Abstract
AIM
To study the causal relationship between obesity-related anthropometric traits and myopia and the mediating role of educational attainment (EA).
METHODS
Univariable Mendelian randomization (UVMR) was performed to evaluate the causal association between body mass index (BMI), height, waist-hip ratio (WHR, adjusted for BMI), and mean spherical equivalent (MSE). BMI was divided into fat and fat-free mass and included in multivariable Mendelian randomization (MVMR) to explore the roles of different BMI components in the causal relationship between BMI and MSE. A mediation analysis based on two-step Mendelian randomization (MR) was carried out. Specifically, UVMR was conducted to estimate the causal effect of BMI on EA. The direct effect of EA on MSE was estimated from MVMR. The mediation effect of EA in the BMI-EA-MSE model was calculated by the product of coefficients method. Expression quantitative trait loci (eQTL)-MR, reverse MR, and Linkage Disequilibrium Score Regression (LDSC) were performed to assess the robustness.
RESULTS
Genetically predicted higher BMI had a positive total effect on MSE (βIVW=0.26 D, 95%CI=0.14 to 0.37 D, P<0.001), whereas there was no significant association between height, WHR, and MSE. Fat mass was found to play a significant role in the effect of body mass on MSE (βIVW=0.50 D, 95%CI=0.21 to 0.78 D, P=0.001), but there was no significant association between fat-free mass and MSE. The causal effect of BMI on EA was -0.14 (95%CI=-0.16 to -0.11, P<0.001), and the direct effect of EA on MSE was -0.63 D (95%CI=-0.81 to -0.44 D, P<0.001). The mediating effect of EA in the BMI-EA-MSE model was 0.09 D (95%CI=0.06 to 0.12 D), with a mediation proportion of 33% (95%CI=22.1% to 44.6%). No reverse causal associations were detected except for BMI on EA. The results of eQTL-MR and LDSC were consistent with each MR analysis.
CONCLUSION
Genetically predicted higher BMI decreases the degree of myopia with a 33% mediation proportion by EA, and fat mass provides a dominant protective role in body mass-myopia. As a supplement to previous observational studies, it provides strong evidence for the relationship between anthropometric traits and refractive errors and offers a theoretical basis for future measures to prevent and control myopia.
Keywords: myopia, anthropometric traits, educational attainment, mediation analysis, Mendelian randomization
INTRODUCTION
Myopia, commonly known as short-sightedness or near-sightedness, is the most prevalent refractive error, typically occurring during childhood and adolescence[1]. The global prevalence of myopia has significantly increased over the past half-century[2], presenting not only challenges of blurred distance vision but also a substantial risk of irreversible vision impairment[3]. The pathogenesis of myopia is intricate, involving a complex interplay of genetic and environmental factors[4].
The potential correlations between myopia and anthropometric measures such as height, weight, and body mass index (BMI) have been extensively studied across diverse populations. However, current evidence presents conflicting conclusions on these complex relationships. Numerous studies suggested a positive correlation between height and axial length in association with myopia, indicating a link between increased axial length during growth and height augmentation[5]–[6]. Some investigations supported a positive association between low BMI and myopia[7]–[9], while contradictory findings have been reported[10]. A recent cross-sectional study involving 1.3 million adolescents suggested that both low and high BMI were correlated with myopia[11].
Educational attainment (EA) is widely considered to be a risk factor for myopia. Previous Mendelian randomization (MR) studies also provided support for the causal effect of EA on myopia[12]. The reciprocal influence between BMI, height, and EA has been validated in both observational and MR studies[13]–[15]. Therefore, it is plausible that anthropometric parameters may affect refractive error, potentially mediated through EA, or EA may act as a confounder.
Traditional observational studies have inherent limitations and cannot completely avoid bias caused by reverse causality and potential confounding factors[16]. MR methods are increasingly used to infer causal relationships between risk factors and disease outcomes, providing results with higher reliability than observational studies and theoretically achieving the credibility of randomized controlled trials (RCTs)[16]. The effectiveness of MR analysis lies in its ability to avoid acquired confounding factors attributed to the random assortment of alleles during gamete formation. Univariable Mendelian randomization (UVMR) was specifically employed to infer causality regarding a single exposure on the outcome. Recent advancements in MR methodology have introduced multivariable Mendelian randomization (MVMR), a powerful approach applicable for investigating multiple exposures' effects on the outcome. MVMR is also adept at exploring mediation pathways in complex relationships[17].
This study investigated the causality between BMI, body mass (categorized into whole fat-free mass and whole fat mass), height, waist-hip ratio (WHR; adjusted for BMI), and refractive error. It further determined whether EA acts as a mediating factor between obesity-related anthropometric traits and refractive error.
MATERIALS AND METHODS
Overall Study Design
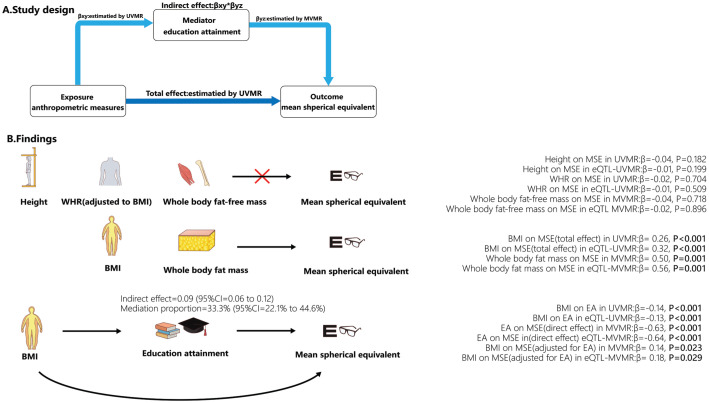
The main study design was depicted in Figure 1A. In this study, we conducted UVMR analysis to investigate the causal effect of BMI, height, and WHR (adjusted for BMI) on autorefraction-measured mean spherical equivalent (MSE). Besides, we categorized body mass into fat and fat-free mass, employing MVMR analysis to study the causal relationship between body mass and MSE. Furthermore, we investigated the potential mediating role of EA in the causal relationship between BMI and MSE through a mediation analysis based on the two-step MR method. Several sensitivity analyses were performed to evaluate whether assumptions were met and to ensure the robustness of the results. The stability of MR results was again assessed using expression quantitative trait loci (eQTL)-MR.
Figure 1. Study design of the mediation role of EA in the relationship between anthropometric traits on refractive error (A) and the main finding of this study (B).
UVMR: Univariable Mendelian randomization; MVMR: Multivariable Mendelian randomization; BMI: Body mass index; EA: Educational attainment; WHR: Waist-hip ratio; MSE: Mean spherical equivalent.
GWAS Dataset
We obtained SNP-trait association data from publicly available Genome-Wide Association Studies (GWAS) for each trait (BMI, height, WHR adjusted for BMI, EA, whole body fat mass, whole body fat-free mass, and MSE), as detailed in Table 1. The anthropometric traits of BMI, height, and WHR GWAS data were obtained from the Genetic Investigation of Anthropometric Traits consortium, with sample sizes of 806 834, 694 649, and 4 080 687 European participants. The EA GWAS data was taken from the Social Science Genetic Association Consortium. In the original GWAS of EA[18], educational levels were defined according to the International Standard Classification of Education 2011[19]. After converting educational level to US years of schooling, 765 283 European participants were enrolled in this study, with each unit representing 4.2 years of schooling. The whole body fat-free mass, whole body fat mass, and MSE were collected from the UK Biobank. The GWAS data for fat mass and fat-free mass were collected in the same cohort, consisting of approximately 450 000 European participants. Body composition was estimated using bioelectrical impedance technique, and fat mass and non-fat mass were measured. Ophthalmic assessments were added at a later stage, with about 25% of the participants receiving ophthalmic assessments. Non-cycloplegic autorefraction detected refractive error using Tomey RC 5000 AutoRefractor Keratometer[20]. A single eye's spherical equivalent (SE) was equal to the sphere power plus half of the cylinder power. The MSE of both eyes was used to represent the autorefraction-measured refractive error of an individual[20]. The final GWAS for MSE was conducted on 95 619 participants of European ancestry, both males and females, aged 40-69y[21].
Table 1. Details of selected traits and associated GWAS.
| Trait | Consortium | Ancestry | Participants | Selected and eQTL SNPs | Unit | PMID |
| BMI | GIANT | European | 806834 | 426/243 | SD (4.8 kg/m2) | 30239722 |
| Height | GIANT | European | 4080687 | 902/618 | SD (9.3 cm) | 36224396 |
| WHR | GIANT | European | 694649 | 275/194 | SD (0.09) | 30239722 |
| EA | SSGAC | European | 765283 | 303/156 | SD (4.2y) | 35361970 |
| Fat mass | UKB | European | 454137 | 511/362 | SD (9.5 kg) | NA |
| Fat-free mass | UKB | European | 454850 | SD (11.5 kg) | NA | |
| MSE | UKB | European | 95619 | NA | 1 diopter | 29808027 |
BMI: Body mass index; WHR: Waits-hip ratio; EA: Educational attainment; Fat mass: Whole body fat mass; Fat-free mass: Whole body fat-free mass; MSE: Mean spherical equivalent; GWAS: Genome-Wide Association Studies; GIANT: Genetic Investigation of Anthropometric Traits; SSGAC: Social Science Genetic Association Consortium; UKB: UK Biobank; SD: Standard deviation.
Genetic Instruments Selected for Exposures
We extracted the selected single nucleotide polymorphisms (SNPs) and their respective associations with exposures from the GWAS resented in Table 1. Initially, we identified genome-wide significant SNPs (P<5×10−8), and subsequently, these primarily selected SNPs underwent further filtering based on the following criteria: 1) exclusion of dependent SNPs through clumping using the PLINK algorithm (r2=0.001, window size=10 MB); 2) removal of palindromic SNPs to prevent potential strand ambiguity; 3) elimination of SNPs associated with the outcome with genome-wide significance (P<5×10−5) to preclude direct effects of the instrumental variables (IVs) on the outcome; 4) eradication of SNPs lacking indispensable information such as beta and P-value for traits in exposure and outcome GWAS. We used F-statistics for each exposure in UVMR and conditional F-statistics in MVMR to assess weak instruments, with F-statistics>10 considered valid instrument strength[22]. The formula for calculating F-statistics is F=β2/se2 (β for the SNP-exposure association effect; se for the variance)[23], and conditional F-statistics were calculated using the “MVMR (version 0.3)” R package.
Univariable MR Method
The causal effects estimation of BMI, height, WHR, and EA on MSE was investigated by the UVMR method. Here, three assumptions were indispensable for UVMR analysis: 1) the selected IVs must have a significant association with the studied exposure, 2) the IVs must be independent of confounding factors, 3) the IVs must affect the outcome only through the exposure rather than other pathways. Based on the above assumptions, seven causal inference methods were employed: MR Egger, weighted median, inverse-variance weighted (IVW), weighted mode, simple mode, MR-PRESSO, and MR-PRESSO (outliers-corrected). A consistent effect across these seven methods, which infer causality under specific IVs assumptions separately, will be more robust against the bias due to horizontal pleiotropy. The IVW method was considered the primary analytical method for the causal estimate, with a Bonferroni-corrected significant P-value less than 0.013 (0.05/4).
Multivariable MR Method
Through UVMR analysis, we found that only BMI exhibits a causal relationship with MSE among the obesity-related anthropometric traits. Subsequently, as part of the two-step MR, we conducted an MVMR analysis to investigate the direct causal effects of EA on MSE adjusted for BMI and the causal effect of BMI on MSE adjusted for EA and explore the model's potential mediating role. Another MVMR analysis of fat and fat-free mass on MSE was conducted to investigate the dominant role. For MVMR, we used the IVW method as the primary method and MR-Egger as a supplementary method.
Mediated Effect Estimation Based on Two-step MR
The total effect of exposure on an outcome can be dissected into direct effect (through the exposure alone) and indirect effect (via the mediator)[24]. The UVMR method can be used to estimate the total effect of an exposure on the outcome. The MVMR method is employed to discern the effect of each exposure on the outcome, considering other exposures. The indirect effect of exposure on the outcome can be estimated by multiplying the causal effect of exposure on the mediator by the mediator on the outcome, referred to as the product of coefficients method[25]. In this study, we apply the product of coefficients method to calculate the indirect effect of the mediator. To be specific, the effect of the exposure (BMI) on the mediator (EA) and the total effect of the exposure (BMI) on the outcome (MSE) was estimated by UVMR, and the effect of the mediator (EA) on the outcome (MSE) by MVMR.
Genetic Correlation Analysis
Linkage disequilibrium score regression (LDSC) is a robust approach for conducting genetic correlation analyses in complex diseases or traits. It quantifies the heritability of a trait through SNPs or assesses the correlation between two traits by utilizing Chi-squared statistics[26]. In this study, we applied the LDSC method to explore the genetic correlation between EA, BMI, height, WHR, fat-free mass, and fat mass with MSE. To maintain statistical rigor, we set a Bonferroni-corrected P-value of 0.007 (0.05/7) as the significance threshold.
Sensitivity Analyses
We conducted several sensitivity analyses to test the robustness of MR results. The stability of the results was assessed using eQTL-MR. To assess potential reverse causality, we conducted reverse UVMR analyses. Cochran's Q-statistic evaluated heterogeneity, and the MR-Egger intercept test was performed for pleiotropy testing. Besides, a leave-one-out analysis and MR-PRESSO model were conducted to test whether the IVs exist as distinct outliers (which may represent pleiotropic variants) and if a fraction of variants contribute to the primary causal effect in the UVMR analyses. Also, the funnel and forest plots of SNPs were undertaken to assess the symmetry of selected SNPs.
Functional Mapping and Annotation Analysis
Functional mapping and annotation (FUMA) is an online analytical platform for annotating and visualizing GWAS results. We conducted an FUMA analysis to delve deeper into the genetic mechanisms underpinning the associations among BMI and EA on MSE. Gene symbols corresponding to eQTL-SNPs were extracted from the Phenoscanner database and subsequently annotated through the GENE2FUNC function of FUMA platform.
The MR analysis and sensitivity analysis in our study were executed using the “TwoSampleMR (version 0.5.6)”, “MVMR (version 0.3)”, and “MRPRESSO (version 1.0)” packages in RStudio (R version 4.2.2, Posit Inc, Boston, USA). Gene symbols corresponding to eQTL-SNPs were extracted from the Phenoscanner database using the “phenoscanner (version 1.0)” R package. Genetic correlation analysis was performed employing the “ldscr (version 0.1.0)” R package. FUMA analysis was carried out using the online tool available at https://fuma.ctglab.nl/.
RESULTS
Genetic Instruments
After strict filtering, 426/243, 902/618, 275/194, and 303/156 independent and significant SNPs associated with BMI, height, WHR, and EA were used as IVs in UVMR and eQTL-UVMR (Table 1). The F-statistic of SNPs in each group was greater than 10, suggesting that causal estimation by UVMR would not be biased due to weak IVs. There were 532/301 and 511/362 SNPs selected as IVs for BMI and EA on MSE and fat mass and fat-free mass on MSE in MVMR and eQTL-MVMR models (Table 1). For IVs used in the MVMR model, the conditional F-statistics were 18, 34, 12, and 11 for EA, BMI, fat-free mass, and fat mass, respectively, all exceeding 10, indicating robust IV strength remained robust even after conditioning on other exposures.
Univariable MR Analysis Results
We observed that BMI and EA were associated with MSE, but there was no evidence of height and WHR affecting MSE. The estimation by the IVW method showed that higher BMI per standard deviation (SD) resulted in a less myopia of 0.26 D (95%CI=0.14 to 0.37 D, P<0.001) and higher EA per 4.2y associated with increased myopic degree of 0.69 D (95%CI=-0.84 to -0.55 D, P<0.001; Figure 2A). The results of the IVW method did not show a significant association between height, WHR, and MSE (βIVW=-0.04, 95%CI=-0.10 to 0.02, P=0.182; βIVW=-0.02, 95%CI=-0.15 to 0.1, P=0.704; Figure 2A).
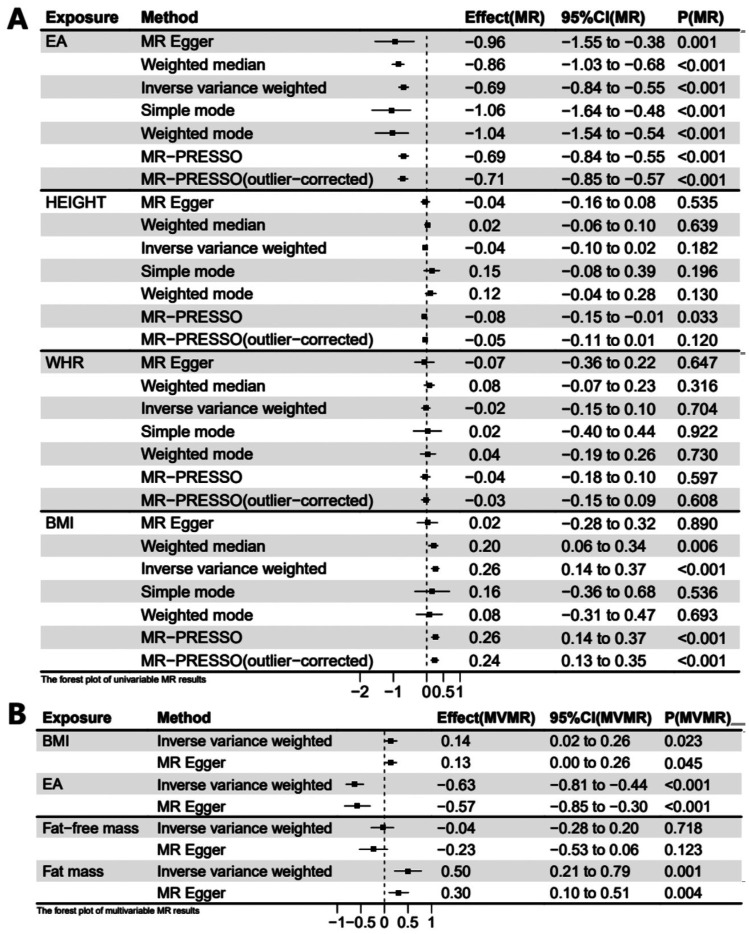
Figure 2. Forest plots of univariable and multivariable MR estimation of exposures on MSE.
A: Forest plot of 1 SD exposure increase resulting in MSE change in the UVMR model; B: Forest plot of 1 SD exposure increase resulting in MSE change in the MVMR model. UVMR: Univariable Mendelian randomization; MVMR: Multivariable Mendelian randomization; BMI: Body mass index; EA: Educational attainment; WHR: Waist-hip ratio; Fat-free mass: Whole body fat-free mass; Fat mass: Whole body fat mass.
The causal relationship between BMI and MSE, as estimated by weighted median and MR-PRESSO approaches, was consistent with the result of the IVW method. While MR-PRESSO detected outliers, the MR-PRESSO (outlier-corrected) also yielded consistent results after removing them. MR-Egger, simple mode, and weighted mode detected the same direction of causal estimation (Figure 2A). In order to ensure result stability, an additional UVMR analysis was conducted using eQTL-SNPs associated with BMI and the results demonstrated similar findings (βIVW=0.32 D, 95%CI=0.17 to 0.47 D, P<0.001). Furthermore, the results from weighted median, MR-PRESSO, and the MR-PRESSO (outlier-corrected) method were consistent with the IVW method (Figure 3A).
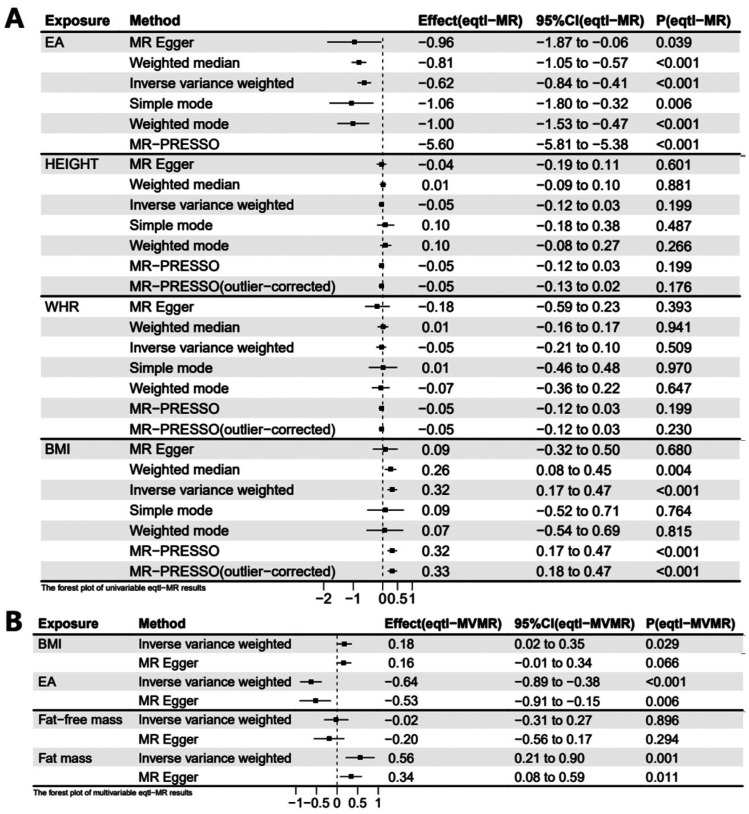
Figure 3. Forest plots of univariable and multivariable eQTL-MR estimation of exposures on MSE.
A: Forest plot of 1 SD exposure increase resulting in MSE change in the UVMR model; B: Forest plot of 1 SD exposure increase resulting in MSE change in the MVMR model. Eqtl: Expression quantitative trait loci; UVMR: Univariable Mendelian randomization; MVR: Multivariable Mendelian randomization; MSE: Mean spherical equivalent; BMI: Body mass index; EA: Educational attainment; WHR: Waist-hip ratio; Fat-free mass: Whole body fat-free mass; Fat mass: hole body fat mass.
Regarding the causal relationship between EA and MSE estimated by the IVW method, the results aligned with those calculated by the MR-Egger, weighted median, simple mode, weighted mode, and MR-PRESSO methods (Figure 2A). MR-PRESSO detected outliers, and after removing them, the result of MR-PRESSO (outlier-corrected) remained consistent with that of the IVW method. For stability, we reanalyzed using eQTL-SNPs associated with EA and obtained consistent results (βIVW=-0.62 D, 95%CI=-0.84 to -0.41 D, P<0.001). MR-PRESSO identified outliers, and the outlier-corrected MR-PRESSO method, along with MR-PRESSO, weighted median, and weighted mode, showed consistent results with those of the IVW method (Figure 3A).
No evidence supporting a causal relationship between height, WHR, and MSE was found in UVMR and eQTL-UVMR analyses. All seven methods did not yield statistically significant results (Figures 2A, 3A).
Multivariable MR Analysis Results
In the above UVMR analyses, we identified BMI as a risk factor for myopia. Therefore, we conducted an MVMR analysis involving BMI and EA on MSE. Additionally, we categorized body mass into fat and fat-free mass, conducting an MVMR analysis on MSE. In the MVMR analysis of BMI and EA on MSE, the direct effect of per SD higher EA on MSE (βIVW=-0.63 D, 95%CI=-0.81 to -0.44 D, P<0.001; Figure 2B) adjusting for BMI was similar to the total effect (βIVW=-0.69 D, 95%CI=-0.84 to -0.55 D, P<0.001) in UVMR analysis. However, the direct effect of per SD higher BMI on MSE (βIVW=0.14 D, 95%CI=0.02 to 0.26 D, P=0.023; Figure 2B) was noticeably reduced after accounting for EA compared to the total effect (βIVW=0.26 D, 95%CI=0.14 to 0.37 D, P<0.001) in UVMR analysis. We also utilized eQTL-SNPs as genetic variables for a reanalysis of the MVMR, obtaining comparable results (βIVW of EA=-0.64 D, 95%CI=-0.89 to -0.38 D, P<0.001; βIVW of BMI=0.18 D, 95%CI=0.02 to 0.35 D, P=0.029; Figure 3B). Another MVMR analysis of fat and fat-free mass on MSE showed that an increase of 1 SD in fat mass resulted in a 0.50 D (95%CI=0.21 to 0.78 D, P=0.001) increase in MSE. Still, there was no significant association between fat-free mass and MSE (βIVW=-0.04 D, 95%CI=-0.28 to 0.20 D, P=0.718; Figure 2B). MVMR based on eQTL-SNPs showed similar results (βIVW of fat mass=0.56 D, 95%CI=0.21 to 0.90 D, P=0.001; βIVW of fat-free mass=-0.02 D, 95%CI=-0.31 to 0.27 D, P=0.896; Figure 3B). The causal effects calculated by MVMR-Egger in two MVMR analyses showed analogous results (Figures 2B, 3B).
Result of Mediation Analysis Based on MR Analyses
In UVMR analysis, we observed a protective effect of BMI against myopia. However, in the MVMR model of EA and BMI on MSE, the protective effect of BMI on MSE decreased after adjusting for EA. We calculated the mediating effect of EA using the product of coefficients method based on IVW estimated effects (Figure 1B). The total effect of BMI on MSE, as calculated by the IVW model in the UVMR analysis, was 0.26 D (95%CI=0.14 to 0.37 D). The indirect effect of EA in the BMI-MSE relationship was 0.09 D (95%CI=0.06 to 0.12 D), obtained by multiplying the effect of BMI on EA (-0.14, 95%CI=-0.16 to -0.11) in the UVMR analysis by the effect of EA on MSE (βIVW=-0.63 D, 95%CI=-0.21 to -0.44 D) in the MVMR model (Figure 2B). The proportion mediated by EA in the total effect of BMI on MSE was 33.3% (95%CI=22.1% to 44.6%). Similarly, we conducted a mediation analysis using the results from eQTL-MR. EA exhibited a mediating effect in the BMI-MSE relationship, with a mediation proportion of 25.4% (95%CI=13.7% to 37.2%).
FUMA Analysis Results
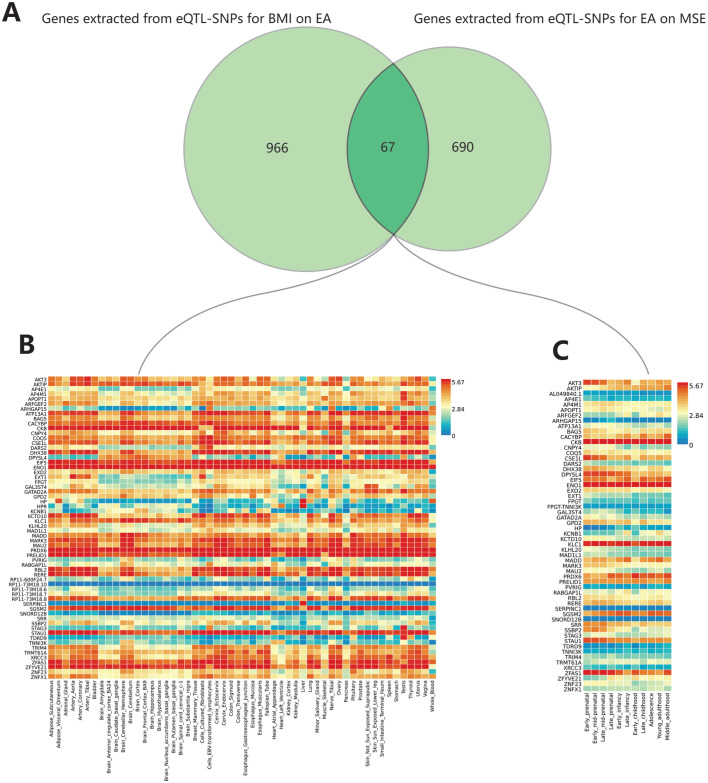
Based on eQTL-SNPs, we extracted 966 and 690 genes from BMI-EA and EA-MSE using the Phenoscanner database, with 67 genes overlapping (Figure 4A). In FUMA analysis, three genes lacked recognized Ensembl IDs (RP11-44F14.11, ZNFX1-AS1, and LOC100506334). The remaining 64 genes, with two missing from background genes (FPGT-TNNI3K and AL049840.1), exhibited expression in 54 tissues (Figure 4B). Fifty-eight genes showed expression in 11 developmental stages of the brain (Figure 4C), with six missing from background genes (RP11-73M18.8, RP11-73M18.7, RP11-73M18.6, RP11-73M18.10, RP11-600F24.7, and FPGT-TNNI3K). Gene sets enrichment analysis was conducted to explore the potential biological mechanisms of these 67 genes in BMI-EA-MSE, revealing 48 significantly enriched gene sets (adjusted P<0.05). We observed strong enrichment signals associated with psychiatric disorders, such as autism spectrum disorders (adjusted P=7.97E-08), schizophrenia (adjusted P=7.93E-17), bipolar disorder (adjusted P=0.001), and depression (adjusted P=0.006). Additionally, we identified strong enrichment signals related to brain structure and function, including general cognitive ability (adjusted P=1.27E-05), brain morphology (adjusted P=1.35E-05), and extremely high intelligence (adjusted P=1.56E-05). We also found strong enrichment signals associated with metabolism, such as response to fenofibrate/LDL cholesterol levels (adjusted P=3.60E-05) and fasting insulin levels (adjusted P=0.03).
Figure 4. The results of FUMA analysis.
A: The overlapping genes of BMI on EA and EA on MSE; B: The expression of overlapping genes in 54 general tissues; C: The expression of overlapping genes in 11 developmental stages of the brain. FUMA: Functional mapping and annotation; eQTL: Expression quantitative trait loci; MSE: Mean spherical equivalent; BMI: Body mass index; EA: Educational attainment.
Genetic Correlation Analysis
The results of LDSC confirmed the above results of MR. LDSC identified significant genetic correlations between MSE and BMI, EA, and fat mass (MSE and BMI: genetic correlation=0.10, P<0.001; MSE and EA: genetic correlation=-0.27, P<0.001; MSE and fat mass: genetic correlation=0.09, P<0.001). Additionally, EA and BMI exhibited a significant genetic correlation: genetic correlation=-0.32, P<0.001. However, no detectable genetic correlations were found between MSE and Height, WHR, or fat-free mass (MSE and Height: genetic correlation=-0.04, P=0.058; MSE and WHR: genetic correlation=0.03, P=0.187; MSE and fat-free mass: genetic correlation=0.02, P=0.440).
Sensitivity Analyses
There were no significant and substantial effects of MSE on all studied exposures from reverse UVMR and eQTL-UVMR analyses. The Cochran's Q statistic results showed substantial heterogeneity of IVs for each exposure to MSE (P of Q statistic<0.05). Therefore, we used the random-effects IVW model for causal estimation to address heterogeneity. For the MR-Egger intercept test, we found no evidence of significant pleiotropy of any UVMR or MVMR analyses (P of intercept>0.05). In addition, leave-one-out analyses for exposures on outcome showed that there was no particular variant dominating the estimate of the causal effect.
DISCUSSION
This study conducted a series of MRs to investigate the causal effects of obesity-related anthropometric traits on MSE and the mediating effect of EA (Figure 1). The results, validated through various sensitivity analyses, eQTL-MR, and LDSC, indicate that higher BMI and fat mass have a protective effect on myopia, with EA serving as a mediator in the BMI-MSE relationship. Additionally, FUMA analysis provides further insights into the model of BMI-EA-myopia.
There are different but seemingly plausible theories about the association between obesity and myopia. In a large cross-sectional study involving 1 784 619 Korean men aged 18-35y, lower BMI was associated with a higher prevalence of myopia[7]. The recent largest nationwide study of 1.3 million male and female adolescents (16-19y) reported that BMI was associated with myopia in a J-shaped pattern (both low BMI and high BMI were risk factors for myopia)[11]. Similarly, Machluf et al[27] supported the J-shaped model, but this correlation is significant only in males. While Wong et al[28] reported that adults with higher BMI were more hyperopic, Shi et al[29] argued that obesity was a risk factor for myopia. The reason for the inconsistency is the presence of numerous confounding factors. For instance, obese individuals tend to be more active indoors and less active outdoors and have lower levels of educational attainment and economic achievement[15]. These confounding factors influence myopia differently, leading to cumulative effects that vary across different populations and groups. Our study agreed that a higher BMI was associated with a lower myopia degree. Besides, our study categorized BMI into fat and fat-free mass and localized the protective effect of BMI on myopia to fat mass. The direct effect of BMI on myopia may be attributed to obese individuals having more retrobulbar fat tissue, potentially restricting the growth of the sclera during the emmetropization process. Scleral overgrowth is an important structural change in myopic eyes[30]. However, insulin resistance, commonly present in obese individuals, is a contributing factor to myopia[31], which contradicts our study. Thus, the effect of obesity on myopia is complex and requires further investigation. In addition, the present study attempted to investigate the correlation between WHR and myopia to explore whether fat distribution affects myopia, but no correlation was found between WHR and myopia.
We are the first to propose the viewpoint that high BMI influences the degree of myopia through EA. The previous MR study identified the causal effect of education on myopia[12], which may be based on SNP-education and SNP-intelligence interactions on myopia[32]. Higher education is associated with lower BMI, but a higher degree only predicts a slight reduction in BMI[13]. While EA and obesity are considered to be associated with socioeconomic status[33], the impact of education on myopia may not be exclusively related to socioeconomic status but rather to factors such as reduced outdoor activities and increased near-work activities, which are core causes of myopia formation. A higher BMI is associated with lower cognitive function[34] and more sensitive psychological characteristics[35], which can reduce an individual's inclination toward higher education. Whereas high schooling itself does not necessarily increase susceptibility to myopia, the near-work associated with the educational process is the contributor to myopia. Therefore, we propose that individuals with higher BMI may be more inclined to receive less education, leading to lower myopia. Of course, this can only partially explain the role of BMI in resisting myopia, and the direct protective role of BMI on myopia requires further exploration. Through FUMA analysis, we further validated 64 overlapping genes and 48 loci. Among the 48 loci, there were strong correlations with brain structure and function, such as intelligence and mental and psychological disorders, corroborating the previously mentioned point. CKB, KLC1, and ENO1 are highly expressed at all brain developmental stages, among which KLC1 is involved in the neurotrophic factor transport of the visual system[36], potentially contributing to myopia development. However, further research is needed to confirm this.
While longer axial lengths and bigger eyeballs of taller individuals indicate a potential association between height and myopia, our study did not find evidence supporting this association[28]. Height, to some extent, can reflect socioeconomic status[15]. Thus, the association between height and myopia could be driven by socioeconomic factors. There is a biological correlation between eye axial growth and body growth[37], but our study found no correlation between height and myopia.
The key strength of our study is the use of MR to infer causality. We used SNPs as instrumental variables to avoid bias from potential confounders and reverse causation. In addition, MVMR has the advantage of considering the combined effects of multiple exposures, even when there are bidirectional relationships among them. In applying mediation analysis, MR analysis generates increased robustness against non-differential measurement error[17]. Several limitations must be considered when explaining these results. First, MR analysis results may be biased by the underlying existence of pleiotropic effects of SNPs (SNPs have a significant effect on confounders and outcome). We conducted several sensitivity analyses to test the potential pleiotropic effect of selected SNPs[38]–[40], and no pleiotropy was detected. Cochran's Q statistics suggested the existence of heterogeneity. To address this concern, we selected the random-effects IVW method as our primary MR approach, which offers robustness against the heterogeneity of SNPs. Besides, the leave-one-out analysis showed that the estimation of causal effects did not depend on one or a small number of variants. Second, a potential limitation of using genetic variables on social traits like EA is the presence of “population phenomena”. As a result, associations between SNPs and EA may be confounded, thus introducing bias in MR estimates[41]–[42]. Further, MR studies using within-family GWAS datasets might generate more reliable effect estimation considering such phenomena. Third, the study was conducted on individuals of European ancestry, so the generalizability of our findings to other populations may be limited. Fourth, due to the interaction between the exposure and the mediator, the estimation of the mediation effect and the direct effect may be biased. Although current MR methods cannot address this issue, MR remains effective for detecting mediators[17]. Finally, one strength of MR analysis was estimating the lifelong effects of exposures. However, the SNPs selected here were associated with BMI, WHR, fat mass, and fat-free mass at a specific time, which may not fully represent these traits over the life span.
In summary, our study suggests that higher BMI and fat mass may contribute to a lower degree of myopia, with EA playing a partial mediating role. However, no causal relationship was observed between height, fat distribution (WHR), and myopia. These findings propose potential public health strategies to mitigate myopia, such as enhancing nutrition to increase fat mass in lean children and implementing educational reforms to reduce study duration. However, these strategies require further research. Further investigations are warranted to unravel the underlying mechanisms linking BMI to myopia to reduce the burden of myopia.
Footnotes
The authors thank the GIANT and SSGAC consortium, the GWAS catalog and the IEU Open GWAS project for providing summary results data for these analyses.
Availability of data: All seven GWAS data can be acquired from the public websites. The outcome-GWAS data was acquired from the GWAS catalog. The number of MSE-GWAS data is GCST009521. And the exposures-GWAS data were acquired from different consortiums. The anthropometric measures of GWAS (BMI, height, WHR) were collected from GIANT (https://portals.broadinstitute.org). The EA-GWAS was obtained from SSGAS (https://www.thessgac.org). The GWAS datasets of fat mass and fat-free mass were collected from the IEU OpenGWAS project (https://gwas.mrcieu.ac.uk/), and the number of fat mass and fat-free mass are ukb-b-19393 and ukb-b-13354.
Authors' contributions: Lu Y conducted the study design and primary data analysis and was the lead author of the manuscript. Zhang CC, Ma RT, and Hu DWJ helped collect the outcome GWAS data associated with myopic refractive error, Li YJ collected the GWAS data related to all exposures, and Li WP proofread the manuscript. All authors read and approved the final manuscript.
Foundations: Supported by Hubei Province Key Research and Development Program Project, Hubei Provincial Department of Science and Technology (No.2022BCA044); Key Scientific Research Projects of Health Commission of Hubei Province in 2023-2024, Health Commission of Hubei Province (No.WJ2023Z006).
Conflicts of Interest: Lu Y, None; Zhang CC, None; Ma RT, None; Li YJ, None; Li WP, None; Hu DWJ, None; Zhou LH, None.
REFERENCES
- 1.Baird PN, Saw SM, Lanca C, Guggenheim JA, Smith Iii EL, Zhou X, Matsui KO, Wu PC, Sankaridurg P, Chia A, Rosman M, Lamoureux EL, Man R, He M. Myopia. Nat Rev Dis Primers. 2020;6(1):99. doi: 10.1038/s41572-020-00231-4. [DOI] [PubMed] [Google Scholar]
- 2.Naidoo KS, Fricke TR, Frick KD, Jong M, Naduvilath TJ, Resnikoff S, Sankaridurg P. Potential lost productivity resulting from the global burden of myopia: systematic review, meta-analysis, and modeling. Ophthalmology. 2019;126(3):338–346. doi: 10.1016/j.ophtha.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Ohno-Matsui K, Wu PC, Yamashiro K, Vutipongsatorn K, Fang Y, Cheung CMG, Lai TYY, Ikuno Y, Cohen SY, Gaudric A, Jonas JB. IMI pathologic myopia. Invest Ophthalmol Vis Sci. 2021;62(5):5. doi: 10.1167/iovs.62.5.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hao J, Yang Z, Zhang R, Ma Z, Liu J, Bi H, Guo D. Crosstalk between heredity and environment in myopia: an overview. Heliyon. 2024;10(8):e29715. doi: 10.1016/j.heliyon.2024.e29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li T, Jiang B, Zhou X. Axial length elongation in primary school-age children: a 3-year cohort study in Shanghai. BMJ Open. 2019;9(10):e029896. doi: 10.1136/bmjopen-2019-029896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tao L, Wang C, Peng Y, Xu M, Wan M, Lou J, Yu X. Correlation between increase of axial length and height growth in Chinese school-age children. Front Public Health. 2021;9:817882. doi: 10.3389/fpubh.2021.817882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee DC, Lee SY, Kim YC. An epidemiological study of the risk factors associated with myopia in young adult men in Korea. Sci Rep. 2018;8(1):511. doi: 10.1038/s41598-017-18926-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahi JS, Cumberland PM, Peckham CS. Myopia over the lifecourse: prevalence and early life influences in the 1958 British birth cohort. Ophthalmology. 2011;118(5):797–804. doi: 10.1016/j.ophtha.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 9.Yue Y, Liu X, Yi S, Liu B, Yi H, Li H. High prevalence of myopia and low hyperopia reserve in 4411 Chinese primary school students and associated risk factors. BMC Ophthalmol. 2022;22(1):212. doi: 10.1186/s12886-022-02436-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S, Lee H, Lee KG, Kim J. Obesity and high myopia in children and adolescents: Korea national health and nutrition examination survey. PLoS One. 2022;17(3):e0265317. doi: 10.1371/journal.pone.0265317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peled A, Nitzan I, Megreli J, Derazne E, Tzur D, Pinhas-Hamiel O, Afek A, Twig G. Myopia and BMI: a nationwide study of 1.3 million adolescents. Obesity. 2022;30(8):1691–1698. doi: 10.1002/oby.23482. [DOI] [PubMed] [Google Scholar]
- 12.Mountjoy E, Davies NM, Plotnikov D, Smith GD, Rodriguez S, Williams CE, Guggenheim JA, Atan D. Education and myopia: assessing the direction of causality by Mendelian randomisation. BMJ. 2018;361:k2022. doi: 10.1136/bmj.k2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benson R, von Hippel PT, Lynch JL. Does more education cause lower BMI, or do lower-BMI individuals become more educated? Evidence from the National Longitudinal Survey of Youth 1979. Soc Sci Med. 2018;211:370–377. doi: 10.1016/j.socscimed.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Ren J, Chen J, Gao R, Bai B, An H, Cai W, Ma A. Lifestyle choices mediate the association between educational attainment and BMI in older adults in China: a cross-sectional study. Front Public Health. 2022;10:1000953. doi: 10.3389/fpubh.2022.1000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyrrell J, Jones SE, Beaumont R, Astley CM, Lovell R, Yaghootkar H, Tuke M, Ruth KS, Freathy RM, Hirschhorn JN, Wood AR, Murray A, Weedon MN, Frayling TM. Height, body mass index, and socioeconomic status: Mendelian randomisation study in UK biobank. BMJ. 2016;352:i582. doi: 10.1136/bmj.i582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richmond RC, Davey Smith G. Mendelian randomization: concepts and scope. Cold Spring Harb Perspect Med. 2022;12(1):a040501. doi: 10.1101/cshperspect.a040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter AR, Sanderson E, Hammerton G, Richmond RC, Davey Smith G, Heron J, Taylor AE, Davies NM, Howe LD. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol. 2021;36(5):465–478. doi: 10.1007/s10654-021-00757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okbay A, Wu Y, Wang N, Jayashankar H, Bennett M, Nehzati SM, Sidorenko J, Kweon H, Goldman G, Gjorgjieva T, Jiang YX, Hicks B, Tian C, Hinds DA, Ahlskog R, Magnusson PKE, Oskarsson S, Hayward C, Campbell A, Porteous DJ, Freese J, Herd P, andMe Research Team, Social Science Genetic Association Consortium. Watson C, Jala J, Conley D, Koellinger PD, Johannesson M, Laibson D, Meyer MN, Lee JJ, Kong A, Yengo L, Cesarini D, Turley P, Visscher PM, Beauchamp JP, Benjamin DJ, Young AI. Polygenic prediction of educational attainment within and between families from genome-wide association analyses in 3 million individuals. Nat Genet. 2022;54(4):437–449. doi: 10.1038/s41588-022-01016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.UNESCO Institute for Statistics. The International Standard Classification of Education (ISCED 2011) Montreal, Canada: http://www.uis.unesco.or . [Google Scholar]
- 20.The UK Biobank Eye and Vision Consortium. Is a large eye size a risk factor for myopia? A Mendelian randomization study. bioRxiv. 2017 doi: 10.1101/240283. [DOI] [Google Scholar]
- 21.Tedja MS, Wojciechowski R, Hysi PG, et al. Genome-wide association meta-analysis highlights light-induced signaling as a driver for refractive error. Nat Genet. 2018;50(6):834–848. doi: 10.1038/s41588-018-0127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. doi: 10.1136/bmj.k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowden J, Del Greco M F, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45(6):1961–1974. doi: 10.1093/ije/dyw220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tönnies T, Schlesinger S, Lang A, Kuss O. Mediation analysis in medical research: part 31 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2023;120(41):681–687. doi: 10.3238/arztebl.m2023.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanderson E. Multivariable Mendelian randomization and mediation. Cold Spring Harb Perspect Med. 2021;11(2):a038984. doi: 10.1101/cshperspect.a038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, Schizophrenia Working Group of the Psychiatric Genomics Consortium. Patterson N, Daly MJ, Price AL, Neale BM. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47(3):291–295. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Machluf Y, Israeli A, Cohen E, Chaiter Y, Mezer E. Dissecting the complex sex-based associations of myopia with height and weight. Eye (Lond) 2024;38(8):1485–1495. doi: 10.1038/s41433-024-02931-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong TY, Foster PJ, Johnson GJ, Klein BE, Seah SK. The relationship between ocular dimensions and refraction with adult stature: the tanjong pagar survey. Invest Ophthalmol Vis Sci. 2001;42(6):1237–1242. [PubMed] [Google Scholar]
- 29.Shi XH, Dong L, Zhang RH, Wei WB. Association between weight-adjusted waist index and myopia in adolescents and young adults: results from NHANES 1999-2008. BMC Ophthalmol. 2024;24(1):14. doi: 10.1186/s12886-024-03282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu Q, Zhou JB. Scleral remodeling in myopia development. Int J Ophthalmol. 2022;15(3):510–514. doi: 10.18240/ijo.2022.03.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peled A, Raz I, Zucker I, Derazne E, Megreli J, Pinhas-Hamiel O, Einan-Lifshitz A, Morad Y, Pras E, Lutski M, Cukierman-Yaffe T, Mosenzon O, Tzur D, Tirosh A, Gerstein HC, Afek A, Twig G. Myopia and early-onset type 2 diabetes: a nationwide cohort study. J Clin Endocrinol Metab. 2022;107(2):e663–e671. doi: 10.1210/clinem/dgab669. [DOI] [PubMed] [Google Scholar]
- 32.Liao X, Tan QQ, Lan CJ. Myopia genetics in genome-wide association and post-genome-wide association study era. Int J Ophthalmol. 2019;12(9):1487–1492. doi: 10.18240/ijo.2019.09.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howe LD, Kanayalal R, Harrison S, Beaumont RN, Davies AR, Frayling TM, Davies NM, Hughes A, Jones SE, Sassi F, Wood AR, Tyrrell J. Effects of body mass index on relationship status, social contact and socio-economic position: Mendelian randomization and within-sibling study in UK biobank. Int J Epidemiol. 2020;49(4):1173–1184. doi: 10.1093/ije/dyz240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mina T, Yew YW, Ng HK, Sadhu N, Wansaicheong G, Dalan R, Low DYW, Lam BCC, Riboli E, Lee ES, Ngeow J, Elliott P, Griva K, Loh M, Lee J, Chambers J. Adiposity impacts cognitive function in Asian populations: an epidemiological and Mendelian randomization study. Lancet Reg Health West Pac. 2023;33:100710. doi: 10.1016/j.lanwpc.2023.100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dakanalis A, Voulgaridou G, Alexatou O, Papadopoulou SK, Jacovides C, Pritsa A, Chrysafi M, Papacosta E, Kapetanou MG, Tsourouflis G, Antonopoulou M, Mitsiou M, Antasouras G, Giaginis C. Overweight and obesity is associated with higher risk of perceived stress and poor sleep quality in young adults. Medicina (Kaunas) 2024;60(6):983. doi: 10.3390/medicina60060983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang M, Esteve-Rudd J, Lopes VS, Diemer T, Lillo C, Rump A, Williams DS. Microtubule motors transport phagosomes in the RPE, and lack of KLC1 leads to AMD-like pathogenesis. J Cell Biol. 2015;210(4):595–611. doi: 10.1083/jcb.201410112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Hur YM, Huang WY, Ding X, Feng K, He M. Shared genetic determinants of axial length and height in children: the Guangzhou twin eye study. Arch Ophthalmol. 2011;129(1):63–68. doi: 10.1001/archophthalmol.2010.323. [DOI] [PubMed] [Google Scholar]
- 38.Butowt R, von Bartheld CS. Conventional kinesin-I motors participate in the anterograde axonal transport of neurotrophins in the visual system. J Neurosci Res. 2007;85(12):2546–2556. doi: 10.1002/jnr.21165. [DOI] [PubMed] [Google Scholar]
- 39.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. 2015;44(2):512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted Median estimator. Genet Epidemiol. 2016;40(4):304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koellinger PD, de Vlaming R. Mendelian randomization: the challenge of unobserved environmental confounds. Int J Epidemiol. 2019;48(3):665–671. doi: 10.1093/ije/dyz138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morris TT, Davies NM, Hemani G, Smith GD. Population phenomena inflate genetic associations of complex social traits. Sci Adv. 2020;6(16):eaay0328. doi: 10.1126/sciadv.aay0328. [DOI] [PMC free article] [PubMed] [Google Scholar]