Abstract
Previous studies have pointed to a potential link between Obstructive Sleep Apnea (OSA) and gastrointestinal diseases, suggesting that this relationship might be influenced by the presence of Metabolic Syndrome. However, the exact role of these factors in determining gastrointestinal diseases has not been thoroughly explored. In our study, we utilized data from the Genome-wide Association Studies (GWAS) database, focusing on OSA, metabolic syndrome characteristics such as Body Mass Index (BMI), waist circumference, triglycerides, cholesterol, hypertension, type 2 diabetes, and common gastrointestinal diseases including chronic gastritis, gastric ulcers, irritable bowel syndrome, colorectal cancer, inflammatory bowel disease, cholecystitis, nonalcoholic fatty liver, and dyspepsia. By applying Single-variable and Multi-variable Mendelian randomization methods, we aimed to assess the correlation between OSA and gastrointestinal diseases and investigate whether this correlation is influenced by metabolic syndrome. Our findings revealed a strong association between OSA and an increased risk of chronic gastritis, gastric ulcers, inflammatory bowel disease, and nonalcoholic fatty liver disease. No significant connections were found with irritable bowel syndrome, colorectal cancer, cholecystitis, or dyspepsia. Additionally, OSA was linked to metabolic syndrome traits like BMI, waist circumference, triglycerides, hypertension, and type 2 diabetes. Further analysis showed that BMI, triglycerides, and hypertension were causally related to inflammatory bowel disease; BMI, waist circumference, hypertension, and type 2 diabetes to nonalcoholic fatty liver disease; and triglycerides, hypertension, and type 2 diabetes to chronic gastritis. The multivariable analysis indicated that hypertension mediates the relationship between OSA and chronic gastritis; BMI, triglycerides, and hypertension mediate the link between OSA and inflammatory bowel disease; and waist circumference mediates the connection between OSA and nonalcoholic fatty liver disease. To wrap up, this finding helps us understand how these issues might be related and stresses the role of metabolic syndrome in preventing them, which could lessen their effect on health.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-77471-x.
Keywords: Obstructive sleep apnea, Gastrointestinal diseases, Metabolic syndrome, Mendelian randomization
Subject terms: Endocrinology, Gastroenterology, Pathogenesis
Introduction
Obstructive sleep apnea (OSA) is a common sleep disorder characterized by the narrowing of the airway due to relaxation of the throat muscles and collapse of soft tissues, leading to disrupted sleep. Symptoms include insomnia, daytime sleepiness, and loud snoring1. The overall prevalence of OSA in the general adult population ranges from 9 to 38%, with rates reaching 78% in certain elderly populations and as high as 90% in men2. OSA is associated with various cardiovascular diseases, including hypertension, arrhythmias, stroke, coronary artery disease, atherosclerosis, and increased overall cardiovascular mortality risk. Additionally, OSA is linked to metabolic dysfunction and is commonly associated with conditions such as Parkinson’s disease (PD), obesity, and hypertension3. In recent years, studies have indicated a correlation between OSA and gastrointestinal disorders such as gastroesophageal reflux disease, Barrett’s esophagus, and gastrointestinal cancers, drawing increasing attention to the impact of OSA on gastrointestinal health4–6.
Sleep disorders often stem from inappropriate lifestyle and dietary habits. Research has shown a correlation between poor sleep quality and functional gastrointestinal disorders and altered gut sensitivity, potentially leading to gastrointestinal symptoms and digestive system diseases in the general population7,8. Sleep patterns are primarily influenced by the circadian rhythm and its associated biological clock, which are regulated by multiple mechanisms directly related to gastrointestinal function. Circadian rhythm disruption is a common factor contributing to sleep issues, and sleep problems play a key role in various gastrointestinal diseases9. For instance, OSA patients have a higher risk of gastrointestinal disorders such as gastric ulcers and gastritis, which may be attributed to factors like chronic inflammation and immune system abnormalities triggered by OSA10. However, due to a lack of high-quality research data and the complexity of assessing the causal bidirectional relationship in traditional epidemiological studies, there is currently no comprehensive study confirming the causal connection between OSA and gastrointestinal diseases.
Metabolic syndrome (MetS) is a collection of metabolic abnormalities associated with increased risks of cardiovascular diseases and diabetes, including hypertension, hyperglycemia, high cholesterol, and obesity. OSA patients often exhibit a higher prevalence of metabolic syndrome11. Accumulating evidence suggests that OSA promotes weight gain, obesity, type 2 diabetes (T2DM), hypertension, and hypercholesterolemia through various mechanisms, forming a vicious cycle between metabolic syndrome and OSA12. Furthermore, studies have shown a close association between metabolic syndrome and gastrointestinal diseases13. Factors such as obesity, high cholesterol, hypertension, and elevated inflammatory markers in metabolic syndrome patients may increase the risk of gastroesophageal reflux, gastrointestinal dysmotility, and gastrointestinal inflammation, thereby contributing to the occurrence of gastrointestinal diseases. As a common risk factor for both OSA and gastrointestinal disorders, metabolic syndrome may play a significant role in their relationship, but further investigation is still needed.
The fundamental principle of Mendelian randomization (MR) is to utilize genetic variations associated with a specific exposure factor (such as lifestyle or physiological indicators) as instrumental variables to assess the causal relationship between the exposure factor and disease or other health outcomes13. Mendelian randomization minimizes confounding effects, and as genetic variations are determined at birth and not influenced by subsequent disease states, it helps avoid problems related to reverse causality. Compared to traditional randomized control trials, Mendelian randomization studies are more cost-effective and faster, utilizing existing large-scale genome-wide association study data for analysis. They simulate the effects of randomized control trials since the distribution of genetic variations is random, which aids in evaluating causal relationships between exposure factors and outcomes14,15. This article aims to explore the causal relationship between obstructive sleep apnea and gastrointestinal diseases using the Mendelian randomization method, as well as to investigate the influence of other factors on this relationship. This research is crucial for providing beneficial diagnosis, treatment, and prevention strategies for related diseases, ensuring comprehensive and accurate medical services for patients.
Materials and methods
Study design
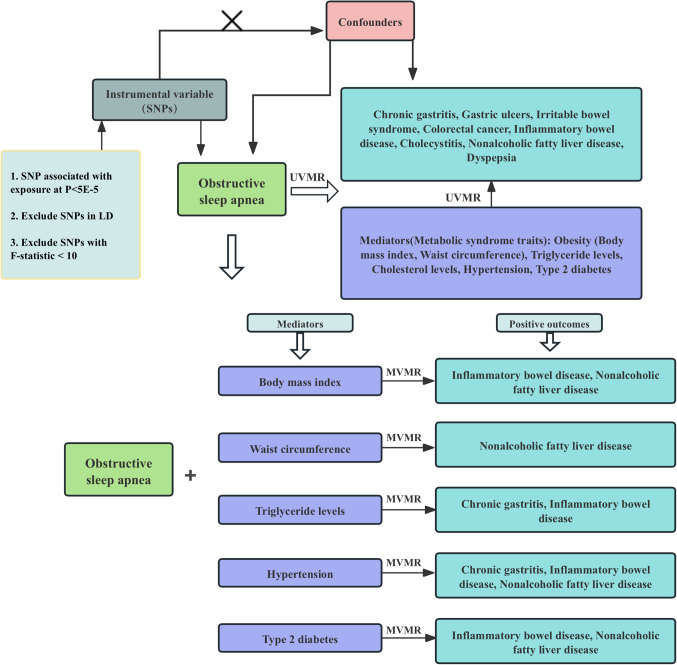
The framework of this Mendelian randomization study is depicted in Fig. 1. The research is divided into two parts. In the first section, we employed univariable Mendelian randomization(UVMR) to investigate potential causal relationships between sleep apnea syndrome and common gastrointestinal diseases: Chronic gastritis, Gastric ulcers, Irritable bowel syndrome, Colorectal cancer, Inflammatory bowel disease, Cholecystitis, Nonalcoholic fatty liver disease, Dyspepsia, as well as candidate mediators associated with metabolic syndrome: Obesity-Body mass index (BMI), Waist circumference, Triglyceride levels, Cholesterol levels, Hypertension, Type 2 diabetes. We further explored whether there were causal relationships between these candidate mediators and positive outcomes. In the second part, we utilized multivariable Mendelian randomization (MVMR) methods to identify mediators that underlie the causal relationship between sleep apnea syndrome and gastrointestinal diseases. In this study, we used genetic variants as instrumental variables (IVs) for MR analysis. The validity of our MR study’s hypothesis is founded on three core assumptions: (1) Relevance assumption: The selected genetic variant (usually single nucleotide polymorphisms, SNPs) must be strongly correlated with the exposure factor being studied. This ensures that the genetic variant can serve as an instrumental variable representing changes in the exposure factor. (2) Independence assumption: The chosen genetic variant should be independent of any other confounding factors that may affect the study outcome. This means that the genetic variant influences the outcome solely through the exposure factor and not through other unconsidered pathways. (3) Exclusion restriction assumption: The effect of the genetic variant on the outcome must be exerted exclusively through the exposure factor and not through any other routes14. These rules out the possibility of the genetic variant directly affecting the outcome, ensuring the validity of the analysis. These assumptions are fundamental for conducting MR studies and help researchers use genetic variants as instrumental variables to explore the causal relationships between specific exposure factors and disease or health outcomes. To mitigate the impact of heterogeneity, this study employed the random-effects model inverse variance weighting (IVW) as the primary method for analysis.
Figure 1.
Flowchart of Mendelian randomization analysis conducted in this study. SVMR analysis investigates the effect of Obstructive Sleep Apnea on gastrointestinal diseases and Metabolic Syndrome traits, MVMR analysis evaluates the roles of Metabolic Syndrome traits mediating the association between Obstructive Sleep Apnea and Gastrointestinal diseases.
Data sources
All genetic instrumental variables are derived from the largest publicly available GWAS summary statistics16 (https://gwas.mrcieu.ac.uk). To avoid population heterogeneity bias, we primarily used summary data from European populations. The datasets for Sleep apnoea, Hypertension, and Type 2 diabetes originate from the FinnGen biobank analysis round 5 (https://www.finngen.fi/fi); Chronic gastritis, Gastric ulcers, Colorectal cancer, Inflammatory bowel disease, Cholecystitis, and Nonalcoholic fatty liver disease data are sourced from the EBI database (https://www.ebi.ac.uk/gwas/downloads/summary-statistics); Body mass index, Waist circumference, Triglyceride, and Cholesterol datasets are from the IEU database (https://gwas.mrcieu.ac.uk/datasets/); Ulcerative colitis and Dyspepsia data are from the UKB database (https://data.bris.ac.uk/data/dataset/pnoat8cxo0u52p6ynfaekeigi). Sample sizes are as indicated in Table 1. All included studies have received ethical approval from their respective institutional review boards, including written informed consent from participants and stringent quality control measures. Since all analyses were conducted using publicly accessible summary data, no additional ethical approval from an institutional review board was required for this study. All details can be found in Table 1.
Table 1.
Details of the phenotypes included in the mendelian randomization analyses.
| Phenotype | Consortium/Author | Ethnicity | Sample size | PubMed ID |
|---|---|---|---|---|
| Obstructive Sleep Apnea | NA | European | 16,761cases, 201,194 controls | 34,017,140 |
| Body mass index | GIANT | European | 322,154 individuals | 25,673,413 |
| Waist circumference | GIANT | European | 232,101 individuals | 25,673,412 |
| Triglyceride | UK Biobank | European | 16,761cases, 201,197 controls | 32,203,549 |
| Cholesterol | GLGC | Mixed | 187,365 individuals | 24,097,068 |
| Hypertension | NA | European | 42,857 cases, 175,935 controls | NA |
| Type 2 diabetes | NA | European | 32,469 cases, 183,185 controls | NA |
| Chronic gastritis | Sakaue S | European | 3,645 cases, 441,451 controls | 34,594,039 |
| Gastric ulcers | NA | European | 3,531 cases, 481,067 controls | 33,959,723 |
| Irritable bowel syndrome | MRC-IEU | European | 10,939 cases, 451,994 controls | NA |
| Colorectal cancer | Sakaue S | European | 6,581 cases, 463,421 controls | 34,594,039 |
| Inflammatory bowel disease | Mbatchou J | European | 404,781 individuals | 34,017,140 |
| Cholecystitis | Sakaue S | European | 9,820 cases, 461,431 controls | 34,594,039 |
| Nonalcoholic fatty liver disease | Ghodsian N | European | 8,434 cases, 770,180 controls | 34,841,290 |
| Dyspepsia | MRC-IEU | European | 7,662 cases, 455,348 controls | NA |
Selection of genetic instrumental variables
To identify qualified genetic IVs that conform to the MR assumptions, a series of tests must be conducted. Firstly, to obtain a sufficient number of instrumental variables and increase statistical power, we set the p-value threshold for IVs at 5E-05 in this study and established independence to eliminate linkage disequilibrium (r^2 < 0.001, window size = 10,000 kb) to select SNPs strongly associated with exposure. Secondly, we calculated the statistical strength of the exposure (F-statistic), where an F-value greater than 10 indicates the absence of weak instrument bias17, with F statistic values for each instrument-exposure association ranging from 19.571 to 81.281 (Table 2). Thirdly, we harmonized exposure and outcome datasets to ensure that the effect alleles belong to the same alleles. The SNPs selected through these rigorous procedures can be used as IVs for subsequent analysis.
Table 2.
The number of SNPs and statistical strength of the exposure (F-statistic).
| Phenotype | nSNPS | F-statistics |
|---|---|---|
| Obstructive Sleep Apnea | 169 | 19.571 |
| Body mass index | 232 | 35.032 |
| Waist circumference | 165 | 30.642 |
| Triglyceride | 632 | 81.281 |
| Hypertension | 266 | 22.926 |
| Type 2 diabetes | 343 | 28.261 |
Statistical analysis and data visualization
The analysis in this study utilized R software packages such as TwoSampleMR, MRPRESSO, MVMR, LASSO, and MendelianRandomization. In univariable Mendelian randomization, IVW was used as the default method to evaluate causal estimates, and was validated using MR-Egger and weighted median methods. The main idea behind the IVW method is to use instrumental variables (IVs) to investigate causality and handle confounding factors. This method is advantageous if the SNPs fully comply with the three principles of MR studies: relevance, consistency, and independence, allowing for correct causal estimates18. Egger’s method is a technique for testing the exogeneity of instrumental variables. It assesses the exogeneity of the IVs by calculating the slope and intercept of the regression coefficients. If the slope is significantly non-zero, there is bias, meaning the IVs are correlated with the error term, necessitating correction with other methods19. The weighted median method is a weight-based approach that multiplies the effect size of each SNP by its corresponding weight and then determines the weighted median of all SNPs as the final causal estimate. This method is beneficial for handling a large number of SNPs and is less affected by outliers20. A p-value less than 0.05 is considered statistically significant across all methods. To ensure the reliability of the results, sensitivity analyses (heterogeneity and pleiotropy tests), MR-Egger intercept tests, and leave-one-out tests were conducted for comparison. Finally, the MR-PRESSO method was employed to detect and remove any outlier SNPs. For multivariable Mendelian randomization, we used IVW and LASSO methods to evaluate causal estimates [citation needed], and applied the MR-Egger method to test whether the outcomes have undetected pleiotropy.
Results
Univariable mendelian randomization
Obstructive sleep apnea and gastrointestinal diseases
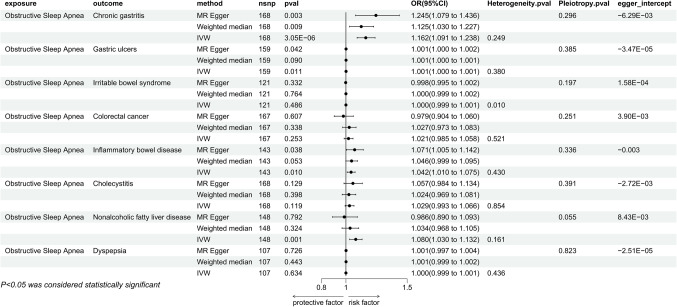
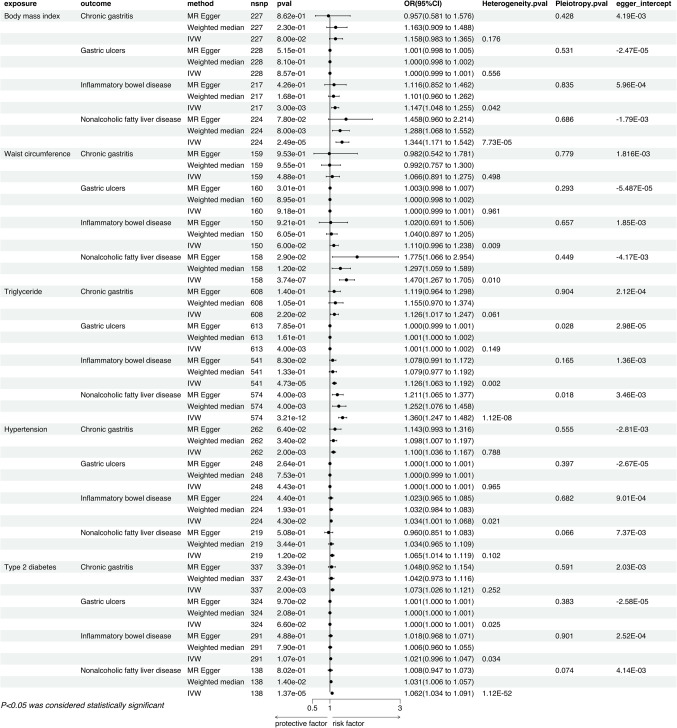
In the analysis employing the IVW method, we discovered a correlation based on genetic predictions between OSA and an increased risk of Chronic Gastritis (P = 3.05E-06, OR = 1.162, 95% CI = 1.091–1.238), Gastric Ulcers (P = 0.011, OR = 1.001, 95% CI = 1.000-1.001), Inflammatory Bowel Disease (P = 0.010, OR = 1.042, 95% CI = 1.010–1.075), and Non-Alcoholic Fatty Liver Disease (P = 0.001, OR = 1.080, 95% CI = 1.030–1.132). Specifically, the causal link between OSA and Chronic Gastritis was significantly validated in both the MR Egger method and the Weighted Median method. The relationship with Gastric Ulcers and Inflammatory Bowel Disease showed significance in the MR Egger method but did not reach significant levels in the Weighted Median method. However, the causal relationship with Non-Alcoholic Fatty Liver Disease did not exhibit significance in either the MR Egger or the Weighted Median method (Fig. 2). Sensitivity analyses indicated no issues with heterogeneity or pleiotropy. The leave-one-out method indicated that there are no individual SNPs solely driving the causal relationships between the exposure and outcomes (Additional file 1), and with no noticeable deviation of the Egger intercept from 0. Results from MR-PRESSO also showed no abnormal SNPs (Additional file 2). Furthermore, our analysis indicated no apparent causal relationships between genetically predicted OSA and Irritable Bowel Syndrome (P = 0.486, OR = 1.000, 95% CI = 0.999–1.001), Colorectal Cancer (P = 0.253, OR = 1.021, 95% CI = 0.985–1.058), Cholecystitis (P = 0.119, OR = 1.029, 95% CI = 0.993–1.066), and Dyspepsia (P = 0.634, OR = 1.000, 95% CI = 0.999–1.001), a finding consistently concluded across three different statistical methods (Fig. 2).
Figure 2.
Mendelian randomization results of the effect of obstructive sleep apnea on gastrointestinal diseases (chronic gastritis, gastric ulcers, irritable bowel syndrome, colorectal cancer, inflammatory bowel disease, cholecystitis, nonalcoholic fatty liver, dyspepsia).
Obstructive sleep apnea and potential mediators
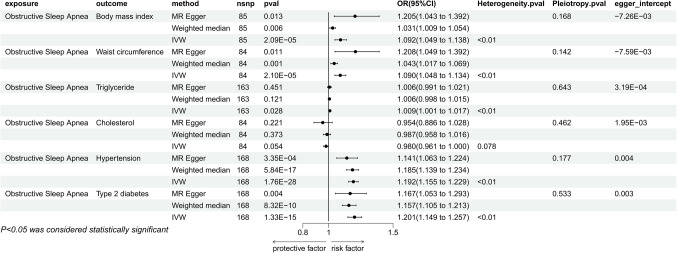
The IVW results indicated that there are causal relationships between genetically predicted OSA and BMI (P = 2.09E-05, OR = 1.092, 95% CI = 1.049–1.138), Waist Circumference (P = 2.10E-05, OR = 1.090, 95% CI = 1.048–1.134), Triglycerides (P = 0.028, OR = 1.009, 95% CI = 1.001–1.017), Hypertension (P = 1.76E-28, OR = 1.192, 95% CI = 1.155–1.229), and T2DM (P = 1.33E-15, OR = 1.201, 95% CI = 1.149–1.257). Moreover, the causal links with BMI, Waist Circumference, Hypertension, and T2DM remained significant in both the MR Egger method and the Weighted Median method, whereas the association with Triglycerides was not significant in these two methods (Fig. 3). Sensitivity analyses revealed the presence of heterogeneity but no pleiotropy. The leave-one-out method indicated that there are no individual SNPs solely driving the causal relationships between the exposure and outcomes (Additional file 1). The Egger intercept did not show a significant deviation from 0. Furthermore, the MR-PRESSO method identified the presence of outlier SNPs (Additional file 2), but the causal relationships remained significant after their removal (Fig. 5). Moreover, our findings revealed no discernible causal connections between genetically forecasted OSA and Cholesterol (P = 0.054, OR = 0.980, 95% CI = 0.961-1.000).
Figure 3.
Mendelian randomization results of the effect of obstructive sleep apnea on metabolic syndrome traits (body mass index, waist circumference, triglycerides, cholesterol, hypertension, type 2 diabetes).
Figure 5.
Mendelian randomization results of MR-PRESSO (after removing outlier SNPs).
Potential mediators and positive exposure
Body mass index and positive exposure
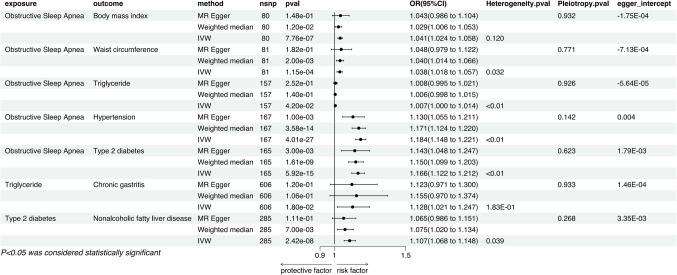
The IVW results indicate that genetically predicted BMI is associated with an increased risk of Inflammatory Bowel Disease (P = 0.003, OR = 1.147, 95% CI = 1.048–1.255) and Nonalcoholic Fatty Liver Disease (P = 2.49E-051, OR = 1.344, 95% CI = 1.171–1.542). The causal relationship with Inflammatory Bowel Disease was not significant in either the MR Egger method or the Weighted Median method, whereas the association with Nonalcoholic Fatty Liver Disease was significant in the Weighted Median method but not in the MR Egger method (Fig. 4). Sensitivity analyses revealed the presence of heterogeneity but no pleiotropy. Leave-one-out results showed no individual SNPs solely driving the causal relationships (Additional file 1), and the Egger intercept did not significantly deviate from 0. MR-PRESSO results showed no outlier SNPs (Additional file 2).
Figure 4.
Mendelian randomization results of the effect of metabolic syndrome traits (body mass index, waist circumference, triglycerides, cholesterol, hypertension, type 2 diabetes) on positive outcomes (chronic gastritis, gastric ulcers, inflammatory bowel disease, cholecystitis, nonalcoholic fatty liver).
Waist circumference and positive exposure
Genetically predicted waist circumference is significantly associated with an increased risk of Nonalcoholic Fatty Liver Disease (P = 3.74E-07, OR = 1.470, 95% CI = 1.267–1.705), a causal relationship confirmed by the IVW method, and found to be significant in both the MR Egger method and the Weighted Median method (Fig. 4). Sensitivity analyses indicated the presence of heterogeneity but no signs of pleiotropy. No single SNPs were found to dominate the causal link through leave-one-out testing, and the Egger intercept did not show a trend deviating from 0 (Additional file 1). Additionally, MR-PRESSO results did not detect any abnormal SNPs (Additional file 2).
Triglycerides and positive exposure
In the IVW method, Triglycerides were associated with an increased risk of Chronic Gastritis (P = 0.022, OR = 1.126, 95% CI = 1.017–1.247) and Inflammatory Bowel Disease (P = 4.73E-05, OR = 1.126, 95% CI = 1.063–1.192), which were not significant in the other two methods. The causal relationships with Gastric Ulcers and Nonalcoholic Fatty Liver Disease may be influenced by pleiotropy and thus were not pursued in subsequent multivariable studies (Fig. 4). Sensitivity analyses showed heterogeneity in the causal relationships with Chronic Gastritis and Inflammatory Bowel Disease but no pleiotropy. Leave-one-out results indicated no individual SNPs driving the causality (Additional file 1), the Egger intercept did not significantly deviate from 0, and MR-PRESSO indicated the presence of outlier SNPs (Additional file 2), however, after their removal, the causal relationships remained significant (Fig. 5).
Hypertension and positive exposure
IVW results suggest that Hypertension is associated with an increased risk of Chronic Gastritis (P = 0.002, OR = 1.100, 95% CI = 1.036–1.167), Inflammatory Bowel Disease (P = 0.043, OR = 1.034, 95% CI = 1.001–1.068), and Nonalcoholic Fatty Liver Disease (P = 0.012, OR = 1.065, 95% CI = 1.014–1.119). Chronic Gastritis was significant in the Weighted Median method but not in the MR Egger method, while Inflammatory Bowel Disease and Nonalcoholic Fatty Liver Disease were not significant in either method (Fig. 4). Sensitivity analyses revealed no heterogeneity for Chronic Gastritis and Nonalcoholic Fatty Liver Disease, with Inflammatory Bowel Disease showing heterogeneity; all three conditions showed no pleiotropy. Leave-one-out results showed no individual SNPs driving the causality (Additional file 1), and the Egger intercept did not significantly deviate from 0. MR-PRESSO results indicated no outlier SNPs (Additional file 2).
Type 2 diabetes and positive exposure
T2DM has a causal relationship with both Chronic Gastritis (P = 0.002, OR = 1.073, 95% CI = 1.026–1.121) and Nonalcoholic Fatty Liver Disease (P = 1.37E-05, OR = 1.062, 95% CI = 1.034–1.091). Nonalcoholic Fatty Liver Disease was significant in the Weighted Median method but not in the MR Egger method, whereas Chronic Gastritis was not significant in either method (Fig. 4). Sensitivity analyses showed no heterogeneity for Chronic Gastritis, with Inflammatory Bowel Disease and Nonalcoholic Fatty Liver Disease presenting heterogeneity; all three conditions showed no pleiotropy. Leave-one-out results indicated no individual SNPs driving the causality, and the Egger intercept did not significantly deviate from 0 (Additional file 1). In the MR-PRESSO method, there were no outlier SNPs detected for T2DM in relation to Chronic gastritis, while outlier SNPs were identified in the association with Nonalcoholic fatty liver disease (Additional file 2). Even after the removal of these outlier SNPs, the relationship between T2DM and Nonalcoholic fatty liver disease remained statistically significant (Fig. 5).
Multivariable mendelian randomization
The mediating role of BMI between OSA and gastrointestinal disease
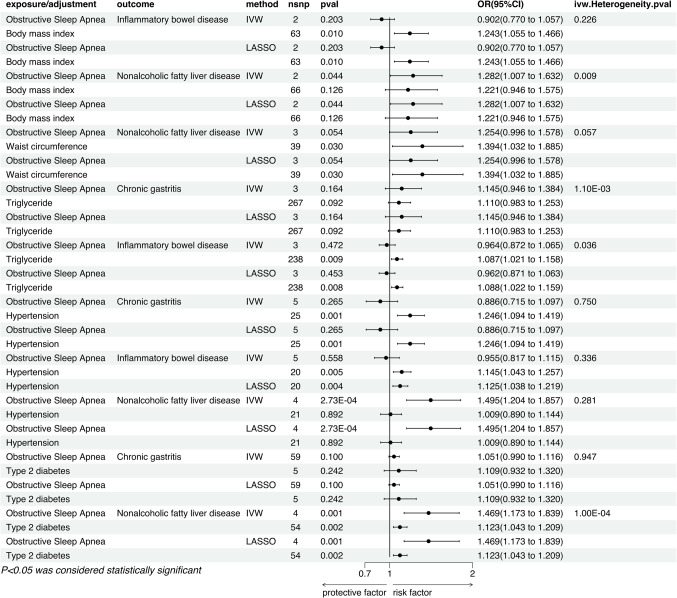
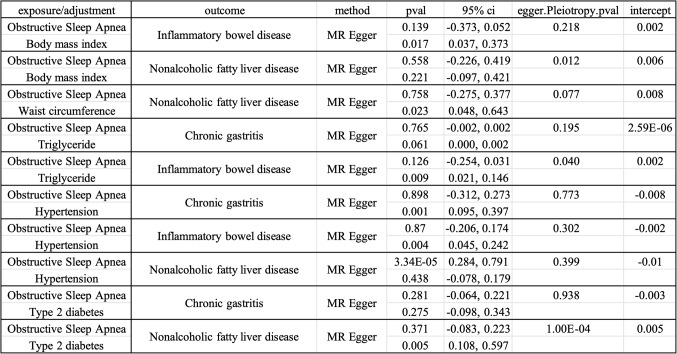
After adjusting for BMI using the IVW method, we observed that the significant association between sleep apnea and inflammatory bowel disease vanished (P = 0.203, OR = 0.902, 95% CI = 0.770–1.057), whereas the connection with non-alcoholic fatty liver disease persisted (P = 0.044, OR = 1.282, 95% CI = 1.007–1.632). Conversely, the causal relationship between BMI and inflammatory bowel disease remained significant (P = 0.010, OR = 1.243, 95% CI = 1.055–1.466), but its association with non-alcoholic fatty liver disease was nullified (P = 0.126, OR = 1.221, 95% CI = 0.946–1.575). This pattern was consistently reflected in both LASSO regression analysis and the MR Egger method (Fig. 6). Additionally, MR Egger intercept analysis did not indicate the presence of horizontal pleiotropy, suggesting that there was no systematic error due to horizontal pleiotropy in the model (Fig. 7).
Figure 6.
Multivariable MR result of causal relationships of obstructive sleep apnea and metabolic syndrome traits on positive outcomes.
Figure 7.
MVMR-Egger results of causal relationships of obstructive sleep apnea and metabolic syndrome traits on positive outcomes.
The mediating role of Waist circumference between OSA and gastrointestinal disease
Upon correcting for waist circumference, the causal relationship between OSA and non-alcoholic fatty liver disease was no longer significant (P = 0.054, OR = 1.254, 95% CI = 0.996–1.578), whereas the association between waist circumference and non-alcoholic fatty liver disease remained pronounced (P = 0.030, OR = 1.394, 95% CI = 1.032–1.885). This result was consistently validated across the IVW, LASSO, and MR-Egger methods (Fig. 6). Additionally, MR-Egger intercept analysis did not reveal signs of horizontal pleiotropy, indicating the absence of systematic bias caused by horizontal pleiotropy (Fig. 7).
The mediating role of triglyceride between OSA and gastrointestinal disease
After adjusting for triglyceride levels and applying the IVW method, we found that the causal relationships between OSA and chronic gastritis (P = 0.164, OR = 1.145, 95% CI = 0.946–1.384), as well as inflammatory bowel disease, were eliminated (P = 0.472, OR = 0.964, 95% CI = 0.872–1.065). Concurrently, the association between triglycerides and chronic gastritis disappeared (P = 0.092, OR = 1.110, 95% CI = 0.983–1.253), while their relationship with inflammatory bowel disease remained significant (P = 0.009, OR = 1.087, 95% CI = 1.021–1.158). These findings were consistently confirmed by LASSO regression analysis and the MR Egger method (Fig. 6). Furthermore, MR Egger intercept analysis did not suggest issues with horizontal pleiotropy, ruling out potential influences of such systematic bias on the study’s results (Fig. 7).
The mediating role of hypertension between OSA and gastrointestinal disease
In the IVW method, after adjustments for hypertension, the causal relationships between OSA and chronic gastritis (P = 0.265, OR = 0.886, 95% CI = 0.715–1.097), as well as inflammatory bowel disease (P = 0.558, OR = 0.955, 95% CI = 0.817–1.115), vanished, while the causal relationships between hypertension and chronic gastritis (P = 0.001, OR = 1.246, 95% CI = 1.094–1.419), inflammatory bowel disease remained significant (P = 0.005, OR = 1.145, 95% CI = 1.043–1.257). However, the association between OSA and non-alcoholic fatty liver remained significant (P = 2.73E-04, OR = 1.495, 95% CI = 1.204–1.857), while the causal relationship between hypertension and non-alcoholic fatty liver disappeared (P = 0.892, OR = 1.009, 95% CI = 0.890–1.144). These results were mirrored in subsequent LASSO and MR-Egger methods (Fig. 6), with no significant evidence of non-zero intercepts in multivariable MR Egger regression, supporting the reliability of the multivariable MR analysis outcomes (Fig. 7).
The mediating role of type 2 diabetes between OSA and gastrointestinal disease
Within the application of the IVW method and following adjustments for type 2 diabetes, the causal links between OSA and chronic gastritis (P = 0.100, OR = 1.051, 95% CI = 0.990–1.116), as well as type 2 diabetes and chronic gastritis (P = 0.242, OR = 1.109, 95% CI = 0.932–1.320), were no longer significant. However, their relationships with non-alcoholic fatty liver disease persisted (OSA: P = 0.001, OR = 1.469, 95% CI = 1.173–1.839, T2DM: P = 0.002, OR = 1.123, 95% CI = 1.043–1.209). This trend was also corroborated by LASSO regression and MR Egger methods (Fig. 6). These results suggest that type 2 diabetes is not a mediating factor in the causal relationships between OSA and chronic gastritis, as well as non-alcoholic fatty liver disease. Moreover, MR Egger regression analysis did not uncover significant evidence of non-zero intercepts, further endorsing the credibility of the multivariable MR analysis results (Fig. 7).
Discussion
In this article, we conducted a study on the relationship between OSA and gastrointestinal diseases, and further explored the potential mediating mechanisms. Univariate positive MR results indicated a causal relationship between OSA and chronic gastritis, gastric ulcers, enteritis, and fatty liver. The multivariate MR analysis revealed that these effects were indirect, suggesting the presence of mediating factors. The causal relationship between OSA and gastrointestinal diseases was influenced by features of metabolic syndrome, which aligns with previous observational studies and hypotheses.
In contemporary society, sleep deprivation and decreased sleep quality have been closely linked to various health issues. Numerous clinical cases have shown that the general population faces sleep disorders, which not only significantly increase the risk of chronic diseases such as hypertension, obesity, stroke, and cardiovascular diseases but also potentially contribute to overall mortality rates21. In recent years, researchers have started investigating the potential mechanisms linking sleep disorders and gastrointestinal disorders. Inflammatory mediators, including tumor necrosis factor (TNF), interleukin-1 (IL-1), and interleukin-6 (IL-6), play crucial roles in regulating sleep and wakefulness cycles. Extensive research has confirmed their involvement in sleep disruption, and these cytokines have also shown abnormal levels in the pathogenesis of gastrointestinal diseases such as gastroesophageal reflux disease (GERD), inflammatory bowel disease, liver disorders, and colorectal cancer. This further emphasizes the potential shared pathophysiological basis between sleep disorders and gastrointestinal diseases22. MetS is a clinical syndrome characterized by multiple metabolic abnormalities, including central obesity, hypertension, hyperglycemia, hypertriglyceridemia, and low high-density lipoprotein cholesterol levels, with obesity being a major precursor of MetS23. Studies have shown that hypoxemia and hypercapnia in OSA patients can lead to increased insulin resistance and inflammatory responses, thereby promoting the development of MetS. Furthermore, OSA can result in excessive activation of the sympathetic nervous system, further exacerbating MetS symptoms. On the other hand, the presence of MetS also increases the risk of OSA. Abdominal obesity and insulin resistance in MetS patients may lead to relaxation of the upper airway muscles, increasing the occurrence of OSA. Additionally, the high blood glucose and cholesterol levels in MetS patients may have negative effects on the respiratory system, further worsening OSA symptoms11. In recent years, research on the association between metabolic syndrome (MetS) and gastrointestinal diseases has also increased. In MetS patients, central obesity and insulin resistance may trigger excessive gastric acid secretion, providing conditions for GERD. Moreover, the sustained presence of high blood glucose and high cholesterol levels poses a threat to the gastric and intestinal mucosa, increasing the likelihood of gastric and duodenal ulcers24. Studies have found that the gut microbiome composition may vary in MetS patients, leading to intensified intestinal inflammation and an increased risk of inflammatory bowel disease25. Considering the various metabolic abnormalities involved in MetS, such as abdominal obesity, hypertension, hyperglycemia, hypertriglyceridemia, and high cholesterol levels, they may collectively contribute to the development of both OSA and gastrointestinal diseases. Therefore, in-depth research on the role of MetS in these two disease categories is crucial for the development of effective prevention and treatment strategies.
Chronic gastritis is a disease characterized by long-term inflammation of the gastric mucosa. Its main features are inflammation and tissue damage to the gastric mucosa, which may be accompanied by abnormal gastric acid secretion, gastric mucosal atrophy, and Helicobacter pylori infection26, In developing countries, 50.8% of the population and 34.7% in developed countries suffer from health problems due to gastritis27. Research has shown that poor sleep quality can lead to an increase in gastrointestinal diseases, with gastritis being 6.935 times more likely in individuals with low sleep quality compared to those with high sleep quality22. This may be because the excessive secretion of pro-inflammatory cytokines in sleep disorders makes the gastric mucosa more susceptible to damage22,28, leading to the development of gastritis, which is consistent with our research findings. In multivariable MR analysis, we revealed that hypertension plays an important mediating role in the link between OSA and chronic gastritis. OSA has been recognized as an important risk factor for hypertension29, and extensive medical research has revealed that hypertension may lead to reduced gastric mucosal blood flow, resulting in mucosal damage and an increased risk of developing chronic gastritis and gastric ulcers. Additionally, Helicobacter pylori infection is a well-known major cause of chronic gastritis. It is worth noting that recent scientific literature has indicated a significant and independent correlation between the seropositivity of Helicobacter pylori and primary hypertension, suggesting that hypertensive patients are more likely to exhibit a seropositive reaction to Helicobacter pylori infection30–32. This association further supports our findings that hypertension may be a key intermediate link connecting OSA and gastrointestinal diseases. In conclusion, hypertension is not only a complication of OSA but may also serve as a biological bridge between OSA and gastrointestinal diseases such as chronic gastritis. This discovery is of great significance for understanding the complex relationship between OSA and gastrointestinal diseases and provides a new perspective for future prevention and treatment strategies. Gastric ulcer is a type of digestive ulcer that occurs in the inner lining of the stomach. When the gastric mucosa is damaged for specific reasons, ulcers can form in that area, leading to the development of gastric ulcers. The occurrence of gastric ulcers is related to various factors, including Helicobacter pylori infection, the use of non-steroidal anti-inflammatory drugs, and excessive gastric acid secretion33. It is worth noting that a study has indicated that patients with sleep apnea syndrome have a 2.4 times higher risk of developing peptic ulcer bleeding compared to normal individuals34. This may be related to the repeated respiratory pauses that occur during sleep apnea. These continuous respiratory interruptions lead to intermittent hypoxia, systemic inflammatory response, and sympathetic nervous system activation, which collectively increase the risk of developing peptic ulcers. Therefore, for patients with sleep apnea syndrome, monitoring and preventive measures for gastric diseases should be strengthened to reduce the risk of peptic ulcers and their complications.
Inflammatory bowel diseases (IBDs), including Crohn’s disease and ulcerative colitis, are a group of chronic inflammatory intestinal diseases that typically result in long-term inflammation and ulcer formation in the intestines, causing symptoms such as diarrhea, abdominal pain, and weight loss. The pathogenesis of IBDs is believed to be the result of interactions between genetic susceptibility and environmental influences on the gut microbiome, leading to impaired intestinal barrier function and inappropriate activation of intestinal immune responses35. Sleep disorders have been shown to affect immune function and the development of inflammation. Sleep deprivation leads to upregulation of immune function, activating pro-inflammatory cytokines (such as IL-1, IL-6, and TNF) and increasing levels of C-reactive protein (CRP), which are markers of IBD activity36. The chronic inflammation of IBDs and the resulting sleep disturbances form a vicious cycle with negative feedback. Sleep deprivation leads to the production of inflammatory cytokines, which, in turn, worsen colitis, creating a recurring cycle37,38. In multivariable Mendelian randomization analysis, we found that BMI, triglyceride levels, and hypertension mediate the impact of OSA on inflammatory bowel disease (IBD). BMI is an internationally used measure of body weight and health status39, and obese individuals often have higher triglyceride levels40. Research has shown a close association between sleep disorders and metabolic syndrome11,13, and metabolic syndrome is also associated with IBDs. They share common pathophysiological features, such as immune imbalance, chronic inflammation, dysregulation of adipokine secretion, and increased risk of cardiovascular diseases41. A large-scale study has indicated that the presence of hypertriglyceridemia is associated with an increased hospitalization rate for inflammatory bowel disease42. It is estimated that up to 40% of individuals with Crohn’s disease and ulcerative colitis (UC) are either obese or overweight43. Obesity can lead to chronic low-grade inflammation, which may impair the intestinal mucosal barrier, leading to dysbiosis and immune system dysregulation. Moreover, obesity can affect the composition and function of the gut microbiome, increasing certain harmful bacterial populations, thereby triggering inflammatory responses in the gut. Additionally, excessive visceral fat accumulation due to obesity is closely related to the characteristics of metabolic syndrome, prothrombotic, and pro-inflammatory states25,all of which can contribute to the onset of IBD. In recent years, epidemiological studies have suggested a connection between blood pressure and IBD, as hypertension can alter tight junction proteins and intestinal permeability, thereby increasing the risk of IBD44. Previous studies have shown that the gut microbial composition in patients with hypertension can change, where a reduction in butyrate-producing bacteria may decrease the concentration of butyrate and weaken its ability to alleviate chronic inflammatory responses, leading to intestinal epithelial diseases45,46. Moreover, hypertension can also activate the proliferation, mobilization, and differentiation of hematopoietic stem cells, thereby increasing peripheral and neuroinflammatory responses, which could potentially induce the development of IBD47. Therefore, we can conclude that BMI, triglyceride levels, and hypertension are key biomarkers for OSA exacerbating the risk of inflammatory bowel disease. Monitoring and managing these indicators may help assess the potential risk of OSA patients developing IBD, thereby enabling more targeted preventive and therapeutic measures.
Nonalcoholic fatty liver disease (NAFLD) is a common liver condition characterized by excessive fat accumulation in the liver in the absence of alcohol consumption. This condition is divided into two types: simple steatosis involving only fat buildup without inflammation or fibrosis, and the more severe nonalcoholic steatohepatitis (NASH), which includes varying degrees of hepatocyte inflammation and fibrosis48. Statistics show that NAFLD has a prevalence rate of up to 75% among the global obese population, with about one-quarter of the world’s population affected49. Research has revealed a close association between OSA and the progression of NAFLD50. Chronic intermittent hypoxia (CIH) caused by OSA has been identified as one of the key factors promoting the progression of NAFLD51,52. Additionally, OSA and CIH can reduce insulin sensitivity and disrupt lipid levels, changes that may contribute to the onset of NAFLD. Under CIH, the level of HIF1α protein in the body increases, activating genes related to lipogenesis and advancing β-oxidation processes in the liver, thereby exacerbating oxidative stress within the liver. Furthermore, OSA disrupts the healthy interaction between the gut and the liver (i.e., the gut-liver axis), increasing gut permeability, which may allow the gut microbiota to play a role in the interaction between OSA and NAFLD, although the specific mechanisms require further investigation53. In MVMR, we found that waist circumference act as mediators for OSA-induced NAFLD. Waist circumference is a common indicator of individual obesity, numerous observational studies consistently suggest that obesity plays a significant role in the relationship between OSA and NAFLD. Research by Jia-Chao Qi et al. indicates that if obesity is excluded, the causal link between OSA and NAFLD may no longer exist54. OSA can lead to increased levels of serum sulfatase 2 (LaSO2), BMI, and triglycerides (TG). Elevated LaSO2, as a marker of inflammation, reflects a state of systemic inflammation and oxidative stress closely linked to the pathophysiology of NAFLD. An increase in BMI is usually associated with obesity, exacerbating intrahepatic fat accumulation by affecting fatty acid metabolism and insulin sensitivity. Elevated TG levels reflect lipid metabolism disorders, potentially leading to more fatty acids being transported to the liver and converted into harmful fatty acid esters, thereby promoting the development of intrahepatic inflammation and fibrosis55. Therefore, for OSA patients, actively managing lipid metabolism, maintaining a healthy weight, and treating hypoxemia caused by OSA are important strategies for reducing or preventing the occurrence of NAFLD. Implementing these measures can significantly reduce the risk of NAFLD and improve overall health and quality of life for patients.
In exploring the broad health implications of OSA, we observe the high prevalence of comorbidities among patients with severe Chronic Obstructive Pulmonary Disease (COPD), which not only significantly hinder the clinical management of COPD but also profoundly impact the health-related quality of life as reflected by COPD Assessment Test scores56. Notably, OSA, as another crucial respiratory disorder, has had its genetic predisposition robustly validated through MR analysis, uncovering an independent and notable causal relationship between genetically predicted OSA and elevated risks of inflammatory gastrointestinal diseases (GDs)57. This finding underscores OSA’s role in facilitating the development of inflammatory conditions, which serve as a common pathological basis for various chronic diseases, including COPD and GDs. In contrast to the aforementioned discovery, however, current research has not observed a direct causal link between OSA and cancer57. This result suggests that while OSA is intimately associated with multiple inflammatory diseases, its specific impact on cancer risk may be more intricate and necessitates further investigation in the future. In summary, OSA is not merely an isolated health concern; its underlying pathophysiological mechanisms may intertwine with those of COPD, GDs, and other disorders through inflammatory pathways, collectively influencing patients’ overall health status.
The strength of this study lies in selecting data from large-scale research, fully incorporating indicators of obstructive sleep apnea syndrome and related gastrointestinal diseases, systematically exploring the relationship between OSA and gastrointestinal diseases through Mendelian randomization for the first time, and using multivariate MR analysis to explore mediation pathways to identify some possible mechanisms, filling the gap left by randomized controlled trials. This study also has certain limitations. First, the Mendelian randomization method used considers the cumulative lifelong impact of genetic variations and should not be inferred to estimate the effects of clinical interventions; second, to obtain a sufficient number of instrumental variables, the p-value threshold for IV selection was set at 5E-05, which may introduce slight instrument bias into the overall estimates. Furthermore, to avoid population heterogeneity bias, we based our analysis solely on GWAS summary statistics from populations of European descent, and the applicability of these results to other ethnic groups requires further exploration.
Conclusion
In summary, after comprehensively analyzing multiple clinical data and biological mechanisms, this study established a causal link between OSA and chronic gastritis, gastric ulcers, inflammatory bowel disease, and fatty liver, revealing the potential mediating role of MetS in the interaction of these diseases, thus providing strong causal evidence for the shared pathophysiological basis of OSA and gastrointestinal diseases. By delving into the association between OSA and gastrointestinal diseases, this study not only strengthens our understanding of the pathogenesis of these clusters of diseases but also identifies MetS as a potential intervention point, which is of significant public health importance for formulating prevention strategies and intervention measures to reduce the burden of gastrointestinal diseases and their related conditions. Further research should be dedicated to elucidating the specific biological pathways of interaction between OSA and gastrointestinal diseases, as well as how MetS regulates these pathways, to provide more precise risk management and treatment targets for clinical practice.
Supplementary Information
Acknowledgements
We gratefully acknowledge the authors and participants of all GWASs from which we used summary statistics data.
Abbreviations
- OSA
Obstructive Sleep Apnea
- MetS
Metabolic syndrome
- PD
Parkinson’s disease
- T2DM
Type 2 diabetes
- MR
Mendelian randomization
- UVMR
Univariable Mendelian randomization
- BMI
Body mass index
- MVMR
Multivariable Mendelian randomization
- IVs
Instrumental variables
- SNPs
Single nucleotide polymorphisms
- IVW
Inverse variance weighting
- TNF
Tumor necrosis factor
- IL-1
Interleukin-1
- IL-6
Interleukin-6
- GERD
gastroesophageal reflux disease
- IBDs
Inflammatory bowel diseases
- IBD
Inflammatory bowel disease
- CRP
C-reactive protein
- UC
Ulcerative colitis
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis
- CIH
Chronic intermittent hypoxia
- COPD
Chronic Obstructive Pulmonary Disease
- GDs
Gastrointestinal diseases
Author contributions
Z.Z proposed the research concept and design, conducted Data analysis and wrote the preliminary draft; CY.J was responsible for Data Curation and Investigation; BS.Y and H.W was responsible for Methodology and the use of software; JW.Z and LX.Z supervised the research process and guided the other authors; while TK, Y, H.W and ZF.D were responsible for the Project Administration; Y.C and XL.Q reviewed and edited the manuscript, while SY.W acquired the funding. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (82074426, 82104864, 82204822), Natural Science Foundation of Liaoning Province (2021-BS-215, 2022-MS-25, 2023-MS-13), Liaoning Revitalization Talents Program (XLYC1802014), Natural Science Foundation of Tibet Autonomous Region (XZ202301ZR0030G, XZ2023ZR-ZY82(Z)).
Data availability
The datasets generated and/or analysed during the current study are available in the [GWAS] repository, [https://gwas.mrcieu.ac.uk]
Declarations
Ethics approval and consent to participate
The data used in this study were all from public databases that can be downloaded directly for research purposes and do not involve the reporting or using of any animal, human, or tissue data.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhe Zhang, Chunyu Jiang, Baosheng Yin and Huan Wang contributed equally to this work.
Contributor Information
Ying Chen, Email: yoursjane1934@126.com.
Shouyu Wang, Email: wangshouyu666@126.com.
Xueling Qu, Email: quxuelingmm@sina.com.
References
- 1.Lévy, P. et al. Obstructive sleep apnoea syndrome. Nat. Reviews Disease Primers. 1, 1–21 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Senaratna, C. V. et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med. Rev.34, 70–81 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Yaggi, H. K. et al. Obstructive sleep apnea as a risk factor for stroke and death. N. Engl. J. Med.353, 2034–2041 (2005). [DOI] [PubMed] [Google Scholar]
- 4.El Hage Chehade, N. et al. Association between obstructive sleep apnea and gastroesophageal reflux disease: a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 38, 1244–1251 (2023). [DOI] [PubMed]
- 5.Elfanagely, Y., Atsawarungruangkit, A., Scharfen, J., Pavlech, L. & Moss, S. F. Association between obstructive sleep apnea and Barrett’s esophagus: a systematic review and meta-analysis. Dig. Dis. Sci.66, 3689–3697 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Teo, Y. H. et al. Obstructive sleep apnea and the incidence and mortality of gastrointestinal cancers: a systematic review and meta-analysis of 5,120,837 participants. J. Gastrointest. Oncol.13, 2789 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vernia, F. et al. Sleep disorders related to nutrition and digestive diseases: a neglected clinical condition. Int. J. Med. Sci.18, 593 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koo, D. L. & Nam, H. The relationship between obstructive sleep apnea and functional dyspepsia. J. Sleep. Med.17, 73–77 (2020). [Google Scholar]
- 9.Orr, W. C., Fass, R., Sundaram, S. S. & Scheimann, A. O. The effect of sleep on gastrointestinal functioning in common digestive diseases. Lancet Gastroenterol. Hepatol.5, 616–624 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Ryan, S., Taylor, C. & McNicholas, W. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Postgrad. Med. J.85, 693–698 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Parish, J. M., Adam, T. & Facchiano, L. Relationship of metabolic syndrome and obstructive sleep apnea. J. Clin. Sleep Med.3, 467–472 (2007). [PMC free article] [PubMed] [Google Scholar]
- 12.Carter, I. I. I., Watenpaugh, D. E. & R. & Obesity and obstructive sleep apnea: or is it OSA and obesity? Pathophysiology. 15, 71–77 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Feakins, R. M. Obesity and metabolic syndrome: pathological effects on the gastrointestinal tract. Histopathology. 68, 630–640 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Burgess, S. & Thompson, S. G. Mendelian randomization: methods for using genetic variants in causal estimation (CRC Press, Boca Raton, 2015).
- 15.Lawlor, D. A., Harbord, R. M., Sterne, J. A. & Timpson, N. Davey Smith, G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat. Med.27, 1133–1163 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Elsworth, B. et al. The MRC IEU OpenGWAS data infrastructure. BioRxiv2020, 2008-2010.244293 (2020). [Google Scholar]
- 17.Bowden, J. et al. Improving the accuracy of two-sample summary-data mendelian randomization: moving beyond the NOME assumption. Int. J. Epidemiol.48, 728–742 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slob, E. A. & Burgess, S. A comparison of robust mendelian randomization methods using summary data. Genet. Epidemiol.44, 313–329 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowden, J., Davey Smith, G. & Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol.44, 512–525 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol.40, 304–314 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altevogt, B. M. & Colten, H. R. Sleep disorders and sleep deprivation: an unmet public health problem (2006). [PubMed] [Google Scholar]
- 22.Ali, T., Choe, J., Awab, A., Wagener, T. L. & Orr, W. C. Sleep, immunity and inflammation in gastrointestinal disorders. World J. Gastroenterology: WJG. 19, 9231 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palaniappan, L. et al. Predictors of the incident metabolic syndrome in adults: the insulin resistance atherosclerosis study. Diabetes care. 27, 788–793 (2004). [DOI] [PubMed] [Google Scholar]
- 24.De Filippis, A. et al. Gastrointestinal disorders and metabolic syndrome: Dysbiosis as a key link and common bioactive dietary components useful for their treatment. Int. J. Mol. Sci.21, 4929 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dragasevic, S. et al. Metabolic syndrome in inflammatory bowel disease: association with genetic markers of obesity and inflammation. Metab. Syndr. Relat. Disord.18, 31–38 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Megha, R., Farooq, U. & Lopez, P. P. Stress-induced gastritis (2018). [PubMed] [Google Scholar]
- 27.Wen, Z. et al. Health related quality of life in patients with chronic gastritis and peptic ulcer and factors with impact: a longitudinal study. BMC Gastroenterol.14, 1–10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khanijow, V., Prakash, P., Emsellem, H. A., Borum, M. L. & Doman, D. B. Sleep dysfunction and gastrointestinal diseases. Gastroenterol. Hepatol.11, 817 (2015). [PMC free article] [PubMed] [Google Scholar]
- 29.Calhoun, D. A. Obstructive sleep apnea and hypertension. Curr. Hypertens. Rep.12, 189–195 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Lip, G., Wise, R. & Beevers, G. Association of Helicobacter pylori infection with coronary heart disease. Study shows association between H pylori infection and hypertension. BMJ: Br. Med. J.312, 250 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunji, T. et al. Helicobacter pylori infection is significantly associated with metabolic syndrome in the Japanese population. Official J. Am. Coll. Gastroenterol.| ACG103, 3005–3010 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Dore, M. P. et al. Increased risk to develop hypertension and carotid plaques in patients with long-lasting Helicobacter pylori gastritis. J. Clin. Med.11, 2282 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woolf, A., Rehman, R. B. & Rose, R. Gastric ulcer (2019). [Google Scholar]
- 34.Shiao, T. H. et al. Sleep apnea and risk of peptic ulcer bleeding: a nationwide population-based study. Am. J. Med.126, 249–255 (2013). e241. [DOI] [PubMed] [Google Scholar]
- 35.Ramos, G. P. & Papadakis, K. A. Mechanisms of disease: inflammatory bowel diseases. In Mayo Clinic Proceedings, Vol. 94, 155–165 (Elsevier, 2019). [DOI] [PMC free article] [PubMed]
- 36.El-Salhy, M. Recent developments in the pathophysiology of irritable bowel syndrome. World J. Gastroenterology: WJG. 21, 7621 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranjbaran, Z. et al. Impact of sleep disturbances in inflammatory bowel disease. J. Gastroenterol. Hepatol.22, 1748–1753 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Tang, Y., Preuss, F., Turek, F. W., Jakate, S. & Keshavarzian, A. Sleep deprivation worsens inflammation and delays recovery in a mouse model of colitis. Sleep Med.10, 597–603 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez, M. C., Correia, M. I. T. & Heymsfield, S. B. A requiem for BMI in the clinical setting. Curr. Opin. Clin. Nutr. Metab. Care. 20, 314–321 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Steinberg, G. R., Kemp, B. E. & Watt, M. J. Adipocyte triglyceride lipase expression in human obesity. Am. J. physiology-Endocrinology Metabolism. 293, E958–E964 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Michalak, A., Mosińska, P. & Fichna, J. Common links between metabolic syndrome and inflammatory bowel disease: current overview and future perspectives. Pharmacol. Rep.68, 837–846 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Fitzmorris, P. S. et al. Impact of metabolic syndrome on the hospitalization rate of Crohn’s disease patients seen at a tertiary care center: a retrospective cohort study. Digestion. 91, 257–262 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Singh, S., Dulai, P. S., Zarrinpar, A., Ramamoorthy, S. & Sandborn, W. J. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat. Reviews Gastroenterol. Hepatol.14, 110–121 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacobs, J. D. et al. Impact of angiotensin II signaling blockade on clinical outcomes in patients with inflammatory bowel disease. Dig. Dis. Sci.64, 1938–1944 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santisteban, M. M. et al. Hypertension-linked pathophysiological alterations in the gut. Circul. Res.120, 312–323 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fasano, A. et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 355, 1518–1519 (2000). [DOI] [PubMed] [Google Scholar]
- 47.Xu, X. et al. Assessing the impact of blood pressure in the development of inflammatory bowel disease. J. Clin. Hypertens.24, 566–572 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Estes, C., Razavi, H., Loomba, R., Younossi, Z. & Sanyal, A. J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 67, 123–133 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dulai, P. S. et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 65, 1557–1565 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mesarwi, O. A., Loomba, R. & Malhotra, A. Obstructive sleep apnea, hypoxia, and nonalcoholic fatty liver disease. Am. J. Respir. Crit Care Med.199, 830–841 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young, T. et al. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med.328, 1230–1235 (1993). [DOI] [PubMed] [Google Scholar]
- 52.Aron-Wisnewsky, J. et al. Chronic intermittent hypoxia is a major trigger for non-alcoholic fatty liver disease in morbid obese. J. Hepatol.56, 225–233 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Aron-Wisnewsky, J., Clement, K. & Pépin, J. L. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 65, 1124–1135 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Qi, J. C. et al. Relationship between obstructive sleep apnea and nonalcoholic fatty liver disease in nonobese adults. Sleep. Breath.20, 529–535 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Drager, L. F. et al. Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity. Obesity. 19, 2167–2174 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Almagro, P. et al. Impact of comorbidities in COPD clinical control criteria. The CLAVE study. BMC Pulm. Med.24, 6 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan, W. et al. Obstructive sleep apnea and 19 gastrointestinal diseases: a mendelian randomization study. Front. Psychiatry. 15, 1256116 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the [GWAS] repository, [https://gwas.mrcieu.ac.uk]