Abstract
AIM
To compare relative peripheral refractive errors (RPREs) in Chinese children with and without myopic anisometropia (MAI) and to explore the relationship between RPRE and myopia.
METHODS
This observational cross-sectional study included 160 children divided into two groups according to the interocular spherical equivalent refraction (SER) difference ≥1.0 D in the MAI group (n=80) and <1.0 D in the non-MAI group (n=80). The MAI group was further divided into two subgroups: ∆SER<2.0 D group and ∆SER≥2.0 D group. Basic ocular biometric parameters of axial length (AL), average keratometry (Ave K), cylinder (CYL), surface regularity index (SRI), and surface asymmetry index (SAI) were recorded. In addition, multispectral refraction topography was performed to measure RPRE, and the parameters were recorded as total refraction difference value (TRDV), refraction difference value (RDV) 0-10, RDV10-20, RDV20-30, RDV30-40, RDV40-53, RDV-superior (RDV-S), RDV-inferior (RDV-I), RDV-temporal (RDV-T) and RDV-nasal (RDV-N).
RESULTS
In the non-MAI group, the interocular differences of all parameters of RPRE were not significant. In the MAI group, the interocular differences of TRDV, RDV10-53, RDV-S, RDV-I, RDV-T, and RDV-N were significant. In subgroup analysis, the interocular differences of TRDV, RDV30-53, RDV-I, and RDV-T were significant in ∆SER<2.0 D group and ∆SER≥2.0 D group, but the interocular differences of RDV10-30, RDV-S and RDV-N were only significant in the ∆SER≥2.0 D group. In correlation analysis, ∆TRDV, ∆RDV 10-53, ∆RDV-S, and ∆RDV-N were negatively correlated with ∆SER but positively correlated with ∆AL.
CONCLUSION
The more myopic eyes have larger hyperopic RPRE in Chinese children with MAI in certain retinal range, and partial ∆RPRE is closely associated with ∆SER and ∆AL.
Keywords: myopia, anisometropia, relative peripheral refractive error, ocular biometric parameters
INTRODUCTION
Myopia is a worldwide eye disease with high prevalence[1]. According to research reports[2], the estimated proportion of the world population with myopia was 28% in 2010 and it is expected to rise to about 50% in 2050. Studies on the prevention and control of myopia are emerging in recent years. Several studies[3]–[5] have proposed the theory of retinal peripheral defocus, which has played an important role in the prevention and control of myopia. According to this theory, products such as orthokeratology[6] and peripheral defocusing lenses[7] which promote transformation from hyperopic relative peripheral refractive errors (RPREs) to myopic RPRE to slow down myopia progression have become effective methods to prevent and control myopia. Taking peripheral defocusing frame glasses as an example, studies showed that self-factors such as age[8], baseline myopia[9], and so on could affect the efficacy of myopia prevention and control.
Some studies showed that both myopic and hyperopic defocus would result in ocular axial changes of the eyes to offset the visual effect caused by defocus[10]–[11]. When the eye was in the state of myopic or hyperopic defocus, the retina would move forward or backward to the ocular imaging plane to get clear focus. This was achieved in two ways: 1) alteration of choroidal thickness; 2) alteration of growth and remodeling rate of sclera[12]. Wallman and Winawer[13] reported that the density of neurons was lower in the peripheral retina compared to the central retina. However, the peripheral retina area was significantly larger than the central retina area. Due to the influence of these two factors, the amount of neurons was higher in the peripheral retina and the effect of these two factors was more pronounced in the peripheral retina. Therefore, they concluded the retinal peripheral signals dominated the progression of myopia. Winawer and Wallman[12] further discovered that wearing lenses with different diopters of defocus on chicks had varying efficacy on myopia progression. Smith Iii et al[14] found that myopic defocus in the near periphery would slow axial growth, but the defocus outside 20° around the central retina wouldn't significantly affect myopia progression. Based on these findings, we thought an investigation of the distribution of retinal peripheral defocus with different degrees of myopia was conducive to clarifying the relationship between myopia and RPRE, exploring the efficacy of RPRE of different regions on myopia progression, and personalizing customized schemes of myopia prevention and control.
There have been studies exploring the distribution of RPRE in different diopters. Sng et al[15] measured the RPRE of Singapore Chinese children at four eccentricities (temporal 30°, temporal 15°, nasal 15°, nasal 30°) and pointed out that moderate and high myopic eyes manifested all hyperopic RPRE at all eccentricities, but the low myopic eyes only manifested hyperopic RPRE at eccentricities of temporal 30° and nasal 30°. Xie et al[16] found that in children aged 4 to 12 years old, low myopia and emmetropia manifested hyperopic RPRE, while low hyperopia manifested myopic RPRE, and RPRE in 15°-45° eccentricity may be closely related to the myopia progression in children. Qi et al[17] found central refraction is significantly correlated with changes of RPRE in 14 to 16 years old non-myopic boys especially RPRE at 20° nasal, 10° nasal, and 20° temporal. It has been found that more myopic eyes have higher hyperopic RPRE to different extents. However, these studies had limitations. First, statistical results were susceptible to differences in age, race, anatomy, and environment. Second, the measurement range of RPRE was limited. On this basis, Du et al[18] compared retinal refraction difference values in adult patients with myopic anisometropia (MAI) compared with those without MAI and found that more myopic eyes in patients with MAI showed more peripheral hyperopic defocus.
Therefore, the present study included Chinese children with MAI as subjects. Anisometropia was defined as interocular spherical equivalent refraction (SER) difference ≥1.0 D. Besides, anisometropia was divided into low anisometropia and high anisometropia with a 2.0 D difference as a limit[19]. MAI is a subgroup of anisometropia. Patients with MAI had consistent genetic factors and very close environmental factors in the paired eyes, which minimized the differences between the eyes with different degrees of myopia. In addition, potential confounding variables such as age and gender were avoided. It provided higher sensitivity in detecting differences between different degrees of myopia and made anisometropia a good disease model for the study of the occurrence and development of myopia[20]. At the same time, multispectral refraction topography (MRT) was introduced to carry out quantitative measurements of RPRE. MRT was a novel advanced equipment that could detect RPRE of 53° range within the retinal area easily. The characteristics of accuracy, reliability, and repeatability of this detection method have been verified[21]. The present study intended to explore the distribution of RPRE in Chinese children with MAI compared with those without MAI by MRT, to clarify the relationship between myopia and RPRE.
SUBJECTS AND METHODS
Ethical Approval
This was an observational cross-sectional study that sampled 160 myopic children from Beijing Ming Vision and Ophthalmology which is the teaching base of Ineye Hospital of Chengdu University of Traditional Chinese Medicine between July 2023 and December 2023. This study got approval from the Medical Ethics Committee of the Ineye Hospital of Chengdu University of Traditional Chinese Medicine (2022yh-024) and conformed to the principles of the Declaration of Helsinki. Written informed consents from all participants and their guardians were obtained.
Subjects
The inclusion criteria: 1) age of 10-18y; 2) binocular myopia (-0.50 to -8.0 D) with astigmatism ≤2.0 D based on objective cycloplegic refraction; 3) best corrected visual acuity ≥20/25; 4) no history of eye disease or surgery; 5) no intervention for myopia was used, such as orthokeratology, soft peripheral defocusing contact lens, and peripheral defocusing frame glasses. The exclusion criteria: 1) poor fitting in examination; 2) low confidence in examination results; 3) accommodation dysfunction or vergence dysfunction/strabismus; 4) atropine or other forms of drug therapy; 5) Not willing to participate in this study.
Participants were divided into two groups according to interocular SER difference based on objective cycloplegic refraction, ∆SER≥1.0 D was recorded as an MAI group; ∆SER<1.0 D was recorded as a non-MAI group. The MAI group was divided into two subgroups: ∆SER<2.0 D group and ∆SER≥2.0 D group. The paired eyes of each subject in all groups were divided into the more myopic eye and the less myopic eye according to the objective cycloplegic refraction, and the interocular differences referred to the differences between the more myopic eye and the less myopic eye.
Examinations
All the children underwent a routine examination which included the following tests: measurement of axial length (AL) using an optical biometer (LS900, Haag-Streit AG, Switzerland), assessment of average keratometry (Ave K), cylinder (CYL), surface regularity index (SRI), and surface asymmetry index (SAI) using corneal topography (TMS-4, Tomey, Japan), and objective cycloplegic refraction. The objective cycloplegic refraction was measured using an autorefractor (KR-9000, Topcon, Japan) after applying a compound tropicamide eye drops (Mydrin-P; Santen, Osaka, Japan) three times at 15-minute intervals. The SER was also calculated, which is the sum of spherical diopters and half of the astigmatic diopters.
For the measurement of RPRE, we used multispectral refraction topography (MSIC2000, Thondar, Shenzhen, China). Each eye was measured at least three times after cycloplegia and the one with the highest confidence (>90%) was chosen for analysis. RPRE was usually used to analyze the refractive state of each peripheral retinal range compared to the macular fovea, which is defined as the refraction value of each retinal peripheral range minus the refraction value of the macular fovea[21]. For a hyperopic RPRE (a positive value), the image was focused behind the retinal plane, while myopic RPRE had the image focused in front of the retinal plane. And according to the range of RPRE, total RPRE from center to peripheral 53° of the retina was recorded as total refraction difference value (TRDV), RPRE from center to 10° of the retina was recorded as refraction difference value (RDV) 0-10, RPRE from 10° to 20° of the retina was recorded as RDV10-20, RPRE from 20° to 30° of the retina was recorded as RDV20-30, RPRE from 30° to 40° of the retina was recorded as RDV30-40, RPRE from 40° to 53° of retina was recorded as RDV40-53, RPRE was divided into RDV-superior (RDV-S), RDV-inferior (RDV-I), RDV-temporal (RDV-T), and RDV-nasal (RDV-N) according to retinal eccentricity direction. All the measurements were operated by the same ophthalmic technician to ensure the accuracy of the examination results.
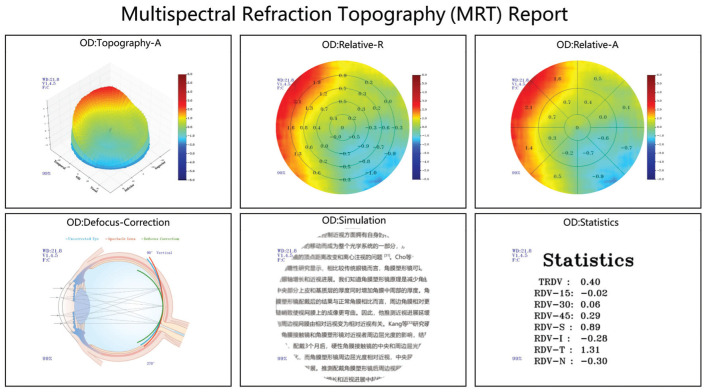
Based on the imaging principle of refraction compensation, MRT used a high-definition zoom camera to directly image the retina of an eye. The calculation system automatically compared and acquired the clearest retinal image, confirmed the actual diopter according to the corresponding compensating lens, and eventually drew a topographic map. MRT used Matrix calculation to calculate and form data reports including Topography-A, Relative-R, Relative-A, Defocus-Correction, Simulation, and Statistics, which could provide data support to meet deep analysis (Figure 1).
Figure 1. The diverse report results of MRT.
MRT: Multispectral refraction topography.
Previously, the “open window” computer optometer (Grand Seiko WAM5500) was mostly performed for the measurement of RPRE. The peripheral retina was exposed by having the patient move their eyes or head to a certain angle, and the optometer was performed from the front to obtain the retinal peripheral diopters at different viewing angles[22]. The optometer cast the incident ray onto the retina of the examined eye and reflected it to the signal-receiving device through the retinal reflection. The refractive states of the examined eye were judged by the difference between the cast lights and the received lights[23]. This method had a long measurement time, a complicated process, few data points, and could not reflect the refractive state of the whole retina.
Compared with the “open window” computer optometer, the characteristic of direct retinal imaging of MRT would avoid errors caused by computer optometer in the process of image transmission. In addition, MRT could complete the monocular 53° range of retinal photography within 5-10s without multi-point fixation. The directness and diversity of data reports of MRT made the clinical application of RPRE more convenient. So far, MRT was the only device that could rapidly detect a retinal defocus topographic map of a 53° range within the retinal area in the world.
Statistical Analysis
Statistical analysis was conducted with SPSS Statistics 25.0 (IBM, Armonk, NY, USA). The Kolmogorov-Smirnov test was initially carried out to evaluate the normality of measurement data. The data that met the assumption of normality was described as mean±standard deviation, an independent sample t-test was used for comparison between groups, and a paired sample t-test was used for comparison between eyes. The data that could not meet the assumption of normality was described as median (25% quartiles -75% quartiles), the Mann-Whitney U test was used for comparison between groups, and the Wilcoxon signed-rank test was used for comparison between eyes. Spearman correlation coefficients were used for further analysis of the relationship between the interocular difference of RPRE and the interocular difference of ocular biometric parameters in the MAI group. P<0.05 was considered statistically significant.
RESULTS
Baseline Characteristics of the Participants
The study contained 169 participants, 9 were excluded because of poor fitting in the examination and low confidence in examination results. One hundred sixty children were divided into MAI group and non-MAI group with a 1.0 D interocular spherical equivalent difference as the limit. In the MAI group, the median age, SER, and AL were 14.5 (11.25 to 17), -3.00 (-5.69 to -1.13), and 24.69 (23.86 to 25.74) respectively. In the non-MAI group, the median age, SER, and AL were 13 (11 to 17), -3.06 (-5.97 to -1.63), and 25.04 (24.01 to 25.86) respectively. Both groups contained 80 participants. In baseline characterization analysis, there was no significant difference in age, SER, and AL between the two groups (P>0.05).
Comparison Between More Myopic Eye and Less Myopic Eye of Diopter and Ocular Biometric Parameters for the Two Groups
In the MAI group, the interocular differences of SER, AL, CYL, and SRI were statistically significant (P<0.05), and the interocular differences of Ave K and SAI weren't significant (P>0.05). In the non-MAI group, the interocular differences of SER, and AL were statistically significant (P<0.05), and the interocular differences of Ave K, CYL, SRI, and SAI weren't significant (P>0.05; Table 1).
Table 1. Comparison of interocular difference of diopter and biometric parameters.
| Parameters | MAI group |
Z | P | Non-MAI group |
Z | P | ||
| More myopic eyes | Less myopic eyes | More myopic eyes | Less myopic eyes | |||||
| SER (D) | -3.88 (-6.56 to 2.50) | -1.44 (-4.94 to -0.79) | -7.749 | <0.001a | -3.56 (-6.47 to -1.75) | -3.13 (-5.88 to -1.50) | -7.261 | <0.001a |
| AL (mm) | 24.92 (24.50 to 26.08) | 24.10 (23.39 to 25.27) | -7.684 | <0.001a | 25.20 (24.17 to 25.99) | 25.11 (24.01 to 25.70) | -5.107 | <0.001a |
| Ave K (D) | 43.91 (43.01 to 44.80) | 43.94 (43.07 to 44.62) | -1.889 | 0.059 | 43.16 (42.33 to 44.12) | 43.31 (42.28 to 44.07) | -0.276 | 0.782 |
| CYL (D) | 1.41 (1.06 to 1.91) | 1.68 (1.19 to 2.38) | -5.487 | <0.001a | 1.34 (0.89 to 1.85) | 1.38 (0.87 to 2.00) | -1.843 | 0.065 |
| SRI | 0.07 (0.06 to 0.23) | 0.13 (0.06 to 0.30) | -0.540 | 0.011a | 0.07 (0.06 to 0.19) | 0.07 (0.06 to 0.16) | -0.396 | 0.692 |
| SAI | 0.30 (0.22 to 0.43) | 0.31 (0.23 to 0.47) | -1.206 | 0.228 | 0.30 (0.22 to 0.50) | 0.29 (0.2 to 0.43) | -1.762 | 0.078 |
MAI: Myopic anisometropia; SER: Spherical equivalent; AL: Axial length; Ave K: Average keratometry; CYL: Cylinder; SRI: Surface regularity index; SAI: Surface asymmetry index. aStatistically significant differences.
Comparison Between More Myopic Eye and Less Myopic Eye of RPRE for the Two Groups
In the MAI group, except for RDV0-10 (P>0.05), the interocular differences of the rest parameters were statistically significant (P<0.05). All the significant differences showed that the more myopic eyes have larger RPRE than the less myopic eyes in certain retinal ranges. In the non-MAI group, there was no significant difference in all parameters of RPRE (P>0.05; Table 2).
Table 2. Comparison of interocular difference of RPRE.
| Parameters | MAI group |
t | P | Non-MAI group |
t | P | ||
| More myopic eyes | Less myopic eyes | More myopic eyes | Less myopic eyes | |||||
| TRDV | 0.723±0.354 | 0.374±0.333 | 7.560 | <0.001a | 0.556±0.333 | 0.500±0.351 | 1.522 | 0.132 |
| RDV0-10 | -0.004±0.036 | 0.001±0.032 | 0.967 | 0.336 | 0.008±0.037 | 0.002±0.030 | 1.062 | 0.292 |
| RDV10-20 | -0.027±0.118 | -0.063±0.099 | 2.381 | 0.020 | -0.028±0.092 | -0.039±0.087 | 0.972 | 0.334 |
| RDV20-30 | 0.214±0.207 | 0.044±0.238 | 5.398 | <0.001a | 0.143±0.207 | 0.111±0.199 | 1.405 | 0.164 |
| RDV30-40 | 0.62±0.36 | 0.288±0.335 | 6.615 | <0.001a | 0.445±0.322 | 0.398±0.320 | 1.298 | 0.198 |
| RDV40-53 | 1.152±0.6 | 0.606±0.534 | 7.696 | <0.001a | 0.866±0.547 | 0.785±0.573 | 1.398 | 0.166 |
| RDV-S | 0.74±0.667 | 0.411±0.695 | 4.031 | <0.001a | 0.49±0.574 | 0.458±0.728 | 0.548 | 0.585 |
| RDV-I | 0.689±0.688 | 0.328±0.711 | 4.563 | <0.001a | 0.588±0.599 | 0.516±0.563 | 1.107 | 0.272 |
| RDV-T | 0.993±0.554 | 0.51±0.657 | 5.820 | <0.001a | 0.881±0.669 | 0.765±0.677 | 1.267 | 0.209 |
| RDV-N | 1.075±0.748 | 0.851±0.762 | 2.061 | 0.043 | 0.893±0.673 | 0.884±0.659 | 0.116 | 0.908 |
RPRE: Relative peripheral refractive error; MAI: Myopic anisometropia; RDV: Refraction difference value; RDV at five retinal eccentricities, from the fovea to 53 degrees recorded as RDV0-10, RDV10-20, RDV20-30, RDV30-40, and RDV40-53; TRDV: Total refraction difference value of center to peripheral 53° of the retina; RDV-S: RDV-superior; RDV-I: RDV-inferior; RDV-T: RDV-temporal; RDV-N: RDV-nasal. The unit of all parameters of RPRE is D (diopter); aStatistically significant differences.
Subgroup Analysis
The MAI group was divided into two subgroups: ∆SER<2.0 D group (n=40) and ∆SER≥2.0 D group (n=40). In the ∆SER<2.0 D group, the median age, SER, and AL were 15 (11 to 17), -2.94 (-6.22 to -1.25), and 24.82 (24.03 to 26.09) respectively. In the ∆SER≥2.0 D group, the median age, SER, and AL were 15 (12 to 17), -3.06 (-5.47 to -1.03), and 24.69 (23.65 to 25.41) respectively. In the baseline analysis, there was no significant difference in age, SER, and AL between the two subgroups (P>0.05).
In the difference analysis for the more myopic eyes and the less myopic eyes for the two subgroups, in the ∆SER<2.0 D group, except for RDV0-10, the rest parameters of RPRE all showed larger in the more myopic eyes. But only the interocular differences of TRDV, RDV30-53, RDV-I, and RDV-T were statistically significant (P<0.05). In the ∆SER≥2.0 D group, all parameters of RPRE except RDV0-10 showed larger in the more myopic eyes and all the interocular differences were statistically significant (P<0.05; Table 3).
Table 3. Comparison of interocular difference of RPRE for the two subgroups.
| Parameters | ∆SER<2.0 D group |
t | P | ∆SER≥2.0 D group |
t | P | ||
| More myopic eyes | Less myopic eyes | More myopic eyes | Less myopic eyes | |||||
| TRDV | 0.646±0.359 | 0.441±0.296 | 3.598 | 0.001a | 0.801±0.336 | 0.307±0.358 | 7.497 | <0.001a |
| RDV0-10 | -0.005±0.034 | -0.002±0.031 | -0.609 | 0.546 | -0.004±0.038 | 0.003±0.034 | -0.757 | 0.454 |
| RDV10-20 | -0.048±0.077 | -0.053±0.09 | 0.331 | 0.743 | -0.005±0.146 | -0.074±0.108 | 2.587 | 0.014a |
| RDV20-30 | 0.168±0.192 | 0.098±0.187 | 1.946 | 0.059 | 0.26±0.213 | -0.01±0.272 | 5.745 | <0.001a |
| RDV30-40 | 0.531±0.353 | 0.368±0.289 | 2.714 | 0.010a | 0.708±0.349 | 0.207±0.362 | 6.993 | <0.001a |
| RDV40-53 | 1.037±0.593 | 0.700±0.504 | 3.600 | 0.001a | 1.268±0.591 | 0.512±0.552 | 7.792 | <0.001a |
| RDV-S | 0.559±0.576 | 0.471±0.612 | 1.008 | 0.320 | 0.921±0.708 | 0.35±0.773 | 4.452 | <0.001a |
| RDV-I | 0.699±0.757 | 0.368±0.669 | 2.465 | 0.018a | 0.678±0.621 | 0.288±0.757 | 4.595 | <0.001a |
| RDV-T | 0.963±0.628 | 0.502±0.666 | 3.427 | 0.001a | 1.023±0.474 | 0.519±0.656 | 5.110 | <0.001a |
| RDV-N | 0.959±0.786 | 1.023±0.813 | -0.475 | 0.638 | 1.198±0.696 | 0.671±0.668 | 3.303 | 0.002a |
RPRE: Relative peripheral refractive error; SER: Spherical equivalent refraction; RDV: Refraction difference value; RDV at five retinal eccentricities, from the fovea to 53 degrees recorded as RDV0-10, RDV10-20, RDV20-30, RDV30-40, and RDV40-53; TRDV: Total refraction difference value of center to peripheral 53° of the retina; RDV-S: RDV-superior; RDV-I: RDV-inferior; RDV-T: RDV-temporal; RDV-N: RDV-nasal. The unit of all parameters of RPRE is D (diopter); aStatistically significant differences.
Correlation Analysis
Spearman correlation analysis was used to investigate the relationship between the interocular difference of RPRE with significant differences and the interocular difference of ocular biometric parameters with significant differences in the MAI group. ∆TRDV, ∆RDV10-53, ∆RDV-S, and ∆RDV-N were negatively correlated with ∆SER, but positively with ∆AL (Table 4).
Table 4. Correlation between ∆RPRE and ∆diopter and ∆biometric parameter.
| Parameters | ∆SER |
∆AL |
∆CYL |
∆SRI |
||||
| rs | P | rs | P | rs | P | rs | P | |
| ∆TRDV | -0.425 | <0.001a | 0.453 | <0.001a | -0.026 | 0.822 | -0.042 | 0.710 |
| ∆RDV10-20 | -0.261 | 0.020a | 0.227 | 0.043a | -0.129 | 0.253 | -0.065 | 0.566 |
| ∆RDV20-30 | -0.412 | <0.001a | 0.411 | <0.001a | -0.026 | 0.819 | -0.046 | 0.687 |
| ∆RDV30-40 | -0.423 | <0.001a | 0.461 | <0.001a | -0.016 | 0.886 | -0.039 | 0.728 |
| ∆RDV40-53 | -0.433 | <0.001a | 0.466 | <0.001a | -0.032 | 0.775 | -0.070 | 0.539 |
| ∆RDV-S | -0.410 | <0.001a | 0.448 | <0.001a | -0.140 | 0.217 | 0.122 | 0.282 |
| ∆RDV-I | -0.139 | 0.218 | 0.107 | 0.343 | 0.137 | 0.226 | -0.088 | 0.440 |
| ∆RDV-T | -0.024 | 0.830 | 0.120 | 0.290 | -0.033 | 0.772 | -0.109 | 0.336 |
| ∆RDV-N | -0.299 | 0.007a | 0.249 | 0.026a | 0.077 | 0.499 | 0.062 | 0.585 |
RPRE: Relative peripheral refractive error; SER: Spherical equivalent; AL: Axial length; CYL: Cylinder; SRI: Surface regularity index; RDV: Refraction difference value; RDV at five retinal eccentricities, from the fovea to 53 degrees recorded as RDV0-10, RDV10-20, RDV20-30, RDV30-40, and RDV40-53; TRDV: Total refraction difference value of center to peripheral 53° of the retina; RDV-S: RDV-superior; RDV-I: RDV-inferior; RDV-T: RDV-temporal; RDV-N: RDV-nasal. The unit of all parameters of RPRE is D (diopter); aStatistically significant differences.
DISCUSSION
In our present study, we investigated the distribution of RPRE and its relationship with diopter and ocular biometric parameters in Chinese children. Our findings showed that there was no difference in all parameters of RPRE in the non-MAI group. Meanwhile, the differences of all parameters of RPRE except RDV0-10 were significant in the MAI group. The differences of RDV0-10 were not significant in both groups and the difference values were close to zero. The reason could be that RDV0-10 was determined as the average refraction value of the center to 10° of the retina minus the refraction value of macular fovea and the measurement range of macular fovea refraction was center to 10° of retina designed by the developer. These two values were close so the difference value was truly small. When it came to subgroup analysis of the MAI group, the interocular difference of TRDV, RDV30-53, RDV-I, and RDV-T were significant in both ∆SER<2.0 D group and ∆SER≥2.0 D group, but the interocular differences of RDV10-30, RDV-S and RDV-N were only significant in the ∆SER≥2.0 D group. We concluded that when the difference in the degree of myopia exceeded 1.0 D, the TRDV, RDV30-53, RDV-I, RDV-T were significantly different, and the RDV10-30, RDV-S, and RDV-N were significantly different when the difference of degree of myopia exceeded 2.0 D. We could infer that as the degree of myopia increased, hyperopic RPRE was in an increasing trend from our study result. The increase of RDV30-53 may appear earlier than the increase of RDV10-30.
There may be reasons for the different distribution of RPRE between the paired eyes in the MAI group. On the one hand, the differences in biological parameters of the anterior ocular segment may partly explain the different distributions of RPRE. The biological parameters of the anterior ocular segment would change in myopia progression. Ametropia was not caused by a single optical aberration but by the unbalanced distribution of various refractive components. Corneal refractive power and AL were the main factors for the ocular refraction state[24]. Chang et al[25] found that the anterior segment of the eyeball has shown flatter corneal curvature, decreased cornea thickness, and decreased endothelial density in myopia progression. Zhang[20] found that AL, pupil diameter, Ave K, and anterior chamber depth were significantly different between the paired eyes by exploring the distribution of ocular biological parameters in adults with MAI. These studies have different results, which may be caused by the different inclusion criteria of the subjects and different examination equipment. In our study, we found that CYL and SRI were significantly different between the paired eyes in the MAI group. The difference of CYL and SRI showed the different symmetry of corneal morphology, which may affect the distribution of RPRE. However, ∆RPRE and ∆biometric parameters did not correlate in our study, which may be due to a small sample. In future studies, we should include more biological parameters of the anterior ocular segment and expand the study sample to explore the effect of biological parameters of the anterior ocular segment on RPRE.
On the other hand, the change of the posterior ocular segment during myopia progression may affect the distribution of RPRE. First, the decrease in sclera thickness was regionally different during myopia progression[26]. Second, the degree of choroidal thinning was different in the retinal region during myopia progression[27]. So the thinning of choroidal thickness may affect the distribution of RPRE to a certain extent. Third, the surface and volume of Bruch's Membrane increased with myopia progression[28]. These reports about the asymmetric change of the posterior ocular segment during myopia progression may reveal the asymmetries and eccentricity-dependent differences in ocular growth patterns. However, there is no way to estimate whether the change of posterior ocular segment is a cause or consequence of the different distributions of RPRE. So we should further collect and follow up the the parameters of the posterior ocular segment and RPRE to explore their relationship.
In correlation analysis, ∆TRDV, ∆RDV10-53, ∆RDV-S, and ∆RDV-N were negatively correlated with ∆SER, and positively correlated with ∆AL. Our finding inferred that the 10°-53° range of RPRE was correlated with myopia development. The result was somewhat different from the study of Zheng et al[29]. Zheng et al[29] found AL was positively correlated with RDV20-45 and this may be due to the different inclusion criteria. Moreover, a study[30] carried on by our team found RPRE in the range of 10°-53° after wearing orthokeratology lenses is closely related to myopia progression, which was consistent with our study result. In correlation analysis of different retinal eccentricity directions, Zhao et al[31] found that the degree of myopia was negatively correlated with RDV-S and Lee and Cho[32] found that the progression of myopia was negatively correlated with peripheral refraction in the nasal field, which was partly consistent with our study results.
We inferred the amount of RPRE after wearing lenses was associated with myopia progression and different amounts of additional myopic RPRE should be designed in children with different degrees of myopia and RPRE to acquire ideal myopic RPRE after wearing lenses. On the one hand, Zhang et al[33] found the myopia progression of eyes of different baseline RPRE in the single vision spectacles group was not different. Besides, eyes of different baseline RPRE made different myopia progression with the same additional myopic RPRE in defocus incorporated multiple segments spectacles group. Shuai et al[30] found that RPRE in the range of 10°-53° after wearing orthokeratology lenses is positively correlated with myopia progression. Lee and Cho[32] thought the baseline RPRE and changes in RPRE could not predict myopia progression. Based on the results, we inferred that the amount of RPRE after wearing lenses instead of the baseline RPRE or the change amount of RPRE was associated with myopia progression. On the other hand, nowadays most peripheral defocusing frame glasses[34]–[35] added the same myopic RPRE but acquired different myopic RPRE after wearing lenses for all patients. To get better myopia prevention and control efficacy, different additional RPRE should be designed in children with different degrees of myopia and our study described the distribution of RPRE in different degrees of myopia which would be helpful to guide the design of lenses.
The relationship between the retinal peripheral defocus theory and myopia development is unclear. On one hand, an earlier study carried on by Mutti et al[36] found that changes in the 30° temporal RPRE before the onset of myopia may be a potential predictor of myopia. Later, Atchison et al[37] tried to establish the functional relationship between central diopter and RPRE by following up the myopia progression and change of RPRE of more than 1700 Chinese children within two years but finally found that RPRE could not predict the onset or progression of myopia in children. In the latest study, Lin et al[38] proposed that RPRE in the superior retina may be a predictor of central myopia shift. Changes in RPRE were more likely to be a result of myopia development than a cause. On the other hand, optical corrected means which promoted transformation from hyperopic RPRE to myopic RPRE were widely agreed as effective myopia prevention and control methods[39]. Our study described the distribution of RPRE during the critical period of myopic development that the more myopic eyes had larger hyperopic RPRE than the less myopic eyes and ∆TRDV, ∆RDV10-53, ∆RDV-S, and ∆RDV-N were negatively correlated with ∆SER, but positively with ∆AL. This study result provided a reference and theoretical basis for revealing the relationship between retinal peripheral defocus theory and myopia.
In conclusion, we included Chinese children with MAI as study subjects to explore the relationship between myopia and the distribution of RPRE to exclude the effect of individual differences. The result confirmed more myopic eyes have larger hyperopic RPRE in certain retinal ranges. It revealed the relationship between RPRE and myopia, which helps achieve the individualized design of defocusing products for myopia prevention and control and explores the myopia prevention and control principle of defocus theory.
Footnotes
Authors' contributions: Tong YT designed the study, analyzed the data, and wrote the manuscript; Ge SS, Du YQ, Ma XQ, Guo YJ, and Chen L collected and analyzed the data; Zhou YH supervised the project.
Conflicts of Interest: Tong YT, None; Du YQ, None; Ge SS, None; Chen L, None; Ma XQ, None; Guo YJ, None; Zhou YH, None.
REFERENCES
- 1.Bremond-Gignac D. Myopia in children. Med Sci (Paris) 2020;36(8-9):763–768. doi: 10.1051/medsci/2020131. [DOI] [PubMed] [Google Scholar]
- 2.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ, Resnikoff S. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Lin Z, Martinez A, Chen X, Li L, Sankaridurg P, Holden BA, Ge J. Peripheral defocus with single-vision spectacle lenses in myopic children. Optom Vis Sci. 2010;87(1):4–9. doi: 10.1097/OPX.0b013e3181c078f1. [DOI] [PubMed] [Google Scholar]
- 4.Sarkar S, Khuu S, Kang P. A systematic review and meta-analysis of the efficacy of different optical interventions on the control of myopia in children. Acta Ophthalmol. 2024;102(3):e229–e244. doi: 10.1111/aos.15746. [DOI] [PubMed] [Google Scholar]
- 5.Erdinest N, London N, Lavy I, Berkow D, Landau D, Levinger N, Morad Y. Peripheral defocus as it relates to myopia progression: a mini-review. Taiwan J Ophthalmol. 2023;13(3):285–292. doi: 10.4103/tjo.TJO-D-22-00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho P, Cheung SW, Edwards M. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: a pilot study on refractive changes and myopic control. Curr Eye Res. 2005;30(1):71–80. doi: 10.1080/02713680590907256. [DOI] [PubMed] [Google Scholar]
- 7.Kanda H, Oshika T, Hiraoka T, Hasebe S, Ohno-Matsui K, Ishiko S, Hieda O, Torii H, Varnas SR, Fujikado T. Effect of spectacle lenses designed to reduce relative peripheral hyperopia on myopia progression in Japanese children: a 2-year multicenter randomized controlled trial. Jpn J Ophthalmol. 2018;62(5):537–543. doi: 10.1007/s10384-018-0616-3. [DOI] [PubMed] [Google Scholar]
- 8.Jones-Jordan LA, Sinnott LT, Chu RH, Cotter SA, Kleinstein RN, Manny RE, Mutti DO, Twelker JD, Zadnik K, CLEERE Study Group Myopia progression as a function of sex, age, and ethnicity. Invest Ophthalmol Vis Sci. 2021;62(10):36. doi: 10.1167/iovs.62.10.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Lu Y, Huang D, Yang J, Fan C, Chen C, Li J, Wang Q, Li S, Jiang B, Jiang H, Li X, Yang Z, Lan W. The efficacy of defocus incorporated multiple segments lenses in slowing myopia progression: results from diverse clinical circumstances. Ophthalmology. 2023;130(5):542–550. doi: 10.1016/j.ophtha.2023.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Arumugam B, Hung LF, To CH, Sankaridurg P, Smith EL., III The effects of the relative strength of simultaneous competing defocus signals on emmetropization in infant rhesus monkeys. Invest Ophthalmol Vis Sci. 2016;57(10):3949–3960. doi: 10.1167/iovs.16-19704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benavente-Perez A, Nour A, Troilo D. The effect of simultaneous negative and positive defocus on eye growth and development of refractive state in marmosets. Invest Ophthalmol Vis Sci. 2012;53(10):6479–6487. doi: 10.1167/iovs.12-9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winawer J, Wallman J. Temporal constraints on lens compensation in chicks. Vis Res. 2002;42(24):2651–2668. doi: 10.1016/s0042-6989(02)00300-0. [DOI] [PubMed] [Google Scholar]
- 13.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43(4):447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Smith Iii EL, Arumugam B, Hung LF, She ZH, Beach K, Sankaridurg P. Eccentricity-dependent effects of simultaneous competing defocus on emmetropization in infant rhesus monkeys. Vision Res. 2020;177:32–40. doi: 10.1016/j.visres.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sng CC, Lin XY, Gazzard G, Chang B, Dirani M, Chia A, Selvaraj P, Ian K, Drobe B, Wong TY, Saw SM. Peripheral refraction and refractive error in Singapore Chinese children. Invest Ophthalmol Vis Sci. 2011;52(2):1181–1190. doi: 10.1167/iovs.10-5601. [DOI] [PubMed] [Google Scholar]
- 16.Xie L, Li YR, Su YY, Chen L. Analysis of peripheral retinal defocus state in children aged 4-12 years. Chinese Journal of Optometry and Vision Science. 2019;25(2):139–145. [Google Scholar]
- 17.Qi LS, Yao L, Wang XF, Zhao J, Liu Y, Wu TY, Yang QH, Zhao C, Zou ZK. Relative peripheral refraction and its role in myopia onset in teenage students. Int J Ophthalmol. 2022;15(7):1108–1115. doi: 10.18240/ijo.2022.07.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du YQ, Zhou YH, Ding MW, Zhang MX, Guo YJ, Ge SS. Observation of peripheral refraction in myopic anisometropia in young adults. Int J Ophthalmol. 2023;16(12):2082–2088. doi: 10.18240/ijo.2023.12.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nunes AF, Batista M, Monteiro P. Prevalence of anisometropia in children and adolescents. F1000Res. 2021;10:1101. doi: 10.12688/f1000research.73657.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang HQ. Study on biological parameters of eyes after femtosecond laser surgery and hyperfocal optical correction for anisometropia. South China University of Technology. 2022.
- 21.Lu W, Ji R, Ding W, Tian Y, Long K, Guo Z, Leng L. Agreement and repeatability of central and peripheral refraction by one novel multispectral-based refractor. Front Med (Lausanne) 2021;8:777685. doi: 10.3389/fmed.2021.777685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore KE, Berntsen DA. Central and peripheral autorefraction repeatability in normal eyes. Optom Vis Sci. 2014;91(9):1106–1112. doi: 10.1097/OPX.0000000000000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gurnani B, Kaur K. StatPearls Internet. Treasure Island (FL): StatPearls Publishing; 2023. Autorefractors. 2023 Jun 11. [Google Scholar]
- 24.Zeng J, Cui Y, Li J, Xie WJ, Li ZM, Zhang L, Meng QL. Correlation of axial length and corneal curvature with diopter in eyes of adults with anisometropia. Int J Clin Exp Med. 2015;8(8):13639–13643. [PMC free article] [PubMed] [Google Scholar]
- 25.Chang SW, Tsai IL, Hu FR, Lin LL, Shih YF. The cornea in young myopic adults. Br J Ophthalmol. 2001;85(8):916–920. doi: 10.1136/bjo.85.8.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maruko I, Iida T, Sugano Y, Oyamada H, Akiba M, Sekiryu T. Morphologic analysis in pathologic myopia using high-penetration optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(7):3834–3838. doi: 10.1167/iovs.12-9811. [DOI] [PubMed] [Google Scholar]
- 27.Hoseini-Yazdi H, Vincent SJ, Collins MJ, Read SA, Alonso-Caneiro D. Wide-field choroidal thickness in myopes and emmetropes. Sci Rep. 2019;9:3474. doi: 10.1038/s41598-019-39653-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonas JB, Bikbov MM, Wang YX, Jonas RA, Panda-Jonas S. Anatomic peculiarities associated with axial elongation of the myopic eye. J Clin Med. 2023;12(4):1317. doi: 10.3390/jcm12041317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng X, Cheng D, Lu X, Yu X, Huang Y, Xia Y, Lin C, Wang Z. Relationship between peripheral refraction in different retinal regions and myopia development of young Chinese people. Front Med (Lausanne) 2021;8:802706. doi: 10.3389/fmed.2021.802706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shuai Y, Yu J, Zhang J, Guo YJ, Liu X. Periretinal defocus in children with myopia after wearing orthokeratology glasses. Recent Advances in Ophthalmology. 2023;43(12):983–986,991. [Google Scholar]
- 31.Zhao Q, Du XL, Yang Y, Zhou YL, Zhao XX, Shan XB, Meng YX, Zhang M. Quantitative analysis of peripheral retinal defocus checked by multispectral refraction topography in myopia among youth. Chin Med J. 2023;136(4):476–478. doi: 10.1097/CM9.0000000000002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee TT, Cho P. Relative peripheral refraction in children: twelve-month changes in eyes with different ametropias. Ophthalmic Physiol Opt. 2013;33(3):283–293. doi: 10.1111/opo.12057. [DOI] [PubMed] [Google Scholar]
- 33.Zhang HY, Lam CSY, Tang WC, Leung M, Qi H, Lee PH, To CH. Myopia control effect is influenced by baseline relative peripheral refraction in children wearing defocus incorporated multiple segments (DIMS) spectacle lenses. J Clin Med. 2022;11(9):2294. doi: 10.3390/jcm11092294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lam CSY, Tang WC, Zhang HY, Lee PH, Tse DYY, Qi H, Vlasak N, To CH. Long-term myopia control effect and safety in children wearing DIMS spectacle lenses for 6years. Sci Rep. 2023;13:5475. doi: 10.1038/s41598-023-32700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bao J, Yang A, Huang Y, Li X, Pan Y, Ding C, Lim EW, Zheng J, Spiegel DP, Drobe B, Lu F, Chen H. One-year myopia control efficacy of spectacle lenses with aspherical lenslets. Br J Ophthalmol. 2022;106(8):1171–1176. doi: 10.1136/bjophthalmol-2020-318367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mutti DO, Hayes JR, Mitchell GL, Jones LA, Moeschberger ML, Cotter SA, Kleinstein RN, Manny RE, Twelker JD, Zadnik K, CLEERE Study Group Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2007;48(6):2510–2519. doi: 10.1167/iovs.06-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atchison DA, Li SM, Li H, Li SY, Liu LR, Kang MT, Meng B, Sun YY, Zhan SY, Mitchell P, Wang NL. Relative peripheral hyperopia does not predict development and progression of myopia in children. Invest Ophthalmol Vis Sci. 2015;56(10):6162–6170. doi: 10.1167/iovs.15-17200. [DOI] [PubMed] [Google Scholar]
- 38.Lin ZH, Xi XY, Wen LB, Luo ZW, Artal P, Yang ZK, Lan WZ. Relative myopic defocus in the superior retina as an indicator of myopia development in children. Invest Ophthalmol Vis Sci. 2023;64(4):16. doi: 10.1167/iovs.64.4.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mak CY, Yam JC, Chen LJ, Lee SM, Young AL. Epidemiology of myopia and prevention of myopia progression in children in East Asia: a review. Hong Kong Med J. 2018;24(6):602–609. doi: 10.12809/hkmj187513. [DOI] [PubMed] [Google Scholar]