Abstract
Background
Preterm infants are at risk of exhausting their body iron stores much earlier than healthy term newborns. It is widespread practice to give enteral iron supplementation to preterm and low birth weight infants to prevent iron deficiency anaemia. However, it is unclear whether supplementing preterm and low birth weight infants with iron improves growth and neurodevelopment. It is suspected that excess exogenous iron can contribute to oxidative injury in preterm babies, causing or exacerbating conditions such as necrotising enterocolitis and retinopathy of prematurity. Additionally, the optimal dose and timing of commencement and cessation of iron supplementation are uncertain.
Objectives
To evaluate the effect of prophylactic enteral iron supplementation on growth and neurodevelopmental outcomes in preterm and low birth weight infants. The secondary objectives were to determine whether iron supplementation results in improved haematological parameters and prevents other causes of morbidity and mortality.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group. We searched Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 8), MEDLINE (1951 to August 2011), CINAHL (1982 to August 2011) and conference proceedings and previous reviews.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐randomised trials that compared enteral iron supplementation with no iron supplementation, or different regimens of enteral iron supplementation in preterm or low birth weight infants or both.
Data collection and analysis
We extracted data using the standard methods of the Cochrane Neonatal Review Group. Both review authors separately evaluated trial quality and data extraction. We synthesised data using risk ratios (RRs), risk differences (RDs) and weighted mean differences (WMDs). Where data about the methodology and results or both were lacking, we made an attempt to contact the study authors for further information.
Main results
We included twenty‐six studies (2726 infants) in the analysis. The heterogeneity of participants, methods and results precluded an extensive quantitative synthesis. Of the 21 studies comparing iron supplementation with controls, none evaluated neurodevelopmental status as an outcome. Of thirteen studies reporting at least one growth parameter as an outcome, only one study of poor quality found a significant benefit of iron supplementation. Regarding haematological outcomes, no benefit for iron supplementation was demonstrated within the first 8.5 weeks of postnatal life (16 trials), except by two poor quality studies. After this age, most studies reported a higher mean haemoglobin in iron‐supplemented infants. We were only able to include a limited number of studies in a quantitative meta‐analysis, which suggested the haemoglobin concentration in iron‐supplemented infants was higher by about 6 g/L at six to nine months. One study comparing high dose and low dose iron supplementation monitored neurodevelopmental outcome for one year, without finding any significant difference between the groups. One study comparing early versus late commencement of iron supplementation found no difference in cognitive outcome, but an increased rate of abnormal neurological examination in the late iron group at five years of age. The studies comparing high and low doses of iron indicated that there was no discernible haematological benefit in exceeding 'standard' doses of iron (i.e. 2 mg/kg/day to 3 mg/kg/day).
Authors' conclusions
The available data suggest that infants who receive iron supplementation have a slightly higher haemoglobin level, improved iron stores and a lower risk of developing iron deficiency anaemia when compared with those who are unsupplemented. However, it is unclear whether iron supplementation in preterm and low birth weight infants has long term benefits in terms of neurodevelopmental outcome and growth. The optimum timing and duration of iron supplementation remains unclear.
Keywords: Humans; Infant; Infant, Newborn; Dietary Supplements; Child Development; Child Development/drug effects; Child Development/physiology; Enteral Nutrition; Erythrocytes; Erythrocytes/cytology; Erythrocytes/drug effects; Hemoglobin A; Hemoglobin A/metabolism; Infant, Low Birth Weight; Infant, Low Birth Weight/blood; Infant, Low Birth Weight/growth & development; Infant, Premature; Infant, Premature/blood; Infant, Premature/growth & development; Iron; Iron/administration & dosage; Iron/blood; Randomized Controlled Trials as Topic
Plain language summary
Enteral iron supplementation in preterm and low birth weight infants
This review examined whether providing iron supplementation is beneficial for preterm and low birth weight infants. The potential benefits included improvements in the level of red blood cells and stored iron in their blood. In the longer term, it was thought that iron supplementation might improve the babies' growth and development. We identified 25 randomised controlled trials (RCTs) which were relevant to this topic. We concluded that the long term benefits of iron supplementation for preterm and low birth weight babies remain uncertain. Regarding red blood cell and iron levels, it was found that in the first year of life, after two months of age, iron supplementation may result in slightly higher iron stores and red blood cell levels, and lower rates of iron deficiency anaemia. However, there was a lot of variability between different studies. More RCTs are needed, using well defined patient groups.
Background
Description of the condition
Most of the healthy term newborn's iron stores have been laid down during the third trimester. Therefore, this important acquisition of iron stores is reduced in preterm infants. The preterm infant has a higher requirement for iron due to proportionally more rapid postnatal growth than that of the term infant. This exacerbates the total body iron deficit of the preterm infant, as iron stores decrease over the first three months of postnatal life. While non‐iron supplemented term infants have not been shown to develop biochemical or haematological iron deficiency before six months of age, there is a high rate of iron deficiency anaemia before this age in preterm infants fed only breast milk (Doyle 1992).
Human milk contains about 0.5 mg/L of elemental iron, while iron fortified formulas contain at least ten times that amount of iron. Despite their limited erythropoiesis, breast fed preterm infants are in negative iron balance for at least the first 30 days after birth, due to obligatory intestinal and insensible skin loss of iron (Shaw 1982).
For these reasons, the American Academy of Pediatrics (AAP) recommends supplementation of preterm neonates with 2 mg/kg/day of enteral iron, either as an iron mixture, or in the form of iron‐fortified formula. It is recommended that this supplementation commence within two months of birth, and be continued until 12 months of age (Baker 2010).
Description of the intervention
Several studies have demonstrated higher haemoglobin or ferritin levels in low or very low birth weight infants who were supplemented with enteral iron compared with breast milk or unfortified cow milk formula (Hall 1993; Lundstrom 1977). However, the evidence is unclear as to whether this improvement in biochemical and haematological parameters is associated with a difference in neurodevelopmental outcomes or growth parameters in preterm and low birth weight infants.
How the intervention might work
Studies in term infants have demonstrated a correlation between iron deficiency anaemia and reduced performance in developmental testing (Lozoff 1987; Walter 1989). It is hypothesised that the provision of enteral iron supplementation to preterm and low birth weight infants, who are at particular risk of iron deficiency, will result in improved neurodevelopmental outcomes by avoiding iron deficiency.
Why it is important to do this review
The potential risks of iron supplementation need to be considered. Iron overload can occur in the setting of multiple blood transfusions (Ng 2001). High transfusion requirements early in life have been shown to be associated with a greater risk of retinopathy of prematurity (Dani 2001; Hesse 1997; Inder 1997). Increased body iron load has also been hypothesised to increase the risk of chronic lung disease (Cooke 1997). Putative risks have been suggested related to iron's ability to cause oxidative injury. In addition to the direct oxidative property of iron, large iron doses decrease the absorption of the anti‐oxidant vitamin E, thus exacerbating anaemia in vitamin E deficient neonates (Doyle 1992).
Iron fortification of formulas has been suspected, but not proven, to cause a range of gastrointestinal symptoms in infants (Hyams 1995). While necrotising enterocolitis (NEC) has not been explicitly linked to enteral iron supplementation, it is recognised that human milk is protective against NEC (McGuire 2003).
If enteral iron supplementation is accepted as being a beneficial intervention, the optimum dose, time of initiation and duration of treatment need to be defined.
Objectives
The primary objective of this systematic review was to evaluate the effect of prophylactic enteral iron supplementation on growth and neurodevelopmental outcomes of preterm and low birth weight infants. The secondary objectives were to determine whether iron supplementation results in improved haematological parameters and prevents other causes of morbidity and mortality.
We planned separate comparisons of:
trials that compared enteral iron supplementation versus no supplementation; and
trials that compared different regimens of enteral iron supplementation (dose, duration and timing of initiation).
Data permitting, the following subgroup analyses were planned.
Type of milk feeding: trials involving exclusively formula‐fed infants and those involving exclusively or partially breast fed infants.
Postnatal age of commencement of iron supplementation: 'early commencement' (less than 28 days postnatal) and 'late commencement' (28 days or more postnatal).
Daily dose of supplemental iron administered: 'low dose' (2 mg/kg/day or less) and 'high dose' (more than 2 mg/kg/day).
Duration of iron supplementation: 'short duration' (six months or less) and 'long duration' (more than six months).
Gestational age and birth weight of participants, or both: less than or equal to 33 completed weeks' or less than 1500 g and more than 33 completed weeks' or 1500 g or more.
Methods
Criteria for considering studies for this review
Types of studies
RCTs and some non‐RCTs (quasi‐randomised) in which individual infants were either:
allocated to receive enteral iron supplementation (of at least 1 mg/kg/day), and compared with a control group (placebo or no drug or < 1 mg/kg/day of iron); or
allocated to different regimens of enteral iron supplementation (in regard to dosage, duration and timing of initiation).
We excluded cross‐over studies.
Types of participants
Infants born preterm (before 37 weeks' completed gestation), or of low birth weight (< 2500 g).
Types of interventions
Enteral iron supplement of at least 1 mg/kg/day versus no supplementation (i.e. < 1 mg/kg/day).
Comparison of different regimens of enteral iron supplementation, in regard to the dose, duration, and timing of initiation of iron supplementation.
We specifically excluded studies in which the subject infants were receiving concurrent treatment with erythropoietin.
Types of outcome measures
Primary outcomes
Standardised measures of neurodevelopmental outcome (e.g. Bayley MDI and PDI), at 12 months or less, two years or less and five years or less.
Length, weight and head circumference at 12 months or less, two years or less and five years or less.
Secondary outcomes
Blood haemoglobin concentration and mean corpuscular volume (MCV), at six to eight weeks, three to four months, six to nine months and 12 months.
Serum ferritin concentration, transferrin saturation and total iron binding capacity (TIBC), at six to eight weeks, three to four months, six to nine months and 12 months.
Severe anaemia (haemoglobin level < 8 g/dL or hematocrit < 0.25).
Mortality (during primary hospitalisation and before two years of life).
Chronic lung disease (persisting oxygen requirement at 36 weeks postmenstrual age).
Retinopathy of prematurity (Stage 3 and above, and all stages).
NEC (Bell's stage 2 or above).
Incidence of invasive infection as determined by culture of bacteria or fungus from blood, cerebrospinal fluid, urine or from a normally sterile body space.
Feed intolerance defined as a requirement to cease enteral feeds and commence parenteral nutrition.
Total duration of primary hospitalisation.
Requirement for readmission to hospital in the first year of life.
Search methods for identification of studies
Electronic searches
We used the standard search method of the Cochrane Neonatal Review Group.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 8), 'Old Medline' (1951 to 1965), MEDLINE (1966 to August 2011), CINAHL (1982 to August 2011) and the Oxford Database of Perinatal Trials. We used the following search strategy.
MeSH search terms 'Iron/tu [Therapeutic use]', 'Iron/ad [Administration and dosage]', 'Iron, Dietary', 'Ferrous compounds/tu [Therapeutic use]', or text words 'ferrous sulphate', 'ferrous sulfate', 'ferrous gluconate' AND MeSH search term 'infant', or text words 'preterm', 'premature', 'low birth weight'
We also searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; controlled‐trials.com; and who.int/ictrp).
Searching other resources
In addition, we included previous reviews (including cross references), abstracts, conference and symposia proceedings published in Pediatric research. We did not restrict searches by language of publication. We contacted lead investigators of included studies, where possible, to clarify methods and results and identify other published or unpublished studies which fulfil the inclusion criteria.
Data collection and analysis
We used the standard methods of the Cochrane Neonatal Review Group.
Selection of studies
We screened the title and abstract of all studies identified by the above search strategy. We obtained the full articles for all potentially relevant trials. We re‐assessed the full text of any potentially eligible reports and excluded those studies that did not meet all of the inclusion criteria. We resolved any disagreements by consensus.
Data extraction and management
We used a data collection form to aid extraction of relevant information from each included study. Both review authors extracted the data separately. We discussed any disagreements until consensus was achieved. We contacted the trialists for further information if data from the trial reports were insufficient.
Assessment of risk of bias in included studies
We used the criteria and standard methods of the Cochrane Neonatal Review Group to independently assess the methodological quality of any included trials in terms of allocation concealment, blinding of parents or caregivers and assessors to intervention, and completeness of assessment in all randomised individuals. We requested additional information from the trial authors to clarify methodology and results as necessary.
We included this information in the Characteristics of included studies and Risk of Bias tables. We used the following criteria to complete the Risk of Bias table:
-
Random sequence generation (checking for possible selection bias). For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
-
Allocation concealment (checking for possible selection bias). For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk of bias.
-
Blinding (checking for possible performance bias). For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes. We categorised the methods as:
low risk, high risk or unclear risk for participants;
low risk, high risk or unclear risk for personnel; and
low risk, high risk or unclear risk for outcome assessors.
-
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts or protocol deviations). For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk of bias.
-
Selective reporting bias. For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcome(s) were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; and the study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk of bias.
-
Other sources of bias. For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at a:
low risk;
high risk; or
unclear risk of bias.
If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
Measures of treatment effect
We used the standard methods of the Cochrane Neonatal Review Group. We performed statistical analyses using Review Manager software (RevMan 2011). We analysed continuous data using weighted mean difference (WMD). Where appropriate data were available, we analysed categorical data using risk ratio (RR), risk difference (RD) and the number needed to benefit or harm (NNTB/NNTH). We reported the 95% Confidence interval (CI) on all estimates.
Unit of analysis issues
Where studies presented data in non‐SI units, we performed conversion to SI units (International System of Units) when extracting the data. Overall, difference in units of analysis was not a significant obstacle in this review.
Dealing with missing data
Where studies published data with insufficient detail to permit their inclusion in quantitative meta‐analysis, we contacted the authors to provide additional detail. If no further detail was received, the studies remained eligible for inclusion in the review, but were not included in the pooled quantitative analysis.
Assessment of heterogeneity
If meta‐analysis was possible, we examined the treatment effects of individual trials and heterogeneity between trial results by inspecting the forest plots. We assessed the impact of heterogeneity in the meta‐analysis using a measure of the degree of inconsistency in the studies' results (I2 statistic). If we found statistical heterogeneity, we explored the possible causes (for example, differences in study quality, participants, intervention regimens or outcome assessments) using post hoc subgroup analyses. We used a fixed‐effect model for meta‐analyses.
Assessment of reporting biases
Where possible, we asked the authors of the included studies to notify us of relevant unpublished data. This would permit an assessment of the likelihood of reporting biases.
Data synthesis
The meta‐analysis was performed using Review Manager software (RevMan 2011), supplied by the Cochrane Collaboration. For estimates of typical RR and RD, we used the Mantel‐Haenszel method. For measured quantities, we used the inverse variance method. We used the fixed‐effect model for all meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We planned separate comparisons of:
trials that compared iron supplementation versus no supplementation; and
trials that compared different regimens of iron supplementation (dose, duration and timing of initiation).
Data permitting, we had planned to conduct the following subgroup analyses.
Type of milk feeding: trials involving exclusively formula‐fed infants and those involving exclusively or partially breast‐fed infants.
Postnatal age of commencement of iron supplementation: 'early commencement' (less than 28 days postnatal) and 'late commencement' (28 days or more postnatal).
Daily dose of supplemental iron administered: 'low dose' (2 mg/kg/day or less) and 'high dose' (more than 2 mg/kg/day).
Duration of iron supplementation: 'short duration' (six months or less) and 'long duration' (more than six months).
Gestational age and birth weight of participants, or both: less than or equal to 33 completed weeks' or less than 1500 g and more than 33 completed weeks' or 1500 g or more.
Results
Description of studies
Results of the search
We identified 64 studies for detailed assessment.
Included studies
We included twenty‐six trials (see table Characteristics of included studies). Twenty‐one trials compared enteral iron supplementation with no supplement or minimal supplementation (less than 1 mg/kg/day). Four trials specifically compared early versus late commencement of iron supplementation (Arnon 2009; Halliday 1983; Jansson 1979; Steinmacher 2007); Arnon 2009 also tested iron versus no or minimal iron. Three studies compared high dose iron supplementation with "standard" dose iron supplementation (Barclay 1991, which also tested iron versus no or minimal iron; Friel 2001; Groh‐Wargo 1990). In addition to comparing with placebo, Berglund 2010 compared a low dose (2 mg/kg/day) with a very low dose (1 mg/kg/day). None of the trials compared the effect of longer duration iron supplementation with shorter duration.
Twelve of the trials were conducted in North America (Berseth 2004; Diamond 1958; Friel 2001; Gorten 1964; Groh‐Wargo 1990; Gross 1985; Hall 1993; Melhorn 1971; Melnick 1988; Quilligan 1954; Reedy 1952; Rudolph 1981) and the remainder in a variety of countries including the United Kingdom (Barclay 1991; Coles 1954; Griffin 1999; Halliday 1983), Germany (Franz 2000; Steinmacher 2007), India (Aggarwal 2005; Sankar 2009), Finland (Hanninen 1961; Lundstrom 1977), Sweden (Berglund 2010; Jansson 1979), Brazil (Ferlin 1998) and Israel (Arnon 2009). The span of publication dates was wide, from 1952 to 2010. The spread of dates was very even, with four from the 1950s, two from the 1960s, three from the 1970s, four from the 1980s, five from the 1990s and eight from 2000 onwards.
Participants
A total of 2726 infants were enrolled in the 26 trials. Some 21 trials involved only preterm infants (Arnon 2009; Berseth 2004; Coles 1954; Diamond 1958; Ferlin 1998; Franz 2000; Gorten 1964; Griffin 1999; Gross 1985; Halliday 1983; Hall 1993; Hanninen 1961; Jansson 1979; Lundstrom 1977; Melhorn 1971; Melnick 1988; Quilligan 1954; Reedy 1952; Rudolph 1981; Sankar 2009; Steinmacher 2007). Another study (Groh‐Wargo 1990) only specified a birth weight cut‐off (< 1500 g), but most of the infants included were likely to have been premature. Three other studies (Barclay 1991; Berglund 2010; Friel 2001) specifically studied low birth weight (< 2500 g) infants of any gestation. A number of the older trials simply recorded their subject infants as 'premature' without specifying the gestation or birth weight cut‐off (Coles 1954; Diamond 1958; Quilligan 1954; Reedy 1952). One trial exclusively enrolled term, low birth weight infants (Aggarwal 2005).
In five trials participating infants were exclusively formula fed (Gorten 1964; Griffin 1999; Hall 1993; Melhorn 1971; Rudolph 1981). In four studies, the feeding method was not stated (Diamond 1958; Hanninen 1961; Melnick 1988; Quilligan 1954). In the remainder, the infants were either fully breast milk fed, or given a combination of breast milk and formula.
Interventions
Most of the studies assessed the effect of iron supplementation (generally between 2 mg/kg/day and 4 mg/kg/day of elemental iron) versus placebo or no supplement. A number of the trials undertaken before the mid‐1960s prescribed much higher doses of iron: Coles 1954, Diamond 1958, Hanninen 1961 and Quilligan 1954 all used more than 10 mg/kg/day, and in one trial up to 44 mg/kg/day (Coles 1954). Iron supplementation was usually commenced between four and six weeks postnatally, although the time of commencement was often defined by the establishment of enteral feeding, such that the target or mean postnatal age of commencement was not published. Four trials compared earlier (from about two to three postnatal weeks) versus later (about eight weeks) introduction of iron supplements (Arnon 2009; Halliday 1983; Jansson 1979; Steinmacher 2007). Three trials compared "high" doses versus "standard" doses of iron supplementation but there was variation in the definition of dose. Specifically, Friel 2001 compared 3.4 mg/kg/day versus 2.1 mg/kg/day; Groh‐Wargo 1990 compared 4 mg/kg/day versus 2 mg/kg/day; and Barclay 1991 compared 7.1 mg/kg/day of iron versus 3.6 mg/kg/day. Berglund 2010 compared a low dose (2 mg/kg/day) with a very low dose (1 mg/kg/day).
For the purpose of comparison between trials, we converted all doses of iron into a dose per kilogram body weight per day. The body weight used for this calculation was the published mean birth weight. If a mean birth weight was not provided, we made an estimate based on the reported weights and gestational ages or both, of the infants in the study. For studies of iron fortified infant formulas, we often had to make an estimate of the daily intake of formula; we applied the estimate of 160 ml/kg/day to all the relevant studies.
Outcomes
Only two of the trials assessed neurodevelopmental outcomes (Friel 2001; Steinmacher 2007). Eight reported anthropometric data, usually weight gain, as an outcome measure (Aggarwal 2005; Barclay 1991; Berglund 2010; Berseth 2004; Ferlin 1998; Hall 1993; Quilligan 1954; Reedy 1952). Most trials reported only short term (up to four to six months corrected age) haematological parameters as the primary outcomes. None of the trials reported neonatal mortality, and quantitative measures of other neonatal morbidities were not reported by most studies.
Excluded studies
We excluded thirty‐six studies because they were not randomised or quasi‐randomised trials, or because the study population was inappropriate (see table, Characteristics of excluded studies). Two studies are awaiting foreign language translation (Hurgoiu 1986; Neimann 1957).
Risk of bias in included studies
Overall, the methodological quality of the studies identified as qualifying for inclusion was fair to poor (see table, Characteristics of included studies). This is partly due to the age of many of the studies. However, several more recent studies continued to use less rigorous methodologies, such as quasi‐randomisation.
Allocation
Of the 26 included studies, only eight are known to have an adequate method of allocation concealment (Aggarwal 2005; Berseth 2004; Friel 2001; Gorten 1964; Griffin 1999; Halliday 1983; Rudolph 1981; Sankar 2009), and in most of these studies we only ascertained the adequacy of allocation concealment by direct communication with the authors. Six of the studies were quasi‐randomised (Diamond 1958; Ferlin 1998; Hanninen 1961; Lundstrom 1977; Quilligan 1954; Reedy 1952). In three cases, the authors admitted to a lack of blinding of the allocation process (Franz 2000; Melnick 1988; Steinmacher 2007). In the remaining studies, a method of allocation concealment is not described.
Blinding
Most of the studies used placebo controls, or at least were comparing two formulas or preparations to which the participants and study personnel were blind. Blinding of the outcome assessors is vitally important for the primary outcomes of this review, namely neurodevelopmental outcome and growth parameters. For the secondary outcomes such as haematological parameters, a natural blinding is likely to have been inherent in the laboratory basis of the tests, even in studies for which we have not been able to confirm blinding of outcome assessment with the authors. Overall, this review did not detect any significant likelihood of systematic bias related to blinding in the included studies.
Incomplete outcome data
Loss to follow‐up, usually due to failure to attend appointments, or due to illness, was a significant problem for many of the included studies. Of the 26 included studies, only eight had documented completion rates of 80% or more (Arnon 2009; Barclay 1991; Berglund 2010; Griffin 1999; Gross 1985; Jansson 1979; Sankar 2009; Steinmacher 2007). Documentation of the recruitment rate of eligible subjects was lacking from all the included studies except Arnon 2009 and Sankar 2009. A number of studies implied complete follow‐up without explicitly stating the number enrolled (Ferlin 1998; Groh‐Wargo 1990; Halliday 1983; Melnick 1988). While the follow‐up was suboptimal in most studies, this review did not detect any significant likelihood of systematic bias in attrition in the included studies.
Selective reporting
If any unpublished data had been received, we would have made an assessment as to the existence of reporting bias in this topic. As we did not receive any unpublished data, there is currently no evidence of reporting biases.
Effects of interventions
Comparison 1: Enteral iron supplementation versus no iron supplementation
Primary outcomes
Neurodevelopmental outcome
None of the trials reported the neurodevelopmental outcome of the participants.
Growth
Thirteen trials reported at least one of the growth parameters (weight, length or head circumference) as an outcome, but often without providing the numerical data. The duration of follow‐up varied from just six weeks postnatal age (Berseth 2004; Melnick 1988), up to 18 months (Gorten 1964). Most trials only monitored growth outcomes until about two months postnatal age. Twelve studies did not find a statistically significant difference in weight gain between the groups (Aggarwal 2005; Barclay 1991; Berglund 2010; Berseth 2004; Coles 1954; Ferlin 1998; Gorten 1964; Hall 1993; Melhorn 1971; Melnick 1988; Quilligan 1954; Sankar 2009). None of the six trials that assessed linear and head growth found a significant difference between the groups (Aggarwal 2005; Barclay 1991; Berseth 2004; Ferlin 1998; Gorten 1964; Hall 1993).
One study found a difference in weight gain between the iron‐treated group and the untreated group. Reedy 1952 reported that the treated infants with birth weight 1000 g to 1500 g had a greater weight gain than the controls at 12 months (mean difference (MD) 1.4 kg; no significance measure was provided). A smaller trend in the same direction was seen in the 1500 g to 2000 g group (MD 794 g; no significance statistic), and the 2000 g to 2250 g group (MD 1.4 kg; no significance statistic). The data from Reedy 1952 is severely limited by the small sample analysed at 12 months (four treated and two untreated patients). We could not conduct a meta‐analysis of the growth outcomes due to a lack of appropriately detailed numerical data (i.e. to enable calculation of means and standard deviations (SDs)).
Secondary outcomes
Blood haemoglobin concentration and mean corpuscular volume (MCV)
Six to eight weeks: a total of 16 trials reported haemoglobin with or without MCV at approximately six to eight weeks postnatal (Barclay 1991; Coles 1954; Diamond 1958; Ferlin 1998; Franz 2000; Gorten 1964; Griffin 1999; Hall 1993; Halliday 1983; Lundstrom 1977; Melhorn 1971; Melnick 1988; Quilligan 1954; Reedy 1952; Rudolph 1981; Sankar 2009). Only Quilligan 1954 and Gorten 1964 reported a significant benefit for the iron supplementation group. Melhorn 1971 found a lower haemoglobin in the iron supplementation group which was maximal at six weeks (MD 6 g/L in 1000 g to 1500 g birth weight; MD 7 g/L in 1501 g to 2000 g birth weight; P < 0.01 for both). This apparent effect of iron was ameliorated by supplementation with vitamin E.
Only nine trials (n = 526) had data which were able to be pooled for meta‐analysis (Barclay 1991; Ferlin 1998; Gorten 1964; Griffin 1999; Hall 1993; Melhorn 1971; Quilligan 1954; Rudolph 1981; Sankar 2009). On meta‐analysis, there was no statistically significant difference in haemoglobin concentration: (WMD 1.4 g/L; 95% CI ‐0.2 to 3.1) (Outcome 1.4). The lack of a statistically significant difference in haemoglobin concentration at six to eight weeks remained in subgroup meta‐analyses of trials of formula‐fed infants (n = 389) (WMD 0.7 g/L; 95% CI ‐1.1 to 2.6). In trials of partially or fully breast fed babies (n = 137) a statistically significant difference in favour of iron supplementation was noted: (WMD 4.1 g/L; 95% CI 0.6 to 7.7). However, there was statistical heterogeneity in all meta‐analyses, most likely due to differences in the participants between the trials. For example, Barclay 1991 included term and preterm infants, while the other studies only included preterm infants.
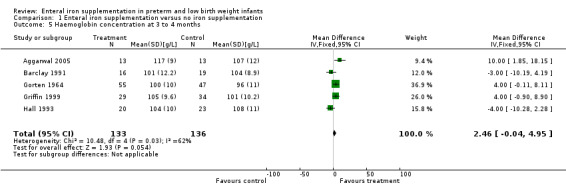
Three to four months : a total of 11 trials reported haemoglobin with or without MCV at approximately three to four months postnatal (Aggarwal 2005; Barclay 1991; Coles 1954; Diamond 1958; Gorten 1964; Griffin 1999; Hall 1993; Halliday 1983; Hanninen 1961; Lundstrom 1977; Reedy 1952). Four trials reported that iron supplementation resulted in a statistically significant higher haemoglobin concentration (Aggarwal 2005; Coles 1954; Hanninen 1961; Lundstrom 1977). Data from five trials were able to be pooled (Aggarwal 2005; Barclay 1991; Gorten 1964; Griffin 1999; Hall 1993). Meta‐analysis found a borderline statistically significant difference in haemoglobin concentration (WMD 2.5 g/L; 95% CI ‐0.04 to 4.95) (Outcome 1.5). There were no statistically significant differences in subgroup meta‐analyses of trials in formula‐fed (WMD 2.4 g/L; 95% CI ‐0.42 to 5.2) or breast fed infants (WMD 2.7 g/L; 95% CI ‐2.7 to 8.1). There was statistical heterogeneity in all meta‐analyses.
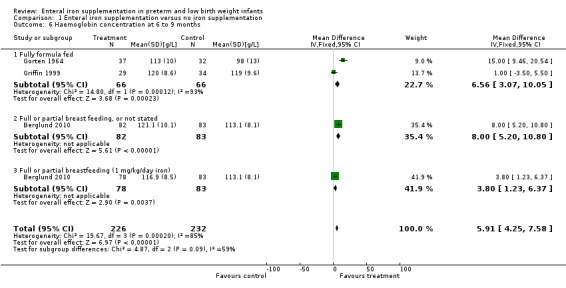
Six to nine months : a total of nine trials reported haemoglobin with or without MCV at approximately six to nine months postnatal (Berglund 2010; Coles 1954; Diamond 1958; Gorten 1964; Griffin 1999; Halliday 1983; Hanninen 1961; Lundstrom 1977; Reedy 1952). Six trials (Berglund 2010; Coles 1954; Gorten 1964; Hanninen 1961; Lundstrom 1977; Reedy 1952) reported a higher haemoglobin in the iron supplementation group at six months; Diamond 1958, Griffin 1999 and Halliday 1983 found no statistically significant difference. Gorten 1964 and Griffin 1999 had data available to pool. Both these studies were of formula‐fed babies. Meta‐analysis showed a statistically significantly higher haemoglobin concentration in the iron supplemented group (WMD 6.6 g/L; 95% CI 3.1 to 10.1) (Outcome 1.6.1), but with significant heterogeneity between the trial estimates of effect. Berglund 2010 was a good quality study that found higher haemoglobin when compared to placebo for both 2 mg/kg/day of elemental iron (MD 8.0g/L; 95% CI 5.2 to 10.8) (Outcome 1.6.2) and 1 mg/kg/day of elemental iron (MD 3.8 g/L; 95% CI 1.2 to 6.4) (Outcome 1.6.3).
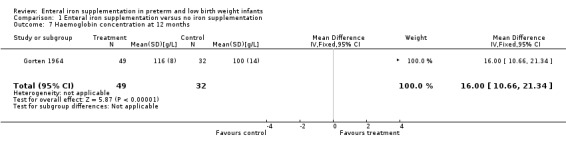
Twelve months : a total of three trials reported haemoglobin with or without MCV at approximately 12 months postnatal (Gorten 1964; Halliday 1983; Reedy 1952). Gorten 1964 reported a statistically significant difference in haemoglobin concentration (WMD 16.0 g/L; 95% CI 10.7 to 21.3) (Outcome 1.7). Halliday 1983 did not find a statistically significant difference. Although Reedy 1952 reported a large difference in haemoglobin between the iron supplementation and no iron supplementation groups (MD 59 g/L in the 1000 g to 1500 g group), the finding should be interpreted with caution due to extremely significant loss to follow‐up (n = 6 at 12 months in this birth weight group, but n = 16 at birth).
MCV was reported as an outcome by Berglund 2010, Hall 1993 and Lundstrom 1977, but not by the other studies. At six to eight weeks, Lundstrom 1977 found no significant difference in MCV, but at three to four months (P < 0.01) and six to nine months (P < 0.05) there was a statistically significant difference in favour of the iron supplementation group (the results were presented graphically rather than numerically). Berglund 2010 also found an increase in MCV compared to placebo at six months for both 2 mg/kg/day of iron (MD 2.5 fL; 95% CI 1.3 to 3.7) (Outcome 1.11.1) and 1 mg/kg/day (MD 1.7 fL; 95% CI 0.5 to 2.9) (Outcome 1.11.2). At six to eight weeks, Hall 1993 found a higher MCV in the iron supplementation group (MD 4 fL; P < 0.05) and likewise at three to four months (MD 6 fL; P < 0.01).
Serum ferritin concentration, transferrin saturation, total iron binding capacity (TIBC)
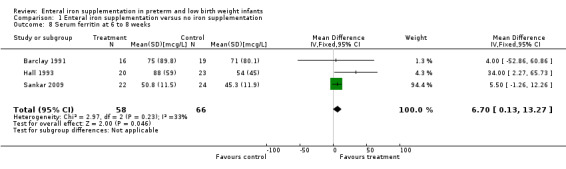
Six to eight weeks : (eight trials: Barclay 1991; Franz 2000; Griffin 1999; Hall 1993; Halliday 1983; Lundstrom 1977; Melnick 1988; Sankar 2009). Three trials reported a higher ferritin level in the iron supplementation group (Hall 1993; Lundstrom 1977; Melnick 1988). The other trials did not find any statistically significant difference. Data on serum ferritin concentration suitable for pooling was reported by Barclay 1991 and Hall 1993. Meta‐analysis did not detect a statistically significant difference (WMD 26.9 mcg/L; 95% CI ‐0.83 to 54.6 mcg/L).
Three trials reported the transferrin saturation at six to eight weeks. Only Hall 1993 reported a statistically significantly difference (MD 7.00%; 95% CI 0.05% to 13.95%) (Outcome 1.12) in favour of iron supplementation.
Three to four months : data on serum ferritin concentration at three to four months postnatal and suitable for pooling was provided by Aggarwal 2005, Barclay 1991 and Hall 1993. The pooled results showed a very small benefit in favour of the no iron supplementation group (WMD ‐4.14 mcg/L; 95% CI ‐5.9 to ‐2.38). A higher ferritin level at three to four months postnatal in the iron supplementation group was found by Lundstrom 1977. No difference in ferritin at three to four months was found by Griffin 1999 and Halliday 1983.
Two trials reported the transferrin saturation at two to four months. Hall 1993 did not detect a statistically significantly difference (MD ‐4.00%; 95% CI ‐9.21 to 1.21) (Outcome 1.13). Halliday 1983 found no difference at three to four months (MD 1% in favour of no iron supplementation, no significance calculation).
Six to nine months : (three trials: Berglund 2010; Griffin 1999; Halliday 1983). Neither Griffin 1999 nor Halliday 1983 found a statistically significant difference in serum ferritin levels. Halliday 1983 reported the data graphically, as did Griffin 1999. Berglund 2010 found a higher serum ferritin at six months compared with placebo for both 2 mg/kg/day elemental iron (MD 30.7 mcg/L; 95% CI 30.0 to 31.4) (Outcome 1.10.1) and 1 mg/kg/day (MD 15.4 mcg/L; 95% CI 14.8 to 16.0) (Outcome 1.10.2). Halliday 1983 reported transferrin saturation at six to nine months, finding no difference (MD 1% in favour of no iron supplementation, no significance calculation). Berglund 2010 found a difference in favour of both 2 mg/kg/day of iron (MD 9.1%; 95% CI 6.0 to 12.2) (Outcome 1.14.1) and 1 mg/kg/day of iron (MD 5.9%; 95% CI 3.4 to 8.4) (Outcome 1.14.2).
Twelve months : (one trial: Halliday 1983). Halliday 1983 did not find a statistically significant difference in serum ferritin levels (data presented graphically rather than numerically).
Only Halliday 1983 reported transferrin saturation at 12 months, finding no significant difference (MD 4% in favour of no iron supplementation; no significance calculation).
No trials reported TIBC.
Severe anaemia (haemoglobin level < 8 g/dL or hematocrit level < 0.25)
Three studies reported the development of anaemia as an outcome measure (Coles 1954; Gorten 1964; Hall 1993). However, only Coles 1954 used a definition which was within the prespecified range of < 8 g/dL; Gorten 1964 defined anaemia as haemoglobin concentration below 9.0 g/dL on two successive tests; and Hall 1993 assessed as an outcome the prevalence of a haemoglobin concentration < 9.0 g/dL at discharge from hospital. Therefore, we did not pool the results for further analysis.
Coles 1954 defined anaemia as haemoglobin concentration below 7 g/dL once (or less than 7.5 g/dL for two consecutive months). No infants (out of 22) in the iron supplementation group developed anaemia, but 3 of 29 in the no iron supplementation group developed anaemia.
Mortality: not reported by any trials.
Chronic lung disease (one trial): Sankar 2009 did not find a statistically significant difference (one occurrence in each group).
Retinopathy of prematurity (one trial): Sankar 2009 did not find a statistically significant difference.
Necrotising enterocolitis (two trials): neither Berseth 2004 nor Sankar 2009 found a statistically significant difference in the incidence of NEC.
Invasive infection (two trials): neither Berseth 2004 nor Sankar 2009 found a statistically significant difference in the incidence of sepsis.
Enteral feed intolerance (four trials: Aggarwal 2005; Berseth 2004; Franz 2000; Melnick 1988).None of the trials reported a statistically significant difference in the incidence of feed intolerance. Aggarwal 2005 reported vomiting in 6% of the iron supplementation group and 0% of the no iron supplementation group at three to four months. Berseth 2004 compared a number of related outcomes (daily residuals, abdominal distention, guaiac‐positive stools, withholding of feeds due to intolerance and clinically significant emesis) and there were no statistically significant differences on any measure. Franz 2000 reported a 16% incidence of feed intolerance in the iron supplementation group, which was said to be similar to that in the no iron supplementation group. Melnick 1988 did not provide numerical data.
Total duration of primary hospitalisation: not reported by any trials.
Requirement for readmission to hospital in the first year of life (1 trial): Sankar 2009 found no significant difference in rehospitalisation rate.
Subgroup analyses
Type of milk feeding : trials involving exclusively formula‐fed infants and those involving exclusively or partially breast fed infants: details provided above.
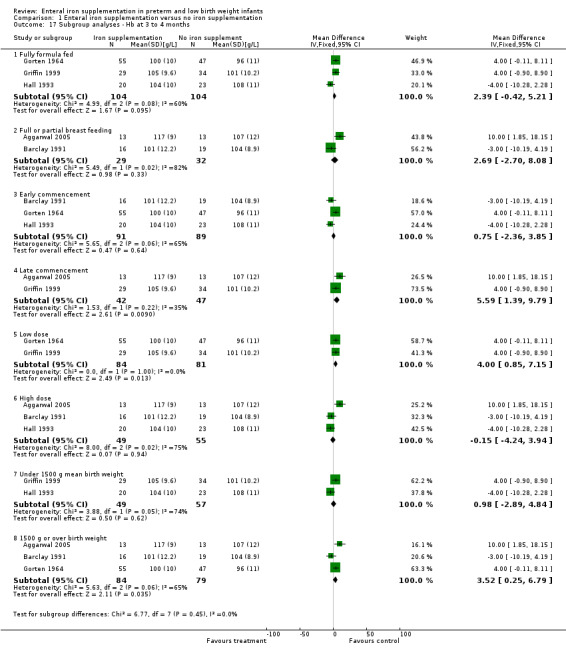
Postnatal age of commencement of iron supplementation : 'early commencement' (less than 28 days postnatal) and 'late commencement' (28 days or more postnatal). Of the 21 studies, only Aggarwal 2005, Berglund 2010, Coles 1954 and Griffin 1999 had a 'late commencement' of iron supplementation. Diamond 1958 and Reedy 1952 did not state the time of commencement. The other 15 studies commenced 'early'. Quantitative meta‐analysis of haemoglobin at six to eight weeks: 'early commencement' (WMD 1.55 g/L; 95% CI ‐0.12 to 3.2), 'late commencement' (WMD ‐1.1 g/L; 95% CI ‐8.8 to 6.6) (Outcome 1.16.4); and haemoglobin at three to four months: 'early' (WMD 0.75 g/L; 95% CI ‐2.4 to 3.9) (Outcome 1.17.3), 'late' (WMD 5.6 g/L; 95% CI 1.4 to 9.8) (Outcome 1.17.4). Daily dose of supplemental iron administered : 'low dose' (2 mg/kg/day or less) and 'high dose' (more than 2 mg/kg/day). The studies using a low dose of iron were Berglund 2010 (including doses of both 2 mg/kg/day and 1 mg/kg/day), Franz 2000, Gorten 1964, Griffin 1999, Gross 1985, Lundstrom 1977 and Rudolph 1981. Reedy 1952 gave a low dose until discharge, followed by a high dose; the other studies used a high dose. Quantitative meta‐analysis of haemoglobin at six to eight weeks: 'low dose' (WMD 3.9 g/L; 95% CI 1.5 to 6.3) (Outcome 1.16.5), 'high dose' (WMD ‐0.76 g/L; 95% CI ‐3.0 to 1.5) (Outcome 1.16.6); and haemoglobin at three to four months: 'low dose' (WMD 4.0 g/L; 95% CI 0.85 to 7.2) (Outcome 1.17.5), 'high dose' (WMD ‐0.15 g/L; 95% CI ‐4.2 to 3.9) (Outcome 1.17.6). Duration of iron supplementation : 'short duration' (six months or less) and 'long duration' (more than six months). Two studies only administered iron for a short period, Coles 1954 ceasing at eight weeks and Melhorn 1971 at six weeks. No numerical data was available for quantitative meta‐analysis after six to eight weeks. Two other studies contained a comparison group ceasing iron early (Griffin 1999 and Reedy 1952) ‐ see Characteristics of included studies tables for description. The remaining 15 studies continued iron supplementation until at least the final outcome measurement. Gestational age or birth weight of participants, or both : less than or equal to 33 completed weeks' or less than 1500 g and more than 33 completed weeks' or 1500 g or more. Only two studies dealt specifically with low birth weight infants (Aggarwal 2005 term LBW infants and Berglund 2010 marginally LBW infants, irrespective of gestation). For a number of studies, mean birth weights could not be obtained, but a reasonable estimate could be made from the provided data. Studies in which the infants had a published or estimated mean birth weight of less than 1500 g were Berseth 2004, Ferlin 1998, Franz 2000, Griffin 1999, Gross 1985, Hall 1993, Melnick 1988, Rudolph 1981 and Sankar 2009. Studies with a published or estimated mean birth weight of over 1500 g were Aggarwal 2005, Barclay 1991, Coles 1954, Gorten 1964, Halliday 1983, Lundstrom 1977. Melhorn 1971 and Reedy 1952. Three studies did not provide enough information to estimate the mean birth weight (Diamond 1958; Hanninen 1961; Quilligan 1954). On quantitative meta‐analysis for haemoglobin at six to eight weeks (8 trials), neither subgroup showed a significant difference: 'under 1500 g' (WMD ‐0.42 g/L; 95% CI ‐4.3 to 3.4) (Outcome 1.16.7); '1500 g or over' (WMD 0.77 g/L; 95% CI ‐1.2 to 2.7) (Outcome 1.16.8). At three to four months (5 trials), the '1500 g or over' subgroup had a slight but statistically significant benefit for haemoglobin in favour of iron supplementation: (WMD 3.5 g/L; 95% CI 0.3 to 6.8) (Outcome 1.17.8). However, the 'under 1500 g' subgroup had no significant difference: (WMD 1.0 g/L; 95% CI ‐2.9 to 4.8) (Outcome 1.17.7).
We could not perform subgroup analyses at later ages than three to four months and for outcomes other than haemoglobin due to the small number of studies with available data.
Comparison 2: Early versus late commencement of iron supplementation ‐ different regimens of iron supplementation (dose, duration and timing of initiation)
(4 trials: Arnon 2009; Halliday 1983; Jansson 1979; Steinmacher 2007).
Primary outcomes
Neurodevelopmental outcome
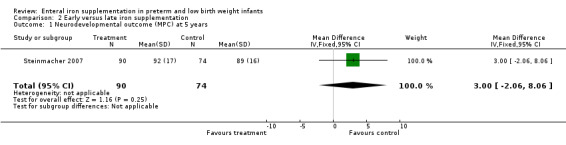
Steinmacher 2007 reported neurodevelopmental outcome at five years of age; there was no significant difference in cognitive function testing at five years of age. There was an increased proportion of children with abnormal clinical neurological examination in the late iron group (35% versus 17%; P = 0.02).
Growth
Steinmacher 2007 reported no significant difference in weight, length and head circumference at five years of age.
Secondary outcomes
Blood haemoglobin concentration and MCV (three trials: Arnon 2009; Halliday 1983; Jansson 1979).
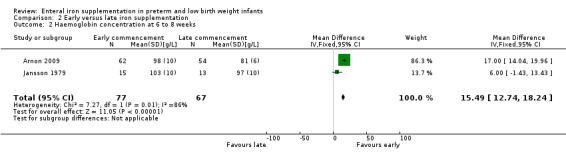
Six to eight weeks: we pooled data from Arnon 2009 and Jansson 1979. Haemoglobin concentration was higher in the early iron group: (WMD 1.7 g/L; 95% CI 1.4 to 2.0). Halliday 1983 reported that there was not a statistically significant difference in haemoglobin concentration between the early and late supplementation groups, but the data were presented graphically rather than numerically.
Three to four months : Jansson 1979 did not report at three to four months. Halliday 1983 reported that there was not a statistically significant difference in haemoglobin concentration between the early and late supplementation groups, but numerical data were not supplied.
Six to nine months : Jansson 1979 did not detect a statistically significant difference in the haemoglobin concentration: (WMD 0 g/L; 95% CI ‐5.43 to 5.43) (Outcome 2.3). Halliday 1983 reported that there was not a statistically significant difference in haemoglobin concentration between the early and late supplementation groups, but numerical data were not supplied.
Twelve months : Jansson 1979 did not report at 12 months. Halliday 1983 reported that there was not a statistically significant difference in haemoglobin concentration between the early and late supplementation groups, but numerical data were not supplied. Neither study reported MCV.
Serum ferritin concentration, transferrin saturation, TIBC (3 trials: Arnon 2009; Halliday 1983; Jansson 1979).
Six to eight weeks : Arnon 2009 found a higher serum ferritin in the early iron group (WMD 25.0 mcg/L; 95% CI 16.9 to 33.1) (Outcome 2.4). Jansson 1979 found no statistically significant difference in serum ferritin (MD ‐16 mcg/L; no significance calculation due to unreported SD). Halliday 1983 reported that there was no statistically significant difference in transferrin saturation, but numerical data were not supplied. Therefore, we were unable to pool the data.
Three to four months : Jansson 1979 did not report at three to four months. Halliday 1983 reported that there was no statistically significant difference in transferrin saturation, but numerical data were not supplied.
Six to nine months : Jansson 1979 found no statistically significant difference in serum ferritin (MD ‐2 mcg/L; no significance calculation). Halliday 1983 reported that there was no statistically significant difference in transferrin saturation, but numerical data were not supplied.
Twelve months : Jansson 1979 did not report at 12 months. Halliday 1983 reported that there was no statistically significant difference in transferrin saturation, but numerical data were not supplied.
Severe anaemia: not reported by any trials.
Mortality: not reported by any trials.
Chronic lung disease: no difference (Arnon 2009).
Retinopathy of prematurity: no difference (Arnon 2009).
Necrotising enterocolitis: no difference (Arnon 2009).
Invasive infection: no difference (Arnon 2009).
Enteral feed intolerance: not reported by any trials.
Total duration of primary hospitalisation: not reported by any trials.
Requirement for readmission to hospital in the first year of life: not reported by any trials.
Comparison 3: High versus low dose iron supplementation
(Three trials: Barclay 1991; Friel 2001; Groh‐Wargo 1990).
Primary outcomes
Neurodevelopmental outcome (one trial)
Friel 2001 did not find a statistically significant difference in the Griffiths Developmental Assessment score at 12 months: 118 in both groups (WMD 0.000; 95% CI ‐6.38 to 6.38) (Outcome 3.1).
Growth (three trials)
None of the trials found any statistically significant differences in growth parameters up to about 20 weeks postnatal age. Numerical data were only provided by Friel 2001, who documented no significant difference in weight and height z scores at approximately 12 months postnatally (z score difference of 0 and 0.1 respectively).
Secondary outcomes
Blood haemoglobin concentration and MCV
Six to eight weeks : (two trials: Barclay 1991; Friel 2001). (Groh‐Wargo 1990 did not supply data for six to eight weeks). Neither individual trial, nor meta‐analysis of both trials, found a statistically significant difference between the groups: (WMD ‐1.4 g/L; 95% confidence interval ‐8.2 to 5.4) (Outcome 3.2.1).
Three to four months : (two trials: Barclay 1991; Friel 2001; Groh‐Wargo 1990). None of the individual trials found a statistically significant difference between the groups. However, meta‐analysis suggested a slight benefit in favour of the higher iron dose: (WMD 4.49 g/L; 95% CI 0.84 to 8.13) (Outcome 3.3). Groh‐Wargo 1990 reported that the MCV was statistically significantly higher in the high dose group: (WMD 3.30 units; 95% CI 0.06 to 6.54) (Outcome 3.6).
Six to nine months: (one trial: Friel 2001). The trial did not find a statistically significant difference in mean haemoglobin concentration between the groups: (MD 4.0 g/L; 95% CI ‐1.6 to 9.6) (Outcome 3.4).
Twelve months : (one trial Friel 2001). The trial did not find a statistically significant difference between the groups: (MD ‐2.0 g/L; (95% CI ‐7.2 to 3.2) (Outcome 3.5).
Serum ferritin concentration, transferrin saturation, TIBC (two trials: Friel 2001; Groh‐Wargo 1990).
Six to eight weeks : Friel 2001 did not detect a statistically significant difference in ferritin concentration between the two groups: (MD ‐5.00; 95% CI ‐33.1 to 23.1) (Outcome 3.7). The transferrin saturation level was statistically significantly lower in the high dose group: (MD ‐10.0%; 95% CI ‐17.7 to ‐2.27) (Outcome 3.11).
Three to four months : neither study detected a statistically significant difference in ferritin concentration. On meta‐analysis, (WMD 4.2; 95% CI ‐3.7 to 12.0). Neither Friel 2001 nor Groh‐Wargo 1990 found a significant difference in transferrin saturation: on meta‐analysis, (WMD 1.74; 95% CI ‐1.2 to 4.7) (Outcome 3.12).
Six to nine months : Friel 2001 reported a statistically significant higher mean ferritin concentration in the high dose group: (MD 4.00; 95% CI 0.12 to 7.88) (Outcome 3.9). The transferrin saturation level was not statistically significantly different: (MD 2.00%; 95% CI ‐2.95 to 6.95) (Outcome 3.13).
Twelve months:Friel 2001 did not detect a statistically significant difference in ferritin concentration (MD ‐2.00; 95% CI ‐7.67 to 3.67) (Outcome 3.10).
Severe anaemia: not reported by any trials.
Mortality: not reported by any trials.
Chronic lung disease: not reported by any trials.
Retinopathy of prematurity: not reported by any trials.
Necrotising enterocolitis: not reported by any trials.
Invasive infection: not reported by any trials.
Enteral feed intolerance: not reported by any trials.
Total duration of primary hospitalisation: not reported by any trials.
Requirement for readmission to hospital in the first year of life: not reported by any trials.
Comparison 4: Duration of iron supplementation
(Two trials: Griffin 1999 and Reedy 1952)
Blood haemoglobin concentration and MCV
Six to eight weeks : Griffin 1999 found no difference in haemoglobin (MD 3.7; 95% CI ‐3.8 to 11.2) (Outcome 4.1). Reedy 1952 found a MD of 6 g/L in favour of short duration in the 1000 g to 1500 g group (no significance calculation possible), and 3 g/L in favour of short duration in the 1500 g to 2000 g group (no comparison available for the 2000 g to 2250 g group). This result is severely limited by the large loss to follow‐up.
Three to four months : Griffin 1999 found no difference in haemoglobin (MD 1.5 g/L; 95% CI ‐3.9 to 6.9 g/L) (Outcome 4.2). Reedy 1952 found a MD of 10 g/L in favour of long duration in the 1000 g to1500 g group (no significance calculation possible), but no difference in the larger birth weight groups. This result is severely limited by the large loss to follow‐up.
Six to nine months : Griffin 1999 found a slight benefit for the longer duration of iron supplementation (MD 5.7 g/L; 95% CI 0.5 to 10.9) (Outcome 4.3). Reedy 1952 found a MD of 5 g/L in favour of short duration in the 1000 g to 1500 g group (no significance calculation possible), 2 g/L in favour of long duration in the 1500 g to 2000 g group and 3 g/L in favour of short duration in the 2000 g to 2250 g group. The results of Reedy 1952 are severely limited by the large loss to follow‐up.
Twelve months : Reedy 1952 found a MD of 28 g/L in the 1000 g to 1500 g group (no significance calculation possible), and of 10 g/L in the 2000 g to 2250 g group (no comparison available for the 1501 g to 2000 g group). The results of Reedy 1952 are severely limited by the large loss to follow‐up.
See Table 1 for summary of results by study.
1. Summary of results by study.
| Study | Primary outcome | Secondary outcomes |
| Aggarwal 2005 |
|
|
| Arnon 2009 |
|
|
| Barclay 1991 |
|
|
| Berglund 2010 |
|
|
| Berseth 2004 |
|
|
| Coles 1954 |
|
|
| Diamond 1958 |
|
|
| Ferlin 1998 |
|
|
| Franz 2000 |
|
|
| Friel 2001 |
|
|
| Gorten 1964 |
|
|
| Griffin 1999 |
|
|
| Groh‐Wargo 1990 |
|
|
| Gross 1985 |
|
|
| Hall 1993 |
|
|
| Halliday 1983 |
|
|
| Hanninen 1961 |
|
|
| Jansson 1979 |
|
|
| Lundstrom 1977 |
|
|
| Melhorn 1971 |
|
|
| Melnick 1988 |
|
|
| Quilligan 1954 |
|
|
| Reedy 1952 |
|
|
| Rudolph 1981 |
|
|
| Sankar 2009 |
|
|
| Steinmacher 2007 |
|
|
Discussion
Summary of main results
There is insufficient evidence regarding the effect of enteral iron supplementation on the neurodevelopmental and long term growth outcomes for preterm and low birth weight infants. No RCT comparing enteral iron supplementation with no iron supplementation examined the comparative neurodevelopmental outcomes.
Enteral iron supplementation of both preterm and term low birth weight infants appears to confer a slight improvement in haemoglobin and ferritin levels after eight weeks postnatal age, and reduces the risk of infants developing anaemia. However, there is significant heterogeneity in the outcomes of the included RCTs, which is likely due to a number of methodological issues outlined below.
This review did not identify any benefit in providing more than 2 mg/kg/day to 3 mg/kg/day of elemental iron. Some recent evidence supports provision of iron supplementation to all low birth weight infants, whether they are born term or preterm (Berglund 2010).
Overall completeness and applicability of evidence
One study compared early commencement of iron supplementation with later commencement, finding no difference in cognitive development at five years of age, but an increase in the rate of abnormal clinical neurological examination in the late commencement group (Steinmacher 2007). One further study comparing a standard dose of iron supplementation with a higher dose examined the neurodevelopmental outcome of infants, and did not find any difference up to 12 months of age (Friel 2001). A number of studies measured weight and/or length and head circumference, but in all cases this was only done for the duration of monitoring of haematological parameters, which for most studies was well under a year postnatally.
Most studies were concerned primarily with assessing the effects of iron supplementation on haematological parameters such as haemoglobin and serum ferritin levels. However, only a small number of studies were able to be quantitatively synthesised, because appropriate data was not available, despite attempting to contact the authors for further data. The studies included were very heterogeneous, in relation to the population, the intervention used, the outcomes measured and the results themselves. Qualitatively, the evidence to date indicates that the provision of enteral iron supplementation to premature and term, low birth weight infants probably confers a slight benefit for the haemoglobin level from approximately two months postnatally. Iron stores, reflected in the serum ferritin, are also slightly benefited by iron supplementation. One recent, relatively large RCT has suggested that an early (two weeks postnatal) commencement of iron supplementation results in improved haematological parameters compared with later (four weeks) introduction of iron (Arnon 2009).
Quality of the evidence
The methodological quality of most of the RCTs was fair at best. For example, only eight of the 26 studies are known to have used allocation concealment of adequate quality. Most failed to report adequate blinding of intervention and outcome measurement, and only eight of 26 had documented follow‐up rates of more than 80% for the key outcomes at the end of the study. Other methodological weaknesses were contamination of non iron‐supplemented groups by iron‐fortified formulas (Barclay 1991; Franz 2000; Jansson 1979) and removal of anaemic infants from analysis (Coles 1954; Gorten 1964; Lundstrom 1977; Melhorn 1971). Since most of these removals came from the control (unsupplemented) groups, this is likely to have resulted in an underestimation of the effect of iron supplementation on haemoglobin levels.
There was also a paucity of outcome data reported for possible morbidity associated with iron supplementation, such as necrotising enterocolitis, retinopathy of prematurity, and chronic neonatal lung disease. Rather, the studies looking at adverse effects of iron supplementation concentrated on haematological measures such as evidence of red cell fragility or haemolysis.
Of the studies comparing enteral iron supplementation versus no iron supplementation, there was some variation in the doses of iron administered. The most common dose of iron (either as a medicinal supplement or as formula fortification) was 2 mg/kg/day to 3 mg/kg/day of elemental iron, which was the dose administered in 11 of those 21 studies. This review did not find any evidence that a dose greater than that recommended by the AAP (2 mg/kg/day) results in improvement of outcomes. This is reflected both in the results of studies that specifically addressed this question (Barclay 1991; Friel 2001; Groh‐Wargo 1990) and in the subgroup analysis of trials using doses of iron of 2 mg/kg/day or less compared with those using a higher dose, meta‐analysis of which actually indicated a small but statistically significant benefit for haemoglobin at six to eight weeks and three to four months in the 'low iron' but not the 'high iron' group.
A few features of the heterogeneity of study methods are worthy of further comment. First, studies varied greatly in their approach to blood transfusion. For example, among the 21 studies comparing enteral iron supplementation with no (or minimal) iron supplementation, four permitted blood transfusions among the participants before and during the study period (Berseth 2004; Franz 2000; Hall 1993; Sankar 2009). Two studies permitted transfusions before, but not after study entry (Barclay 1991; Griffin 1999). The other studies either excluded all infants who required transfusion (7 studies), or did not state any policy (eight studies). The effect of this heterogeneity could be important, given the iron content of blood, and previous research which has suggested that premature babies who receive multiple transfusions are at risk of iron overload (Ng 2001). It is feasible that extremely preterm infants who receive one or more blood transfusions may not receive as much benefit from iron supplementation as more mature infants who do not receive any transfusions (Ng 2001; Rao 2002).
One author suggests that the measurement of ferritin levels in preterm neonates who have received transfusions may be useful to guide the initiation of iron therapy, but again this remains untested (Brown 1996). Of the nine studies comparing iron with no iron that were known or estimated to have a mean birth weight of less than 1500 g (Aggarwal 2005; Berseth 2004; Ferlin 1998; Franz 2000; Griffin 1999; Hall 1993; Melnick 1988; Rudolph 1981; Sankar 2009), five of these studies Berseth 2004; Franz 2000; Griffin 1999; Hall 1993; Sankar 2009) included infants who had received blood transfusions prior to commencement of the intervention and two studies did not state the policy on transfusions (Melnick 1988; Rudolph 1981).
Second, the provision or otherwise of vitamin E supplementation varied between the studies. Apart from the two studies which investigated the supplementation of vitamin E as a controlled intervention (Ferlin 1998; Melhorn 1971), five other studies reported vitamin E supplementation of both the intervention and control groups, ranging from 2 IU/kg/day to 20 IU/kg/day (Barclay 1991; Berseth 2004; Gross 1985; Lundstrom 1977; Rudolph 1981; Sankar 2009). The heterogeneity and uncertainty around the provision or otherwise of vitamin E in these studies is unlikely to have resulted in a significant adverse impact on the review's validity. For example, the finding by Melhorn 1971 that iron supplementation exacerbates the 'early' anaemia of prematurity (i.e. at six to eight weeks postnatally) was not supported by the other trials in this review. In the studies in which the presence or absence of vitamin E supplementation was not documented, it is unlikely, particularly in the studies from the 1950s and 1960s, that it had been given. In most modern neonatal units, a small vitamin E supplement is routinely given to preterm infants, in accordance with AAP recommendations of 5 IU to 25 IU per day (AAP 1985). In Melhorn 1971, the apparent deleterious effect of iron supplementation was ameliorated by vitamin E supplementation. Therefore, any residual concern that iron might exacerbate the early anaemia of pregnancy in non‐vitamin E supplemented infants is unlikely to be relevant today.
Third, the provision of enteral cobalt was examined in three studies from the 1950s. No clear picture of its utility for haematological outcome emerged. The study by Coles 1954 suggested the conferring of a slight benefit for haemoglobin level by the addition of cobalt supplementation at two months, but this was smaller in magnitude, duration and statistical significance than that given by iron supplementation. The study of Diamond 1958 appeared to show a slight benefit also, but was of poor quality. In Quilligan 1954 there was no comparison with iron alone, and so no conclusion can be drawn about the additive effect of cobalt.
Authors' conclusions
Implications for practice.
There is a paucity of evidence from RCTs about how enteral iron supplementation of preterm and low birth weight infants affects growth and neurodevelopmental outcomes. However, there appear to be slight haematological benefits after eight weeks postnatal age. Doses of more than 2 mg/kg/day to 3 mg/kg/day of iron do not appear to confer extra benefit. One recent trial suggests that commencing iron early (at two weeks of age) results in improved haematological parameters from as early as eight weeks of age. The RCT evidence to date does not suggest a particular threshold of birth weight or gestational age at which iron supplementation becomes beneficial, and two of the more recent and methodologically sound trials suggest a benefit even for marginally low birth weight infants, whether term or preterm.
Implications for research.
There is a need for new RCTs of enteral iron supplementation in preterm infants that stratify according to birth weight and gestation, feeding method (breast milk or formula) and prior blood transfusion. This is because it remains unclear from RCT evidence to date whether enteral iron supplementation is required, or indeed might cause harm, in the very low birth weight or extremely low birth weight preterm infant who has already received a blood transfusion. There are strong arguments on a physiological basis that supplementation might only be required in non‐transfused infants, but this has not been satisfactorily tested to date.
What's new
| Date | Event | Description |
|---|---|---|
| 17 April 2012 | Amended | Table 1 'Summary of results by study' linked to text. |
History
Protocol first published: Issue 1, 2005 Review first published: Issue 3, 2012
| Date | Event | Description |
|---|---|---|
| 4 February 2010 | Amended | Converted to new review format |
| 4 December 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Dr William Maguire edited a draft of the review and provided invaluable advice. The following researchers kindly provided further information regarding aspects of their publications which are included in this review: Prof H.P.S. Sachdev, Cheryl Harris, M.L.S. Ferlin MD, Dr Axel Franz, Dr James Friel, Dr Ian Griffin, Prof Henry Halliday, Greg Melnick MD, Prof H. Devlieger, Nathan Rudolph MD and Shmuel Arnon MD.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Data and analyses
Comparison 1. Enteral iron supplementation versus no iron supplementation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight at 12 months or less | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Full or partial breast feeding, or not stated | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Length at 12 months or less | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Full or partial breast feeding, or not stated | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Head circumference at 12 months or less | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Full or partial breast feeding, or not stated | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Haemoglobin concentration at 6 to 8 weeks | 9 | 526 | Mean Difference (IV, Fixed, 95% CI) | 1.43 [‐0.20, 3.07] |
| 5 Haemoglobin concentration at 3 to 4 months | 5 | 269 | Mean Difference (IV, Fixed, 95% CI) | 2.46 [‐0.04, 4.95] |
| 6 Haemoglobin concentration at 6 to 9 months | 3 | 458 | Mean Difference (IV, Fixed, 95% CI) | 5.91 [4.25, 7.58] |
| 6.1 Fully formula fed | 2 | 132 | Mean Difference (IV, Fixed, 95% CI) | 6.56 [3.07, 10.05] |
| 6.2 Full or partial breast feeding, or not stated | 1 | 165 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [5.20, 10.80] |
| 6.3 Full or partial breastfeeding (1 mg/kg/day iron) | 1 | 161 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [1.23, 6.37] |
| 7 Haemoglobin concentration at 12 months | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 16.0 [10.66, 21.34] |
| 8 Serum ferritin at 6 to 8 weeks | 3 | 124 | Mean Difference (IV, Fixed, 95% CI) | 6.70 [0.13, 13.27] |
| 9 Serum ferritin at 3 to 4 months | 3 | 104 | Mean Difference (IV, Fixed, 95% CI) | 11.13 [0.74, 21.52] |
| 10 Serum ferritin at 6 to 9 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 2 mg/kg/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 1 mg/kg/day | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 MCV at 6 to 9 months | 1 | 326 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [1.24, 2.96] |
| 11.1 2 mg/kg/day iron | 1 | 165 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [1.28, 3.72] |
| 11.2 1 mg/kg/day iron | 1 | 161 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [0.49, 2.91] |
| 12 Transferrin saturation at 6 to 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13 Transferrin saturation at 3 to 4 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14 Transferrin saturation at 6 to 9 months | 1 | 326 | Mean Difference (IV, Fixed, 95% CI) | 7.14 [5.19, 9.08] |
| 14.1 2 mg/kg/day iron | 1 | 165 | Mean Difference (IV, Fixed, 95% CI) | 9.10 [5.97, 12.23] |
| 14.2 1 mg/kg/day iron | 1 | 161 | Mean Difference (IV, Fixed, 95% CI) | 5.90 [3.42, 8.38] |
| 15 Subgroup analyses ‐ Hb at 6 to 8 weeks | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.1 Fully formula fed | 5 | 389 | Mean Difference (IV, Fixed, 95% CI) | 0.71 [‐1.14, 2.55] |
| 15.2 Full or partial breast feeding, or not stated | 4 | 137 | Mean Difference (IV, Fixed, 95% CI) | 4.14 [0.59, 7.70] |
| 15.3 Early commencement | 8 | 463 | Mean Difference (IV, Fixed, 95% CI) | 1.55 [‐0.12, 3.23] |
| 15.4 Late commencement | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐8.82, 6.62] |
| 15.5 Low dose | 3 | 209 | Mean Difference (IV, Fixed, 95% CI) | 3.92 [1.53, 6.32] |
| 15.6 High dose | 6 | 317 | Mean Difference (IV, Fixed, 95% CI) | ‐0.76 [‐3.01, 1.49] |
| 15.7 Under 1500 g mean birth weight | 5 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐4.27, 3.43] |
| 15.8 1500 g or over mean birth weight | 3 | 305 | Mean Difference (IV, Fixed, 95% CI) | 0.77 [‐1.16, 2.69] |
| 16 Enteral feed intolerance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 17 Subgroup analyses ‐ Hb at 3 to 4 months | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 17.1 Fully formula fed | 3 | 208 | Mean Difference (IV, Fixed, 95% CI) | 2.39 [‐0.42, 5.21] |
| 17.2 Full or partial breast feeding | 2 | 61 | Mean Difference (IV, Fixed, 95% CI) | 2.69 [‐2.70, 8.08] |
| 17.3 Early commencement | 3 | 180 | Mean Difference (IV, Fixed, 95% CI) | 0.75 [‐2.36, 3.85] |
| 17.4 Late commencement | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | 5.59 [1.39, 9.79] |
| 17.5 Low dose | 2 | 165 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [0.85, 7.15] |
| 17.6 High dose | 3 | 104 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐4.24, 3.94] |
| 17.7 Under 1500 g mean birth weight | 2 | 106 | Mean Difference (IV, Fixed, 95% CI) | 0.98 [‐2.89, 4.84] |
| 17.8 1500 g or over birth weight | 3 | 163 | Mean Difference (IV, Fixed, 95% CI) | 3.52 [0.25, 6.79] |
1.1. Analysis.
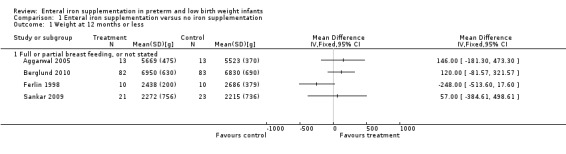
Comparison 1 Enteral iron supplementation versus no iron supplementation, Outcome 1 Weight at 12 months or less.
1.2. Analysis.
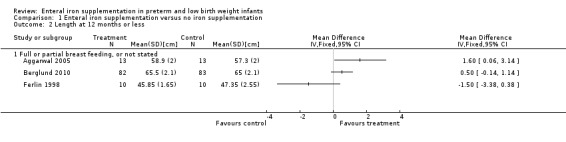
Comparison 1 Enteral iron supplementation versus no iron supplementation, Outcome 2 Length at 12 months or less.
1.3. Analysis.
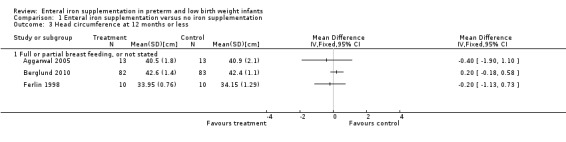
Comparison 1 Enteral iron supplementation versus no iron supplementation, Outcome 3 Head circumference at 12 months or less.
1.4. Analysis.
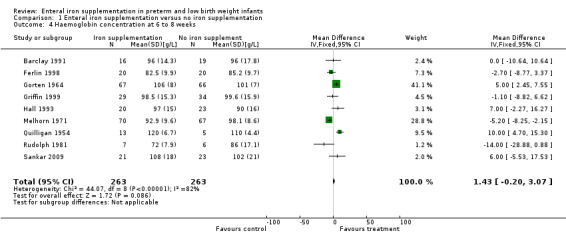
Comparison 1 Enteral iron supplementation versus no iron supplementation, Outcome 4 Haemoglobin concentration at 6 to 8 weeks.
1.5. Analysis.
Comparison 1 Enteral iron supplementation versus no iron supplementation, Outcome 5 Haemoglobin concentration at 3 to 4 months.
1.6. Analysis.
Comparison 1 Enteral iron supplementation versus no iron supplementation, Outcome 6 Haemoglobin concentration at 6 to 9 months.
1.7. Analysis.
Comparison 1 Enteral iron supplementation versus no iron supplementation, Outcome 7 Haemoglobin concentration at 12 months.
1.8. Analysis.
Comparison 1 Enteral iron supplementation versus no iron supplementation, Outcome 8 Serum ferritin at 6 to 8 weeks.
1.9. Analysis.
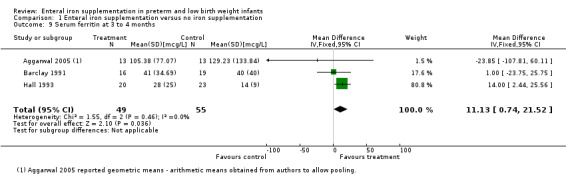
Comparison 1 Enteral iron supplementation versus no iron supplementation, Outcome 9 Serum ferritin at 3 to 4 months.
1.10. Analysis.
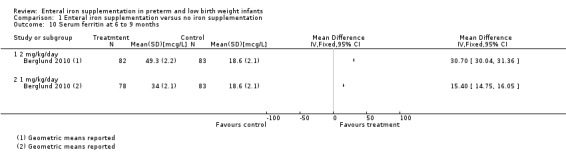
Comparison 1 Enteral iron supplementation versus no iron supplementation, Outcome 10 Serum ferritin at 6 to 9 months.
1.11. Analysis.
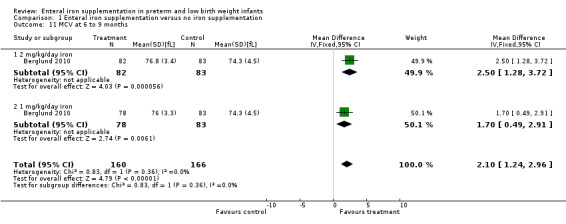
Comparison 1 Enteral iron supplementation versus no iron supplementation, Outcome 11 MCV at 6 to 9 months.
1.12. Analysis.
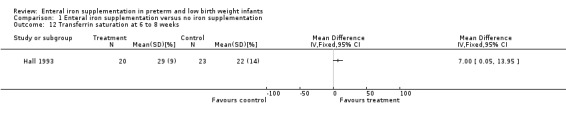
Comparison 1 Enteral iron supplementation versus no iron supplementation, Outcome 12 Transferrin saturation at 6 to 8 weeks.
1.13. Analysis.
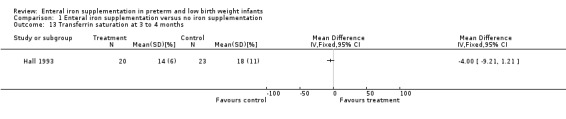
Comparison 1 Enteral iron supplementation versus no iron supplementation, Outcome 13 Transferrin saturation at 3 to 4 months.
1.14. Analysis.
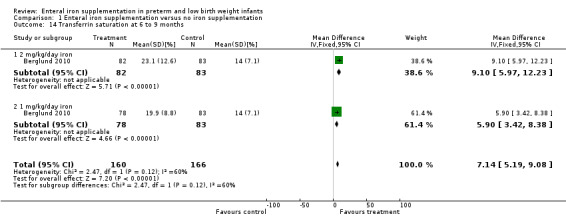
Comparison 1 Enteral iron supplementation versus no iron supplementation, Outcome 14 Transferrin saturation at 6 to 9 months.
1.15. Analysis.
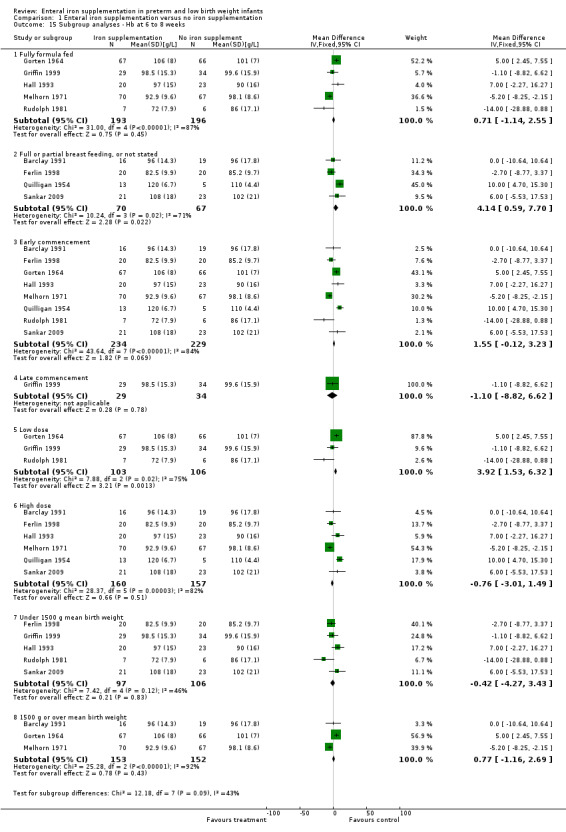
Comparison 1 Enteral iron supplementation versus no iron supplementation, Outcome 15 Subgroup analyses ‐ Hb at 6 to 8 weeks.
1.16. Analysis.
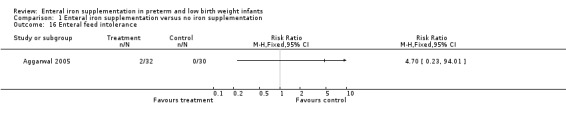
Comparison 1 Enteral iron supplementation versus no iron supplementation, Outcome 16 Enteral feed intolerance.
1.17. Analysis.
Comparison 1 Enteral iron supplementation versus no iron supplementation, Outcome 17 Subgroup analyses ‐ Hb at 3 to 4 months.
Comparison 2. Early versus late iron supplementation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neurodevelopmental outcome (MPC) at 5 years | 1 | 164 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐2.06, 8.06] |
| 2 Haemoglobin concentration at 6 to 8 weeks | 2 | 144 | Mean Difference (IV, Fixed, 95% CI) | 15.49 [12.74, 18.24] |
| 3 Haemoglobin concentration at 6 to 9 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Ferritin level at 6 to 8 weeks | 1 | 116 | Mean Difference (IV, Fixed, 95% CI) | 25.0 [16.94, 33.06] |
2.1. Analysis.
Comparison 2 Early versus late iron supplementation, Outcome 1 Neurodevelopmental outcome (MPC) at 5 years.
2.2. Analysis.
Comparison 2 Early versus late iron supplementation, Outcome 2 Haemoglobin concentration at 6 to 8 weeks.
2.3. Analysis.
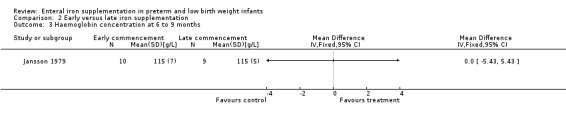
Comparison 2 Early versus late iron supplementation, Outcome 3 Haemoglobin concentration at 6 to 9 months.
2.4. Analysis.
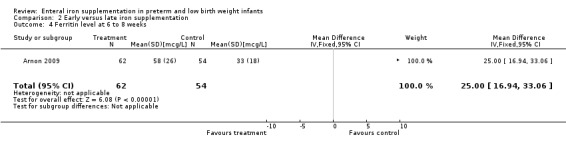
Comparison 2 Early versus late iron supplementation, Outcome 4 Ferritin level at 6 to 8 weeks.
Comparison 3. High dose versus low dose iron supplementation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neurodevelopmental outcome (Grifiths Developmental Assessment score) at 12 months or less | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Haemoglobin concentration at 6 to 8 weeks | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐1.41 [‐8.24, 5.42] |
| 2.1 Full formula feeding | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐17.86, 7.86] |
| 2.2 Full or partial breast feeding, or not stated | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐8.06, 8.06] |
| 3 Haemoglobin concentration at 3 to 4 months | 3 | 117 | Mean Difference (IV, Fixed, 95% CI) | 4.49 [0.84, 8.13] |
| 3.1 Full formula feeding | 2 | 81 | Mean Difference (IV, Fixed, 95% CI) | 4.34 [0.21, 8.47] |
| 3.2 Full or partial breast feeding, or not stated | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [‐2.79, 12.79] |
| 4 Haemoglobin concentration at 6 to 9 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Haemoglobin concentration at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Mean corpuscular volume at 3 to 4 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Serum ferritin at 6 to 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Serum ferritin at 3 to 4 months | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 4.15 [‐3.71, 12.02] |
| 9 Serum ferritin at 6 to 9 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Serum ferritin at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Transferrin saturation at 6 to 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 Transferrin saturation at 3 to 4 months | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | 1.74 [‐1.23, 4.71] |
| 13 Transferrin saturation at 6 to 9 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14 Transferrin saturation at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.

Comparison 3 High dose versus low dose iron supplementation, Outcome 1 Neurodevelopmental outcome (Grifiths Developmental Assessment score) at 12 months or less.
3.2. Analysis.
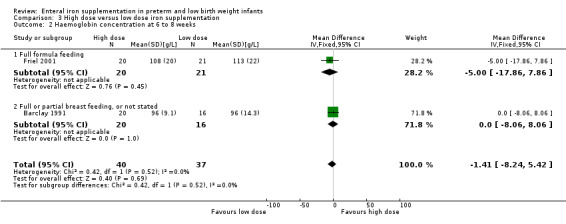
Comparison 3 High dose versus low dose iron supplementation, Outcome 2 Haemoglobin concentration at 6 to 8 weeks.
3.3. Analysis.
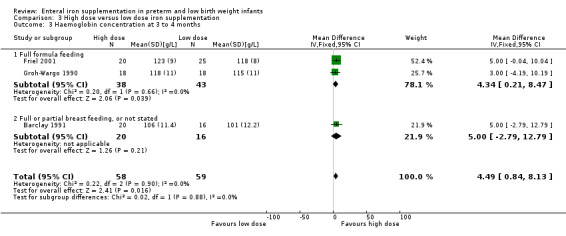
Comparison 3 High dose versus low dose iron supplementation, Outcome 3 Haemoglobin concentration at 3 to 4 months.
3.4. Analysis.

Comparison 3 High dose versus low dose iron supplementation, Outcome 4 Haemoglobin concentration at 6 to 9 months.
3.5. Analysis.

Comparison 3 High dose versus low dose iron supplementation, Outcome 5 Haemoglobin concentration at 12 months.
3.6. Analysis.
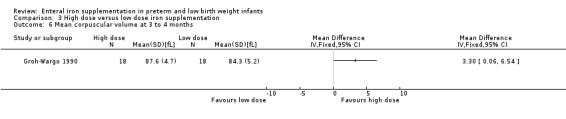
Comparison 3 High dose versus low dose iron supplementation, Outcome 6 Mean corpuscular volume at 3 to 4 months.
3.7. Analysis.
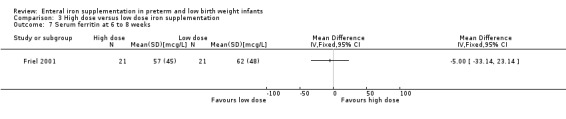
Comparison 3 High dose versus low dose iron supplementation, Outcome 7 Serum ferritin at 6 to 8 weeks.
3.8. Analysis.
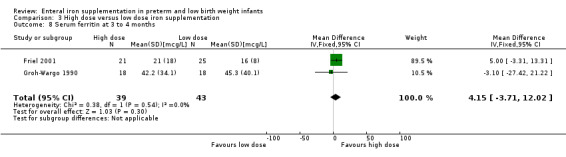
Comparison 3 High dose versus low dose iron supplementation, Outcome 8 Serum ferritin at 3 to 4 months.
3.9. Analysis.
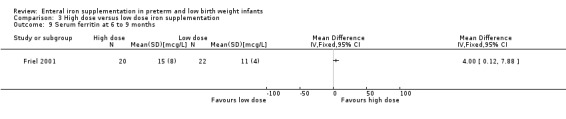
Comparison 3 High dose versus low dose iron supplementation, Outcome 9 Serum ferritin at 6 to 9 months.
3.10. Analysis.
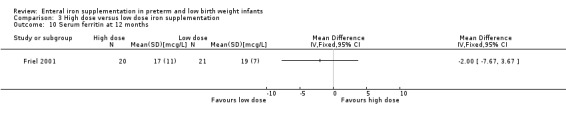
Comparison 3 High dose versus low dose iron supplementation, Outcome 10 Serum ferritin at 12 months.
3.11. Analysis.
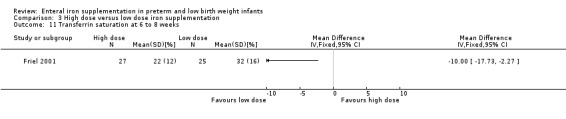
Comparison 3 High dose versus low dose iron supplementation, Outcome 11 Transferrin saturation at 6 to 8 weeks.
3.12. Analysis.
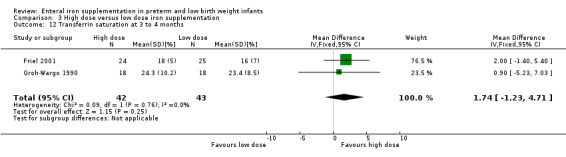
Comparison 3 High dose versus low dose iron supplementation, Outcome 12 Transferrin saturation at 3 to 4 months.
3.13. Analysis.
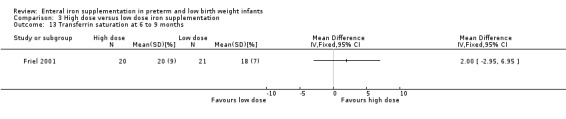
Comparison 3 High dose versus low dose iron supplementation, Outcome 13 Transferrin saturation at 6 to 9 months.
3.14. Analysis.
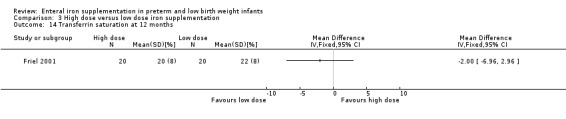
Comparison 3 High dose versus low dose iron supplementation, Outcome 14 Transferrin saturation at 12 months.
Comparison 4. Short duration versus long duration iron supplementation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Haemoglobin at 6 to 8 weeks | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 3.70 [‐3.79, 11.19] |
| 2 Haemoglobin at 3 to 4 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [‐3.92, 6.92] |
| 3 Haemoglobin at 6 to 9 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 5.70 [0.50, 10.90] |
4.1. Analysis.
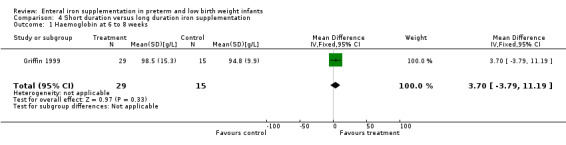
Comparison 4 Short duration versus long duration iron supplementation, Outcome 1 Haemoglobin at 6 to 8 weeks.
4.2. Analysis.
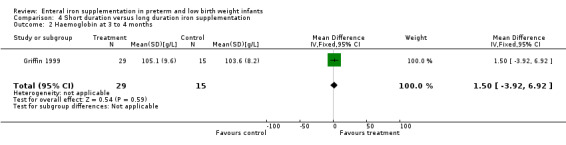
Comparison 4 Short duration versus long duration iron supplementation, Outcome 2 Haemoglobin at 3 to 4 months.
4.3. Analysis.
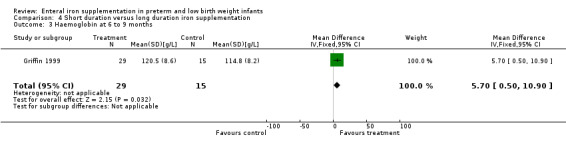
Comparison 4 Short duration versus long duration iron supplementation, Outcome 3 Haemoglobin at 6 to 9 months.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aggarwal 2005.
| Methods | Blinding of randomisation: yes (author correspondence) Blinding of intervention: yes Complete follow‐up: 4 weeks ‐ yes (85%); 8 weeks ‐ no (36%) Blinding of outcome measurement: yes | |
| Participants | Gestation: term Birth weight: low birth weight (< 2500 g) Feeding: breast fed Age of enrolment: 50 to 80 days Exclusion criteria: twinning, congenital malformations, > 10 mL of blood sampling in the past, those already on iron supplementation, significant morbidity and maternal antepartum haemorrhage Blood transfusions: excluded if previously received | |
| Interventions | Iron supplementation group: 3 mg/kg/day as ferric ammonium citrate drops (n = 37), commencing at 50 to 80 days of age No iron supplementation group: indistinguishable placebo mixture (n = 36) | |
| Outcomes | Primary: haemoglobin concentration Secondary: peripheral smear, serum ferritin, weight, length, head circumference Timing: baseline (7 to 11weeks postnatal), 4 weeks (11 to 15 weeks postnatal) and 8 weeks (15 to 19 weeks postnatal) | |
| Notes | 73 infants were randomised Randomisation was performed using computer generated random numbers Protocol for treatment of anaemia: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generation |
| Allocation concealment (selection bias) | Low risk | Adequate allocation concealment (author correspondence) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8 week follow‐up 36% |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selectivity |
| Other bias | Low risk | No other risk of bias identified |
Arnon 2009.
| Methods | Blinding of randomisation: unclear Blinding of intervention: no (author communication) Complete follow‐up: Yes (87%) Blinding of outcome measurement: Yes (author communication) | |
| Participants | Gestation: <= 32 weeks Birth weight: no limits (mean < 1300 g) Feeding: breast milk Exclusion criteria: major anomalies, haemolytic disease, twin‐twin transfusion syndrome, illness requiring cessation of supplementation for over 3 days Blood transfusions: permitted prior to study entry (when enteral intake >= 100 mL/kg/day); excluded if requiring transfusion during study period. Restrictive transfusion policy | |
| Interventions | Early iron group: Iron polymaltose complex, 5mg/kg/day, from 2 weeks of age Late iron group: Iron polymaltose complex, 5mg/kg/day, from 4 weeks of age (after receiving placebo from 2 weeks) Both groups received 25 IU of vitamin E from 2 to 8 weeks of age | |
| Outcomes | Primary outcome: vitamin E (alpha‐tocopherol) levels at 2, 4 and 8 weeks of age Secondary outcomes: serum iron, serum ferritin, haemoglobin level and reticulocyte count at 2, 4 and 8 weeks of age Not published for full cohort, but received from author: requirement for transfusion and soluble transfusion receptor level (at 2, 4 and 8 weeks of age) | |
| Notes | Requirement for transfusion and soluble transfusion receptor level (at 2, 4 and 8 weeks of age) were also reported in Arnon 2007 in a subset of this cohort | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generation |
| Allocation concealment (selection bias) | Unclear risk | Allocation known to pharmacist but concealed from treating staff using envelopes (author communication). |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: No (author communication) Blinding of outcome measurement: Yes (author communication) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: Yes (87%) |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selectivity |
| Other bias | Low risk | No other risk of bias identified |
Barclay 1991.
| Methods | Blinding of randomisation: not known Blinding of intervention: not known Complete follow‐up: yes (89%) Blinding of outcome measurement: not known | |
| Participants | Gestation: term or preterm Birth weight: less than 2500 g Feeding: 11 infants were exclusively fed breast milk, and the remainder received infant formula containing 5 to 6.7 mg/l (0.8 mg/kg/day) of iron Exclusion criteria: congenital or chromosomal abnormalities, haemolytic disease, gastrointestinal disorders and intravenous nutrition for more than 5 days Blood transfusion: excluded if occurring after day 14 | |
| Interventions | High iron group (HiFe): approximately 7.1 mg/kg/day (13.8 mg of iron daily as iron edetate) (n = 20) Low iron group (MidFe): approximately 3.6 mg/kg/day (7 mg iron daily as iron edetate) (n = 16) (this group used as iron supplementation group for comparison with no iron supplementation) No iron supplementation group (NatFe): no additional iron (n = 19) All supplements began at 28 days postnatally | |
| Outcomes | Primary: erythrocyte superoxide dismutase levels Secondary: haemoglobin concentration, reticulocyte count; plasma copper, zinc and ferritin levels; weight, length and head circumference Timing: baseline (27 days), 8 weeks, 12 weeks and 20 weeks postnatal age | |
| Notes | 62 infants were randomised Infants were randomised within each stratum of gestational age (< 32 weeks, 33 to 35 weeks and > 36 weeks) The infants received different vitamin regimens based on their birth weight, which did not necessarily correlate strictly with the gestational age groups The data for those who failed to attend follow‐up visits were not included in the analysis Treatment of anaemia: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Blinding of randomisation: not known |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention: not known Blinding of outcome measurement: not known |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes (89%) |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other risk of bias identified |
Berglund 2010.
| Methods | Blinding of randomisation: yes Blinding of intervention: yes Complete follow‐up: yes (91%) Blinding of outcome measure: yes |
|
| Participants | Gestation: term or preterm, enrolment at 6 weeks postnatal age Birth weight: 2000 g to 2500 g Feeding: breast milk or iron‐fortified formula Exclusion criteria: disease symptoms at 6 weeks postnatal, chronic disease, previous blood transfusion, previous iron supplementation and anaemia or other haematological disorder at 6 weeks postnatal Blood transfusions: excluded |
|
| Interventions | High iron group (HiFe): 2 mg/kg/day of elemental iron (as ferrous succinate) from 6 weeks to 6 months postnatal age Low iron group (MidFe): 1 mg/kg/day of elemental iron (as ferrous succinate) from 6 weeks to 6 months postnatal age No iron group: placebo mixture from 6 weeks to 6 months postnatal age |
|
| Outcomes | Haematological: at baseline (6 weeks), 12 weeks and 6 months ‐ complete blood count, haemoglobin level, mean cell volume, serum ferritin, serum transferrin, serum iron, C‐reactive protein, transferrin saturation, transferrin receptor concentration Growth: at baseline (6 weeks), 12 weeks, 19 weeks and 6 months ‐ weight, length, head circumference, knee‐heel length |
|
| Notes | Infants were randomised within stratification for gender and study centre | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation |
| Allocation concealment (selection bias) | Low risk | Blinding of treatment assignment was reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | As reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow up 91% |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other risk of bias identified |
Berseth 2004.
| Methods | Blinding of randomisation: yes (author communication) Blinding of intervention: yes Complete follow‐up: no (52%) Blinding of outcome measurement: yes | |
| Participants | Gestation: less than 33 weeks Birth weight: less than 1500 g Feeding: breast milk (fortified) Exclusion criteria: conditions expected to affect growth or feed tolerance; 5‐minute Apgar score < 4; major surgery; IVH of grade 3 or 4; 4 or more days of steroid therapy before or within 72 hours of day 0; had received any HMF before day 0; had received EPO, vitamin D, minerals or iron by day 0; still mechanically ventilated by day 0 Blood transfusion: permitted | |
| Interventions | Iron supplementation group: approximately 2.4 mg/kg/day of iron (in powdered fortifier which providing 15.3 mg/L of iron (n = 96), commencing when enteral intake > 100 mL/kg/day No iron supplementation group: approximately 0.64 mg/kg/day of iron (in another fortifier providing 4.4 mg/L of iron (n = 85) The first day of fortification was at half strength, and from the second day at full strength | |
| Outcomes | Primary outcome: weight gain Secondary outcomes: Length and head circumference; hematocrit, albumin, transthyretin, alkaline phosphatase, BUN, triglycerides, sodium, potassium, chloride, bicarbonate, calcium, phosphorus, magnesium, zinc, copper, ferritin and vitamin D Incidence of feed intolerance (gastric residuals, abdominal distention and emesis), respiratory outcomes (supplemental oxygen, mechanical ventilation), and incidence of NEC. Need for blood transfusion | |
| Notes | 185 infants were randomised Infants were randomised when they had achieved an enteral intake of 100 ml/kg/day of unfortified human milk Randomisation occurred within predetermined stratification groups (by gender and birth weight above or below 1000 g) Blinding was achieved by otherwise identical, coded labels | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated (author communication) |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes (author communication). |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 52% follow‐up to completion |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other identified bias |
Coles 1954.
| Methods | Blinding of randomisation: not known Blinding of intervention: not known Complete follow‐up: no (58.7% at 6 months) Blinding of outcome measurement: not known | |
| Participants | Gestation: premature (specific gestation not specified) Birth weight: not stated Feeding: breast or formula fed Exclusion criteria: severe haemorrhage, death Blood transfusion policy: not stated | |
| Interventions | Iron supplementation group (Group IV): Approx 44 mg/kg/day of iron (as 4.5 grains of ferrous sulphate) and cobalt sulphate 20 mg daily (n = 22) from 4 to 8 weeks of life No iron supplementation group (Group III): Cobalt sulphate orally, 20 mg daily from 4 to 8 weeks (n = 29) Other groups not included in systematic review: Group I: controls (n = 53) Group II: cobalt sulphate orally, 10 mg daily from day 1 to 12 of life (n = 22) | |
| Outcomes | Primary outcomes: haemoglobin concentration and red blood cell count Timing: birth, 1 week, 2 weeks, 1 month, monthly to 6 months, at 9 months and 1 year postnatal | |
| Notes | 51 infants were randomised to the groups included in this review (groups IV and III) Iron and cobalt supplements or both were administered from 4 to 8 weeks postnatally Management of anaemia: ferrous sulphate 4.5 grains (approximately 44 mg/kg/day) when haemoglobin < 70 g/L (< 6 months), or < 75 g/L (> 6 months) and cobalt sulphate 20 mg if RCC < 2.5 m/cmm and haemoglobin < 70 g/L Vitamin supplementation: vitamins A, D and C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Blinding of randomisation: not known |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention: not known Blinding of outcome measurement: not known |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow‐up: no (58.7% at 6 months) |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other risk of bias identified |
Diamond 1958.
| Methods | Blinding of randomisation: no (quasi‐randomised) Blinding of intervention: not known Complete follow‐up: not known (100% implied) Outcome assessment blind: not known | |
| Participants | Gestation: not stated Birth weight: not stated Feeding: not stated | |
| Interventions | Iron supplementation group (Group 2): approximately 12 mg/kg/day of iron (as 75 mg per day of ferrous sulfate) (n = 12) No iron supplementation group (Group 3): no supplement (n = 16) Time of commencement of supplementation is not stated Additional study group not included in systematic review: Group 1: approximately 12 mg/kg/day of iron (as 75 mg per day of ferrous sulfate) and 2 mg/kg/day of cobalt chloride (n = 16) | |
| Outcomes | Primary outcome: haemoglobin level Timing of outcome measurement: monthly, up to 6 months postnatal | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised |
| Allocation concealment (selection bias) | High risk | Blinding of randomisation: no (quasi‐randomised) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention: not known Outcome assessment blind: not known |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete follow‐up: not known (100% implied) |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other known bias |
Ferlin 1998.
| Methods | Blinding of randomisation: no (author communication ‐ quasi‐randomised) Blinding of intervention: yes (placebo used) Complete follow‐up: not known (100% implied) Blinding of outcome measurement: yes (author communication) | |
| Participants | Gestation: up to 35 weeks gestation Birth weight: less than 1600 g birth weight Feeding: all started on breast milk, then changed to a non‐iron supplemented formula at unspecified intervals Exclusions: those who failed to attend follow‐up Blood transfusion policy: excluded from study | |
| Interventions | Iron supplementation group: iron supplementation of 4 mg/kg/day of iron (as ferrous sulfate), with 25 IU/day of vitamin E supplementation (Group III, n = 10), or without vitamin E supplementation (Group II, n = 10). Iron supplementation was commenced on the 15th day of life No iron supplementation group: placebo (Group I, n = 10) or 25 IU/day of vitamin E supplementation (Group IV, n = 10) | |
| Outcomes | Primary: haemoglobin concentration and hematocrit
Secondary: weight, length and head circumference; reticulocytes, platelets and red cell resistance to hydrogen peroxide
Timing: 24 to 72 hours of age and 2 months of age |
|
| Notes | Although the infants included in the analysis were followed to completion of the study, it is stated that regular attendance at follow‐up appointments was a prerequisite for inclusion in the study; it is not stated how many infants were initially randomised but later excluded due to loss to follow‐up or other prespecified events such as the need for blood transfusion, or other 'interfering events' that resulted in cessation of treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised |
| Allocation concealment (selection bias) | High risk | Blinding of randomisation: no (author communication ‐ quasi‐randomised) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes (placebo used) Blinding of outcome measurement: yes (author communication) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete follow‐up: not known (100% implied) |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other known bias |
Franz 2000.
| Methods | Blinding of randomisation: no (author communication) Blinding of intervention: yes; but allocation became obvious due to stool colour Complete follow‐up: no (65%) Blinding of outcome measurement: yes | |
| Participants | Gestation: no limit Birth weight: less than 1301 g Feeding: either breast fed or received an iron‐fortified formula (12 mg/L, or approximately 1.9 mg/kg/day of iron) Exclusion criteria: major anomalies, haemolytic disease and twin‐to‐twin transfusion Blood transfusion policy: permitted, before and after entry; restrictive transfusion policy | |
| Interventions | Iron supplementation group: 2 mg/kg/day of iron as ferrous sulfate, commenced as soon as enteral feedings were tolerated (n = 105) No iron supplementation group: 2 mg/kg/day of iron as ferrous sulfate, commenced at 61 days of life (n = 99) Management of anaemia: when the hematocrit level fell below 0.30, the dose of iron was increased to 4 mg/kg/day. The policy for the administration of blood transfusions was clearly outlined | |
| Outcomes | Primary outcome: serum ferritin and incidence of iron deficiency Secondary outcomes: transferrin, transferrin saturation, serum iron, reticulocytes, hematocrit; number of transfusions required and blood volume transfused Timing: birth; at 1.6 times birth weight; at 61 days of life; and opportunistically when the hematocrit level was < 0.25 | |
| Notes | Randomised on day 7 of life. The infants either received breast milk or iron‐fortified formula (12 mg/L, approximately 1.9 mg/kg/day) The iron intake due to formula was reported to be similar between the two groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated. Reported in Steinmacher 2007 (continuation of this study) |
| Allocation concealment (selection bias) | High risk | Blinding of randomisation: no (author communication) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes; but allocation became obvious due to stool colour Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow‐up to completion: 65% |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other risk of bias identified |
Friel 2001.
| Methods | Blinding of randomisation: yes (author communication) Blinding of intervention: yes Complete follow‐up: no (72.4%) Blinding of outcome measurement: yes | |
| Participants | Gestation: term or preterm Birth weight: low birth weight (< 2500 g) Feeding: formula Exclusions: bronchopulmonary dysplasia, hydrocephalus, liver dysfunction or had congenital malformations Blood transfusion policy: not stated | |
| Interventions | High iron group (HiFe): approximately 3.4 mg/kg/day of iron (in formula containing 21 mg/L of iron) (n = 29) Low iron group (MidFe): approximately 2.1 mg/kg/day of iron (in formula containing 13.4 mg/L of iron) (n = 29) Infants were assigned to groups at close to 2000 g weight and when on full oral feeds | |
| Outcomes | Primary outcomes: Griffith Developmental Assessment score (at 3, 6, 9 and 12 months corrected), weight, and height. Haemoglobin level, hematocrit, ferritin, transferrin, transferrin saturation, and plasma iron Secondary outcomes: malondialdehyde level, plasma zinc and copper levels, red blood cell fragility, catalase level, superoxide dismutase level and glutathione peroxidase level Timing: baseline, discharge, and 3, 6, 9 and 12 months corrected age | |
| Notes | Randomisation was achieved using a random number table and concealed allocation was performed using opaque sealed envelopes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table (author communication) |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes (author communication) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow‐up to completion: 72.4% |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other risk of bias identified |
Gorten 1964.
| Methods | Blinding of randomisation: yes (sealed envelopes) Blinding of intervention: yes Complete follow‐up: no (55.9% at 12 months) Blinding of outcome measurement: yes | |
| Participants | Gestation: premature Birth weight: mean birth weight was 1.84 kg (29.9 weeks gestation) in the study group; and 1.89 kg (33.5 weeks gestation) in the control group Feeding: formula Blood transfusion policy: not stated | |
| Interventions | Iron supplementation group: approximately 2 mg/kg/day of iron (as formula containing 12 mg per quart (13 mg/L) of iron (n = 69) No iron supplementation group: formula containing no added iron (n = 76) Enrolled in the first week of life | |
| Outcomes | Primary outcome: incidence of iron deficiency (haemoglobin level below 9.0 g/dL, hematocrit below 0.32) Secondary outcomes: haemoglobin concentration, red blood cell count, white blood cell count, hematocrit, red cell indices. Weight and length Timing: first week of life, as required during admission, and at each clinic visit after discharge (up to 80 weeks postnatal) | |
| Notes | Management of anaemia: when Hb < 9 g/dL and HcT < 0.32, changed to iron‐containing formula. Infants in the control group removed from further analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes (sealed envelopes) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow‐up: no (55.9% at 12 months) |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other risk of bias identified |
Griffin 1999.
| Methods | Blinding of randomisation: yes (author communication) Blinding of intervention: yes Complete follow‐up: yes (96.2%) Blinding of outcome measurement: yes | |
| Participants | Gestation: 32 weeks or less Birth weight: 1750 g or less Feeding: formula feeding after commencement of intervention Exclusions: systemically unwell, requiring supplemental oxygen, or on any medications other than vitamin drops Blood transfusion policy: permitted before enrolment (at discharge), but infants excluded from study if required after enrolment | |
| Interventions | Iron supplementation group: approximately 1.5 mg/kg/day of iron (in formula containing 9 mg/L of iron) (n = 29) No iron supplementation group: approximately 0.8 mg/kg/day of iron (in formula containing 5 mg/L of iron) (n = 34) Iron supplementation commenced at discharge from hospital Short duration group: 9 mg/L (approximately 1.5 mg/kg/day) of iron (as high iron formula) from discharge, then changed to the lower iron formula at term (n = 15) Prior to discharge the infants received the high‐iron formula or a combination of breast milk and formula feeds | |
| Outcomes | Primary outcomes: haemoglobin concentration and plasma ferritin Timing: every 2 weeks from discharge to term, then monthly to 6 months corrected age (approximately 8 months postnatal age) | |
| Notes | Infants were randomised using sealed envelopes 48 hours before discharge. Three infants were excluded from analysis because they received blood transfusions after discharge. The outcome statistics are presented in graphical form, with statistical significance data derived by ANOVA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated (author communication) |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes (author communication) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes (96.2%) |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other risk of bias identified |
Groh‐Wargo 1990.
| Methods | Blinding of randomisation: not known Blinding of intervention: yes (double‐blind) Complete follow‐up: not known Blinding of outcome assessment: not known | |
| Participants | Gestation: no limit stated Birth weight: less than 1500 g birth weight Feeding: low‐iron formula Exclusions: none stated Blood transfusion policy: permitted | |
| Interventions | High iron group (HiFe) (Group 2): iron supplementation (as Fer‐in‐Sol) of 4 mg/kg/day (n = 18) Low iron group (MidFe) (Group 1): iron supplementation (as Fer‐in‐Sol) of 2 mg/kg/day (n = 18) Iron supplementation was commenced at 2 weeks postnatal age | |
| Outcomes | Primary outcomes: weight, haemoglobin, MCV, FEP, % saturation and ferritin Timing: 0.5 months, 2 months, and 4 months | |
| Notes | There is no description of the number of eligible patients who were not included in the trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Blinding of randomisation: not known |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention: yes (double‐blind) Blinding of outcome assessment: not known |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete follow‐up: not known |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Unclear risk | Insufficient information to inform assessment of risk of other bias |
Gross 1985.
| Methods | Blinding of randomisation: not known Blinding of intervention: not known Complete follow‐up: yes (100%) Blinding of outcome assessment: not known | |
| Participants | Gestation: 27 to 33 weeks Birth weight: < 1500 g (AGA) Feeding: breast milk or formula Exclusions: any major disease or haemolytic disease, hematocrit level < 0.40, unable to begin enteral feeds in first week of life Transfusions: nil received | |
| Interventions | Iron supplementation group: 2 mg/kg/day of iron as ferrous sulfate, from 2 weeks of age (fed preterm human milk, mature human milk or formula) No iron supplementation group: no ferrous sulfate (fed preterm human milk, mature human milk or formula) | |
| Outcomes | Primary outcome: serum vitamin E concentration Secondary outcomes: vitamin E/lipid ratio, hydrogen peroxide haemolysis, hematocrit, reticulocyte count Timing: day 1 and after 6 weeks of feeding (0 to 7 weeks postnatal) (vitamin E measures) and 2 weeks and 4 weeks of feeding (2 to 5 weeks postnatal) (other measures) | |
| Notes | The infants were assigned to 1 of 6 groups by means of a random number table. Follow‐up was complete | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Blinding of randomisation: not known |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention: not known Blinding of outcome assessment: not known |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes (100%) |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other risk of bias identified |
Hall 1993.
| Methods | Blinding of randomisation: not known Blinding of intervention: yes (double‐blind) Complete follow‐up: no (37.3%) Blinding of outcome measurement: yes | |
| Participants | Gestation: less than 35 weeks Birth weight: less than 1800 g (AGA) Feeding: formula or breast fed Exclusions: chronic lung disease, NEC, neurological disease interfering with nutrition, other conditions affecting nutrient intake Blood transfusions: permitted | |
| Interventions | Iron supplementation group: approximately 2.4 mg/kg/day of iron (in formula containing 15 mg/L of iron) (n = 36)
No iron supplementation group: approximately 0.5 mg/kg/day of iron (in formula containing 3 mg/L of iron) (n = 43)
The above formulas were used from study entry to discharge
At the time of hospital discharge, all formula‐fed infants were changed to a cow‐milk based formula containing 12 mg/L of iron Futher group not included in systematic review: 'Comparison group' receiving breast milk fortified with iron to provide 1.7 mg/L of iron (n = 71) |
|
| Outcomes | Primary outcomes: plasma iron, ferritin, transferrin, transferrin saturation Secondary outcomes: haemoglobin, hematocrit, red blood cell count, MCV, MCH, MCHC and reticulocyte count. Weight, length, head circumference, arm circumference and triceps skinfold thickness Timing: haematological outcomes: study entry (up to 21 days postnatal), hospital discharge (approximately 5 to 6 weeks postnatal), and 8 weeks after discharge (approximately 13 to 14 weeks postnatal). Anthropomorphic outcomes: study entry, hospital discharge and 2, 4, 8 and 12 weeks postdischarge | |
| Notes | The method of randomisation was not described. Infants were enrolled within 21 days of birth. 56 infants completed the study (37.3%) ‐ 20/36 in the high iron (HiFe) formula group, 23/43 in the low iron (MidFe) formula group, and 13/71 in the breast milk group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Blinding of randomisation: not known |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes (double‐blind) Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow up to completion: 37.3% |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other risk of bias identified |
Halliday 1983.
| Methods | Blinding of randomisation: yes (author communication) Blinding of intervention: yes (placebo used) Complete follow‐up: not known (100% follow‐up implied) Blinding of outcome measurement: yes (author communication) | |
| Participants | Gestational age 28 to 36 weeks Birth weight: no limit stated Feeding: mostly fed evaporated milk supplemented with a multivitamin preparation Exclusions: significant respiratory problems, SGA Blood transfusion: nil received | |
| Interventions | Iron supplementation group (also early iron group) (Group A): approximately 6 mg/kg/day (12 mg) of elemental iron (as ferrous sulphate with ascorbic acid) until one year of age (n = 16) No iron supplementation group (Group B): ascorbic acid solution without iron until 1 year of age (n = 17) Late iron group (Group C): placebo until 8 weeks, then 6 mg/kg/day (12 mg) of elemental iron daily until 1 year of age (n = 16) Iron supplementation was commenced on the 7th day of life and increased from 2 mg to 12 mg daily by the 6th week | |
| Outcomes | Primary outcomes: iron intake, haemoglobin concentration, serum iron, serum transferrin and serum ferritin Timing: day 3 postnatally, then 3 weekly until 12 weeks and then at 18, 24, 36 and 54 weeks postnatal | |
| Notes | The method of randomisation and the means of allocation concealment are not described. However, the author indicated in correspondence that the randomisation was generated by a statistician and allocation was by sealed envelopes. No loss to follow‐up was recorded, although the number of babies analysed at each time interval is not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Author communication |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes (author communication) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes (placebo used) Blinding of outcome measurement: yes (author communication) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete follow‐up: not known (100% follow‐up implied) |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other risk of bias identified |
Hanninen 1961.
| Methods | Blinding of randomisation: no (quasi‐randomised) to one of three groups Blinding of intervention: not known Complete follow‐up: not known Blinding of outcome measurement: not known | |
| Participants | Gestation: preterm Birth weight: between 1060 g to 2400 g Feeding: not stated Management of anaemia: excluded from analysis | |
| Interventions | Iron supplementation group (Group 1): approximately 27 mg/kg/day of iron (as ferricholincitrate equivalent to 24 mg of iron twice daily) from the fourth week of life No iron supplementation group (Group 3): no supplemental iron Further group (Group 2) not included in the systematic review: approximately 64 mg/kg/day of iron (as ferrogluconate equivalent to 29 mg of iron 4 times a day) from the fourth week of life Total n = 95: breakdown of infant numbers into study groups is not given | |
| Outcomes | Primary outcome: haemoglobin, MCH Timing: day 1 postnatal, 3 weeks of age, 3 months and six months postnatal | |
| Notes | Infants were assigned on the basis of their order of admission. It is not clear whether the feeding method was the same between the two groups. It is stated that some of the babies in the control group were excluded because of anaemia. However, the rate of study completion was not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised |
| Allocation concealment (selection bias) | High risk | Blinding of randomisation: no (quasi‐randomised) to one of three groups |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention: not known Blinding of outcome measurement: not known |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete follow‐up: not known |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other known risk of bias |
Jansson 1979.
| Methods | Blinding of randomisation: not known Blinding of intervention: not known Complete follow‐up: yes (90.3%) Blinding of outcome measurement: not known | |
| Participants | Gestation: 35 weeks or less Birth weight: 2000 g or less Feeding: human milk until at least 2 weeks of age or a weight of 2100 g. From this point, they either continued to receive their mother's milk, or a formula containing 10 mg/L of ferrous sulphate Exclusions: initial haemoglobin level below 150 g/L or above 260 g/L; signs or haemolysis Blood transfusions: excluded | |
| Interventions | Early iron group (Group A) : 2 to 3 mg/kg/day of iron (as ferrous succinate) from 3 weeks of age (n = 15) Late iron group (Group B): 2 to 3 mg/kg/day of iron (as ferrous succinate) from 2 months of age (n = 13) Both groups received the same vitamin supplement | |
| Outcomes | Primary outcome: serum ferritin Secondary outcomes: haemoglobin concentration, reticulocyte count, platelet count Timing: 24hours, 8 to 10 weeks and 6 months of age | |
| Notes | 3 infants were subsequently excluded for not following the feeding regimen, leaving 28 infants for analysis (90.3%). It is recorded that 2 infants were partially breast fed while receiving iron‐fortified formula; which group these belonged to is not documented, nor the possible effect of this on the results taken into account. Both groups received iron‐fortified formula in addition to the intervention (early or late enteral iron supplementation) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Blinding of randomisation: not known |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention: not known Blinding of outcome measurement: not known |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes (90.3%) |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other risk of bias identified |
Lundstrom 1977.
| Methods | Blinding of randomisation: no (quasi‐randomised) Blinding of intervention: not known Complete follow‐up: yes (117/125, 93.6%), but 53 excluded from analysis Blinding of outcome measurement: not known | |
| Participants | Birth weight: 1050 g to 2000 g Feeding: all received breast milk in hospital. The continuation of breastfeeding was encouraged after discharge, but some were weaned to formula. Exclusions: if clinical course had been complicated before 2 weeks of age. Any infant requiring an exchange transfusion was excluded Transfusions: those requiring transfusion after enrolment were subsequently excluded | |
| Interventions | Iron supplementation group: ferrous sulfate 2 mg/kg/day (as ferrous sulfate) from 2 weeks of age (n = 60). Some of these were weaned to a formula containing 11 mg/L of iron (providing approximately 1.8 mg/kg/day of iron), and thus had their oral iron supplement ceased No iron supplementation group: no iron supplementation (n = 57) | |
| Outcomes | Primary outcomes: haemoglobin concentration, serum iron, total iron binding capacity, serum ferritin, reticulocyte count, platelet count Timing: 2 weeks, 1 month postnatal, then monthly until 6 months postnatal | |
| Notes | Allocation to groups was based on birth date. 7 infants were excluded for receiving blood transfusions, and one for being diagnosed with hereditary spherocytosis. 44 of the unsupplemented group were excluded from analysis as they developed anaemia requiring iron therapy. A further 9 were inadvertently started too early on iron therapy, and also excluded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised |
| Allocation concealment (selection bias) | High risk | Blinding of randomisation: no (quasi‐randomised) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention: not known Blinding of outcome measurement: not known |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up to completion: 93.6% |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other risk of bias identified |
Melhorn 1971.
| Methods | Blinding of randomisation: not known Blinding of intervention: not known Complete follow‐up: yes (79%) Blinding of outcome measurement: not known | |
| Participants | Gestation: before 40 weeks Birth weight: below 2400 g Feeding: formula Exclusions: pre‐existing haematological abnormalities, infections or surgery, SGA or LGA | |
| Interventions | Iron supplementation group: approximately 8.7 mg/kg/day of iron as 10 mg, 15 mg or 20 mg of oral iron daily, depending on birth weight (< 1500 g, 1501 g to 2000 g and 2001 g to 2400 g respectively), with (Group 4, n = 50) or without (Group 3, n = 44) the addition of vitamin E, 25 IU/day orally
No iron supplementation group: no supplement (Group 1, n = 45) or vitamin E, 25 IU/day orally (Group 2, n = 47) Vitamin E was given from the 8th to the 42nd day of life, and iron from the 15th to the 42nd day. All infants were fed a low iron formula (1.5 mg/L, or approximately 0.2 mg/kg/day of iron) while in hospital, but on discharge the babies in the iron supplementation group received a high iron formula (8.4 mg/L, or approximately 1.3 mg/kg/day of iron) |
|
| Outcomes | Primary outcomes: haemoglobin, hematocrit, reticulocyte count, platelet count, red cell fragility, serum tocopherol, serum iron Timing: day 1 postnatal, then weekly until 4 weeks, then 2 to 4 weekly until 16 weeks postnatal | |
| Notes | Infants in the groups receiving iron supplementation received an iron‐fortified formula between hospital discharge and 4 months of age (8 mg/quart or 8.4 mg/L of iron), and the others a low iron formula (1.4 mg/quart or 1.5 mg/L). As planned, infants who developed a haemoglobin concentration below 75 g/L were removed from the study. Of the 234 infants enrolled, 186 were studied. 38 infants were removed from the study, 10 because of haemoglobin levels below 75 g/L | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Blinding of randomisation: not known |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention: not known Blinding of outcome measurement: not known |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes (79%) |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other risk of bias identified |
Melnick 1988.
| Methods | Blinding of randomisation: no (author communication) Blinding of intervention: no (author communication) Complete follow‐up: not known (implied to be complete) Blinding of outcome measurement: no (author communication) | |
| Participants | 38 LBW (AGA) infants | |
| Interventions | Iron supplementation group: approximately 2.4 mg/kg/day of iron (in formula containing 15 mg/L of iron) No iron supplementation group: approximately 0.8 mg/kg/day of iron (in formula containing 3.5 mg/L of iron) Total n = 19 | |
| Outcomes | Primary outcomes: haemoglobin, serum ferritin, transferrin concentration Timing: study entry, day 15 and day 29 (postnatal age uncertain but estimated to be approximately 2 weeks, 4 weeks and 6 weeks postnatal) | |
| Notes | The number of infants in each study group is not recorded, nor is the adequacy of follow‐up. No loss to follow‐up was recorded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | High risk | Blinding of randomisation: no (author communication) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: no (author communication) Blinding of outcome measurement: no (author communication) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete follow‐up: not known (implied to be complete) |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other risk of bias identified |
Quilligan 1954.
| Methods | Blinding of randomisation: no (quasi‐randomised) Blinding of intervention: not known Complete follow‐up: no (approximately 50%) Blinding of outcome measurement: not known | |
| Participants | 38 premature infants. The mean weight and gestation of the cohort was not reported Feeding method: not known Blood transfusion policy: not stated | |
| Interventions | Iron supplementation group (Group 3): approximately 10 mg/kg/day of iron (as ferrous sulfate, 22.5 mg/day), and 40 mg of cobalt chloride daily No iron supplementation group (Group 1): no supplement (n = 12) Further group, not included in systematic review (Group 2): 75 mg of ferrous sulfate mixture daily (n = 10) (no data on this group because of loss to follow‐up) | |
| Outcomes | Primary outcomes: haemoglobin, hematocrit, red blood cell count, reticulocyte count Secondary outcome: weight Timing: weekly while the infants were inpatients, approximately every 10 days after discharge, up to 60 days postnatal | |
| Notes | Divided on a quasi‐randomised fashion (based on 'serial admissions'). It is not clear whether the groups were treated equally in all other respects; for example, the method of feeding is not documented. The authors reported a poor follow‐up rate, but did not publish exact numbers. Attendence at the follow‐up visits was variable, with as few as 5 of 12 infants in the control group presenting in day 41 to 50 and 8 of 16 in the treatment group being tested on day 51 to 60 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised |
| Allocation concealment (selection bias) | High risk | Blinding of randomisation: no (quasi‐randomised) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention: not known Blinding of outcome measurement: not known |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow‐up: no (approximately 50%) |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Unclear risk | Insufficient information |
Reedy 1952.
| Methods | Blinding of randomisation: no (quasi‐randomised) Blinding of intervention: no (no placebo) Complete follow‐up: no (20.7%) Blinding of outcome assessment: not known | |
| Participants | Gestation: premature Birth weight: 2268 g or below Feeding: breast milk initially, with most changed later to a formula Exclusions: poor clinic attendance Blood transfusion: excluded from study | |
| Interventions | Iron supplementation group (Group 1): approximately 1.3 mg/kg/day of iron until discharge, then approximately 5.4 mg/kg/day of iron, as iron mixture. The iron mixture consisted of a mixture of liver concentrate, copper sulfate, iron and ammonium citrate (elemental iron 66 mg per ounce, or approximately 2.3 mg/mL), at a dose of 15 drops (approximately 1.4 mg iron) per day as inpatients, and 1 teaspoon (approximately 11.5 mg) per day from discharge (at standard discharge weight of 2126 g) (n = 30) Control group: no supplement or placebo (n = 32) Short duration group: iron mixture for the first three months only (n = 17) | |
| Outcomes | Primary outcomes: red cell count, haemoglobin, reticulocyte count, leukocyte count, eosinophil count Secondary outcome: weight Timing: 24 to 48 hours postnatal, every 7 days while inpatients, then monthly as outpatients until 12 months postnatal | |
| Notes | Infants were assigned on the basis of their order of admission to the nursery (i.e. quasi‐randomised), with alternate infants receiving iron supplementation from the 7th day of life. 382 babies were commenced on the study; 24 discontinued because of need for blood transfusion; 10 were excluded because of poor clinic attendance; and 269 were lost to follow‐up or died. Thus, 79 infants were included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised |
| Allocation concealment (selection bias) | High risk | Blinding of randomisation: no (quasi‐randomised) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: no (no placebo) Blinding of outcome assessment: not known |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow‐up: no (20.7%) |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other risk of bias identified |
Rudolph 1981.
| Methods | Blinding of randomisation: yes (author communication) Blinding of intervention: no Complete follow‐up: no (60% at 6 weeks) Blinding of outcome measurement: yes | |
| Participants | Gestation: 32 weeks or less Birth weight: 1001 to 1600 g Feeding: formula Exclusions: haemolytic disease, clinical illness, or a hematocrit level > 0.38 on day 5 to 6 Transfusions: policy not stated | |
| Interventions | Iron supplementation group: approximately 1.9 mg/kg/day of iron (in formula containing 12 mg/L of iron) (formula A1) (n = 10) No iron supplementation group: cow's milk‐based formula containing only a trace of iron (formula A) (n = 10) Further group not analysed in systematic review: soy‐based formula containing 12 mg/L of iron (n = 10) | |
| Outcomes | Primary outcomes: haemoglobin, hematocrit, reticulocytes, hydrogen peroxide haemolysis, serum cholesterol, serum vitamin E Secondary outcomes: red cell and plasma selenium Timing: weekly until 6 weeks postnatal | |
| Notes | Assignment was at 5 to 6days of age. There was no cross‐over in groups for analysis. 30 infants were entered into the study. Of these 26 were followed up until 6 weeks of age, or hospital discharge at approximately 5 weeks of age. 18/30 (60%) were analysed at 6 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes (author communication) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: no
Blinding of outcome measurement: yes Patient care staff were not blinded; laboratory personnel were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow‐up to completion: 60% |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other risk of bias identified |
Sankar 2009.
| Methods | Blinding of randomisation: yes Blinding of intervention: no Complete follow‐up: yes (91%) Blinding of outcome measurement: yes | |
| Participants | Gestation: mean 32 weeks, all preterm Birthweight: < 1500 g (mean approximately 1200 g) Feeding: > 90% exclusively or predominantly breast fed at enrolment Exclusions: major anomalies; rhesus haemolytic disease Transfusions: permitted (restrictive policy) | |
| Interventions | Iron supplementation group (enteral colloidal iron preparation): 3 mg/kg/day (birth weight 1000 g to 1500 g) or 4 mg/kg/day (birth weight < 1000 g); preparation also contained folic acid (200 mcg/mL) and vitamin B12 (5 mcg/mL). Formula fed babies received iron‐fortified formula (13.6 mg/L), with equivalent iron amount subtracted from daily supplement Control group: no iron supplement. No placebo control. Formula fed babies received iron‐fortified formula (13.6 mg/L) | |
| Outcomes | Primary outcome: serum ferritin at 60 days Secondary outcomes (at 60 days): haemoglobin concentration; hematocrit; weight; requirement for blood transfusion; re‐hospitalisation; composite of morbidities (NEC, PVL, ROP, CNLD); sepsis/pneumonia | |
| Notes | All infants received recommended intake of vitamins including folic acid and B12 through breast milk fortification or infant formula | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generation |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: no Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up to completion: 91% |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other risk of bias identified |
Steinmacher 2007.
| Methods | Blinding of randomisation: no (author communication) Blinding of intervention: yes; but allocation became obvious due to stool colour Complete follow‐up: yes (85%) Blinding of outcome measurement: yes | |
| Participants | Gestation: no limit Birth weight: less than 1301 g Feeding: either breast fed, or received an iron‐fortified formula (12 mg/L, or approximately 1.9 mg/kg/day of iron) Exclusion criteria: major anomalies, haemolytic disease and twin‐to‐twin transfusion Blood transfusion policy: permitted, before and after entry; restrictive transfusion policy | |
| Interventions | Iron supplementation group: 2 mg/kg/day of iron as ferrous sulfate, commenced as soon as enteral feedings were tolerated (n = 105) No iron supplementation group: 2 mg/kg/day of iron as ferrous sulfate, commenced at 61 days of life (n = 99) Management of anaemia: when the hematocrit level fell below 0.30, the dose of iron was increased to 4 mg/kg/day. The policy for the administration of blood transfusions was clearly outlined | |
| Outcomes | Primary outcome: serum ferritin and incidence of iron deficiency Secondary outcomes: transferrin, transferrin saturation, serum iron, reticulocytes, hematocrit; number of transfusions required and blood volume transfused Additional outcomes at 5 years of age: clinical neurological examination; mobility assessment (Gross Motor Functioning Classification Scale (GMFCS)); motor co‐ordination (Lincoln‐Oseretzky Scale (short form) 18); cognitive function (Kaufmann Assessment Battery for Children, including MPC); visual assessment; behaviour (Child Behaviour Check List) | |
| Notes | This study was a continuation of Franz 2000, using the original randomised cohort | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generation |
| Allocation concealment (selection bias) | High risk | Blinding of randomisation: no (author communication) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention: yes; but allocation became obvious due to stool colour Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes (85%) |
| Selective reporting (reporting bias) | Unclear risk | No known selectivity |
| Other bias | Low risk | No other risk of bias identified |
ANOVA: analysis of variance AGA: appropriate for gestational age BUN: blood urea nitrogen EPO: erythropoietin FEP: free erythrocyte porphyrin Hb: haemoglobin HcT: hematocrit HMF: human milk fortifiers IU: international units IVH: intraventricular haemorrhage MCH: mean cell haemoglobin MCHC: mean cell haemoglobin concentration (or mean erythrocyte haemoglobin concentration) MCV: mean corpuscular volume NEC: necrotising enterocolitis RCC: red cell count SGA: small for gestational age
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arnon 2007 | All study subjects were included in Arnon 2009. All outcomes are included in Arnon 2009 (published and unpublished data) |
| Brozovic 1974 | Not a controlled trial of iron |
| Carnielli 1998 | Oral versus IV iron |
| Creutz 1958 | Not an RCT |
| Del Mundo 1964 | Not preterm or low birth weight infants |
| Dewey 2004 | Intervention is not iron supplementation, and 4 to 6 month old infants |
| Friel 1995 | IV iron administration |
| Friel 2003 | Study population is term infants of normal birth weight |
| Fydryk 1962 | Not a controlled trial |
| Garry 1981 | Study population is term infants of normal birth weight |
| Graeber 1977 | IV iron only |
| Greer 1988 | Not a trial of iron therapy |
| Gross 1974 | Method of allocation not stated |
| Heese 1990 | Compares IM and oral iron |
| James 1960 | IV iron administered |
| Jobert 1975 | Not an RCT |
| Kivivuori 1999 | Oral versus IM iron |
| Lindberg 1973 | Not an RCT |
| Meyer 1996 | Oral versus IV iron |
| Miller 2006 | Not an RCT |
| Naude 2000 | Two groups received same iron dose, but different oral formulations |
| Nazir 2002 | Subjects were on erythropoietin treatment |
| Nelson 1988 | Study population is term infants |
| Niccum 1953 | Not an RCT |
| Olivares 1992 | Not an RCT |
| Oski 1972 | Letter to the editor ‐ not an RCT |
| Owen 1981 | Study population is term infants |
| Picciano 1980 | Study population is term infants |
| Rothe‐Meyer 1953 | Not an RCT |
| Saarinen 1978 | Study population is term infants |
| Siep 1956 | Not an RCT |
| Singhal 2000 | Study population term babies with normal birth weight |
| Sitarz 1960 | IV iron administered |
| Tevetoglu 1958 | Not an RCT |
| Tuttle 1952 | Not an RCT |
| Victorin 1984 | Not an RCT |
IM: intramuscular IV: intravenous RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Hurgoiu 1986.
| Methods | Awaiting translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Neimann 1957.
| Methods | Awaiting translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Contributions of authors
Ryan Mills: Background, search strategy and search, data extraction and assessment of methodological quality. Initial write‐up of methods, description of studies, results and discussion.
Mark Davies: Review question, search strategy and search, data extraction and assessment of methodological quality.
Sources of support
Internal sources
Grantley Stable Neonatal Unit, Royal Women's Hospital, Brisbane, Australia.
Dept of Paediatrics and Child Health, University of Queensland, Brisbane, Australia.
Dept of Paediatrics, Logan Hospital, Australia.
External sources
No sources of support supplied
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Aggarwal 2005 {published data only}
- Aggarwal D, Sachdev HP, Nagpal J, Singh T, Mallika V. Haematological effect of iron supplementation in breast fed term low birth weight infants. Archives of Disease in Childhood 2005;90:26‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arnon 2009 {published data only}
- Arnon S, Regev RH, Bauer S, Shainkin‐Kestenbaum R, Shiff Y, Bental Y, et al. Vitamin E levels during early iron supplementation in preterm infants. American Journal of Perinatology 2009;26:387‐92. [DOI] [PubMed] [Google Scholar]
Barclay 1991 {published data only}
- Barclay SM, Aggett PJ, Lloyd DJ, Duffty P. Reduced erythrocyte superoxide dismutase activity in low birth weight infants given iron supplements. Pediatric Research 1991;29:297‐301. [DOI] [PubMed] [Google Scholar]
Berglund 2010 {published data only}
- Berglund S, Westrup B, Domellöf M. Iron supplements reduce the risk of iron deficiency anemia in marginally low birth weight infants. Pediatrics 2010;126:e874‐83. [DOI] [PubMed] [Google Scholar]
Berseth 2004 {published data only}
- Berseth CL, Aerde JE, Gross S, Stolz SI, Harris CL, Hansen JW. Growth, efficacy, and safety of feeding an iron‐fortified human milk fortifier. Pediatrics 2004;114:e699‐706. [DOI] [PubMed] [Google Scholar]
Coles 1954 {published data only}
- Coles BL, James U. The effect of cobalt and iron salts on the anaemia of prematurity. Archives of Disease in Childhood 1954;29:85‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diamond 1958 {published data only}
- Diamond EF, Gonzales FG, Pisani A. The effect of cobalt‐iron therapy on the blood picture in premature infants. Illinois Medical Journal 1958;113:154‐6. [PubMed] [Google Scholar]
Ferlin 1998 {published data only}
- Ferlin MLS, Chuan LS, Jorge SM, Vannucchi H. Early anemia of prematurity. Nutrition Research 1998;18:1161‐73. [Google Scholar]
Franz 2000 {published data only}
- Franz AR, Mihatsch WA, Sander S, Kron M, Pohlandt F. Prospective randomized trial of early versus late enteral iron supplementation in infants with a birth weight of less than 1301 grams. Pediatrics 2000;106:700‐6. [DOI] [PubMed] [Google Scholar]
Friel 2001 {published data only}
- Andrews WL, Friel JK, Simmons B, Aziz K, Kwa PG, Downton G. Effect of two levels of iron supplementation of infant formulas on developmental outcome and iron nutrition in very low birth weight infants. Pediatric Research 1998;43:255A. [Google Scholar]
- Friel JK, Andrews WL, Aziz K, Kwa PG, Lepage G, L'Abbe MR. A randomized trial of two levels of iron supplementation and developmental outcome in low birth weight infants. Journal of Pediatrics 2001;139:254‐60. [DOI] [PubMed] [Google Scholar]
Gorten 1964 {published data only}
- Gorten MK, Cross ER. Iron metabolism in premature infants: II. Prevention of iron deficiency. Journal of Pediatrics 1964;64:509‐20. [DOI] [PubMed] [Google Scholar]
Griffin 1999 {published data only}
- Griffin IJ, Cooke RJ, Reid MM, McCormick KP, Smith JS. Iron nutritional status in preterm infants fed formulas fortified with iron. Archives of Disease in Childhood Fetal and Neonatal Edition 1999;81:45‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Groh‐Wargo 1990 {published data only}
- Groh‐Wargo SL, Danish EH, Super DM. Iron therapy in the premature infant. Pediatric Research 1990;27:284A. [Google Scholar]
Gross 1985 {published data only}
- Gross SJ, Gabriel E. Vitamin E status in preterm infants fed human milk or infant formula. Journal of Pediatrics 1985;106:635‐9. [DOI] [PubMed] [Google Scholar]
Hall 1993 {published data only}
- Hall RT, Wheeler RE, Benson J, Harris G, Rippetoe L. Feeding iron‐fortified premature formula during initial hospitalization to infants less than 1800 grams birth weight. Pediatrics 1993;92:409‐14. [PubMed] [Google Scholar]
Halliday 1983 {published data only}
- Halliday HL, Lappin TR, McClure BG. Do all pre‐term infants need iron supplements?. Irish Medical Journal 1983;76:430‐2. [PubMed] [Google Scholar]
Hanninen 1961 {published data only}
- Hanninen P, Peltonen T, Salmi T. On the use of iron in the treatment of premature infants. Kinderarztliche Praxis 1961;29:521‐4. [PubMed] [Google Scholar]
Jansson 1979 {published data only}
- Jansson L, Holmberg L, Ekman R. Medicinal iron to low birth weight infants. Acta Paediatrica Scandinavica 1979;68:705‐8. [DOI] [PubMed] [Google Scholar]
Lundstrom 1977 {published data only}
- Lundstrom U, Siimes MA, Dallman PR. At what age does iron supplementation become necessary in low‐birth‐weight infants?. Journal of Pediatrics 1977;91:878‐83. [DOI] [PubMed] [Google Scholar]
Melhorn 1971 {published data only}
- Melhorn DK, Gross S. Vitamin E‐dependent anemia in the premature infant. I. Effects of large doses of medicinal iron. Journal of Pediatrics 1971;79:569‐80. [DOI] [PubMed] [Google Scholar]
Melnick 1988 {published data only}
- Melnick G, Crouch JB, Caksackkas HL, Churella HR. Iron status of low‐birth‐weight infants fed formulas containing high or low iron content. Pediatric Research 1988;23:488A. [Google Scholar]
Quilligan 1954 {published data only}
- Quilligan JJ Jr. Effect of a cobalt‐iron mixture on the anemia of prematurity. Texas State Journal of Medicine 1954;50:294‐6. [PubMed] [Google Scholar]
Reedy 1952 {published data only}
- Reedy ME, Schwartz SO, Plattner EB. Anemia of the premature infant: a two‐year study of the response to iron medication. Journal of Pediatrics 1952;41:25‐39. [DOI] [PubMed] [Google Scholar]
Rudolph 1981 {published data only}
- Rudolph N, Preis O, Bitzos E, Reale MM, Wong SL. Hematologic and selenium status of low‐birth‐weight infants fed formulas with and without iron. Journal of Pediatrics 1981;99:57‐62. [DOI] [PubMed] [Google Scholar]
Sankar 2009 {published data only}
- Sankar MJ, Saxena R, Mani K, Agarwal R, Deorari AK, Paul VK. Early iron supplementation in very low birth weight infants ‐ a randomized controlled trial. Acta Paediatrica 2009;98:953‐8. [DOI] [PubMed] [Google Scholar]
Steinmacher 2007 {published data only}
- Steinmacher J, Pohlandt F, Bode H, Sander S, Kron M, Franz AR. Randomized trial of early versus late enteral iron supplementation in infants with a birth weight of less than 1301 grams: neurocognitive development at 5.3 years' corrected age. Pediatrics 2007;120:538‐46. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arnon 2007 {published data only}
- Arnon S, Shiff Y, Litmanovitz I, Regev RH, Bauer S, Shainkin‐Kestenbaum R, et al. The efficacy and safety of early supplementation of iron polymaltose complex in preterm infants. American Journal of Perinatology 2007;24:95‐100. [DOI] [PubMed] [Google Scholar]
Brozovic 1974 {published data only}
- Brozovic B, Burland WL, Simpson K, Lord J. Iron status of preterm low birthweight infants and their response to oral iron. Archives of Disease in Childhood 1974;49:386‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carnielli 1998 {published data only}
- Carnielli VP, Riol R, Montini G. Iron supplementation enhances response to high doses of recombinant human erythropoietin in preterm infants. Archives of Disease in Childhood Fetal and Neonatal Edition 1998;79:F44‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Creutz 1958 {published data only}
- Creutz E, Steinhoff A. Prevention of anemia in the newborn with cobalt‐ferrlecit. Kinderarztliche Praxis 1958;26:17‐20. [PubMed] [Google Scholar]
Del Mundo 1964 {published data only}
- Mundo F, Adiao AC, Sta. Ana P. A study of iron depletion prophylaxis by the use of iron‐containing milk formulas. Journal of the Philippine Medical Association 1964;40:779‐86. [PubMed] [Google Scholar]
Dewey 2004 {published data only}
- Dewey KG, Cohen RJ, Brown KH. Exclusive breast‐feeding for 6 months, with iron supplementation, maintains adequate micronutrient status among term, low‐birthweight, breast‐fed infants in Honduras. Journal of Nutrition 2004;134:1091‐8. [DOI] [PubMed] [Google Scholar]
Friel 1995 {published data only}
- Friel JK, Andrews WL, Hall MS, Rodway MS, McCloy UC, Matthew JD, et al. Intravenous iron administration to very‐low‐birth‐weight newborns receiving total and partial parenteral nutrition. Journal of Parenteral and Enteral Nutrition 1995;19:114‐8. [DOI] [PubMed] [Google Scholar]
Friel 2003 {published data only}
- Friel JK, Aziz K, Andrews WL, Harding SV, Courage ML, Adams RJ. A double‐masked, randomized control trial of iron supplementation in early infancy in healthy term breast‐fed infants. Journal of Pediatrics 2003;143:582‐6. [DOI] [PubMed] [Google Scholar]
Fydryk 1962 {published data only}
- Fydryk J, Lacki L, Cieslak E. Treatment of hypochromic anemia in underweight infants with oral iron preparations. Pediatria Polska 1962;37:919‐26. [PubMed] [Google Scholar]
Garry 1981 {published data only}
- Garry PJ, Owen GM, Hooper EM, Gilbert BA. Iron absorption from human milk and formula with and without iron supplementation. Pediatric Research 1981;15:822‐8. [PubMed] [Google Scholar]
Graeber 1977 {published data only}
- Graeber JE, Williams ML, Oski FA. The use of intramuscular vitamin E in the premature infant. Optimum dose and iron interaction. Journal of Pediatrics 1977;90:282‐4. [DOI] [PubMed] [Google Scholar]
Greer 1988 {published data only}
- Greer FR, McCormick A. Growth and calcium metabolism in premature infants fed varying amounts of protein and minerals during the first year of life. Pediatric Research 1988;23:492A. [Google Scholar]
Gross 1974 {published data only}
- Gross S, Melhorn DK. Vitamin E‐dependent anemia in the premature infant. Journal of Pediatrics 1974;85:753‐9. [DOI] [PubMed] [Google Scholar]
Heese 1990 {published data only}
- Heese HD, Smith S, Watermeyer S, Dempster WS, Jakubiec L. Prevention of iron deficiency in preterm neonates during infancy. South African Medical Journal 1990;77:339‐45. [PubMed] [Google Scholar]
James 1960 {published data only}
- James JA, Combes M. Iron deficiency in the premature infant: Significance, and prevention by the intramuscular administration of iron‐dextran. Pediatrics 1960;26:368‐74. [PubMed] [Google Scholar]
Jobert 1975 {published data only}
- Jobert J, Bachelot C, Jerome JM, Sele M, Bost M. Trial treatment of early anemia in premature infants. Pediatrie 1975;30:809‐20. [PubMed] [Google Scholar]
Kivivuori 1999 {published data only}
- Kivivuori SM, Virtanen M, Raivio KO, Viinikka L, Siimes MA. Oral iron is sufficient for erythropoietin treatment of very low birth‐weight infants. European Journal of Pediatrics 1999;158:147‐51. [DOI] [PubMed] [Google Scholar]
Lindberg 1973 {published data only}
- Lindberg T. Supply of vitamins and iron to premature babies. Lakartidningen 1973;70:3260‐2. [PubMed] [Google Scholar]
Meyer 1996 {published data only}
- Meyer MP, Haworth C, Meyer JH, Commerford A. A comparison of oral and intravenous iron supplementation in preterm infants receiving recombinant erythropoietin. Journal of Pediatrics 1996;129:258‐63. [DOI] [PubMed] [Google Scholar]
Miller 2006 {published data only}
- Miller SM, McPherson RJ, Juul SE. Iron sulfate supplementation decreases zinc protoporphyrin to heme ratio in premature infants. Journal of Pediatrics 2006;148:44‐8. [DOI] [PubMed] [Google Scholar]
Naude 2000 {published data only}
- Naude S, Clijsen S, Naulaers G, Daniels H, Vanhole C, Devlieger H. Iron supplementation in preterm infants: a study comparing the effect and tolerance of a Fe2+ and a nonionic FeIII compound. Journal of Clinical Pharmacology 2000;40:1447‐51. [PubMed] [Google Scholar]
Nazir 2002 {published data only}
- Nazir S, Peverini RL, Derning DD, Hopper AO, Vyhmeister NR. Comparison of 2 iron doses in infants receiving recombinant human erythropoietin therapy. Archives of Pediatrics and Adolescent Medicine 2002;156:540‐4. [DOI] [PubMed] [Google Scholar]
Nelson 1988 {published data only}
- Nelson SE, Ziegler EE, Copeland AM, Edwards BB, Fomon SJ. Lack of adverse reactions to iron‐fortified formula. Pediatrics 1988;81:360‐4. [PubMed] [Google Scholar]
Niccum 1953 {published data only}
- Niccum WL, Jackson RL, Stearns G. Use of ferric and ferrous iron in the prevention of hypochromic anemia in infants. American Journal of Diseases in Children 1953;86:553‐67. [DOI] [PubMed] [Google Scholar]
Olivares 1992 {published data only}
- Olivares M, Llaguno S, Marin V, Hertrampf E, Mena P, Milad M. Iron status in low‐birth‐weight infants, small and appropriate for gestational age. Acta Paediatrica 1992;81:824‐8. [DOI] [PubMed] [Google Scholar]
Oski 1972 {published data only}
- Oski FA. Iron‐fortified formulas in infancy: best form of iron supplementation?. Journal of Pediatrics 1972;80:524‐5. [DOI] [PubMed] [Google Scholar]
Owen 1981 {published data only}
- Owen GM, Garry PJ, Hooper EM, Gilbert BA, Pathak D. Iron nutriture of infants exclusively breast‐fed the first five months. Journal of Pediatrics 1981;99:237‐40. [DOI] [PubMed] [Google Scholar]
Picciano 1980 {published data only}
- Picciano MF, Deering RH. The influence of feeding regimens on iron status during infancy. American Journal of Clinical Nutrition 1980;33:746‐53. [DOI] [PubMed] [Google Scholar]
Rothe‐Meyer 1953 {published data only}
- Rothe‐Meyer A, Kreutzfeldt H. Iron therapy for the premature infant. Ugeskrift for Laeger 1953;115:331‐3. [PubMed] [Google Scholar]
Saarinen 1978 {published data only}
- Saarinen UM. Need for iron supplementation in infants on prolonged breast feeding. Journal of Pediatrics 1978;93:177‐80. [DOI] [PubMed] [Google Scholar]
Siep 1956 {published data only}
- Halvorsen S, Seip M. Erythrocyte production and iron stores in premature infants during the first months of life; the anemia of prematurity‐etiology, pathogenesis, iron requirement. Acta Paediatrica 1956;45:600‐17. [DOI] [PubMed] [Google Scholar]
Singhal 2000 {published data only}
- Singhal A, Morley R, Abbott R, Fairweather‐Tait S, Stephenson T, Lucas A. Clinical safety of iron‐fortified formulas. Pediatrics 2000;105:E38. [DOI] [PubMed] [Google Scholar]
Sitarz 1960 {published data only}
- Sitarz AL, Wolff JA, Hofe FH. Comparison of oral and intramuscular administration of iron for prevention of the late anemia of premature infants. Pediatrics 1960;26:375‐86. [Google Scholar]
Tevetoglu 1958 {published data only}
- Tevetoglu F, Ozkaragoz K. Cobalt‐iron therapy in the treatment and prevention of the anemia of prematurity. Medical Times 1958;86:81‐7. [PubMed] [Google Scholar]
Tuttle 1952 {published data only}
- Tuttle AH, Etteldorf JN. Molybdenized ferrous sulfate (mol‐iron) in the treatment of the anemia of premature infants. Journal of Pediatrics 1952;41:170‐5. [DOI] [PubMed] [Google Scholar]
Victorin 1984 {published data only}
- Victorin LH, Olegard R. Iron in the preterm infant: a pilot study comparing Fe2+ and Fe3+ tolerance and effect. Journal of Pediatrics 1984;105:151‐2. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Hurgoiu 1986 {published data only}
- Hurgoiu V, Rub‐Saidac A, Filipas V, Marcu A, Adam M. Test of maternal milk supplemented with iron in the feeding of premature infants. Revista de Pediatrie, Obstetrica Si Ginecologie Pediatria 1986;35:91‐5. [PubMed] [Google Scholar]
Neimann 1957 {published data only}
- Neimann N, Vezeaux De Lavergne E, Pierson M, Stehlin S, Daubinet G. Iron metabolism in infants and the problem of physiological hypochromic anemia in infancy, results of iron therapy. Semaine des Hopitaux 1957;33:4007‐18P. [PubMed] [Google Scholar]
Additional references
AAP 1985
- American Academy of Pediatrics Committee on Nutrition. Nutritional needs of low‐birth‐weight infants. Pediatrics 1985;75:976‐86. [PubMed] [Google Scholar]
Baker 2010
- Baker RD, Greer FR, Committee on Nutrition American Academy of Pediatrics. Diagnosis and prevention of iron deficiency and iron‐deficiency anemia in infants and young children (0‐3 years of age). Pediatrics 2010;126:1040‐50. [DOI] [PubMed] [Google Scholar]
Brown 1996
- Brown MS. Effect of transfusion and phlebotomy on serum ferritin levels in low birth weight infants. Journal of Perinatology 1996;16:39‐42. [PubMed] [Google Scholar]
Cooke 1997
- Cooke RW, Drury JA, Yoxall CW, James C. Blood transfusion and chronic lung disease in preterm infants. European Journal of Pediatrics 1997;156:47‐50. [DOI] [PubMed] [Google Scholar]
Dani 2001
- Dani C, Reali MF, Bertini G, Martelli E, Pezzati M, Rubaltelli FF. The role of blood transfusions and iron intake on retinopathy of prematurity. Early Human Development 2001;62:57‐63. [DOI] [PubMed] [Google Scholar]
Doyle 1992
- Doyle JJ, Zipursky A. Neonatal blood disorders. In: Sinclair JC, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992:425. [Google Scholar]
Hesse 1997
- Hesse L, Eberl W, Schlaud M, Poets CF. Blood transfusion. Iron load and retinopathy of prematurity. European Journal of Pediatrics 1997;156:465‐70. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hyams 1995
- Hyams JS, Treem WR, Etienne NL, Weinerman H, MacGilpin D, Hine P, et al. Effect of infant formula on stool characteristics of young infants. Pediatrics 1995;95:50‐4. [PubMed] [Google Scholar]
Inder 1997
- Inder TE, Clemett RS, Austin NC, Graham P, Darlow BA. High iron status in very low birth weight infants is associated with an increased risk of retinopathy of prematurity. Journal of Pediatrics 1997;131:541‐4. [DOI] [PubMed] [Google Scholar]
Lozoff 1987
- Lozoff B, Brittenham GM, Wolf AW, McClish DK, Kuhnert PM, Jimenez E, et al. Iron deficiency anaemia and iron therapy effects on infant developmental test performance. Pediatrics 1987;79:981‐95. [PubMed] [Google Scholar]
McGuire 2003
- McGuire W, Anthony MY. Donor human milk versus formula for preventing necrotising enterocolitis in preterm infants: systematic review. Archives of Disease in Childhood Fetal & Neonatal Edition 2003;88:F11‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ng 2001
- Ng PC, Lam CW, Lee CH, To KF, Fok TF, Chan IH, et al. Hepatic iron storage in very low birthweight infants after multiple blood transfusions. Archives of Disease in Childhood Fetal & Neonatal Edition 2001;84:F101‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 2002
- Rao R, Georgieff RR. Perinatal aspects of iron metabolism. Acta Paediatrica Suppl 2002;91:124‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- Copenhagen: The Nordic Cochrane Centre. Review Manager (RevMan). Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011, Version 5.1.
Shaw 1982
- Shaw JC. Iron absorption by the premature infant. The effect of transfusion and iron supplements on the serum ferritin levels. Acta Paediatrica Scandinavica Suppl 1982;299:83‐9. [DOI] [PubMed] [Google Scholar]
Walter 1989
- Walter T, Andraca I, Chadud P, Perales CG. Iron deficiency anemia: adverse effects on infant psychomotor development. Pediatrics 1989;84:7‐17. [PubMed] [Google Scholar]


