Abstract
AIM
To observe the changes in corneal subepithelial nerve fibers (CNFs) and Langerhans cells (LCs) in patients with type 2 diabetes using corneal laser confocal microscopy (CLCM).
METHODS
A total of 60 patients (64 eyes), including 40 patients with type 2 diabetes (DM group) and 20 subjects without diabetes (control group) were included with CLCM. Neuron J plugin of Image J software were used for quantitative analysis of CNF length (CNFL), CNF density (CNFD), corneal nerve branch fiber density (CNBD), main branch length density, branch length density, corneal nerve fiber tortuosity (NT) score, and LCs density. An independent samples t-test to analyze the variability between the two groups was performed, and Pearson correlation analysis was used to analyze the relationships between CNF and multiple biochemical indicators in the DM group. The predictive power of CNF for type 2 diabetes was assessed using the receiver operating characteristic (ROC) curve.
RESULTS
There were significant differences in the CNFL, CNFD, and main branch length density between two groups. The results of Pearson correlation analysis showed a significant negative correlation between CNFD and the duration of diabetes as well as triglyceride levels and total cholesterol, and a significant positive correlation between CNFD and serum albumin. In addition, the NT score showed a positive correlation and urea nitrogen, similar to the positive correlation observed between LC density and glycosylated hemoglobin (HbA1c) levels. CNFD showed the highest area under the curve (AUC of ROC) value, followed by main branch length density and CNFL. The AUC of the ROC curve under the logistic regression model also demonstrated good predictive values. The cut-off values of CNFD, CNFL, and main branch length density for diabetes showed 31.25, 18.85, and 12.56, respectively.
CONCLUSION
In patients with type 2 diabetes, there is a notable reduction in both CNFL and CNFD. These measurements can be influenced by various blood biochemical factors. However, the compromised nerve fibers can serve as valuable indicators for predicting the onset of type 2 diabetes and also as biomarkers for detecting diabetic neuropathy and its related complications.
Keywords: corneal subepithelial nerve, diabetes, glycated hemoglobin, Langerhans cells, diabetic neuropathy
INTRODUCTION
According to the statistics from the International Diabetes Federation (IDF) in 2017, the global prevalence of diabetes in the age group of 20 to 79 was 8.8%, with an estimated over 640 million people worldwide affected by diabetes by 2040[1]–[2]. Diabetes, as a systemic disease with multi-organ involvement, not only delays corneal wound healing, increases the risk of infection, and causes diabetic retinopathy in the retina, but also raises concerns regarding corneal nerve damage. In recent years, studies have found that corneal nerve alterations in diabetes can serve as a new potential indicator for early detection of diabetic neuropathy. Corneal laser confocal microscopy (CLCM) plays a crucial role in assisting the diagnosis of various corneal conditions such as fungal keratitis[3], acanthamoeba keratitis[4], keratoconus[5] and endothelial dystrophy[6]. It has also gained increasing importance in the evaluation of corneal nerve and dry eye disease. In this study, we observed corneal subepithelial nerve fibers (CNFs) and Langerhans cells (LCs) in patients with type 2 diabetes. The Pearson correlation between subepithelial nerve fibers and biochemical indicators was conducted. We further emphasized the strong correlation between corneal nerve fibers and type 2 diabetes by utilizing receiver operating characteristic (ROC) curves. We validated the potential role of corneal nerve fibers as biomarkers for diabetic neuropathy.
SUBJECTS AND METHODS
Ethical Approval
The study was approved by the Ethics Committee of the Chinese PLA General Hospital (Approval No.S2023-199-01). All image data is carefully evaluated anonymously to ensure the protection of patients' personal privacy.
Subjects
This study included 60 age-related cataract patients (64 eyes) scheduled for phacoemulsification cataract surgery at the ophthalmology outpatient clinic of our hospital from May 2023 to July 2023.
Inclusion criteria: All patients in the diabetes group met the diagnostic criteria for type 2 diabetes[7], and all patients had good cognitive ability and compliance. Exclusion criteria: 1) glaucoma, corneal inflammation, conjunctivitis, scleritis, blepharitis; 2) uveitis, history of ocular trauma; 3) lacrimal duct disease, lacrimal gland diseases; 4) high myopia, history of corneal contact lens wear; 5) long-term oral steroid use, systemic connective tissue disease, immune-related diseases; 6) history of diabetic retinopathy laser treatment; 7) diseases of the entire nervous system, such as stroke, Parkinson's disease, multiple system atrophy, etc.; 8) malignant tumors, nephritis, or other secondary renal failure.
Observation Indicators
A venous blood sample of 2 mL was collected from all patients to assess the levels of biochemical indicators, including fasting blood glucose, glycosylated hemoglobin (HbA1c), triglycerides, total cholesterol, renal function (creatinine, blood urea nitrogen), uric acid and serum albumin (ALB).
All patients underwent CLCM using the HRT III confocal microscope (Heidelberg, Germany) with a laser wavelength of 670 nm. The examination included imaging of the entire corneal depth with an average magnification of 800 times and a theoretical resolution of 1 µm. A clear and high-resolution image of a 400 µm×400 µm area was successfully obtained. Before the examination, 0.4% oxybuprocaine hydrochloride eye drops (Sanxin Pharmaceutical, Japan) were instilled twice to ensure effective surface anesthesia of the eye being examined. Next, 0.2% Carbomer eye drops (Bausch & Lomb, Germany) was applied to the corneal microscope surface, followed by placing a sterile corneal contact cap. The patient's lower jaw and forehead were fixed on a holder, and they were instructed to fixate on a target light. The laser scanning camera position was adjusted to align the laser beam with the corneal pupil area. The camera was gradually advanced, maintaining a distance of approximately 5-10 mm from the contact cap. It was then carefully fine-tuned to align the central cornea cap with the reflected laser beam spot in the pupil area. The camera was gently advanced until it made slight contact with the cap. The focal plane was set to 0 for the contact between the cap and the cornea, and the focal plane was precisely adjusted by turning the laser scanning camera adjustment ring, allowing for the acquisition of corneal images at various depths. Over 100 images were captured for each patient and securely stored. Three high-quality images of the subepithelial corneal nerve fibers were successfully obtained for each eye, and all procedures were performed by the same skilled and experienced ophthalmic technician. At the same time, the grouping information of all participants has been kept confidential from the technician.
Image Analysis
Three clear images of the CNF layer were selected for each eye. Neuron J plugin of Image J software were used to trace the nerve fiber morphology (Add tracings) and calculate the total length of all main and branch fibers (Measure tracings). CNF length (CNFL), CNF density (CNFD), corneal nerve branch fiber density (CNBD), nerve fiber tortuosity (NT), and the number of LCs were observed and analyzed. The above operations were repeated for three images, and the results were averaged. CNFL (mm/mm2) represents the total length of nerve fiber trunks and branches within a unit area. CNFD (n/mm2) represents the number of nerve fiber trunks within a unit area. CNBD (n/mm2) represents the number of nerve fiber branches within a unit area. NT score: 0 points indicate nerve fibers that are mostly straight; 1 point indicates slightly curved nerve fibers; 2 points indicate moderately curved nerve fibers with frequent directional changes, but all changes are small; 3 points indicate significantly curved nerve fibers with larger changes in fiber direction; 4 points indicate highly curved nerve fibers with sudden and frequent changes in fiber direction. LCs: the number of dendritic cells was counted in each image.
Statistical Methods
Statistical analysis was conducted using the SPSS AU online software (https://spssau.com). The data are presented as mean±standard deviation (SD). After testing for normality, independent sample t-tests were used for comparisons between the two groups. Pearson's correlation analysis was performed to assess the correlation between corneal subepithelial nerve parameters with various biochemical indicators in patients with diabetes and a correlation heatmap corresponding was plotted. The predictive power of corneal nerve fiber parameters for type 2 diabetes was evaluated using the ROC curves analysis. A P-value <0.05 was considered statistically significant.
RESULTS
A total of 60 patients (64 eyes), with 40 patients (43 eyes) in the type 2 diabetes group (DM group) including 22 males and 18 females, with an age range of 44 to 85 years (mean age 67.28±9.84y), and 20 patients (21 eyes) in the non-diabetic (control group) including 7 males and 13 females, with an age range of 50 to 82 years (mean age 68.33±8.61y). Among the diabetic patients included in the study, 7 diabetic patients controlled their blood sugar through exercise and dietary therapy, 20 patients took only oral hypoglycemic drugs, 3 patients received only subcutaneous insulin injections, and 8 patients took both oral hypoglycemic drugs and subcutaneous insulin injections. All patients had stable blood sugar control in 1mo before the surgery. There were no statistically significant differences in terms of age (t=-0.85, P=0.40) or gender (χ2=0.78, P=0.38) between the DM group and the control group.
When comparing various parameters of nerve fibers between the DM group and the control group, the DM group showed a series of significant declines in CNFL (16.60±5.14 mm/mm2) compared to the control group (19.65±3.32 mm/mm2; t=-2.48, P=0.016), CNFD (34.01±10.14 n/mm2) compared to the control group (44.05±10.91 n/mm2; t=-3.63, P=0.001), and main branch length density (11.92±3.45 n/mm2) compared to the control group (15.00±3.33 n/mm2; t=-3.39, P=0.001; Figure 1 and Table 1). There was no statistically significant difference in CNBD, branch length density, NT score and LCs density between the two groups.
Figure 1. CNF images of patients in the DM group and the control group observed under CLCM and traced using Neuron J plugin of Image J software.
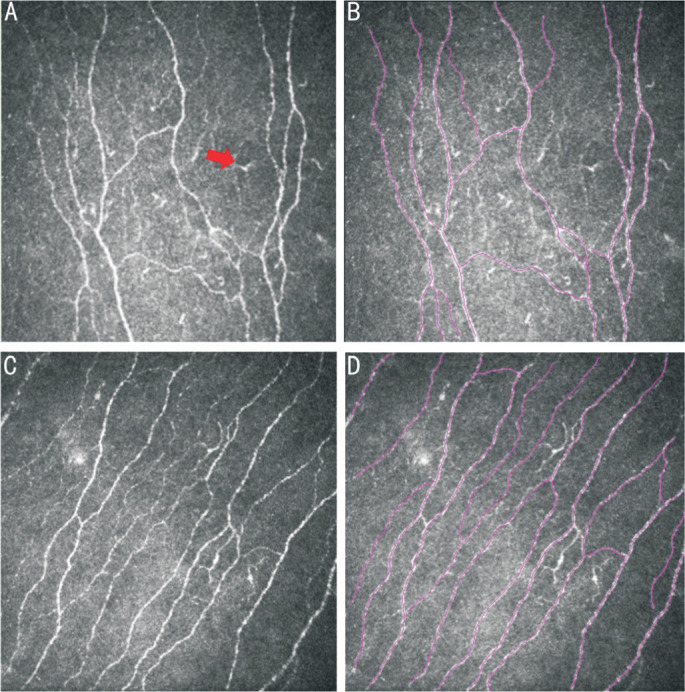
A: CNF images of patients in the DM group observed under CLCM showed lower CNF density, irregular and tortuous morphology, and abundant LC cells (the red arrow pointing to the increased and matured LCs under corneal epithelium in diabetes). B: Analysis and tracing of CNF in patients from the DM group observed under CLCM using Neuron J plugin. C: CNF images of patients in the control group observed under CLCM showed higher CNF density, straighter morphology, and fewer LC cells. D: Analysis and tracing of CNF in patients from the control group observed under CLCM using Neuron J plugin (Purple lines indicate the automatically recognized fiber morphology by Neuron). CNF: Corneal nerve fiber; DM: Diabetes mellitus; CLCM: Corneal laser confocal microscopy; LC: Langerhans cell.
Table 1. Comparison of corneal nerve fiber parameters between the DM group and the control group.
| Parameters | Diabetes group (n=43) | Control group (n=21) | t | P |
| CNFL (mm/mm2) | 16.60±5.14 | 19.65±3.32 | -2.48 | 0.016a |
| CNFD (n/mm2) | 34.01±10.14 | 44.05±10.91 | -3.63 | 0.001b |
| CNBD (n/mm2) | 34.88±22.04 | 38.39±18.68 | -0.63 | 0.533 |
| Main branch length density (mm/mm2) | 11.92±3.45 | 15.00±3.33 | -3.39 | 0.001b |
| Branch length density (mm/mm2) | 4.68±3.00 | 4.65±1.78 | 0.04 | 0.966 |
| NT score | 1.77±0.90 | 1.76±1.04 | 0.022 | 0.983 |
| LCs density (n/frame) | 2.95±3.02 | 1.67±1.59 | 1.83 | 0.07 |
aP<0.05; bP<0.01. SD: Standard deviation; CNFL: Corneal nerve fiber length; CNFD: Corneal nerve fiber density; CNBD: Corneal nerve branch fiber density; NT: Nerve fiber tortuosity; LC: Langerhans cell; DM: Diabetes mellitus.
mean±SD
Pearson correlation analysis revealed a significant correlation between CNFD with the duration of diabetes, triglyceride levels, total cholesterol, and serum ALB (r=-0.31, P=0.043; r=-0.42, P=0.005; r=-0.31, P=0.041; r=0.31 P=0.04). Additionally, NT scores are positively correlated with urea nitrogen levels (r=0.34, P=0.026), while HbA1c showed a positive correlation with LCs density (r=0.30, P=0.048; Table 2 and Figure 2).
Table 2. Pearson correlation analysis between corneal nerve parameters and biochemical indicators in the diabetes group.
| Parameters | CNFD (n/mm2) |
CNBD (n/mm2) |
Main branch length density (mm/mm2) |
Branch length density (mm/mm2) |
CNFL (mm/mm2) |
NT score | LCs density |
| DM duration | -0.310a | -0.255 | -0.199 | -0.268 | -0.291 | 0.022 | 0.195 |
| Glucose | -0.099 | 0.012 | 0.003 | -0.218 | -0.126 | -0.047 | 0.166 |
| HbA1c | -0.115 | -0.036 | 0.032 | -0.119 | -0.048 | -0.103 | 0.304a |
| TG | -0.423b | -0.165 | -0.279 | -0.076 | -0.232 | 0.099 | -0.128 |
| TCHO | -0.313a | -0.244 | -0.155 | -0.101 | -0.163 | 0.288 | -0.086 |
| Crea | -0.122 | 0.025 | -0.157 | -0.072 | -0.147 | 0.148 | 0.112 |
| Urea nitrogen | -0.222 | 0.054 | -0.101 | 0.089 | -0.016 | 0.340a | 0.09 |
| Alb | 0.314a | 0.147 | 0.181 | 0.042 | 0.146 | 0.016 | 0.073 |
| Uric acid | -0.106 | -0.143 | 0.053 | 0.065 | 0.073 | 0.154 | 0.017 |
aP<0.05; bP<0.01. CNFD: Corneal nerve fiber density; CNBD: Corneal nerve branch fiber density; CNFL: Corneal nerve fiber length; NT: Nerve fiber tortuosity; LC: Langerhans cell; DM: Diabetes mellitus; HbA1c: Glycated hemoglobin; TG: Triglyceride; TCHO: Total cholesterol; Crea: Creatinine; Alb: Albumin.
Figure 2. Correlation heatmap between corneal nerve parameters and biochemical indicators in diabetes (red represents a positive correlation between two indicators and blue represents a negative correlation).
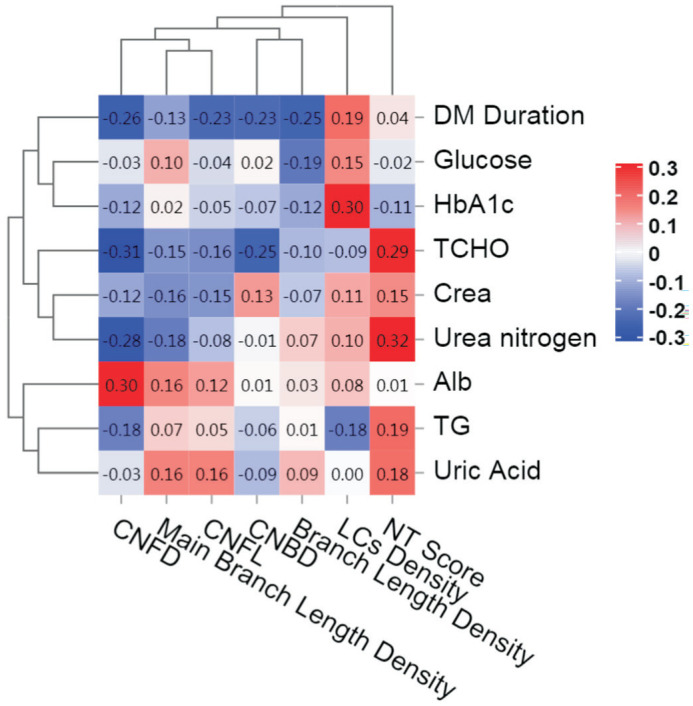
DM: Diabetes mellitus; HbA1c: Glycated hemoglobin; TCHO: Total cholesterol; Crea: Creatinine; Alb: Albumin; TG: Triglycerides.
Among all the 5 corneal nerve indices, CNFD exhibited the highest predictive value of area under the curve (AUC) for type 2 diabetes (AUC=0.758). The AUC values of main branch length density, CNFL, CNBD, and branch length density were respectively 0.726, 0.715, 0.592, and 0.533 (Figure 3A and Table 3), the former two predicted better diagnosis performance. The AUC of the ROC curve under a logistic regression model is 0.788, suggesting a relatively high predictive value for diagnosing type 2 diabetes (Figure 3B). The cut-off values of CNFD, CNFL, and main branch length density were respectively 31.25, 18.85, and 12.56 (Table 3).
Figure 3. The ROC curves of corneal nerve parameters for type 2 diabetes.
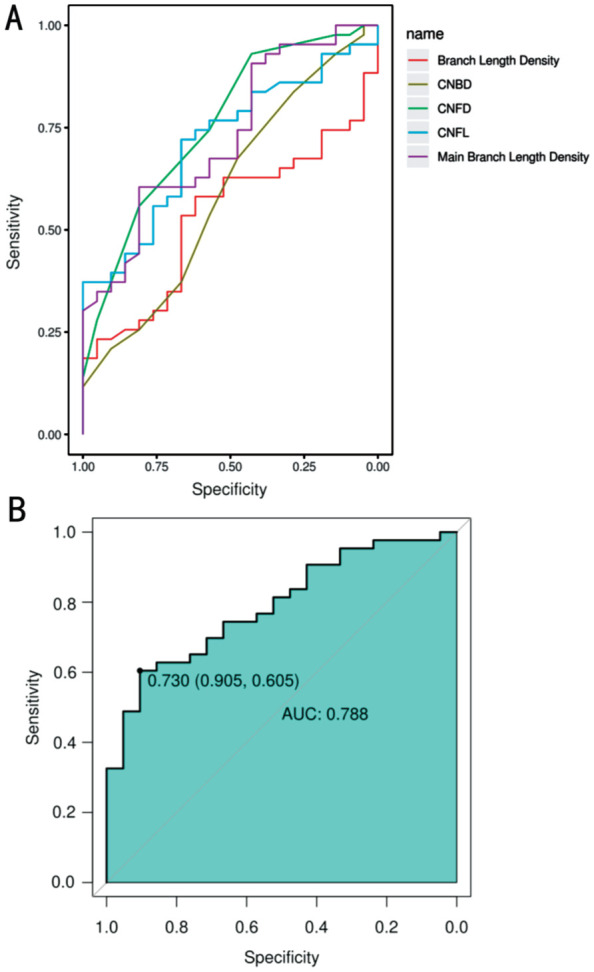
A: The ROC curves of CNFD, CNBD, CNFL, main branch length density, and branch length density for type 2 diabetes; B: The ROC curve under a logistic regression model for type 2 diabetes (the ROC curve for CNFD showed the highest AUC value, followed by main branch length density and CNFL). ROC: Receiver operating characteristic; CNFD: Corneal nerve fiber density; CNBD: Corneal nerve fiber branch density; CNFL: Corneal nerve fiber length; AUC: Area under the curve.
Table 3. The optimal threshold for the ROC curves.
| Parameters | AUC (95%CI) | Optimal threshold | Sensitivity | Specificity | Cut-off |
| CNFD (n/mm2) | 0.758 (0.634-0.882) | 0.368 | 0.810 | 0.558 | 31.250 |
| CNBD (n/mm2) | 0.592 (0.443-0.741) | 0.151 | 0.476 | 0.674 | 37.500 |
| CNFL (mm/mm2) | 0.715 (0.589-0.841) | 0.388 | 0.667 | 0.721 | 18.850 |
| Main branch length density (mm/mm2) | 0.726 (0.599-0.854) | 0.414 | 0.810 | 0.605 | 12.560 |
| Branch length density (mm/mm2) | 0.533 (0.391-0.675) | 0.202 | 0.667 | 0.535 | 4.060 |
ROC: Receiver operating characteristic; AUC: Area under the curve; CNFD: Corneal nerve fiber density; CNBD: Corneal nerve fiber branch density; CNFL: Corneal nerve fiber length; CI: Confidence interval.
DISCUSSION
Diabetes not only poses risks to blood vessels and nerves, but it can also have detrimental effects on the eyes. In addition to diabetic retinopathy, diabetes can also cause damage to the corneal nerves, leading to decreased corneal sensitivity, dry eye syndrome, and even neurotrophic keratitis[8]–[9]. Consistent with other studies[10]–[13], our experiment demonstrated significant reductions in various parameters of corneal nerve fibers among patients with diabetes, these included nerve fiber length, nerve fiber density, and main branch length density. Diabetic patients often exhibit adverse associations between corneal nerve parameters and both the duration of diabetes and HbA1c levels[14]–[15]. Long-term blood glucose control, as reflected by HbA1c levels, can have a detrimental impact on the cornea, leading to the activation of the corneal sub-basal LCs and an increase in nerve tortuosity scores[16]. Our analysis revealed a significant negative correlation between the duration of diabetes and CNFD, as well as a positive correlation between HbA1c levels[17] and LCs[10],[16],[18]–[19] (Table 2), which is consistent with previous research findings. Importantly, our study revealed that corneal nerve fibers have shown promising predictive value for type 2 diabetes in the ROC curve analysis. In summary, subepithelial nerve fibers in the cornea play a crucial role in facilitating early diagnosis and monitoring of complications in type 2 diabetes. Therefore, we believe that corneal nerve fiber parameters hold significant potential as a biomarker for diabetic neuropathy.
Furthermore, total cholesterol, low-density lipoprotein cholesterol, triglycerides[1],[20]–[22] and renal function impairment[23]–[24] have been identified as risk factors for corneal nerve fibers, which is consistent with our findings. Recently, some scholars have proposed that targeting hypertriglyceridemia could be a potential strategy for treating nerve damage[21],[25], as elevated triglycerides can induce oxidative and nitrosative stress, leading to nerve damage[26]. What is particularly noteworthy is that we have identified a novel blood marker, serum ALB, exhibits a positive correlation with CNFD. ALB, a small molecule weight, non-glycosylated serum protein, serves as an indicator of the body's nutritional status and plays a vital role in maintaining plasma osmotic pressure, substance binding and transformation, antioxidant functions, and neuroprotective effects[27]–[28]. Previous research has indicated that ALB plays a beneficial role in preserving frozen nerves and the addition of ALB in the calcein-AM and 4′,6-diamidino-2-phenylindole (CAM-DAPI) staining has been found to significantly enhance the vitality of Schwann cells[12], which are known for protective and nourishing effects on neurons. The potential mechanisms underlying this association may align with our findings and require further exploration.
Recently, in the research of corneal nerve evaluation of diabetes neuropathy, scholars combine functional evaluation with structural evaluation[29]. Researchers from different studies emphasized that distal peripheral neuropathy in diabetes patients showed decreased corneal sensitivity and corneal neuropathy[30]. The corneal nerve plays an important role in providing corneal protection by regulating tear production and eyelid closure, stimulating corneal collagen expression, and protecting epithelial cell function[31]. Patients with diabetes corneal neuropathy usually have corneal hypoesthesia, eye irritation or pain[32], and reduced corneal nerve density and corneal sensitivity can lead to defects in the healing process of corneal epithelial wounds and lead to persistent epithelial defects[33]. In our study, we have emphasized the comparison of differences in CNFs between diabetic and non-diabetic patients. This result not only highlights the damage of corneal nerve fibers by diabetes through objective data but also underscores the promoting effect of diabetes on corneal lesions. Certainly, we also aim to conduct a more in-depth and specific analysis of the clinical signs and manifestations of corneal lesions in diabetic patients in the future.
In conclusion, corneal nerve damage and LC activation in patients with diabetes can be identified by CLCM[34]–[35], representing early keratopathy and neuropathy in diabetes. Subepithelial nerve fibers in the cornea have the potential to be valuable biomarkers for diabetic neuropathy, offering a new avenue for early diagnosis and treatment of diabetic keratopathies, such as reduced corneal sensation, ocular surface irritation, dry eye syndrome, and delayed corneal epithelial healing. However, it is important to note that this study has certain limitations, which may have contributed to the absence of significant findings. The sample size of our study was predominantly composed of older individuals, which may limit the generalizability of our findings to the broader population. Meanwhile, early research has already reported a potential link between corneal nerve damage in elderly individuals and dementia[36]–[37]. Therefore, it would be more remarkable clinical and research value to explore this branch of the study with a larger more defined research, stratifying patients by age and duration of diabetes. In any case, we are well aware of the negative impact of diabetes on the cornea. Corneal nerve fibers can serve as early predictors of diabetic keratopathy and also as biomarkers for diabetic neuropathy. They play a crucial role in the early diagnosis and treatment of diabetic keratopathy and diabetic neuropathy, providing important auxiliary value. Predicting corneal and nerve damage through corneal nerve fibers, can improve the early diagnosis rate of diabetic complications and assist in early clinical intervention and treatment. This can also provide valuable guidance for diabetic patients to understand their blood sugar control status and improve blood sugar control targets. By timely adjusting hypoglycemic treatment plans in the endocrinology department and providing nutritional repair of the cornea through local medications in the ophthalmology clinic, it can ultimately enhance the overall quality of life and prognosis for diabetic patients.
Footnotes
Authors' contributions: Meng LR participated in the design of the study and was the major contributor to writing the manuscript; Meng LR and Chen H performed the data collection and analysis; Meng LR and Li ZW interpreted the data and produced the draft of the manuscript. Chen WQ and Gao Y contributed to the inclusion of subjects; Ye Z and Li ZH guided and revised the manuscript. All authors read and approved the final version of the manuscript.
Conflicts of Interest: Meng LR, None; Chen H, None; Chen WQ, None; Gao Y, None; Li ZW, None; Ye Z, None; Li ZH, None.
REFERENCES
- 1.Kneer K, Green MB, Meyer J, Rich CB, Minns MS, Trinkaus-Randall V. High fat diet induces pre-type 2 diabetes with regional changes in corneal sensory nerves and altered P2X7 expression and localization. Exp Eye Res. 2018;175:44–55. doi: 10.1016/j.exer.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markoulli M, Flanagan J, Tummanapalli SS, Wu J, Willcox M. The impact of diabetes on corneal nerve morphology and ocular surface integrity. Ocul Surf. 2018;16(1):45–57. doi: 10.1016/j.jtos.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Ali Tabatabaei S, Soleimani M, Tabatabaei SM, Beheshtnejad AH, Valipour N, Mahmoudi S. The use of in vivo confocal microscopy to track treatment success in fungal keratitis and to differentiate between Fusarium and Aspergillus keratitis. Int Ophthalmol. 2020;40(2):483–491. doi: 10.1007/s10792-019-01209-2. [DOI] [PubMed] [Google Scholar]
- 4.Lee HJ, Alipour F, Cruzat A, Posarelli M, Zheng LX, Hamrah P. Utility of in vivo confocal microscopy in diagnosis of acanthamoeba keratitis: a comparison of patient outcomes. Cornea. 2023;42(2):135–140. doi: 10.1097/ICO.0000000000003184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teo AWJ, Mansoor H, Sim N, Lin MT, Liu YC. In vivo confocal microscopy evaluation in patients with keratoconus. J Clin Med. 2022;11(2):393. doi: 10.3390/jcm11020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reinprayoon U, Jermjutitham M, Kasetsuwan N. Rate of cornea endothelial cell loss and biomechanical properties in Fuchs' endothelial corneal dystrophy. Front Med. 2021;8:757959. doi: 10.3389/fmed.2021.757959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan RMM, Chua ZJY, Tan JC, Yang YY, Liao ZH, Zhao Y. From pre-diabetes to diabetes: diagnosis, treatments and translational research. Medicina. 2019;55(9):546. doi: 10.3390/medicina55090546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu FX, Lee PSY, Yang LL, Gao N, Zhang YY, Ljubimov AV, Yang E, Zhou QJ, Xie LX. The impact of sensory neuropathy and inflammation on epithelial wound healing in diabetic corneas. Prog Retin Eye Res. 2022;89:101039. doi: 10.1016/j.preteyeres.2021.101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto T, Otake H, Hiramatsu N, Yamamoto N, Taga A, Nagai N. A proteomic approach for understanding the mechanisms of delayed corneal wound healing in diabetic keratopathy using diabetic model rat. Int J Mol Sci. 2018;19(11):3635. doi: 10.3390/ijms19113635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roszkowska AM, Licitra C, Tumminello G, Postorino EI, Colonna MR, Aragona P. Corneal nerves in diabetes-the role of the in vivo corneal confocal microscopy of the subbasal nerve plexus in the assessment of peripheral small fiber neuropathy. Surv Ophthalmol. 2021;66(3):493–513. doi: 10.1016/j.survophthal.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Petropoulos IN, Ponirakis G, Ferdousi M, et al. Corneal confocal microscopy: a biomarker for diabetic peripheral neuropathy. Clin Ther. 2021;43(9):1457–1475. doi: 10.1016/j.clinthera.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 12.González Porto SA, Domenech N, et al. The addition of albumin improves Schwann cells viability in nerve cryopreservation. Cell Tissue Bank. 2018;19(4):507–517. doi: 10.1007/s10561-018-9700-7. [DOI] [PubMed] [Google Scholar]
- 13.Han JX, Wang H, Liang HH, Guo JX. Correlation of the retinopathy degree with the change of ocular surface and corneal nerve in patients with type 2 diabetes mellitus. Int J Ophthalmol. 2021;14(5):750–758. doi: 10.18240/ijo.2021.05.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Onofrio L, Kalteniece A, Ferdousi M, et al. Small nerve fiber damage and Langerhans cells in type 1 and type 2 diabetes and LADA measured by corneal confocal microscopy. Invest Ophthalmol Vis Sci. 2021;62(6):5. doi: 10.1167/iovs.62.6.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pupe C, Dieckmann G, Dornas R, Nascimento O. Corneal confocal microscopy in patients with distal symmetric polyneuropathy compared to controls. Arq Neuropsiquiatr. 2022;80(8):812–821. doi: 10.1055/s-0042-1755231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.So WZ, Qi Wong NS, Tan HC, Yu Lin MT, Yu Lee IX, Mehta JS, Liu YC. Diabetic corneal neuropathy as a surrogate marker for diabetic peripheral neuropathy. Neural Regen Res. 2022;17(10):2172–2178. doi: 10.4103/1673-5374.327364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferdousi M, Romanchuk K, Mah JK, Virtanen H, Millar C, Malik RA, Pacaud D. Early corneal nerve fibre damage and increased Langerhans cell density in children with type 1 diabetes mellitus. Sci Rep. 2019;9(1):8758. doi: 10.1038/s41598-019-45116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chao C, Wang R, Jones M, Karson N, Jussel A, Smith J, Richdale K, Harrison W. The relationship between corneal nerve density and hemoglobin A1c in patients with prediabetes and type 2 diabetes. Invest Ophthalmol Vis Sci. 2020;61(12):26. doi: 10.1167/iovs.61.12.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu ML, Hill LJ, Downie LE, Chinnery HR. Neuroimmune crosstalk in the cornea: The role of immune cells in corneal nerve maintenance during homeostasis and inflammation. Prog Retin Eye Res. 2022;91:101105. doi: 10.1016/j.preteyeres.2022.101105. [DOI] [PubMed] [Google Scholar]
- 20.Khan A, Pasquier J, Ramachandran V, et al. Altered circulating microRNAs in patients with diabetic neuropathy and corneal nerve loss: a pilot study. J Clin Med. 2022;11(6):1632. doi: 10.3390/jcm11061632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azmi S, Ferdousi M, Liu YF, et al. Bariatric surgery leads to an improvement in small nerve fibre damage in subjects with obesity. Int J Obes. 2021;45:631–638. doi: 10.1038/s41366-020-00727-9. [DOI] [PubMed] [Google Scholar]
- 22.Iqbal Z, Bashir B, Ferdousi M, Kalteniece A, Alam U, Malik RA, Soran H. Lipids and peripheral neuropathy. Curr Opin Lipidol. 2021;32(4):249–257. doi: 10.1097/MOL.0000000000000770. [DOI] [PubMed] [Google Scholar]
- 23.Tummanapalli SS, Issar T, Yan A, Kwai N, Poynten AM, Krishnan AV, Willcox MDP, Markoulli M. Corneal nerve fiber loss in diabetes with chronic kidney disease. Ocul Surf. 2020;18(1):178–185. doi: 10.1016/j.jtos.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Celiker H, Erekul G, Turhan SA, Kokar S, Yavuz DG, Gunduz OH, Tavakoli M, Toker E. Early detection of neuropathy in patients with type 2 diabetes with or without microalbuminuria in the absence of peripheral neuropathy and retinopathy. J Fr Ophtalmol. 2021;44(4):485–493. doi: 10.1016/j.jfo.2020.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Adam S, Azmi S, Ho JH, et al. Improvements in diabetic neuropathy and nephropathy after bariatric surgery: a prospective cohort study. Obes Surg. 2021;31(2):554–563. doi: 10.1007/s11695-020-05052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Aqaba MA, Dhillon VK, Mohammed I, Said DG, Dua HS. Corneal nerves in health and disease. Prog Retin Eye Res. 2019;73:100762. doi: 10.1016/j.preteyeres.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Chen CB, Hammo B, Barry J, Radhakrishnan K. Overview of albumin physiology and its role in pediatric diseases. Curr Gastroenterol Rep. 2021;23(8):11. doi: 10.1007/s11894-021-00813-6. [DOI] [PubMed] [Google Scholar]
- 28.Cain LD, Nie LH, Hughes MG, Johnson K, Echetebu C, Xu GY, Hulsebosch CE, McAdoo DJ. Serum albumin improves recovery from spinal cord injury. J Neurosci Res. 2007;85(7):1558–1567. doi: 10.1002/jnr.21265. [DOI] [PubMed] [Google Scholar]
- 29.Lv Y, Zhao SZ. What is the best strategy on detection of cornea neuropathy in people with diabetes? Recent advances in potential measurements. Diabetes Res Clin Pract. 2018;142:203–212. doi: 10.1016/j.diabres.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 30.Pritchard N, Edwards K, Vagenas D, Russell AW, Malik RA, Efron N. Corneal sensitivity is related to established measures of diabetic peripheral neuropathy. Clin Exp Optom. 2012;95(3):355–361. doi: 10.1111/j.1444-0938.2012.00729.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhao H, He Y, Ren YR, Chen BH. Corneal alteration and pathogenesis in diabetes mellitus. Int J Ophthalmol. 2019;12(12):1939–1950. doi: 10.18240/ijo.2019.12.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon KC, Im SK, Seo MS. Changes of tear film and ocular surface in diabetes mellitus. Korean J Ophthalmol. 2004;18(2):168–174. doi: 10.3341/kjo.2004.18.2.168. [DOI] [PubMed] [Google Scholar]
- 33.Good KL, Maggs DJ, Hollingsworth SR, Scagliotti RH, Nelson RW. Corneal sensitivity in dogs with diabetes mellitus. Am J Vet Res. 2003;64(1):7–11. doi: 10.2460/ajvr.2003.64.7. [DOI] [PubMed] [Google Scholar]
- 34.Perkins BA, Lovblom LE, Bril V, et al. Corneal confocal microscopy for identification of diabetic sensorimotor polyneuropathy: a pooled multinational consortium study. Diabetologia. 2018;61(8):1856–1861. doi: 10.1007/s00125-018-4653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gad H, Petropoulos IN, Khan A, Ponirakis G, MacDonald R, Alam U, Malik RA. Corneal confocal microscopy for the diagnosis of diabetic peripheral neuropathy: a systematic review and meta-analysis. J Diabetes Investig. 2022;13(1):134–147. doi: 10.1111/jdi.13643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ponirakis G, Al Hamad H, Omar DAM, et al. Corneal nerve loss predicts dementia in patients with mild cognitive impairment. Ann Clin Transl Neurol. 2023;10(4):599–609. doi: 10.1002/acn3.51747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Majeed A, Marwick B, Yu HQ, Fadavi H, Tavakoli M. Ophthalmic biomarkers for Alzheimer's disease: a review. Front Aging Neurosci. 2021;13:720167. doi: 10.3389/fnagi.2021.720167. [DOI] [PMC free article] [PubMed] [Google Scholar]


