T, McClintock C, Nasveld PE, Treloar SA (2011) Self-reported tinnitus and ototoxic exposures among deployed Australian Defence Force personnel. Mil Med 176(4):461–467 [DOI] [PubMed] [Google Scholar]
- 71.Meaney MJ, Szyf M (2005) Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci 7(2):103–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khouja JN, Suddell SF, Peters S, Taylor AE, Munafò MR (2019) Does e-cigarette use in non-smoking young adults act as a gateway to smoking? A systematic review and meta-analysis. medRxiv 19007005 [DOI] [PMC free article] [PubMed]
- 73.Wiegand A, Kreifelts B, Munk MH, Geiselhart N, Ramadori KE, MacIsaac JL et al (2021) DNA methylation differences associated with social anxiety disorder and early life adversity. Transl Psychiatry 11(1):1–10 [DOI] [PMC free article] [PubMed]
- 74.Ziegler C, Grundner-Culemann F, Schiele MA, Schlosser P, Kollert L, Mahr M et al (2019) The DNA methylome in panic disorder: a case-control and longitudinal psychotherapy-epigenetic study. Transl Psychiatry 9(1):1–11 [DOI] [PMC free article] [PubMed]
- 75.Wu X, Freeze HH (2002) GLUT14, a duplicon of GLUT3, is specifically expressed in testis as alternative splice forms. Genomics 80(6):553–557 [DOI] [PubMed] [Google Scholar]
- 76.Lerner F, Niere M, Ludwig A, Ziegler M (2001) Structural and functional characterization of human NAD kinase. Biochem Biophys Res Commun 288(1):69–74 [DOI] [PubMed] [Google Scholar]
- 77.Zeng Z, Huo X, Zhang Y, Hylkema MN, Wu Y, Xu X (2019) Differential DNA methylation in newborns with maternal exposure to heavy metals from an e-waste recycling area. Environ Res 171:536–545 [DOI] [PubMed] [Google Scholar]
- 78.Li S, Wong EM, Bui M, Nguyen TL, Joo JHE, Stone J, et al (2019) Inference about causation between body mass index and DNA methylation in blood from a twin family study. Int J Obesity 43(2):243–252 [DOI] [PubMed]
- 79.Brown KD, Maqsood S, Huang JY, Pan Y, Harkcom W, Li W et al (2014) Activation of SIRT3 by the NAD+ precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metabolism 20(6):1059–1068 [DOI] [PMC free article] [PubMed]
- 80.Sharma S, Grudzien-Nogalska E, Hamilton K, Jiao X, Yang J, Tong L, Kiledjian M (2020) Mammalian Nudix proteins cleave nucleotide metabolite caps on RNAs. Nucleic Acids Res 48(12):6788–6798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pilvar D, Reiman M, Pilvar A, Laan M (2019) Parent-of-origin-specific allelic expression in the human placenta is limited to established imprinted loci and it is stably maintained across pregnancy. Clin Epigenetics 11(1):1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Debette S, Ibrahim Verbaas CA, Bressler J, Schuur M, Smith A, Bis JC et al (2015) Genome-wide studies of verbal declarative memory in nondemented older people: the Cohorts for Heart and Aging Research in Genomic Epidemiology consortium. Biol Psychiatry 77(8):749-763 [DOI] [PMC free article] [PubMed]
- 83.Chi G, Liu Y, MacDonald JW, Reynolds L, Enquobahrie DA, Fitzpatrick A et al (2022). Epigenome-wide analysis of long-term air pollution exposure and DNA methylation in monocytes: results from the Multi-Ethnic Study of Atherosclerosis. Epigenetics 17(3):297-313 [DOI] [PMC free article] [PubMed]
- 84.Mao C, Guan Z, Wu C, Li Y, Qi X (2021) Analysis of DNA methylation in peripheral blood of patients with coal-burning-borne endemic fluorosis. Chin J Endemiol 93–98
- 85.Ladd-Acosta C, Hansen KD, Briem E, Fallin MD, Kaufmann WE, Feinberg AP (2014) Common DNA methylation alterations in multiple brain regions in autism. Mol Psychiatry 19(8):862–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shi H, Wang H, Zhang C, Lu Y, Yao J, Chen Z et al (2022) Mutations in OSBPL2 cause hearing loss associated with primary cilia defects via Sonic Hedgehog signaling. JCI insight. 10.1172/jci.insight.149626 [DOI] [PMC free article] [PubMed]
- 87.Moon KH, Ma JH, Min H, Koo H, Kim H, Ko HW, Bok J (2020) Dysregulation of sonic hedgehog signaling causes hearing loss in ciliopathy mouse models. Elife 9:e56551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lakmini BC, Suntharesan J, de Silva UAMD, Atapattu N (2021) Ring chromosome 15 syndrome presenting with gonadotropin dependent precocious puberty. Sri Lanka Journal of Child Health 50(4):718–720 [Google Scholar]
- 89.Shamseldin HE, Shaheen R, Ewida N, Bubshait DK, Alkuraya H, Almardawi E et al (2020) The morbid genome of ciliopathies: an update. Genetics Med 22(6):1051–1060 [DOI] [PubMed] [Google Scholar]
- 90.Sood R, Kamikubo Y, Liu P (2017) Role of RUNX1 in hematological malignancies. Blood J Am Soc Hematol 129(15):2070–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krishnan V, Ito Y (2017) A regulatory role for RUNX1, RUNX3 in the maintenance of genomic integrity. Adv Exp Med Biol 962:491–510 [DOI] [PubMed] [Google Scholar]
- 92.Suzuki T, Shimizu Y, Furuhata E, Maeda S, Kishima M, Nishimura H et al (2017) RUNX1 regulates site specificity of DNA demethylation by recruitment of DNA demethylation machineries in hematopoietic cells. Blood Adv 1(20):1699–1711 [DOI] [PMC free article] [PubMed]
- 93.Inoue M, Wakayama Y, Jimi T, Shibuya S, Hara H, Unaki A, Kenmochi K (2008) Skeletal muscle syntrophin interactors revealed by yeast two-hybrid assay. Nagoya J Med Sci 70(3–4):117–126 [PubMed] [Google Scholar]
- 94.Yamakawa H, Oyama S, Mitsuhashi H, Sasagawa N, Uchino S, Kohsaka S, Ishiura S (2007) Neuroligins 3 and 4X interact with syntrophin-γ2, and the interactions are affected by autism-related mutations. Biochem Biophys Res Commun 355(1):41–46 [DOI] [PubMed] [Google Scholar]
- 95.Larder R, Sim MFM, Gulati P, Antrobus R, Tung YCL, Rimmington D et al (2017) Obesity-associated gene TMEM18 has a role in the central control of appetite and body weight regulation. Proc Nat Acad Sci USA 114(35):9421–9426 [DOI] [PMC free article] [PubMed]
- 96.Rask-Andersen M, Jacobsson JA, Moschonis G, Chavan RA, Sikder MAN, Allzén E et al (2012) Association of TMEM18 variants with BMI and waist circumference in children and correlation of mRNA expression in the PFC with body weight in rats. Eur J Human Gen 20(2):192–197 [DOI] [PMC free article] [PubMed]
- 97.Rohde K, Keller M, Klös M, Schleinitz D, Dietrich A, Schön MR et al (2014) Adipose tissue depot specific promoter methylation of TMEM18. J Mol Med 92(8):881–888 [DOI] [PubMed]
- 98.Amaral PP, Mattick JS (2008) Noncoding RNA in development. Mammalian genome 19(7):454–492 [DOI] [PubMed] [Google Scholar]
- 99.Mahishi LH, Hart RP, Lynch DR, Ratan RR (2012) miR-886-3p levels are elevated in Friedreich ataxia. J Neurosci 32(27):9369–9373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang Y, Wang ZG, Tang L, Gong SG, Sun YY, Wang L et al (2021) Plasma exosomal miR-596: A novel biomarker predicts survival in patients with idiopathic pulmonary artery hypertension. J Int Med Res 49(3):3000605211002379. [DOI] [PMC free article] [PubMed]
- 101.Endo H, Muramatsu T, Furuta M, Uzawa N, Pimkhaokham A, Amagasa T, Inazawa J, Kozaki KI (2013) Potential of tumor-suppressive miR-596 targeting LGALS3BP as a therapeutic agent in oral cancer. Carcinogenesis 34(3):560–569 [DOI] [PubMed]
- 102.Park DY, Kim HJ, Kim CH, Lee JY, Han K, Choi JH (2018) Prevalence and relationship of olfactory dysfunction and tinnitus among middle-and old-aged population in Korea. PLoS ONE 13(10):e0206328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou G, Lane G, Noto T, Arabkheradmand G, Gottfried JA, Schuele SU et al (2019) Human olfactory-auditory integration requires phase synchrony between sensory cortices. Nature Commun 10(1):1–12 [DOI] [PMC free article] [PubMed]
- 104.Pidsley R, Zotenko E, Peters TJ, Lawrence MG, Risbridger GP, Molloy P et al (2016) Critical evaluation of the Illumina MethylationEPIC BeadChip microarray for whole-genome DNA methylation profiling. Genome Biol 17:1–17 [DOI] [PMC free article] [PubMed]
- 105.Suzuki M, Liao W, Wos F, Johnston AD, DeGrazia J, Ishii J et al (2018) Whole-genome bisulfite sequencing with improved accuracy and cost. Genome Res 28(9):1364–1371 [DOI] [PMC free article] [PubMed]
- 106.Bhatt IS, Ramadugu SK, Goodman S, Bhagavan SG, Ingalls V, Dias R, Torkamani A (2023) Polygenic Risk Score-Based Association Analysis of Speech-in-Noise and Hearing Threshold Measures in Healthy Young Adults with Self-reported Normal Hearing. J Assoc Res Otolaryngol 24(5):513–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The deidentified data will be shared on dbGaP after the completion of the project (R21DC016704-01A1).
The codes used for data processing and statistical analysis are available at https://github.com/Bhatt-lab-bioinfo-tools/ewas_tinnitus.