Abstract
Meitan Cuiya (MTCY), a representative green tea from Guizhou, China, may exhibit lower quality in summer due to increased bitterness and astringency. Spreading is a common method to enhance tea quality, but its impact on summer MTCY remains unclear. This study combined transcriptomics and volatile metabolomics to investigate the effects of spreading duration on quality of summer fresh tea leaves and MTCY. Results showed that spreading time shortened to 4 h improved the taste of MTCY, due to lower catechins and higher theanine levels. This duration also yielded woody floral scent in MTCY, marked by high levels of trans-Cubebol, linalool, (Z)-linalool oxide. Transcriptomic analysis linked the 4-h spreading to proteasome activities. Aroma formation was related to diterpenoid and flavonoid biosynthesis. Additionally, gibberellins and auxin were associated with quality formation in fresh tea leaves. This research lays a foundation for improving quality of fresh tea leaves and MTCY in summer.
Keywords: Meitan Cuiya, Spreading, Green tea, Summer tea, Transcriptomics, GC–MS, Hormone
Highlights
-
•
Shorten spreading time in summer, make catechin less and theanine more, in order to improve Meitan Cuiya taste.
-
•
4 h spreading Meitan Cuiya has a better taste and nice aroma.
-
•
Gibberellins and auxin may participate in quality formation of Meitan Cuiya Spreading duration.
1. Introduction
Green tea is one of China's oldest and most esteemed beverages, highly valued both domestically and globally. Meitan Cuiya (MTCY), a renowned flat green tea, represents the geographic legacy of Meitan County in Zunyi City, Guizhou Province. MTCY, produced from fresh spring leaves, is prized for its sweet, robust, and smooth flavor, attributed to its high amino acid content and lower levels of catechins and caffeine, compared to summer-harvested leaves (Liu et al., 2015). However, during the summer, MTCY production experiences a downturn, leading to the wastage of significant quantities of fresh leaves and subsequent revenue loss. Hence, there exists an imperative to enhance the quality of MTCY during the summer season to improve summer tea quality.
Numerous variables within the realm of production practices exert significant influence on the quality of dry tea. These factors encompass the caliber of raw materials, the ambient conditions of processing environments, as well as the methodologies employed. For example, nano‑selenium application reduces tea polyphenol, catechin, and caffeine levels, improving summer green tea quality (Huang et al., 2023). Moderate shading increases chlorophyll and reduces caffeine and tea polyphenol content, enhancing matcha quality (Hu et al., 2024). Extended wilting, reduced fixation time, and higher drying temperatures improve the aroma of Taiping Houkui tea (Wang et al., 2024). However, there are currently no studies on the effects of spreading duration on the quality of summer green tea.
In traditional MTCY processing, spreading, fixation, rolling, and drying are key stages influencing flavor, with spreading gaining increased attention. During spreading, volatile compounds, amino acids, and glycosides undergo significant transformations, potentially increasing amino acids, inhibiting catechin and flavonoid synthesis, and reducing the phenol-to-amine ratio (Ni et al., 2021). For Longjing tea, optimal flavor is achieved when the tea is spread until it reaches 70 % water content, which is associated with malic acid, succinic acid, quinic acid, theanine, and glucoside (Shan et al., 2023). Different wilting methods also affect aroma, with sun-withered white tea exhibiting stronger floral notes, while trough-withered tea has a grassier scent (Wu et al., 2022). Additionally, light quality during spreading impacts tea quality; for instance, white tea under 5000 lx yellow light shows better taste and aroma (Tian, Zhou, Yao, & Lu, 2024).
In this study, fresh summer tea leaves were subjected to varying spreading durations, followed by a standardized manufacturing process to produce MTCY. The effects of different spreading times on the quality of both fresh leaves and MTCY were systematically analyzed, incorporating sensory evaluation, quantification of key quality-related compounds (catechins, caffeine, theanine), and the profiling of volatile substances. Differential gene expression analysis revealed that plant hormones, including gibberellins (GAs), methyl jasmonate (MeJA), auxin (IAA), and abscisic acid (ABA), were closely related to spreading time. By combing transcriptomic and metabolomic approaches, we gained insights into the mechanisms underlying these quality differences. This study provides a theoretical basis for improving the quality of MTCY in summer.
2. Materials and methods
2.1. Tea sample preparation
One bud with one leaf from the ten-year-old tea plant cultivar ‘Fuding Dabai,’ grown in Meitan County, Zunyi City, Guizhou Province, was used as the raw material. Samples were taken at 0, 2, 4, 6, 8, and 10 h of spreading. The ambient temperature was 23 ± 2 °C, and the relative humidity was 65–70 %, maintaining a thickness of 8 cm for the tea spread. According to the local standard of Meitan Cuiya (DB52/T478–2015), fresh tea leaves were processed into MTCY. Fresh leaf samples were frozen in liquid nitrogen, stored at −80 °C for total RNA extraction and transcriptome sequencing, and then made into MTCY. Each spreading duration had 3 biological replicates.
2.2. Chemical reagents
The standards of L-Theanine, the caffeine and the cathine components: (−)-Epigallocatechin gallate (EGCG), (−)-epicatechin gallate (ECG), (−)-epigallocatechin (EGC), (−)-epicatechin (EC), (−)-catechin (C), and caffeine, were liquid chromatography (HPLC, 98 %) grade obtained from Sigma-Aldrich (Shanghai, China). Other regents were obtained from Solarbio (Beijing, China).
2.3. Sensory assessment
The sensory evaluation to assess the tea's flavor profile was conducted at GuiZhou University by twenty nationally accredited tea evaluators from China, following the Tea Sensory Evaluation Method (GB/T 23776–2018). The sensory panel research has passed our institution's ethical permission, and the rights and health of all involved are guaranteed. All evaluators give informed consent to the review process, volunteer to participate, and have the right to withdraw at any time. Firstly, the appearance of dried tea was evaluated. Then the tea soup was brewed to evaluate the taste. Placed 3.0 g of dry tea samples into teacups, then add 150 mL boiling water and cover the cup. After a steeping time of 4 min, pour out all the tea liquid and leave the tea leaves in the cup. The expert panel then describes and ranks flavors and aroma.
2.4. Extraction and quantification of main flavor components of MTCY and fresh tea leaves
The major catechin components EGCG, (EC, ECG, EGC) and C, theanine and caffeine were extracted using existing methods and detected using high-performance liquid chromatography (HPLC) (Huang et al., 2022), without modification. The soluble sugar content was detected according to the instructions of the plant soluble sugar extraction kit (Nanjing Jiancheng Bioengineering Institute, Nanjing).
2.5. Detection, characterization and relative quantification of volatile substances
Volatiles in MTCY and fresh leaves were extracted and determined by headspace solid phase microextraction coupled with gas chromatography/mass spectrometry (HS-SPME-GC/MS). Volatiles were extracted using distillation and headspace techniques, referring to existing methods (Qiao et al., 2023) without modification. After 5 min of adsorption, a 50 μm DVB/CAR/PDMS extraction fiber (Supelco, USA) was inserted into the triple quadrupole gas chromatography-mass spectrometer (Agilent 7000D GC/TQ, Agilent 7693 A Autosampler). The volatile compounds were identified by comparing their mass spectra with the NIST data system library and their linear retention indices (RI) with those of standard solutions of n-alkanes (C7–C40), RI for reference is shown in Table S2. The obtained ion-flow mass spectrometry was qualitatively compared with NIST database (match degree greater than 80) and relative retention index, and the relative percentage of volatile organic compounds was calculated by peak area normalization method.
2.6. RNA sequencing and transcriptome data analysis
The RNA extraction and construction of cDNA library were performed by Novogene company (Beijing, China). The Tieguanyin genome was used as the reference genome. An index of the reference genome was constructed using HISAT2 v2.0.5, and the recombinant clean reads were aligned to the reference genome using HISAT2 v2.0.5. Differential expression analysis (DEGs) between the two comparison groups was performed using DESeq2 software (1.20.0). Gene Ontology (GO) enrichment analysis of DEGs was conducted using cluster Profiler (3.8.1) software. Additionally, the local version of the GSEA analysis tool (http://www.broadinstitute.org/gsea/index.jsp) was employed to analyze the GO and Kyoto Ency-clopedia of Genes and Genomes (KEGG) datasets in the library, performing Gene Set Enrichment Analysis (GSEA). Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) analysis uses the method of our previous study (Jiang, Zhu, Yang, Zhi, & Ren, 2022) without modification, and the primer sequence is shown in Table S1.
2.7. Determination of endogenous hormone content
The contents of gibberellin A3 (GA3), gibberellin A4 (GA4), MeJA, IAA and ABA were detected according to enzyme-linked immunoassay (Yang, Zhang, Wang, Zhu, & Wang, 2001) without modification.
2.8. Statistical analysis
The data used in this study were derived from at least three biological replicates. Differences with P < 0.05 were considered significant, P < 0.01 were considered highly significant. Unless otherwise stated, comparisons were analyzed using one-way analysis of variance (ANOVA).
3. Result and discussion
3.1. Effects of spreading times on the sensory characteristics of summer MTCY
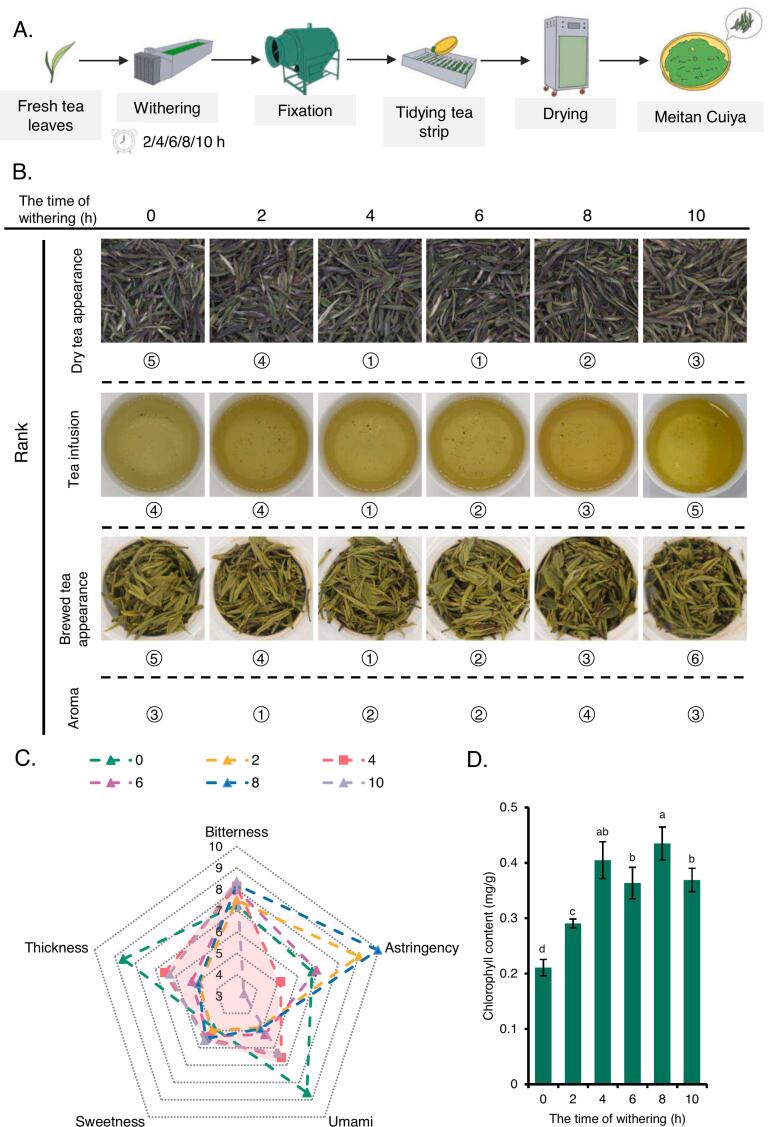
To explore the effects of spreading time on the flavor profile of MTCY, we processed the fresh tea leaves into MTCY from different spreading time (0, 2, 4, 6, 8 and 10 h) treatments under identical conditions (Fig. 1A). Sensory assessments of MTCY samples subjected to varying spreading durations were conducted by trained evaluators. As shown in Fig. 1B, with the increase of spreading time, the phenotype of dry tea, tea infusion and brewed tea appearance of tea samples had some differences, but adjacent time samples not significant. In the sensory evaluation of tea aromas, the 2 h had the better MTCY fragrance, presenting a distinct fresh floral and fruity aroma. The 4 h and 6 h MTCY followed, 4 h exhibiting mature floral and woody notes, 6 h exhibiting more refreshing fruity aroma. The 10 h and 0 h MTCY is in the third tier, characterized by strong woody and light floral scents, with the 0 h MTCY having a more pronounced grassy scent. The 8 h MTCY had a strong grassy scent, ranking it last.
Fig. 1.
Effect of fresh leaf spreading time (0, 2, 4, 6, 8, 10 h) on the quality in Meitan Cuiya.
(A) Schematic representation illustrating the manufacturing process employed for the production of MTCY tea in this study. (B) The aroma and taste of MTCY tea with different spreading time were evaluated and ranked. (C) Radar chart of sensory evaluation for the MTCY processed from the tea leaves from the different spreading time. Soluble sugar content (D) and chlorophyll content (E) of MTCY treated with different spreading time. Different letters in front of the same com-pounds and bar graphs denote significant differences (one-way ANOVA test; P < 0.05).
Sensory evaluations of taste were further delineated according to five key attributes of MTCY, encompassing thickness, bitterness, astringency, umami, and sweetness. As shown in Fig. 1C, the overall sweetness of the MTCY prepared during the six time periods was lower and the bitterness was similar, but the 4 h MTCY have significantly lower astringency, prominent umami taste and moderate thickness. To investigate the effect of spreading time on the color of dry tea, we measured the chlorophyll content and found that MTCY increased with prolonged spreading duration (Fig. 1D). Specifically, the highest content observed at 4 h and 8 h did not exhibit a statistically significant difference between the two, whereas the content observed at 6 h and 10 h was marginally lower compared to the former pair with a significant discrepancy compared with 8 h. These results once again indicate that from a sensory evaluation perspective, in summer MTCY made from 4 h spreading fresh leaves exhibits higher quality.
3.2. Effects of spreading time on the main quality components of tea leaves and MTCY
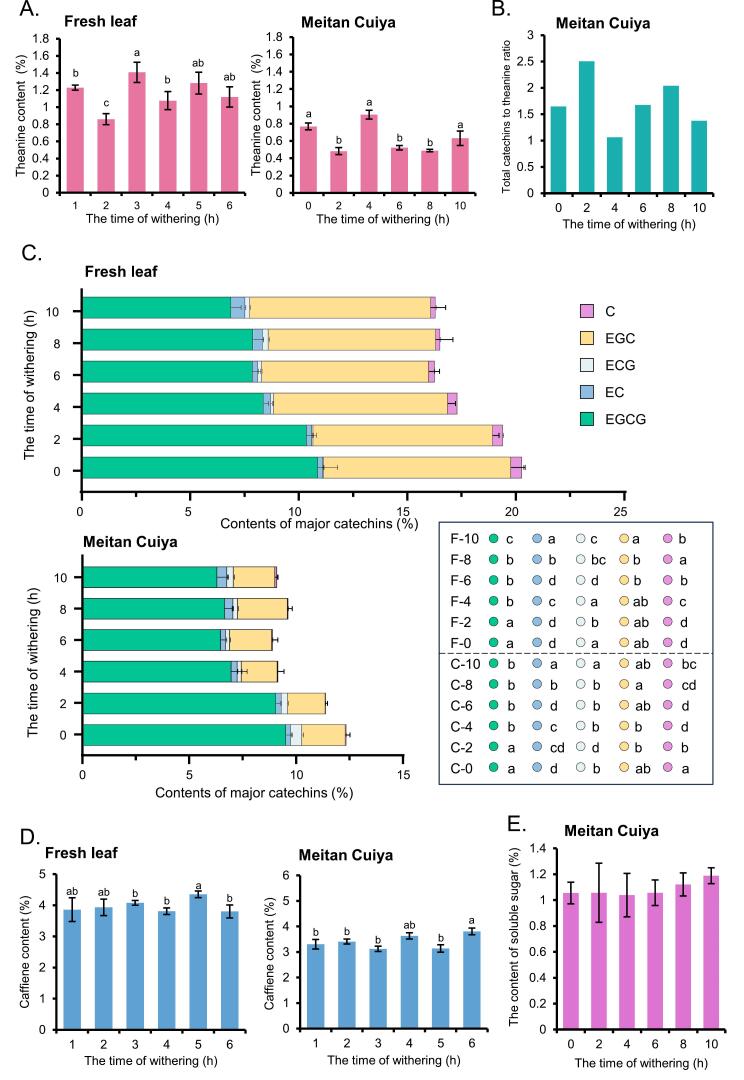
To investigate the impact of spreading time on the quality of fresh leaves and MTCY, this study measured several representative components related to tea quality. As spreading time increased, theanine content in fresh leaves increased from 0.78 %, reached a peak of 1.41 % at 4 h, and then decreased (Fig. 2A). In the MTCY made from these fresh leaves, the theanine content was consistently lower than that in the fresh leaves at the same time points, approximately halving. The theanine content in 4 h MTCY was the highest at 9.04 %, but there was no significant difference compared to 0 h and 10 h. The theanine content at 2 h, 6 h, and 8 h were 0.48 %, 0.52 % and 0.49 %, respectively, with no significant difference among the three, but was significantly lower than at other time points.
Fig. 2.
Effects of spreading times on the main chemical components in fresh leaves and Meitan Cuiya.
(A, C, D) Contents of theanine (%), major catechins (%), and caffeine (%). F means fresh leaves, C means MTCY, the number means spreading hours. (B) The total catechins to theanine of MTCY. (E) The soluble sugar content of MTCY. Measurements were standardized to dry weight. Different letters indicate significant differences (one-way ANOVA test; P < 0.05). (For color references, see web version.)
The catechin content in fresh leaves decreased with longer spreading times (Fig. 2C), dropping from 21.08 % at 0 h to 16.63 % at 10 h. The catechin content in 4-h fresh leaves were 17.64 %, with no significant difference compared to 6 h (17.15 %), 8 h (16.98 %), and 10 h (16.63 %). The trend for individual catechins was similar to that of the total catechins. EGCG content was 11.94 % at 0 h, and then decreased with spreading time, reaching the lowest value of 7.03 % at 10 h. Among them, 4 h (8.66 %) was significantly lower than 0 h and 2 h (9.99 %), but had no significant difference from 6 h (9.23 %) and 8 h (7.42 %). Other catechin components EC, ECG, EGC and C showed similar trends to EGCG. In MTCY, the total catechin content decreased after processing, with 12.66 % at 0 h, slightly lower at 2 h with no significant difference, and significantly lower at 4 h (9.62 %) compared to the first two, but with no significant difference from 6 h, 8 h, and 10 h. Additionally, the ratio of total catechins to theanine was calculated (Fig. 2B), showing the lowest value at 4 h, followed by 10 h, 0hs, 6 h and 8 h, and the highest at 2 h.
Fig. 2D indicates that caffeine content in fresh leaves had little difference, varying between 3.80 % and 4.35 %, and reaching a peak at 8 h. The caffeine content in MTCY was slightly lower than in fresh leaves, with the highest values at 6 h (3.63 %) and 10 h (3.81 %) and the lowest at 4 h (3.12 %) and 8 h (3.14 %). The soluble sugar content in MTCY was also analyzed (Fig. 2E). With increasing spreading time, soluble sugar content increased from 0 h (1.05 %) to 10 h (1.19 %), but the difference between samples was not significant.
Theanine, a key amino acid in tea, is known for its calming effects and contributes significantly to the fresh, umami taste of tea, adding a slight sweetness that balances bitterness and enhances the smoothness of the infusion (L. Zhang, Cao, Granato, Xu, & Ho, 2020). In MTCY, the 4 h sample exhibited the highest theanine content, which aligns with its superior umami taste. Catechins, polyphenolic compounds renowned for their antioxidant properties, are divided into non-ester catechins (C, EC, EGC) and ester catechins (ECG, EGCG), with EGCG being the most abundant in green tea and contributing to bitterness and astringency (Zhang et al., 2020). The 4 h sample had relatively lower total catechin and EGCG content, correlating with a less bitter and astringent taste, as observed in the sensory evaluation. Caffeine, a central nervous system stimulant, is primarily responsible for tea's bitter taste, though in MTCY, caffeine levels showed minimal variation across different spreading times (Zhang et al., 2020). This suggests that the differences in bitterness between samples are more likely due to variations in catechin content. Soluble sugars, which contribute sweetness and a smooth, full-bodied mouthfeel, showed a slight increase with spreading time, but the differences were not significant enough to affect sweetness perception (Zhang et al., 2020). Overall, the 4 h MTCY sample had a more balanced and superior taste profile, characterized by higher theanine and lower catechin levels, consistent with the sensory evaluation. These findings indicate that spreading time significantly influences theanine and catechin levels, while it has less impact on caffeine and soluble sugar content.
3.3. Effects of spreading time on volatile substance of MTCY
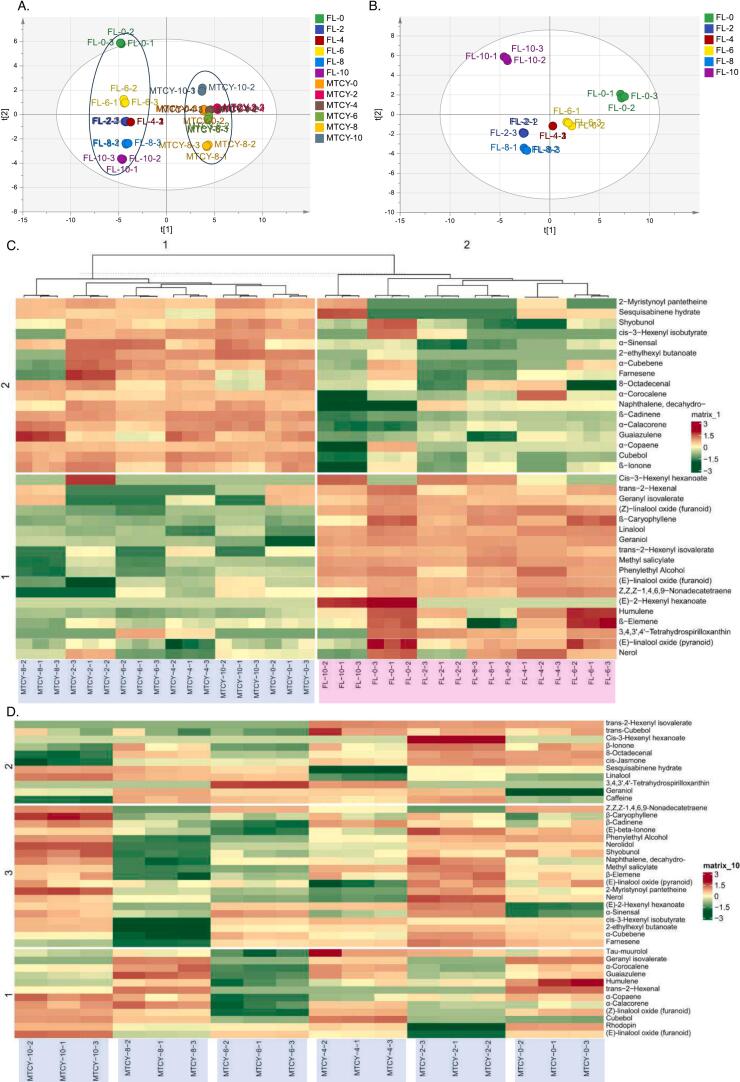
In this study, we utilized GC–MS to relatively quantify the dynamic changes of metabolites. A comprehensive analysis revealed the presence of 44 distinct Volatile Organic Compounds (VOCs). The principal component analysis (PCA) plot (Fig. 3A) demonstrates a clear separation between fresh leaf samples and MTCY samples. The fresh leaf samples cluster in the left quadrant, while the MTCY samples concentrate in the right quadrant. This distinct demarcation highlights the significant differences between the two groups, indicating that fresh leaves and MTCY samples exhibit markedly different characteristics. The PCA analysis of fresh leaf samples (Fig. 3B) reveals distinct separations among the sample groups based on spreading time. The 0 h samples are located in the upper right quadrant, 4 h and 6 h samples in the lower right quadrant, 2 h and 8 h samples in the lower left quadrant, and 10 h samples in the upper left quadrant. This clear clustering patterns corresponding to different spreading times, suggesting that fresh leaf samples at different time points have distinct characteristics. Notably, the fresh leaves sampled at 2, 4, 6, and 8 h were positioned closer together in the PCA plot, indicating that their VOCs are similar to some extent. This clustering suggests a significant association between the spreading times and the composition of VOCs in the leaves.
Fig. 3.
Dynamic Analysis of Metabolites and Differential Analysis of Volatile Organic Compounds (DAVOCs).
(A) Principal Component Analysis (PCA) plot illustrating the separation between fresh leaf samples and those subjected to MTCY processing. (B) PCA plot illustrating the separation between fresh leaf samples with varying spreading times (C) DAVOCs between fresh leaves and MTCY across different spreading durations. (D) Heatmap of VOCs of MTCY samples. FL means fresh leaves. The relative contents of DAVOCs and VOCs are log-transformed (log2) and normalized.
We then performed the Differential Analysis of Volatile Organic Compounds (DAVOCs) between fresh leaves and MTCY tea samples across various spreading durations (Fig. 3C). The analysis revealed a total of 35 DAVOCs between the two groups, with 18 found primarily in fresh leaves samples and 17 primarily in MTCY samples. Categorizing the DAVOCs, the fresh leaves contained 4 alcohols, 1 aldehyde, 4 esters, 3 terpenes, 2 ketones, 1 hydrocarbon and 1 acid; whereas the MTCY samples had 9 alcohols, 2 aldehydes, 2 ketones, 2 terpenes, and 2 esters. Most fresh leaves DAVOCs (such as cis-3-Hexenyl hexanoate, trans-2-Hexenal, methyl salicylate and (E)-2-Hexenyl hexanoate, etc) have a prominent fresh herb aroma, featuring lemon citrus and light floral notes. In contrast, MTCY DAVOCs (such as α-Sinensal, β-Cadinene, β-Ionone and α-Copaene, etc) exhibit a primarily woody, floral, and fruity aroma. And, the increase in alcohols in MTCY samples could be associated with enhanced aromatic qualities, as many alcohols (such as α-Sinensal, 2-ethylhexyl butanoate and guaiazulene, etc) contribute to floral and fruity aromas (Chan, Tan, Chan, Lee, & Goh, 2016; Han et al., 2019).
Further analysis of the differences in VOCs of MTCY is illustrated in the heat map (Fig. 3D). Representative floral green tea aroma substances, including geraniol, phenylethyl alcohol, nerol, β-ionone, linalool, nerolidol, and cis-3-Hexenyl hexanoate, exhibit a fresh floral and fruity aroma. The 2 h MTCY was noted to have the better fragrance, showcasing a distinct fresh floral and fruity aroma, which matches the presence of volatile organic compounds such as cis-3-Hexenyl hexanoate, geraniol, phenylethyl alcohol, nerol, β-ionone, and linalool. And, different from 2 h, the 4 h MTCY contains relatively rich trans-cubebol, geraniol, tau-muurolol, α-Corocalene, geranyl isovalerate and linalool oxide (furanoid) for more woody notes and a dull floral note. These findings support the sensory evaluation, indicating that the chemical composition at 2 h and 4 h contributes to their better aroma profile. Since 4 h MTCY has a better taste and a second-ranked aroma, 2 h has a better aroma but a heavier astringency, and overall 4 h spreading MTCY has a better quality.
3.4. Transcriptome profiles and DEGs annotations
We generated 18 cDNA libraries (three biological repeats for each group) from tea leaves with different spreading duration for sequencing to build the transcriptome profiles. A total of about 77 million clean reads were obtained and screened from 18 libraries, with an average of 42.80 million clean reads per library, accounting for 97.13 % of the total raw reads, and the Q30, Q20 and GC content were 98.10 %, 94.53 %, 43.82 %, respectively. And the unique localization rate ranged from 84.57 % to 82.80 %.
To investigate the differences in the expression of metabolite biosynthesis genes in tea leaves during different spreading durations, we selected specific time points for analysis. The selected time points were 0 h (the time when spreading starts), 2 h (the spreading time of better aroma), 6 h (the representing the traditional spreading time for MTCY), and 10 h (the maximum spreading time set in this study), all compared with 4 h (the spreading time for overall better quality). The DEGs in the four comparisons, “4h vs. 0h”, “4h vs. 2h” “6h vs. 4h” and “10h vs. 4h” were analyzed.
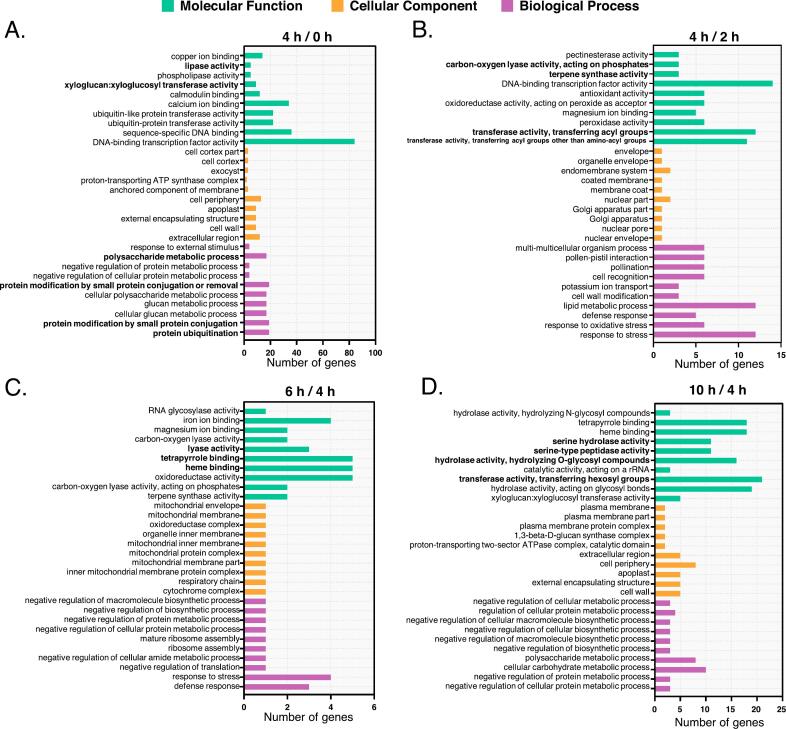
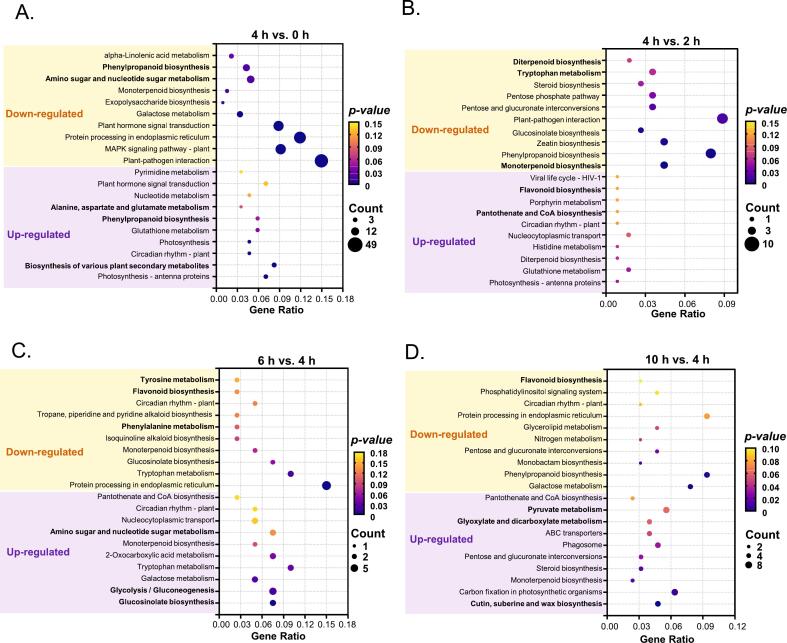
The top 10 enriched GO terms of each cluster for the four comparisons were presented in Fig. 4. In the “4h vs. 0h” comparison (Fig. 4A), 16 GO terms were significantly enriched, including 9 GO MF (molecular functions), 5 GO cellular components (CC), and 2 GO BP (biological processes). Notably, “xyloglucan: xyloglucosyltransferase activity”, “lipase activity”, “polysaccharide metabolic process”, “protein modification by small protein conjugation or removal”, and “protein ubiquitination” were up-regulated. These enzymes are involved in the synthesis and degradation of secondary metabolites in tea, such as lipids, aromatic compounds, and terpenoids. In the “4h vs. 2h” comparison (Fig. 4B), GO terms “transferase activity, transferring acyl groups”, “other than amino-acyl groups”, “terpene synthase activity” and “carbon-oxygen lyase activity, acting on phosphates” were down-regulated. These enzymes are strongly associated with the biosynthesis of lipids and terpenoids. For the “6h vs. 4h” comparison (Fig. 4C), GO terms related to “lyase activity” were down-regulated, reflecting a likely decrease in enzymes involved in the formation of volatile compounds. In the “10h vs. 4h” comparison (Fig. 4D), 8 GO terms showed significant enrichment, including 5 GOCC and 3 GOBP. Among these, “hydrolase activity, hydrolyzing O-glycosyl compounds”, “serine-type peptidase activity”, and “serine hydrolase activity” were up-regulated, typically associated with the degradation of glycosides and proteins. While “transferase activity, transferring hexosyl groups” was down-regulated, which is generally involved in the synthesis of glycosylated compounds.
Fig. 4.
The top 10 significantly enriched GO terms (P-value <0.05) for each of the three main GO categories (molecular function, cellular component, and biological process) in the (A) “4h vs. 0h”, (B) “4h vs. 2h”, (C) “6h vs. 4h”, and (D) “10h vs. 4h” comparison groups.
The GO enrichment analysis underscores the critical role of withering in the formation of MTCY quality. At the 2-h mark, enzymes associated with aroma synthesis were notably active. By 4 h, enzymes involved in the synthesis and degradation of secondary metabolites, including lipids, aromatic compounds, and terpenoids, exhibited widespread activity. However, after 6 h of spreading, the activity of enzymes responsible for volatile compound synthesis began to decline. By 10 h, the observed enzymatic changes suggested a potential reduction in aroma precursors and sweet-tasting components, with excessive hydrolysis possibly leading to a loss of previously accumulated secondary metabolites. These findings suggest that a 4-h spreading, period may be more conducive to the formation and accumulation of key quality components in MTCY, optimizing both aroma and flavor attributes.
We further performed KEGG enrichment analysis to gain insight into the difference of the influence of spreading duration on metabolites in tea leaves (Fig. 5). The top 10 up-regulated and top 10 down-regulated enriched KEGG pathways for each comparison were visualized. In the “4h vs. 0h” (Fig. 5A) comparison, “amino sugar and nucleotide sugar metabolism” were down-regulated, while “biosynthesis of various plant secondary metabolites”, “phenylpropanoid biosynthesis”, and “plant hormone signal transduction” were up-regulated. In the “4h vs. 2h” comparison (Fig. 5B), “monoterpenoid biosynthesis”, “phenylpropanoid biosynthesis”, “tryptophan metabolism”, and “diterpenoid biosynthesis” were down-regulated, while “flavonoid biosynthesis”, “pantothenate and CoA biosynthesis”, and “plant hormone signal transduction” were up-regulated. In the “6h vs. 4h” comparison (Fig. 5C), “tyrosine metabolism”, “flavonoid biosynthesis” and “phenylalanine metabolism” were down-regulated, while “glycolysis/gluconeogenesis”, “amino sugar and nucleotide sugar metabolism” and “glucosinolate biosynthesis” were up-regulated. In the “10 h/ 4h” comparison (Fig. 5D), “flavonoid biosynthesis”, while “pyruvate metabolism”, “glyoxylate and dicarboxylate metabolism”, and “cutin, suberine and idea for biosynthesis” were up-regulated.
Fig. 5.
The top 10 enriched KEGG pathways for both up- and down-regulated pathways in the (A right) ‘4 h vs. 0 h“, (B right) ‘6 h vs. 4 h”, and (C right) ‘10 h vs. 4 h” comparison groups. (For color references, see web version.)
These KEGG enrichment analysis indicated that the secondary metabolism of 4 h was more active than that of 0 h, but the aroma synthesis pathway was weaker than that of 2 h. As spreading extends from 6 to 10 h, secondary metabolism remains active but shifts towards degradation processes, including a continued accumulation of specific metabolites. This metabolic shift could lead to the breakdown of previously synthesized compounds, particularly those linked to flavor and aroma. These results align closely with the GO analysis, suggesting that a 4-h spreading duration strikes an optimal balance between the accumulation of key metabolites, including flavonoids and other secondary compounds, while preventing excessive degradation of important precursors. The consistency of these findings with our quality tests further highlights the importance of this specific spreading duration for achieving the desired tea characteristics, particularly in preserving a balance between flavor, aroma, and bioactive compounds.
3.5. The transcriptome time-series analysis was used to study the spreading representative genes in different spreading time
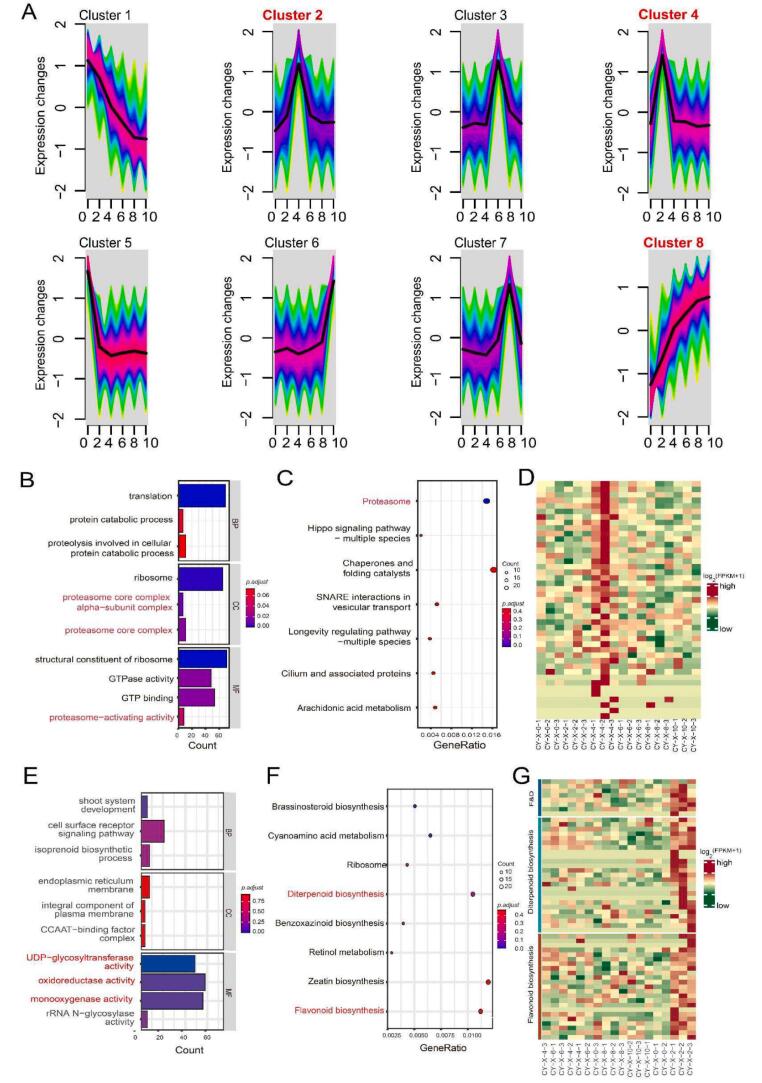
Upon scrutinizing the expression patterns of DEGs, an exhaustive time series analysis has divulged a nuanced classification into eight distinct clusters, as illustrated in Fig. 6A. Notably, within cluster 2, a substantial upregulation of DEGs emerged at the 4-h time point. GO analysis uncovered a conspicuous association with the proteasome core complex, alpha-subunit complex, and proteasome-activating activity (Fig. 6B). And the KEGG enrichment analysis depicted in Fig. 6C underscores the categorization of approximately 20 genes as integral components of the proteasome complex, revealing a discernible discrepancy between the 4-h time point and others, as evidenced by the heatmap (Fig. 6D). Proteasomes are multi-enzyme complexes primarily functioning through the ubiquitin-proteasome pathway (UDP), the main protein degradation mechanism responsible for breaking down most intracellular proteins (Tanaka, 2009; Xu & Xue, 2019). Particularly in the plant response to biotic and abiotic stresses, such as pathogen infection, temperature fluctuations, and drought, proteasomes play a vital role. They assist plants in regaining homeostasis by degrading damaged or misfolded proteins. For instance, overexpressing the RING membrane-anchor 1 homologue 1 gene in Arabidopsis has been shown to significantly improve the plant's drought resistance (Lee et al., 2009). During the spreading process of fresh tea leaves, which involves continuous water loss and abiotic stress, proteasome-related activities were significantly upregulated at the 4-h mark. This suggests that fresh leaves in 4 h spreading may primarily rely on active proteasome activity to combat stress, helping maintain normal physiological states. This physiological state may be conducive to the accumulation and balance of tea quality components, and lay a material foundation for the production of 4 h MTCY.
Fig. 6.
Transcriptome Analysis and Gene Expression Clustering during Spreading.
(A) Transcriptome Analysis and Gene Expression Clustering during Spreading. Y-axis representing expression changes and the X-axis representing the spreading time points. (B) GO and (C) KEGG enrichment analysis of Cluster 2. (D) Heatmap of gene expression for proteasome pathway. (log10 FPKM+1, row z-score) (E) GO and (F) KEGG enrichment of Cluster 4. (G) Heatmap of gene expression for diterpenoid biosynthesis and flavonoid biosynthesis, D&F means intersection of diterpenoid biosynthesis and flavonoid biosynthesis. (log10 FPKM+1, row z-score)
In Cluster 4 (Fig. 6A), DEGs were significantly upregulated at the 2-h time point. GO enrichment analysis (Fig. 6E) revealed a substantial number of DEGs annotated with oxidoreductase activity、monooxygenase activity and UDP-glycosyltransferase activity. The major aroma compounds in tea, including terpenoids, aromatic alcohols, and ketones, are often formed through oxidation-reduction reactions, such as cis-3-hexenyl hexanoate, cis-jasmone, geraniol, and phenylethyl alcohol. Monooxygenase activity is also closely linked to the synthesis of terpenoids and phenolic compounds, such as β-ionone, (E)-β-ionone, and nerolidol (Han et al., 2019). In addition, the sweet floral aroma of the aroma substances in tea, such as linalool, geraniol, phenylethyl alcohol and β-Ionone, comes from their glycoside form formed by UDP-glycosyltransferase glycosylation (Ilc, Parage, Boachon, Navrot, & Werck-Reichhart, 2016; Zhou et al., 2017).
Additionally, KEGG pathway enrichment analysis (Fig. 6F) indicated that, in cluster 4, approximately 40 DEGs are involved in the biosynthesis pathways of diterpenoids and flavonoids. Analyzing the gene expression profiles of these two pathways, as shown in heatmap (Fig. 6G), reveals that these genes are significantly highly expressed in the fresh leaves at the 2-h mark, particularly the genes at the intersection of these two pathways. Numerous studies have shown that many volatile terpenoids contributing to the aroma of tea, such as geranyl diterpenoids, are synthesized via the diterpenoid biosynthesis pathway and released during tea processing, and can further form carotenoids (Junze, Wu, & Zhanpin, Chen, & Zhang, 2022). Flavonoids not only affect the taste and color of tea but also produce volatile aroma compounds upon degradation, which contribute to the production of flower and fruit flavor of tea (Saputri, Chien, Lin, Yanti, & Agrawal, 2023). The upregulation of these two pathways contributes to the generation of the pleasant aroma detected in fresh leaves, laying the foundation for subsequent processing into MTCY, which produces a fresh and floral aroma. These result helps to understand the reason why MTCY aroma is better formed at 2 h spreading time.
3.6. Hormone participated in the formation of tea quality during withering
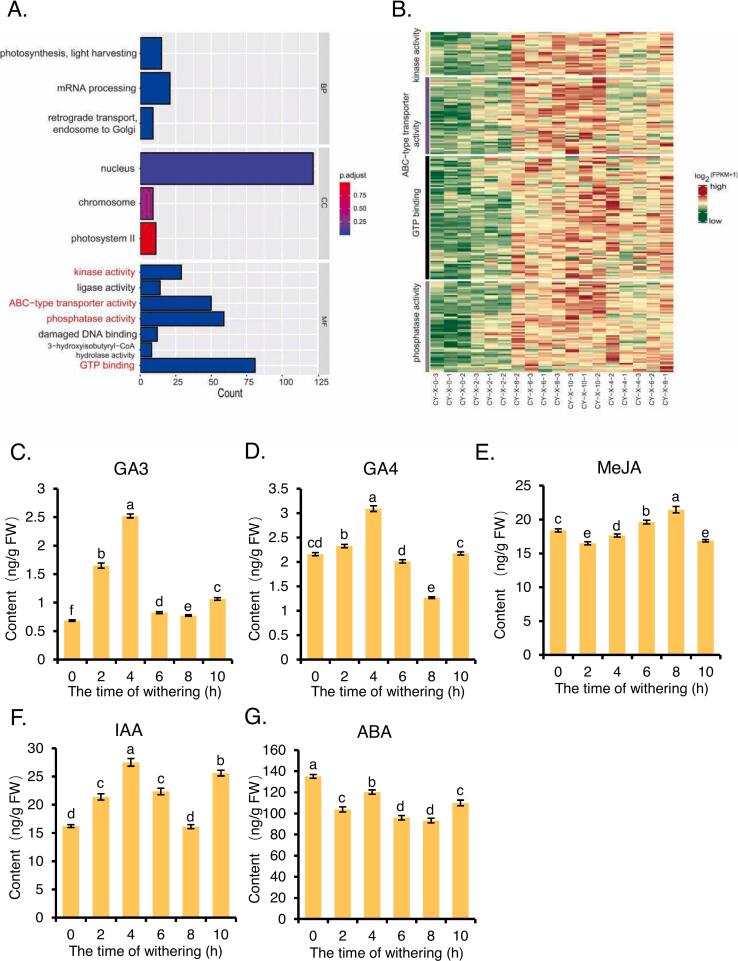
In the time-series transcriptome analysis, cluster 8 (Fig. 6A) exhibited a continuously rising trend. Further GO enrichment analysis (Fig. 7A) revealed that there were a large number of annotations related to kinase activity, phosphatase activity, ABC-type transporter activity, and GTP binding within the GOMF. Heatmap analysis of gene expression associated with these annotations showed that the expression levels of genes under these four annotations increased with spreading time (Fig. 7B). Kinases, particularly protein kinases, play pivotal roles in hormone signal transduction by phosphorylating proteins, thus regulating key cellular activities. These kinases are integral to pathways involving plant hormones such as IAA, GAs, and ABA (Jagodzik, Tajdel-Zielinska, Ciesla, Marczak, & Ludwikow, 2018; Zhang, Zhou, Zhang, Su, & Xu, 2023). For example, transmembrane kinase 4 specifically phosphorylates tryptophan aminotransferase, inhibiting its activity and impacting auxin synthesis and plant development (Zhang et al., 2023). GA signaling promotes the degradation of phosphorylated GA signal inhibitor SLR1, thereby enhancing GA accumulation (Sasaki et al., 2003). In ABA signaling, the pyrabactin resistance receptor inhibits PP2C phosphatase, activating SnRK2s (SNF1-associated protein kinase) (Fujii et al., 2009). Protein phosphatases like PP1 and PP2A regulate ABA- and MeJA-mediated stomatal movement (Hossain, Munemasa, Nakamura, Mori, & Murata, 2011), while phosphatases in general modulate the intensity and duration of hormone responses through dephosphorylation (Graves & Krebs, 1999). ABC transporters facilitate the transmembrane transport of hormones, influencing their distribution and concentration in plants (Do, Martinoia, & Lee, 2018). Similarly, GTP-binding proteins, such as G proteins, play key roles in hormone signal transduction and regulate multiple hormone pathways (Neves, Ram, & Iyengar, 2002). From this, we speculate that plant hormones are highly correlated with 2 h spreading. We measured the levels of several representative plant hormones to further investigate these findings.
Fig. 7.
Characteristics of changes in the content of major hormones in fresh leaves at different spreading times.
(A) GO enrichment analysis of Cluster 8. (B) Heatmap of gene expression for Kinase activity, Phosphatase activity, ABC-type transporter activity, and GTP binding pathway. (log10 FPKM+1, row z-score) (C-G) Contents of GA3, GA4, MeJA, IAA and ABA in fresh leaves. Different letters in front of the same compound and in bar graphs denote significant differences (one-way ANOVA test; P < 0.05).
We conducted a comprehensive examination of the accumulation of representative GAs, GA3 and GA4, in fresh leaves at different stages of spreading (Fig. 7C, D). Notably, the concentration of GA3 consistently increased throughout the spreading process, peaking at 2.52 ng/g FW at 4 h, then decreasing to 0.83 ng/g FW at 6 h. Subsequent time points at 8 h and 10 h showed slight increases, remained significantly higher than the initial time point. In contrast, Fig. 7D shows that the concentration of GA4 in 0 h samples was nearly four times higher than that of GA3. Its concentration surged to 3.09 ng/g FW at 4 h, dropped to 1.27 ng/g FW at 8 h, and then returned to similar levels at 6 h. GAs primarily regulate plant growth and developmental processes. GAs can regulate secondary metabolism, not only affect plant growth, flowering, and fruit development but also may indirectly influence may impact the synthesis of secondary metabolites such as terpenoids, phenolics, and flavonols (Hartweck, 2008; Tyagi et al., 2022). Tea tree is rich in terpenoids such as linalool, geraniol and nerol, as well as rich catechins, tea polyphenols, flavonoids and other polyphenols, which may be regulated by GAs. The increase in GA3 and GA4 during the early stages of spreading, particularly peaking at 4 h, suggests their involvement in the accumulation of quality components, potentially contributing to the enhanced quality observed at this time point.
Next, we measured the content of MeJA, in fresh leaves, as depicted in Fig. 7E. MeJA exhibited slight fluctuations in the early stages of spreading, significantly rising to 19.63 ng/g FW at 6 h, peaking at 21.45 ng/g FW at 8 h, and then returning to baseline levels. JAs is an important plant hormone that mainly plays a role in regulating defense responses and secondary metabolism. (Dong & Lin, 2021). MeJA activates multiple secondary metabolic pathways related to the production of volatile compounds, such as the terpenoid biosynthesis pathway, the phenylpropanoid pathway, and the lipoxygenase pathway (Kim, Chen, Wang, & Rajapakse, 2006). Additionally, MeJA itself is a volatile compound with a floral scent. The content of MeJA increases initially with the spreading time, peaks at 8 h, and then decreases to the lowest level at 10 h. This indicates that in this study, MeJA did not play a dominant role in the synthesis and release of early-stage aroma compounds.
We also measured the content of IAA (representative auxin) and ABA in fresh leaves. As shown in Fig. 7F, IAA content peaked at 27.52 ng/g FW at 4 h, then decreased to 16.11 ng/g FW at 8 h, and finally returned to 25.60 ng/g FW at 10 h. In contrast, ABA content decreased after spreading, showed an increase at 4 h, but remained below the initial 0 h level of 135.10 ng/g FW (Fig. 7G). IAA is known to regulate plant growth and development, as well as influence the production of VOCs by controlling the expression of phenylalanine ammonia-lyase and terpene synthase (Do et al., 2018). Similarly, ABA can increase VOC content by promoting phenylpropanoid metabolism and terpene synthesis (Murcia et al., 2017). The rise in IAA levels during the early stages of spreading, peaking at 4 h, suggests a role in boosting VOC production at this critical time point, potentially contributing to the desirable floral and fruity aroma noted in sensory evaluations. On the other hand, the decline in ABA throughout the spreading process indicates a negative correlation with VOC production, especially in relation to the woody and grassy notes associated with longer spreading times. These results suggest that the dynamic interplay between IAA and ABA may significantly influence the aroma profile of MTCY during different spreading durations, with IAA promoting more favorable VOC production early on, while ABA suppression may coincide with a reduction in less desirable volatile compounds.
The collective findings underscore the dynamic fluctuations in GA3, GA4, and IAA concentrations, peaking at 4 h of spreading. This temporal pattern suggests their potential pivotal roles in mediating the summer MTCY spreading process. In addition, in Fig. 6A, it was observed that cluster 2 showed a significant increase in proteasome-related protein activity at 4 h. Proteasomes degrade regulatory proteins in signaling pathways, thereby modulating the intensity and duration of plant hormone signal transduction, such as auxin and gibberellin (Dharmasiri & Estelle, 2002; Hartweck, 2008). The observed increase in proteasome activity at 4 h aligns with the elevated hormone levels, suggesting a close relationship between proteasome function and hormone regulation during this stage of processing. However, while these correlations between hormone fluctuations and proteasome activity provide a strong indication of their involvement, the precise mechanisms by which these hormones influence tea quality formation remain unclear and warrant further investigation. Understanding how these hormonal dynamics translate into the biochemical and sensory properties of MTCY could offer valuable insights into optimizing the tea processing steps, particularly the spreading duration.
3.7. qRT-PCR analysis of genes related to differential metabolite synthesis
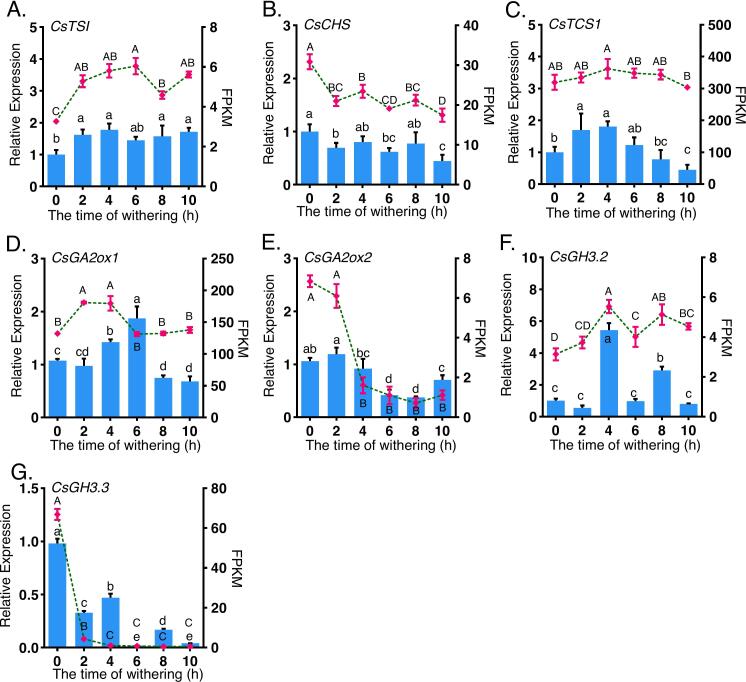
To validate the reliability of the transcriptome data, seven DEGs were selected for qRT-PCR analysis, including tea theanine synthase (CsTSI), chalcone synthase (CsCHS), caffeine synthase (CsTCS1), gibberellin 2-oxidase 1 (CsGA2ox1), gibberellin 2-oxidase 2 (CsGA2ox2), and two GRETCHEN HAGEN3 (GH3) genes (CsGH3.2 and CsGH3.3). As shown in Fig. 8, transcriptome data are represented by the line graphs, while qRT-PCR results are depicted as bar charts. The high consistency between these two sets of results indicates that the transcriptomic data are accurate and reliable. CsTSI, CsCHS, and CsTCS1 are key genes involved in the biosynthesis of theanine, catechins, and caffeine, respectively, and their gene expression levels were positively correlated with the metabolite contents. The expression patterns of these genes were consistent with the measured levels of the corresponding metabolites. CsGA2ox1 and CsGA2ox2 are highly correlated with the levels and activities of GAs and exhibit different expression patterns during various stages of plant growth (Li et al., 2020). In this study, the expression pattern of CsGA2ox1 was similar to the trend of GA3 content, while CsGA2ox2 expression aligned with the trend of GA4 content. This suggests that these two genes differentially influence GAs during the spreading process in tea leaves. While CsGH3.2 and CsGH3.3 are typically highly correlated with IAA levels, their expression trends did not align with IAA content in this study(Chen, Zhao, & Huang, 2022). This indicates that these genes may not be the primary factors influencing IAA levels during the spreading process in tea leaves.
Fig. 8.
qRT-PCR validation of DEGs related to the biosynthesis pathways of theanine (A), catechin (B), caffeine (C), gibberellins (D-E), and indole-3-acetic acid (IAA) (F-G) in tea leaves under different spreading durations. Bar graphs represent qRT-PCR results, while dashed line graphs show the fragments per kilobase of transcript per million mapped reads (FPKM) values from RNA-Seq analysis. Different uppercase letters indicate significant FPKM differences, and lowercase letters indicate significant qRT-PCR differences (one-way ANOVA test; P < 0.05).
4. Conclusion
This study integrates transcriptomic and metabolomic approaches to elucidate the impact of different spreading durations on the quality of summer Meitan Cuiya (MTCY) tea. The findings revealed that 2-h spreading resulted in a superior aroma, characterized by compounds such as cis-3-Hexenyl hexanoate, geraniol, β-ionone, and linalool, which together create a fresh, floral-fruity scent. The 4-h spreading produced a woody-floral scent, with compounds like trans-cubebol, tau-muurolol, geranyl isovalerate contributing to its unique profile. Although spreading time had no significant effect on caffeine and soluble sugar content, the 4-h spreading led to higher theanine and lower catechin levels, resulting in a fresher taste. Overall, the 4-h spreading was identified as optimal for enhancing tea quality. Transcriptomic time-course analysis indicated that increased oxidoreductase, UGT, and monooxygenase activities, along with the upregulation of diterpenoid and flavonoid biosynthesis, likely play key roles in aroma formation. Elevated proteasome activity further supported cellular homeostasis, likely aiding flavor compound synthesis. Plant hormones such as gibberellins and auxins were implicated in regulating quality attributes during spreading. These insights offer a valuable foundation for improving summer tea quality through precise control of spreading time. Future studies will build on these findings to explore the regulatory networks and hormonal pathways that govern the biochemical and sensory properties of tea, aiming to further optimize processing conditions.
CRediT authorship contribution statement
Yihe Jiang: Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Dayu Huang: Investigation. Cui Lu: Formal analysis, Data curation. Shenyuan Ye: Investigation. Linlin Li: Investigation. Tong Li: Writing – review & editing. Xiaohua Liu: Data curation. Benguo Chen: Investigation. Jun Guo: Investigation. Litang Lu: Writing – review & editing, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Guizhou Province Science and Technology Planning Project (Qiankehe Support [2021] General 111); the Earmarked Fund for GZMARS-Tea; Guizhou Province High-Level Innovative Talents “Hundred” Level Talent Project (Qiankehe Platform Talent)(GCC [2023] 014); the National Natural Science Foundation of China (32360774); Guizhou University Doctoral Fund (grant [2022] 56); Guizhou University (SYSKF 2024-16, SYSKF2024-18).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101878.
Contributor Information
Yihe Jiang, Email: yhjiang@gzu.edu.cn.
Dayu Huang, Email: 3366784326@qq.com.
Shenyuan Ye, Email: syuany218@163.com.
Linlin Li, Email: 1429200392@qq.com.
Tong Li, Email: lit@gzu.edu.cn.
Xiaohua Liu, Email: gzmtlxh@163.com.
Benguo Chen, Email: 603558831@qq.com.
Jun Guo, Email: 1427297287@qq.com.
Litang Lu, Email: ltlv@gzu.edu.cn.
Appendix A. Supplementary data
Supplementary tables
Data availability
Data will be made available on request.
References
- Chan W.K., Tan L.T., Chan K.G., Lee L.H., Goh B.H. Nerolidol: A Sesquiterpene alcohol with multi-faceted pharmacological and biological activities. Molecules. 2016;21(5) doi: 10.3390/molecules21050529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Zhao D., Huang X. Transcriptome analysis of easy- and hard-to-root tea plants uncovers roles for CsGH3.2 and CsGH3.3 in adventitious root formation. Plant Cell, Tissue and Organ Culture (PCTOC) 2022;150 doi: 10.1016/j.gene.2020.145247. [DOI] [Google Scholar]
- Dharmasiri S., Estelle M. The role of regulated protein degradation in auxin response. Plant Molecular Biology. 2002;49(3–4):401–409. doi: 10.1023/A:1015203013208. [DOI] [PubMed] [Google Scholar]
- Do T.H.T., Martinoia E., Lee Y. Functions of ABC transporters in plant growth and development. Current Opinion in Plant Biology. 2018;41:32–38. doi: 10.1016/j.pbi.2017.08.003. [DOI] [PubMed] [Google Scholar]
- Dong N.Q., Lin H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. Journal of Integrative Plant Biology. 2021;63(1):180–209. doi: 10.1111/jipb.13054. [DOI] [PubMed] [Google Scholar]
- Fujii H., Chinnusamy V., Rodrigues A., Rubio S., Antoni R., Park S.Y.…Zhu J.K. In vitro reconstitution of an abscisic acid signalling pathway. Nature. 2009;462(7273):660–664. doi: 10.1038/nature08599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves J.D., Krebs E.G. Protein phosphorylation and signal transduction. Pharmacology & Therapeutics. 1999;82(2):111–121. doi: 10.1016/S0163-7258(98)00056-4. [DOI] [PubMed] [Google Scholar]
- Han Y., Wang H., Wang X., Li K., Dong M., Li Y.…Shang F. Mechanism of floral scent production in Osmanthus fragrans and the production and regulation of its key floral constituents, β-ionone and linalool. Horticulture Research. 2019;6(1):106. doi: 10.1038/s41438-019-0189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartweck L.M. Gibberellin signaling. Planta. 2008;229(1):1–13. doi: 10.1007/s00425-008-0830-1. [DOI] [PubMed] [Google Scholar]
- Hossain M.A., Munemasa S., Nakamura Y., Mori I.C., Murata Y. K252a-sensitive protein kinases but not okadaic acid-sensitive protein phosphatases regulate methyl jasmonate-induced cytosolic Ca2+ oscillation in guard cells of Arabidopsis thaliana. Journal of Plant Physiology. 2011;168(16):1901–1908. doi: 10.1016/j.jplph.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Hu Z., Yao X., Chen H., Li F., Zhao H., Tang H., Jiao Y., Jiang Y., Tian J., He Y., Lu L. Changes and dynamics of the main quality components in tea leaves of 4 tea cultivars during the shading process. Scientia Horticulturae. 2024;333 doi: 10.1016/j.scienta.2024.113242. [DOI] [Google Scholar]
- Huang X., Tang Q., Chen C., Li Q., Lin H., Bai S., Zhao J., Li J., Wang K., Zhu M. Combined analysis of transcriptome and metabolome provides insights into nano-selenium foliar applications to improve summer tea quality (Camellia sinensis) LWT. 2023;175 doi: 10.1016/j.lwt.2023.114496. [DOI] [Google Scholar]
- Huang X., Tang Q., Li Q., Lin H., Li J., Zhu M., Liu Z., Wang K. Vol. 185. 2022. Integrative analysis of transcriptome and metabolome reveals the mechanism of foliar application of Bacillus amyloliquefaciens to improve summer tea quality (Camellia sinensis) pp. 302–313. [DOI] [PubMed] [Google Scholar]
- Ilc T., Parage C., Boachon B., Navrot N., Werck-Reichhart D. Monoterpenol oxidative metabolism: Role in plant adaptation and potential applications. Frontiers in Plant Science. 2016;7:509. doi: 10.3389/fpls.2016.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagodzik P., Tajdel-Zielinska M., Ciesla A., Marczak M., Ludwikow A. Mitogen-activated protein kinase cascades in plant hormone signaling. Frontiers in Plant Science. 2018;9:1387. doi: 10.3389/fpls.2018.01387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Zhu Q., Yang H., Zhi T., Ren C. Phenylalanine suppresses cell death caused by loss of fumarylacetoacetate hydrolase in Arabidopsis. Scientific Reports. 2022;12(1):13546. doi: 10.1038/s41598-022-17819-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junze R., Wu Y., Zhanpin Z., Chen R., Zhang L. Diterpenoid biosynthesis and regulation in medicinal plants. Chinese Journal of Natural Medicines. 2022;20:761–772. doi: 10.1016/S1875-5364(22)60214-0. [DOI] [PubMed] [Google Scholar]
- Kim H., Chen F., Wang X., Rajapakse N. Effect of methyl Jasmonate on secondary metabolites of sweet basil (Ocimum basilicum L.) Journal of Agricultural and Food Chemistry. 2006;54:2327–2332. doi: 10.1021/jf051979g. [DOI] [PubMed] [Google Scholar]
- Lee H.K., Cho S.K., Son O., Xu Z., Hwang I., Kim W.T. Drought stress-induced Rma1H1, a RING membrane-anchor E3 ubiquitin ligase homolog, regulates aquaporin levels via ubiquitination in transgenic Arabidopsis plants. Plant Cell. 2009;21(2):622–641. doi: 10.1105/tpc.108.061994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wang Y., Liu H., Lin S.-J., Han M.-H., Zhuang J. Genomic analyses of the crosstalk between gibberellins and brassinosteroids metabolisms in tea plant (Camellia sinensis (L.) O. Kuntze) Scientia Horticulturae. 2020;268 doi: 10.1016/j.scienta.2020.109368. [DOI] [Google Scholar]
- Liu M., Tian H.L., Wu J.H., Cang R.R., Wang R.X., Qi X.H.…Chen X.H. Relationship between gene expression and the accumulation of catechin during spring and autumn in tea plants (Camellia sinensis L.) Horticulture Research. 2015;2:15011. doi: 10.1038/hortres.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murcia G., Fontana A., Pontin M., Baraldi R., Bertazza G., Piccoli P. ABA and GA3 regulate the synthesis of primary and secondary metabolites related to alleviation from biotic and abiotic stresses in grapevine. Phytochemistry. 2017;135:34–52. doi: 10.1016/j.phytochem.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Neves S.R., Ram P.T., Iyengar R. G Protein Pathways. Science. 2002;296(5573):1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- Ni T., Xu S., Wei Y., Li T., Jin G., Deng W.-W., Ning J. Understanding the promotion of withering treatment on quality of postharvest tea leaves using UHPLC-orbitrap-MS metabolomics integrated with TMT-based proteomics. LWT. 2021;147 doi: 10.1016/j.lwt.2021.111614. [DOI] [Google Scholar]
- Qiao D., Zhu J., Mi X., Xie H., Shu M., Chen M., Li R., Liu S., Wei C. Effects of withering time of fresh leaves on the formation of flavor quality of Taiping Houkui tea. LWT. 2023;182 doi: 10.1016/j.lwt.2023.114833. [DOI] [Google Scholar]
- Saputri D., Chien C., Lin H.-Y., Yanti S., Agrawal D. Flavonoid and Main aroma-active compounds identification of Taiwan Citrus Depressa Hayata peels. Food Research. 2023;7:257–267. doi: 10.26656/fr.2017.7(s1).38. [DOI] [Google Scholar]
- Sasaki A., Itoh H., Gomi K., Ueguchi-Tanaka M., Ishiyama K., Kobayashi M.…Matsuoka M. Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science. 2003;299(5614):1896–1898. doi: 10.1126/science.1081077. [DOI] [PubMed] [Google Scholar]
- Shan X., Yu Q., Chen L., Zhang S., Zhu J., Jiang Y.…Li J. Analyzing the influence of withering degree on the dynamic changes in non-volatile metabolites and sensory quality of Longjing green tea by non-targeted metabolomics. Frontiers in Nutrition. 2023;10 doi: 10.3389/fnut.2023.1104926/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. The proteasome: Overview of structure and functions. Proceedings of the Japan Academy. Series B, Physical and Biological Sciences. 2009;85(1):12–36. doi: 10.2183/pjab.85.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S., Zhou H., Yao X., Lu L. Finding the optimal light quality and intensity for the withering process of Fuding Dabai tea and its impact on quality formation. LWT. 2024;193 doi: 10.1016/j.lwt.2023.115713. [DOI] [Google Scholar]
- Tyagi K., Maoz I., Lapidot O., Kochanek B., Butnaro Y., Shlisel M.…Lichter A. Effects of gibberellin and cytokinin on phenolic and volatile composition of Sangiovese grapes. Scientia Horticulturae. 2022;295 doi: 10.1016/j.scienta.2021.110860. [DOI] [Google Scholar]
- Wang Y., Liu N., Yu T., Gao J., Fan Y., Wang W., Wang J., Wu Y., Zhang J., Ning J. The enhancement of flowery-like aroma in green tea under optimized processing conditions by sensory-directed flavor analysis. Food Chemistry: X. 2024;22 doi: 10.1016/j.fochx.2024.101427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Chen Y., Feng W., Shen S., Wei Y., Jia H.…Ning J. Effects of three different withering treatments on the aroma of white tea. Foods. 2022;11(16):2502. doi: 10.3390/foods11162502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F.Q., Xue H.W. The ubiquitin-proteasome system in plant responses to environments. Plant, Cell & Environment. 2019;42(10) doi: 10.1111/pce.13633. [DOI] [PubMed] [Google Scholar]
- Yang J., Zhang J., Wang Z., Zhu Q., Wang W. Hormonal changes in the grains of Rice subjected to water stress during grain filling. Plant Physiology. 2001;127(1) doi: 10.1104/pp.127.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Cao Q.-Q., Granato D., Xu Y.-Q., Ho C.-T. Association between chemistry and taste of tea: A review. Trends in Food Science & Technology. 2020;101:139–149. doi: 10.1016/j.tifs.2020.05.015. [DOI] [Google Scholar]
- Zhang W.J., Zhou Y., Zhang Y., Su Y.H., Xu T. Protein phosphorylation: A molecular switch in plant signaling. Cell Reports. 2023;42(7) doi: 10.1016/j.celrep.2023.112729. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Zeng L., Gui J., Liao Y., Li J., Tang J., Meng Q., Dong F., Yang Z. Functional characterizations of β-glucosidases involved in aroma compound formation in tea (Camellia sinensis) Food Research International. 2017;96:206–214. doi: 10.1016/j.foodres.2017.03.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables
Data Availability Statement
Data will be made available on request.