Abstract
Background:
There is a great public health need to identify novel treatment strategies for opioid use disorder (OUD) in order to reduce relapse and overdose. Noninvasive brain stimulation (NIBS) has demonstrated preliminary effectiveness for substance use, but little is known about its use in OUD. Neuromodulation may represent a potential adjunctive treatment modality for OUD, so we conducted a systematic review to understand the state of the current research in this field.
Methods:
We conducted a systematic review of studies using noninvasive brain stimulation to affect clinical outcomes related to substance use for adults with opioid use disorder. We searched the following online databases: PubMed, The Cochrane Library, PsycINFO (EBSCOhost, 1872-present), and Science Citation Index Expanded (ISI Web of Science, 1945-present). All studies that measured clinical outcomes related to substance use, including cue-induced craving, were included. We assessed risk of bias using the Cochrane Handbook.
Results:
The initial search yielded 5590 studies after duplicates were removed. After screening titles and abstracts, 14 full-text studies were assessed for eligibility. Five studies were determined to meet inclusion criteria with a combined total subjects of N = 150. Given the paucity of studies and small number of total subjects, no quantitative analysis was performed. These studies used TMS (n = 3), tDCS (n = 1), and the BRIDGE device (n = 1), a noninvasive percutaneous electrical nerve field stimulator, to reduce cue-induced craving (n = 3), reduce clinical withdrawal symptoms (n = 1), or measure substance-use-related cortical plasticity (n = 1).
Conclusions:
There is a dearth of research in the area of noninvasive brain stimulation for OUD. NIBS represents a novel treatment modality that should be further investigated for OUD.
Keywords: Addiction, noninvasive brain stimulation, opioid use disorder, transcranial direct current stimulation, transcranial magnetic stimulation
INTRODUCTION
Opioid use disorder (OUD) affects more than 2 million people each year (1). Opioid overdose resulted in over 42,000 fatalities in 2016 (2). Medications have proven to be effective for the treatment of OUD, but relapse remains common, affecting up to 50% of those in treatment (3,4).
According to the Substance Abuse and Mental Health Services Administration (SAMHSA), the goal of treatment for OUD is remission of the disorder leading to lasting recovery (5). To help facilitate the recovery process, the Food and Drug Administration (FDA) has approved three medications, methadone, buprenorphine, and extended-release naltrexone, to treat OUD. All three medications have been shown to help individuals reduce or stop opioid misuse and achieve long-term recovery (6-9). There is strong evidence in support of both methadone and buprenorphine for reduction of opioid use, retention of patients in treatment (10), and reduction in mortality risk from overdose (6).
Nonpharmacologic treatment modalities for OUD include psychotherapy and neuromodulation. Individuals with OUD frequently benefit from counseling, case management, motivational interviewing, and family therapy (11-14). Behavioral counseling such as motivational interviewing (MI) is especially effective at the entry phase of treatment when engagement and retention are paramount. Whereas therapies that emphasize improvement in coping and relapse prevention skills, such as cognitive behavioral therapy (CBT), are best implemented during primary treatment phase (15), in terms of efficacy, previous studies have demonstrated that MI is effective at improving retention for outpatient substance use disorder treatment in general (16). Multiple studies have shown that CBT is effective specifically for the treatment of OUD especially in combination with buprenorphine (17,18).
Functional neuroimaging in substance use disorders has demonstrated disrupted connectivity in the prefrontal cortex and limbic system in abstinent (19-22) and current users (23). Several studies have demonstrated decreased connectivity in the prefrontal cortex (PFC) (19-22), and increased connectivity in the nucleus accumbens (21,22). However, others have demonstrated greater connectivity to the dorsomedial PFC (24) and decreased connectivity to the insula, nucleus accumbens, and amygdala (23). There is also evidence for impaired structural connectivity (25-30) in frontal regions in OUD. Indeed, hypofrontality has been causally linked to substance use, as one animal study demonstrated that optogenetically compensating for hypoactivity of the dorsolateral PFC (DLPFC) decreased drug-seeking behavior (31).
Neuromodulatory techniques have been proposed as adjunctive therapies to target this altered neurocircuitry (32-34). Repetitive transcranial magnetic stimulation (rTMS) to the left DLPFC stimulates dopamine release in the striatum (35,36), anterior cingulate cortex, and medial prefrontal cortex (37). Neuromodulatory activation of the reward circuitry facilitates synaptic plasticity (38), which can lead to changes in cortical regions associated with behavioral inhibition and decision-making (39-42). Neuromodulation techniques have potential to ameliorate this disrupted connectivity. Noninvasive brain stimulation (NIBS) are forms of neuromodulation that are applied externally or percutaneously. These include TMS, transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), and some applications of vagus nerve stimulation (VNS). These techniques can be subdivided into depolarizing (i.e., TMS, implanted VNS) or nondepolarizing (i.e. tDCS and tACS), depending on their ability to depolarize neurons.
TMS involves placement of an external electromagnetic coil on the head, while a large current (~8000 amps) is passed through an insulated wire coil held flat on the surface of a subject’s scalp in a fraction of a millisecond. This pulse of current creates a magnetic field, which induces a weak electrical current within the surface brain cortex (43). TMS is currently approved by the U.S. Food and Drug Administration (FDA) for the treatment of major depressive disorder (MDD) and obsessive compulsive disorder (OCD) and has shown promise for modulation of neural reward circuitry. Stimulation protocols for MDD and OCD vary but generally involve daily treatment for four to six weeks. Repetitive TMS (rTMS) has been shown to induce analgesia via release of endogenous opioids (44,45). Further, positron emission tomography demonstrated decreased availability of μ-opioid receptor following TMS (46) and TMS-induced analgesia was reversed by administration of naloxone (47), suggesting TMS modulates pain via opioid receptors (48).
In tDCS, low intensity direct current at a constant rate is applied to the scalp via two or more electrodes that can be “wet” (e.g. utilizing electrolyte gel or saline) or “dry.” Typical treatment parameters include current amplitude (in milliamps [mA]), stimulation duration (usually 40 min or fewer per session), number of sessions, as well as electrode size, placement, and orientation (the “montage”). The application of this low intensity current (0.5–2.0 mA) modulates the neuronal resting membrane potential and cortical excitability in targeted brain regions. Beneath the anodal electrode, resting membrane potential decreases, thereby increasing cortical excitability, while beneath the cathodal electrode, the membrane is hyperpolarized, which decreases excitability (49-51). tDCS is not approved by the FDA for any indication but has been used experimentally and off-label to treat acute (52) and chronic pain (53), depression (54), Parkinson’s disease, and other disorders (49-51). tDCS has also been studied for the treatment of other substance use disorders, including alcohol, tobacco, cocaine, methamphetamine, and cannabis (55).
A related form of transcranial electrical stimulation is tACS. Similar to tDCS, instead of using a fixed current amplitude for the entire stimulation period, it utilizes a varying current waveform, usually sinusoidal. While with tDCS, one electrode is designated the anodal while the other is cathodal, in tACS, the alternating nature of the current means that during one half of the wave oscillation, one electrode is anodal and the other cathodal, while in the second half of the cycle, this pattern is inverted. tACS is believed to affect brain oscillations, whereby increasing the intensity of stimulation modulates the excitability of cortical tissue nonlinearly. Low-intensity stimulation decreases the amplitude of motor evoked potentials (MEPs), while high intensity stimulation increases MEP amplitude (49,51,56). Transcranial random noise stimulation (tRNS) uses broadband noise waveforms. As one of the newer forms of NIBS, little is known about its precise mechanism. Application of tRNS to the motor cortex increases MEP amplitude and may induce temporal summation of neuronal activity. tACS has been used to study perception, motor function, and cognition (51,57).
VNS differs from other forms of neurostimulation in that it involves stimulation of the peripheral nervous system. VNS is traditionally administered invasively, whereby the device is implanted under the skin of the chest wall and connected to two electrodes that are tunneled under the skin and wrapped around the left cervical branch of the vagus nerve. The device contains a lithium battery, which generates an electrical current (0.25 to 3.0 mA) at a given frequency (20 to 50 Hz) and pulse width (130 to 500 ms) across a specified cycle (usually 30 sec on to 5 min off). However, newer forms of noninvasive VNS are being investigated for a number of applications, including headache (58), anxiety (59), and Parkinson’s disease (60,61). The cervical region of the vagus nerve is comprised primarily afferent unmyelinated C fibers with low stimulation thresholds, which allows for low current stimulation to trigger an upstream response to the brain, rather than downstream motor effects. VNS was first identified as a potential treatment for depression after individuals receiving VNS for drug-resistant epilepsy reported improvements in mood. Subsequently, VNS was approved by the FDA in 2005 for use in treatment refractory depression, but in 2007, the Centers for Medicare and Medicaid Services declined to reimburse the procedure, so its use has been limited (62-64). VNS is also approved for treatment-resistant epilepsy. Preclinical studies also suggest VNS may facilitate fear extinction, but this has not been studied in humans (65).
There is a small body of the literature of highly variable quality demonstrating that NIBS can reduce craving, substance use, and relapse for substance use disorders (SUDs). However, these studies have largely been limited to alcohol, cocaine, tobacco, and methamphetamine (55). The primary outcome of these studies is cue-induced craving, which has been shown to predict relapse and substance use (66,67). Meta-analyses (68,69) have demonstrated that TMS and tDCS reduce craving across multiple SUDs, including alcohol (70-77), nicotine (78-85), and cannabis (86). Given the therapeutic potential of NIBS for the treatment of OUD, we conducted a systematic review to assess the current state of the evidence of NIBS for OUD.
METHODS
Protocol and Registration
This systematic review is reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) Statement (87) and is registered in the PROSPERO database (http://www.crd.york.ac.uk/PROSPERO/) under the number CRD42018115572.
Eligibility Criteria
Articles that utilized any form of noninvasive brain stimulation to affect clinical outcomes related to substance use for adults (≥18 years old) with OUD were included. Examples of clinical outcomes related to substance use could include subjective report of craving and withdrawal symptoms, retention in substance use treatment, adherence to substance use medication, toxicology results, and reported illicit drug use. Studies involving children (<18 years old) were excluded. Studies comparing noninvasive brain stimulation with a control treatment (sham or no treatment) were included, but not all studies had a comparison group. Types of noninvasive brain stimulation included transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), and percutaneous forms of vagus nerve stimulation (VNS). The search strategy included descriptors for noninvasive brain stimulation and opioid use disorder (Appendix 1). Randomized controlled trials (RCTs), cohort studies, case–control studies, cross-sectional studies, time-series, and systematic chart reviews published in peer-reviewed journals, letters to the editor, and conference abstracts and posters were considered for this systematic review. Articles that were not primary research studies, including literature reviews, case reports, and meta-analyses, were excluded. Publications without outcome variables, such as protocols or without published quantitative data, were also excluded.
Outcomes
All clinical outcomes related to substance use, including cue-induced craving, were included. Examples of clinical outcomes related to substance use could include subjective report of craving and withdrawal symptoms, retention in substance use treatment, adherence to substance use medication, toxicology results, and reported illicit drug use.
Search Strategy
We searched the following online databases: PubMed, The Cochrane Library, PsycINFO (EBSCOhost, 1872-present), and Science Citation Index Expanded (ISI Web of Science, 1945-present). We utilized a wide-ranging search strategy using broad search terms with the goal of including all types of noninvasive brain stimulation. The initial search contained the MeSH terms “opioid-related disorders,” “transcranial magnetic stimulation,” and “transcranial direct current stimulation” as well as synonyms for opioid use disorder and noninvasive brain stimulation. There were no restrictions on publication date or language. The detailed search strategy for each of the databases is shown in Appendix 1.
Study Selection
Titles and abstracts of the retrieved articles were independently evaluated by two reviewers (HBW, MJM). Studies clearly not meeting inclusion criteria were excluded based on title and abstract. The remaining studies were assessed based on full-text articles and selected if they fully met the inclusion and exclusion criteria (Fig. 1). All screens were performed by two separate reviewers (HBW, MJM) with discrepancies resolved by a third reviewer (TYM). The search strategy is shown in Appendix 1. Following this initial search and screen, we manually searched the references and performed a citation analysis of the included studies to identify any additional articles that met inclusion criteria.
Figure 1.
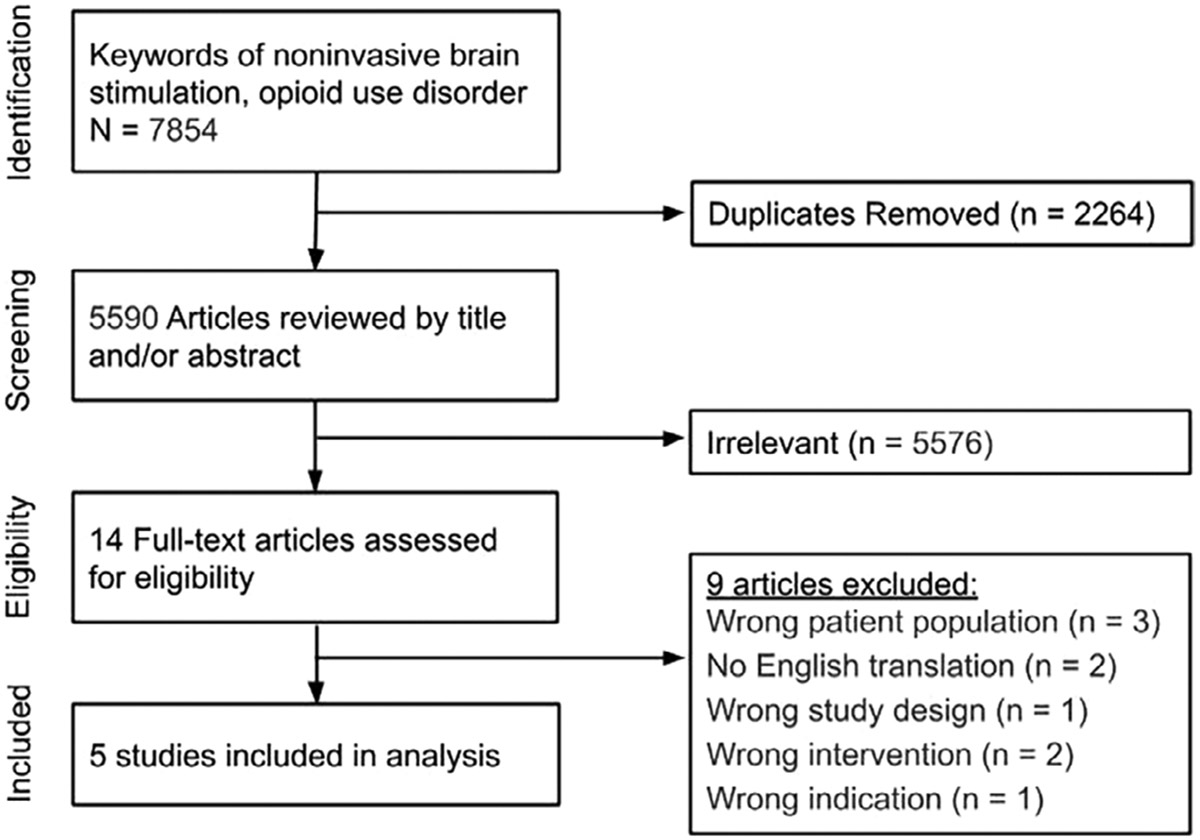
PRISMA Diagram
Data Extraction
Two reviewers (HBW and MJM) independently conducted the data extraction, and disagreements were resolved by a third reviewer (TYM). General characteristics of the studies were collected such as year of publication, location of study, study design, type of noninvasive brain stimulation, stimulation treatment parameters, number of brain stimulation treatments, sample size, and clinical outcome measures. For the primary outcome of interest, pretreatment and posttreatment craving scores were collected for the intervention and control groups, when available.
Qualitative Summary
Due to the heterogeneity of study methods and the small number of included studies (see Results), extracted data were analyzed qualitatively by summarizing the main results of each included paper. Treatment effect was reported based on the data and statistical analysis presented in each study. Assessment of methodologic quality of the studies and risk of bias was performed for each included study as suggested by the Cochrane Handbook (88) and Cochrane Risk of Bias tools (methods.cochrane.org): 1) high risk when more than one indicator of bias was present across all scales and 2) low risk when one or no indicator was present. The risk of bias was classified by two independent reviewers (HBW and MJM), and differences were resolved by a third reviewer (TYM). No studies were excluded based on degree of risk of bias.
RESULTS
Study Characteristics
The initial search yielded 5590 studies after duplicates were removed. After screening titles and abstracts, 14 full-text studies were assessed for eligibility. Five studies were determined to meet inclusion criteria (Fig. 1) (89-93). Given the paucity of studies identified, no quantitative analysis was performed.
The study populations were heterogeneous and incompletely characterized. Subjects ranged in age from 29 to 57 years old, with an average age in the 30s (89,92,93). None of the studies reported urine toxicology testing. In one study, subjects were treatment-seeking (89), while in another, they were not (90). Three other studies did not provide this information (91-93). Duration of opioid use was highly variable. In two studies, subjects’ duration of opioid use ranged from 5 to 25 years, with an average of 16 years of use (92,93), while in a third study, opioid use ranged from 2 to 20 years (91). In another, duration of use only averaged 5.8 years, and no range of use was provided (89). Sahlem et al. did not provide any information on age or duration of opioid use (90).
The included studies used TMS (n = 3) (90,92), tDCS (n = 1) (93), and the BRIDGE device (n = 1) (89), a noninvasive percutaneous electrical nerve field stimulator believed to modulate branches of the vagus nerve, to reduce cue-induced craving (n = 3) (90,92,93), reduce clinical withdrawal symptoms (n = 1) (89), or measure substance-use-related cortical plasticity (91). In three studies, cue-elicited craving was assessed by asking subjects to watch a real video of heroin use (both injection and inhalation) for 5 minutes, then rate their craving on a scale of 0 (not at all) to 100 (very likely to use). None of these studies used neutral cues to compare reactivity to heroin cues (91-93). Two of these studies measured craving before and after the intervention (92,93), but the third only measured craving before TMS (91). The last study reported using a validated cue paradigm and visual analogue scale but did not publish details of cue-induction or range of the scale used (90). These details were clarified in a personal communication (G. Sahlem, personal communication, 7/31/2019). Across all five studies, there was a combined N of 150 participants. There were three randomized controlled trials (90,92,93), one retrospective cohort study (89), and one case control study (91). Three studies compared a noninvasive brain stimulation technique to control (90,92,93), and in all three, the active intervention was found to be superior to the control condition for reduction in cue-induced craving. Two studies included multiple stimulation sessions (89,92). In Shen’s study (2016), participants received 5 daily treatments, while in Miranda’s study, the number of sessions was variable, ranging from 1 to 5 days of treatment, based on clinical response. However, no data are provided on average number of sessions. The other three studies only examined a single treatment session (90,91,93). All five studies included pretreatment and posttreatment outcome measurements (89-93), but none included a follow-up timepoint (Table 1).
Table 1.
Data Extraction From Included Studies.
| Study | Participants | Intervention | Sham control? | Stimulation parameters |
Primary outcome | Minimum abstinence | Craving mean (SD)* | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||||
| Pre- | Post- | Pre- | Post- | |||||||
| Shen, 2016 | 20 heroin-using males | TMS | Y | L DLPFC, 10 Hz, 100% RMT, 2000 pulses, 5 sessions | Cue-induced craving | unknown | 60 (11.2) | 25 (9.2) | 62 (9.5) | 55 (9.2) |
| Wang, 2016 | 20 individuals with history of OUD | tDCS | Y | BL occipital lobes (anodal) and BL FTP areas (cathodal), 1.5 mA, 20 min, 1 session | Cue-induced craving | 1.5-2 y | 68 (8.4) | 43 (7.6) | 62 (5.5) | 62 (5.5) |
| Shen, 2017 | 12 heroin-using males and 12 healthy controls | TMS | N | L Primary Motor Cortex, 10 Hz, 90% RMT, 2000 pulses, 1 session | MEP | 2 weeks | - | - | - | - |
| Miranda, 2018 | 73 outpatients with OUD transitioning to MAT | BRIDGE | N | Dorsal and ventral aspects of the ear to vagus nerve, 3.2 V, “alternating frequencies,” 1–5 sessions | Withdrawal symptoms | unknown | 20.1 (6.1) | 3.1 (3.4) | - | - |
| Study | Participants | Intervention | Sham control? | Stimulation parameters | Primary outcome | Last use? | Craving change from baseline (SD)† | |||
| Sahlem, 2017 | Nontreatment-seeking opioid users | TMS | Y | L DLPFC, 10 Hz, 110–120% RMT, 3000 pulses, 1 session | Cue-induced craving | unknown | −1.7 (1.5) | 0.9 (0.1) | ||
Cue-induced craving was rated on a scale of 0 to 100.
Cue-induced craving was rated on a scale of 0 to 10.
Qualitative Summary of Results
In Shen et al.’s study (2016), twenty males with OUD (heroin only) were randomized to receive active or sham rTMS (92). However, the authors do not specify if subjects were actively using, on MAT, or abstinent and do not provide any urine toxicology for verification. Active rTMS consisted of daily stimulation for 5 days to the left dorsolateral prefrontal cortex (DLPFC) at 10 Hz, 100% resting motor threshold (RMT), 5 s on, 10 s off, for a total of 2000 pulses. For the control condition, the coil was turned 90 degrees away from the skull so that no stimulation was applied to the cortex. Cue-induced craving significantly decreased for individuals who received one session of active rTMS (60 ± 11.2 pretreatment, 40 ± 11.4 posttreatment, p < 0.05) but did not change in the control condition (62 ± 9.5 pretreatment, 62 ± 9.5 posttreatment, p > 0.05). After an additional four rTMS treatments, cue-induced craving decreased further for active rTMS (25 ± 9.2, p = 0.004 compared to baseline) but did not significantly change for sham treatment (55 ± 9.2, p > 0.05).
In the second rTMS study, Sahlem and colleagues applied rTMS to 13 nontreatment-seeking individuals with OUD (90). In a crossover design, participants received active and sham TMS. Active treatment consisted of one session of rTMS applied to the left DLPFC at 10 Hz, goal of 110–120% RMT, for 3000 pulses. This study was a published abstract, so many details of study design were not included, such as the range of the craving scale used. Authors provided additional information in a personal communication. Cues were presented using a three-part cue paradigm consisting of an audio script, physical cues, and a video (94). Craving was rated on a 0–10 visual analogue scale consisting of two items averaged for a final score (“How much do you WANT to use (opiate of choice) right now?” and “HOW HARD would it be for you TO RESIST USING (opiate of choice) right now if it was offered to you?”). Craving was assessed just after the cue paradigm was administered and 15 min after rTMS. Last reported opioid use and urine toxicology were collected but not reported (G. Sahlem, personal communication, 7/31/2019). Active rTMS reduced cue-induced craving more than sham (−1.7 ± 1.5 vs. −0.9 ± 0.1), but did not achieve statistical significance (p = 0.45, Effect size 0.15). They also observed thermal pain thresholds significantly increased more for active treatment than sham (0.5°C ± 1.2°C vs. - 0.8°C ± 1.7°C, p < 0.05, Effect size 0.87), although there were no changes in sensory thresholds or pain tolerance.
In another study by Shen et al. (2017), the investigators applied one session of TMS to 12 male heroin users and 12 male healthy controls in order to study the effect of opioids on cortical plasticity, which the authors believe might predict relapse or craving. Investigators administered 10 Hz intermittent TMS at 90% RMT, 5 s on, 10 s off for 10 min, 2000 pulses, over the left primary motor cortex using a figure-8 coil. Motor evoked potentials (MEPs) were measured from the abductor pollicis brevis muscle on the right hand before and after TMS (5, 10, 15, and 30 min). Twenty MEPs were triggered by single pulse TMS stimulation at the primary motor cortex, and the average peak value was recorded. Cue-induced craving was assessed prior to TMS administration. Authors did not observe a correlation between duration of abstinence and loss of plasticity. TMS rapidly potentiated MEPs in the control group for 30 min but not in the heroin group. However, plasticity 10 min after TMS was correlated with craving in the heroin group (Pearson’s r = −0.25) (91).
Wang et al. conducted a study of tDCS for heroin use (93). Twenty individuals with a history of “heroin addiction,” defined as more than 3 years of continuous compulsive drug seeking, who were abstinent from heroin for at least 18 months, were randomized to active or sham tDCS. For active tDCS, 5 × 7 cm electrodes were placed over the bilateral occipital lobes (anodal) and bilateral frontal–parietal–temporal areas (cathodal stimulation) at 1.5 mA for 20 min. Authors did not provide details on electrode shape or type (dry, saline, or carbon-rubber with electrolyte gel). Sham tDCS was applied as in the active condition but turned off after 30 s. No ramp-up or ramp-down of sham tDCS was described. Individuals who received active tDCS (n = 10) reported a significant decrease in cue-induced craving (68 ± 8.4 prestimulation vs. 43 ± 7.6 poststimulation, p = 0.003), while the reported craving for individuals who received the control intervention (n = 10) did not significantly change (p > 0.05). ANOVA revealed a significant interaction between stimulation group (active vs. sham) and treatment condition (pre vs. post), F1,9 = 12.097, all p < .05, η2p = 0.573, which demonstrated a significant reduction in craving following real tDCS treatment as compared to sham.
Lastly, Miranda et al. conducted an open-label retrospective cohort study of the BRIDGE device, a noninvasive percutaneous electrical nerve field stimulator comprised four leads that are inserted into the dorsal and ventral aspects of the ear (89) and is intended to stimulate branches of the vagus nerve. However, the proposed mechanism of “electrical field nerve stimulation” is vague and is not well characterized. Participants were 73 individuals in outpatient drug treatment clinics who met criteria for DSM-IV opioid dependence and were transitioning to medication-assisted treatment (MAT). All subjects received active treatment with the BRIDGE device whereby voltage-controlled stimulation (3.2 V) was applied to the leads at “alternating frequencies” for one to five days as a bridge to MAT. Stimulation frequencies used were not provided. Application of the BRIDGE device significantly reduced clinical ratings of withdrawal symptoms at each measured timepoint (20.1 ± 6.1 pre-device placement, 7.5 ± 5.9 after 20 min, 4 ± 4.4 after 30 min, 3.1 ± 3.4 after 60 min, and 0.6 after 5 days). As a result, 88.8% of participants successfully transitioned to MAT (Tables 1 and 2).
Table 2.
Assessment of Methodologic Quality of the Studies and Risk of Bias was Performed for Each Included Study as Suggested by the Cochrane Handbook [89] and Cochrane Risk of Bias Tools (methods.cochrane.org): 1) High Risk When More Than One Indicator of Bias Was Present Across All Scales and 2) Low Risk When One or No Indicator Was Present. Studies: Risk of Bias*.
| Study | Allocation concealment |
Blinding of outcome assessors for all outcomes |
Blinding of participants and personnel for all outcomes |
Incomplete outcome data for all outcomes |
Other sources of bias | Selective outcome reporting |
Sequence generation |
|---|---|---|---|---|---|---|---|
| Shen, 2016 (92) | Low | Low | Low | Low | Low | Low | Low |
| Wang, 2016 (93) | High | Low | High | Low | Low | Low | Low |
| Shen, 2017 (91) | High | High | High | Low | Low | Low | High |
| Sahlem, 2017 (90) | Unclear | Unclear | Unclear | High | Low | Low | Low |
| Miranda, 2018 (89) | High | High | High | Low | High | High | High |
Quality assessment was performed according to the Cochrane Handbook by assessing risk of bias for each assigned category. In the table, “Low” indicates low risk of bias, whereas “High” indicates high risk of bias.
DISCUSSION
Given the current epidemic of opioid use and overdoses, there is great public health need to identify novel treatment strategies for OUD. These few studies suggest potential effectiveness of NIBS to reduce craving for OUD, which is consistent with the existing literature regarding its use for other substance use disorders, including alcohol, cocaine, tobacco, and methamphetamine (55). However, as we have identified, there are little good-quality data in very few participants, highlighting a significant knowledge gap and the need for further research to investigate NIBS for OUD. The results of our review preliminarily suggest that one session of rTMS or tDCS may reduce craving immediately after treatment—but larger, well-controlled replication studies with well-defined study populations are needed before any definitive conclusions can be reached. Indeed, there are a number of ongoing studies registered on ClinicalTrials.gov of TMS, including theta burst stimulation, for opioid use disorder (NCT03538444, NCT03821337, NCT03229642, NCT03804619, and NCT03576781).
In our systematic review of noninvasive brain stimulation techniques for the treatment of opioid use disorder, we identified five studies that met our inclusion criteria. The included studies were heterogeneous in methods and quality (Tables 1 and 2). One study was described as a “crossover” design, but authors stated that 20 subjects were randomized to active treatment (n = 10) or sham (n = 10), and the active and sham groups were compared (92). Given no within-subjects analyses were performed, it is most likely that this was in fact not a crossover study design. Authors confirmed that although this study was initially designed as a crossover study, due to subject drop-out, only the initial phase was published. Thus, it is more appropriately described as a parallel arm study (T. Yuan, personal communication, 10/15/2019). Furthermore, analysis in which the difference between pre and postcraving values are compared in separate treatment groups is weaker than assessing the interaction between treatment and craving (95). This approach was used by two studies (92,93). Across studies, assessments of quality revealed most of the studies were low quality (Table 2) often due to poor blinding and randomization. In addition, the study populations were often poorly defined (Table 1). Three of the studies did not specify if the subjects were active users or taking MAT. Furthermore, none of the studies included self-reported last use or biochemical verification (i.e., urine toxicology) of use or abstinence. In two studies, subjects were reported abstinent for at least two weeks (91) and 1.5 to 2 years (93) but did not provide negative urine toxicology results. The study by Sahlem specifies that subjects were non-treatment seeking individuals but does not report last use or urine toxicology results (90). In Miranda et al., subjects were individuals initiating MAT and were presumably using actively, as they experienced withdrawal symptoms that were mitigated by the brain stimulation device (89). While they did provide urine toxicology of concomitant substance use, it is unclear if the reported opioid use was based on urine toxicology or self-report, and they do not include self-reported last use.
Given the heterogeneous and poorly defined subject populations, the methods of eliciting craving are questionable for these studies. Both Wang and Shen (2016 and 2017) use the same cue-induction paradigm (91-93). The demographics and baseline craving scores between the two studies (92,93) are almost identical, but Wang’s study population was reportedly abstinent for 1.5 to 2 years, which raises concern that Shen’s (2016) participants may have also been abstinent. Furthermore, if these subjects were abstinent, it is important to know if they are experiencing withdrawal symptoms based on their last reported use, urine toxicology results, and if they are taking MAT. Additionally, it is ethically questionable to induce craving in individuals in extended recovery from opioid use disorder.
There are many questions regarding methodology and quality in Miranda & Taca (89). First, the BRIDGE device is poorly described, as authors do not describe the baseline waveform or stimulation frequency of the device, nor do they identify a precise mechanism of action, proposing that their stimulator modulates branches of the cranial nerves that innervate the external ear and ultimately communicate with the amygdala. There is little empirical evidence provided to support this mechanism. Second, the pattern of opioid withdrawal symptoms seems highly atypical. First, subjects’ baseline COWS score was 23, which is especially high. For reference, in a case of severe precipitated withdrawal, these authors reported a patient with a maximum COWS score of 17 (96). Authors also report that 0% of the subjects received any rescue medications, including opioid or benzodiazepine during the 5-day study period, which is both highly unusual and inconsistent with the standard of care (97). This also raises concern that subjects may have been using opioids during this time, but there is no repeat urine toxicology provided as supporting evidence. Furthermore, it should be noted that one of the authors is the patent holder for the BRIDGE device and a paid consultant for Alkermes, the manufacturer of extended-release injectable naltrexone (Vivitrol), which may represent conflicts of interest.
Although NIBS has demonstrated preliminary effects for other substance use disorders and represents a novel treatment that should be investigated for OUD, there are basic questions that need to be answered, including the type of NIBS that may be effective (e.g., TMS, tDCS, tACS), treatment parameters, and number of treatment sessions. Shen demonstrated that 5 days of treatment reduced craving more than one session, suggesting that multiple treatment sessions may have a cumulative effect. However, the durability of these effects are yet unknown. Furthermore, there are differences in durability of effects for depolarizing (TMS) and nondepolarizing NIBS (tDCS), as evidence suggests tDCS may have cumulative and delayed effects on cortical plasticity (49,53,98). Preliminary evidence suggests theta burst stimulation (a type of higher frequency rTMS) may reduce craving in a mixed sample of substance use disorders (99) and therefore should also be investigated for OUD, which could reduce the length of treatment sessions. However, for any study of NIBS, participants should be randomized and include adequate sham conditions.
Future studies should also focus on modulating networks that have been empirically identified. The literature we identified used standard stimulation sites for rTMS (left DLPFC) and tDCS (fronto-parieto-temporal region). Some studies suggest the medial prefrontal cortex is responsible for cue-reactivity across substance use disorders and should be a treatment target (100). Other groups have used functional connectivity analysis to empirically identify network deficiencies and then modulate these networks using rTMS in order to determine causality (101), an approach that should be applied to the study of substance use disorders as well.
We also propose considerations for study population and outcome measures in order to ensure high-quality studies with reliable results. Further research is needed in real-world clinical population of opioid users. The current standard of care for OUD includes treatment with MAT, including buprenophrine, methadone, or naltrexone (5). Given the effectiveness of MAT in increasing treatment retention and reducing illicit opioid use and overdose (102,103) future studies of brain stimulation should include individuals on MAT, as none of the current studies included individuals already on MAT (Table 1). Outcome measures should include self-reported opioid use corroborated by urine toxicology as well as treatment retention in order to measure clinically-significant outcomes, which could demonstrate effectiveness of the treatment. These outcomes were lacking in the literature we identified (Table 1). Currently, cue-induced craving is used as the primary outcome for many NIBS studies, as it predicts risk of relapse (104). However, NIBS should be investigated as an adjunctive treatment to both reduce craving and relapse as well as to increase adherence to MAT. NIBS may also serve as a bridging technique to mitigate withdrawal symptoms during initiation of antagonist treatment, when exogenous opioids cannot be used, as used by Miranda (89). NIBS should also be investigated for this scenario, potentially starting stimulation during a bridge period and continuing it afterward. Given its subthreshold neuromodulation property, evidence suggests that tDCS may be more effective when coupled with a desired behavior (51,56). Thus, NIBS should also be studied as adjunctive treatment to psychosocial interventions, such as CBT, to determine if there is a synergistic effect of combination treatment.
CONCLUSIONS
Opioid use poses a significant public health challenge. While medications are effective, relapse remains common. Substance use disorders are characterized by increased response to reward and impaired top-down executive function. NIBS techniques have been utilized to alter both of these circuits in animal and human studies. Preliminary studies have demonstrated NIBS can be used to reduce craving for individuals with substance use disorders. The present systematic review identified very few published studies of NIBS techniques for OUD. The studies that have been performed have suffered from small sample sizes and poor characterization of the study population and their substance use patterns, as well as inadequate attempts at participant masking and controlling sources of bias. As such, there is a paucity of high-quality, rigorously-conducted research. There is great need for future studies to determine if these brain stimulation techniques can be effective treatments for OUD and what the optimal treatment parameters should be.
Source(s) of financial support:
This work was supported by NIH K23DA042326 (Joji Suzuki) and a 2017 Harvard Medical School Norman E. Zinberg Fellowship in Addiction Psychiatry Research (Timothy Y. Mariano).
APPENDIX 1. SEARCH STRATEGY
Table A1.
| Pubmed | ||
|---|---|---|
| 1 | “Opioid-Related Disorders”[MeSH] | 23,583 |
| 2 | (opioid*[tiab] OR opiat*[tiab] OR opium[tiab] OR narcot*[tiab] OR heroin*[tiab]) AND (abuse*[tiab] OR addict*[tiab] OR dependen*[tiab] OR disorder*[tiab] OR misuse*[tiab] OR use*[tiab]) | 47,875 |
| 3 | 1 OR 2 | 57,461 |
| 4 | (“Transcranial Magnetic Stimulation”[MeSH] OR “Transcranial Direct Current Stimulation”[Mesh]) | 11,439 |
| 5 | (“transcranial magnetic”[tiab] OR “transcranial direct current”[tiab] OR “transcranial alternating current”[tiab] OR “transcranial random noise”[tiab] OR “vagus nerve stimulation”[tiab] OR TMS[tiab] OR tDCS[tiab] OR tACS[tiab] OR rTMS[tiab] OR tRNS[tiab] OR VNS[tiab] OR theta burst stimulat*[tiab] OR iTBS[tiab] OR cTBS[tiab]) | 25,919 |
| 6 | (brain*[tiab] OR cortex[tiab] OR cortical[tiab] OR transcranial[tiab] OR cranial[tiab] OR magneti*[tiab]) AND (stimulat*[tiab] OR electrostim*[tiab] OR electro-stim*[tiab] OR electrotherap*[tiab] OR electro-therap*[tiab] OR excitation[tiab]) | 147,523 |
| 7 | ((noninvasive[tiab] OR noninvasive[tiab]) AND stimulat*[tiab]) | 8533 |
| 8 | 4 OR 5 OR 6 OR 7 | 160,330 |
| 9 | 3 AND 8 | 1134 |
| Embase | ||
| 1 | “narcotic dependence”/exp OR “opioid use disorder”/exp | 29,106 |
| 2 | ((opium:ab,ti OR opioid*:ab,ti OR opiate*:ab,ti OR heroin*:ab,ti OR narcot*:ab,ti) AND (abuse*:ab,ti OR addict*:ab,ti OR dependen*:ab,ti OR disorder*:ab,ti OR misuse*:ab,ti OR use*:ab,ti)) | 104,005 |
| 3 | 1 OR 2 | 114,270 |
| 4 | “noninvasive brain stimulation”/exp OR “brain depth stimulation”/exp OR “transcranial magnetic stimulation”/exp OR “transcranial electrical stimulation”/exp OR “nerve stimulation”/exp | 132,172 |
| 5 | “transcranial magnetic”:ab,ti OR “transcranial direct current”:ab,ti OR “transcranial alternating current”:ab,ti OR “transcranial random noise”:ab,ti OR “vagus nerve stimulation”:ab,ti OR TMS:ab,ti OR tDCS:ab,ti OR tACS:ab,ti OR rTMS:ab,ti OR tRNS:ab,ti OR VNS:ab,ti OR theta burst stimulat*:ab,ti OR iTBS:ab,ti OR cTBS:ab,ti | 2470 |
| 6 | OR ((brain:ab,ti OR cortex:ab,ti OR cortical:ab,ti OR transcranial:ab,ti OR cranial:ab,ti OR magneti*:ab,ti) AND (stimulat*:ab,ti OR electrostim*:ab,ti OR electro-stim*:ab,ti OR electrotherapy*:ab,ti OR electro-therap*:ab,ti OR excitation:ab,ti)) | 189,689 |
| 7 | ((“noninvasive”:ab,ti OR “noninvasive”:ab,ti) AND stimulat*:ab,ti) | 12,418 |
| 8 | 4 OR 5 OR 6 OR 7 | 275,375 |
| 9 | 3 AND 8 | 3263 |
| PsycINFO | ||
| 1 | (DE “Drug Addiction” OR DE “Addiction” OR DE “Heroin Addiction” OR DE “Drug Abuse” OR DE “Substance Use Disorder” OR DE “Drug Dependency”) AND (DE “Opiates”) | 5243 |
| 2 | TI((“Opioid-Related Disorders” OR opioid* OR opiate* OR opium OR narcot* OR heroin*) AND (abuse OR addict* OR dependen* OR disorder* OR misuse* OR use*))) | 6646 |
| 3 | AB((“Opioid-Related Disorders” OR opioid* OR opiate* OR opium OR narcot* OR heroin*) AND (abuse OR addict* OR dependen* OR disorder* OR misuse* OR use*))) | 26,894 |
| 4 | 1 OR 2 OR 3 | 27,783 |
| 5 | (DE “Brain Stimulation”) OR (DE “Transcranial Magnetic Stimulation”) | 12,610 |
| 6 | TI((“transcranial magnetic” OR “transcranial direct current” OR “transcranial alternating current” OR “transcranial random noise” OR “vagus nerve stimulation” OR TMS OR tDCS OR tACS OR rTMS OR tRNS OR VNS OR theta burst stimulat* OR iTBS OR cTBS) OR ((brain* OR cortex OR cortical OR transcranial OR cranial OR magneti*) AND (stimulat* OR electrostim* OR electro-stim* OR electrotherap* OR electro-therap* OR excitation)) OR ((non-invasive[tiab] OR non*invasive[tiab]) AND stimulat*[tiab]) | 98,508 |
| 7 | AB((“transcranial magnetic” OR “transcranial direct current” OR “transcranial alternating current” OR “transcranial random noise” OR “vagus nerve stimulation” OR TMS OR tDCS OR tACS OR rTMS OR tRNS OR VNS OR theta burst stimulat* OR iTBS OR cTBS) OR ((brain* OR cortex OR cortical OR transcranial OR cranial OR magneti*) AND (stimulat* OR electrostim* OR electro-stim* OR electrotherap* OR electro-therap* OR excitation)) OR ((non-invasive[tiab] OR non*invasive[tiab]) AND stimulat*[tiab]) | 100,283 |
| 8 | 5 OR 6 OR 7 | 100,344 |
| 9 | 4 AND 8 | 843 |
| Web of Science—Science Citation Index Expanded | ||
| 1 | TS = (“OpioidRelated Disorders” OR ((opium OR opioid* OR opiate* OR heroin* OR narcot*) AND (abuse OR addict* OR dependen* OR disorder* OR misuse* OR use*))) | 65,157 |
| 2 | TS = (“transcranial magnetic” OR “transcranial direct current” OR “transcranial alternating current” OR “transcranial random noise” OR “vagus nerve stimulation” OR TMS OR tDCS OR tACS OR rTMS OR tRNS OR VNS OR theta burst stimulat* OR iTBS OR cTBS OR ((brain* OR cortex OR cortical OR transcranial OR cranial OR magneti*) AND (stimulat* OR electrostim* OR electro-stim* OR electrotherap* OR electro-therap* OR excitation)) OR ((non-invasive OR non*invasive) AND stimulat*) OR “noninvasive brain stimulation” OR “brain depth stimulation” OR “transcranial electrical stimulation” OR “nerve stimulation”) | 259,687 |
| 3 | 1 AND 2 | 2615 |
Footnotes
Conflict of Interest: Timothy Y. Mariano is an employee of Sage Therapeutics, Inc., has served on an advisory board for Janssen Pharmaceuticals and has been a consultant for Ad Scientiam-all outside the scope of the present work. The other authors have no conflicts of interest to disclose. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
REFERENCES
- 1.Center for Behavioral Health Statistics and Quality. Key substance use and mental health indicators in the United States: Results from the 2015 national survey on drug use and health. Substance Abuse and Mental Health Services Administration, editor. Rockville, MD: U.S. Department of Health and Human Services, 2016. [Google Scholar]
- 2.Jones CM, Einstein EB, Compton WM. Changes in synthetic opioid involvement in drug overdose deaths in the United States, 2010-2016. JAMA 2018;319:1819–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark RE, Baxter JD, Aweh G, O’Connell E, Fisher WH, Barton BA. Risk factors for relapse and higher costs among medicaid members with opioid dependence or abuse: Opioid agonists, comorbidities, and treatment history. J Subst Abuse Treat 2015;57:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferri M, Finlayson AJ, Wang L, Martin PR. Predictive factors for relapse in patients on buprenorphine maintenance. Am J Addict 2014;23:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Substance Abuse and Mental Health Services Administration. Medications for opioid use disorder. U.S. Department of Health and Human Services, editor. Rockville, MD: U.S. Department of Health and Human Services, 2018. [Google Scholar]
- 6.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2014;2:CD002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merlo LJ, Greene WM, Pomm R. Mandatory naltrexone treatment prevents relapse among opiate-dependent anesthesiologists returning to practice. J Addict Med 2011;5:279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev 2011;CD001333. [DOI] [PubMed] [Google Scholar]
- 9.White WL. Medication-assisted recovery from opioid addiction: Historical and contemporary perspectives. J Addict Dis 2012;31:199–206. [DOI] [PubMed] [Google Scholar]
- 10.Timko C, Schultz NR, Cucciare MA, Vittorio L, Garrison-Diehn C. Retention in medication-assisted treatment for opiate dependence: A systematic review. J Addict Dis 2016;35:22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbott PJ. Case management: Ongoing evaluation of patients’ needs in an opioid treatment program. Prof Case Manag 2010;15:145–152. [DOI] [PubMed] [Google Scholar]
- 12.National Institute on Drug Abuse. Principles of drug addiction treatment: A research-based guide. 3rd ed. Bethesda, MD: National Institutes of Health, 2012. [Google Scholar]
- 13.Substance Abuse and Mental Health Services Administration. In: DoHaH S, editor. Enhancing motivation for change in substance abuse treatment. Rockville, MD, 1999. [PubMed] [Google Scholar]
- 14.Roberts J, Annett H, Hickman M. A systematic review of interventions to increase the uptake of opiate substitution therapy in injecting drug users. J Public Health (Oxf) 2011;33:378–384. [DOI] [PubMed] [Google Scholar]
- 15.Copenhaver MM, Bruce RD, Altice FL. Behavioral counseling content for optimizing the use of buprenorphine for treatment of opioid dependence in community-based settings: A review of the empirical evidence. Am J Drug Alcohol Abuse 2007;33:643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll KM, Ball SA, Nich C et al. Motivational interviewing to improve treatment engagement and outcome in individuals seeking treatment for substance abuse: A multisite effectiveness study. Drug Alcohol Depend 2006;81:301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling W, Hillhouse M, Ang A, Jenkins J, Fahey J. Comparison of behavioral treatment conditions in buprenorphine maintenance. Addiction 2013;108:1788–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McHugh RK, Hearon BA, Otto MW. Cognitive behavioral therapy for substance use disorders. Psychiatr Clin North Am 2010;33:511–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Li Z, Li W et al. Disrupted default mode network and basal craving in male heroin-dependent individuals: A resting-state fMRI study. J Clin Psychiatry 2016;77:e1211–e1217. [DOI] [PubMed] [Google Scholar]
- 20.Lin HC, Wang PW, Wu HC, Ko CH, Yang YH, Yen CF. Altered gray matter volume and disrupted functional connectivity of dorsolateral prefrontal cortex in men with heroin dependence. Psychiatry Clin Neurosci 2018;72:435–444. [DOI] [PubMed] [Google Scholar]
- 21.Ma N, Liu Y, Li N et al. Addiction related alteration in resting-state brain connectivity. Neuroimage 2010;49:738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhai T, Shao Y, Chen G et al. Nature of functional links in valuation networks differentiates impulsive behaviors between abstinent heroin-dependent subjects and nondrug-using subjects. Neuroimage 2015;115:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Upadhyay J, Maleki N, Potter J et al. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain 2010;133:2098–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Q, Liu J, Wang W et al. Disrupted coupling of large-scale networks is associated with relapse behaviour in heroin-dependent men. J Psychiatry Neurosci 2018;43:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh A, Basu D, Khandelwal N, Ahuja CK, Bn S, Rana D. Risk, reversibility and resilience of brain circuitries linked to opioid dependence: A diffusion tensor imaging study of actively opioid-using subjects and three comparison groups. Asian J Psychiatr 2019;40:107–115. [DOI] [PubMed] [Google Scholar]
- 26.Li W, Li Q, Zhu J et al. White matter impairment in chronic heroin dependence: A quantitative DTI study. Brain Res 2013;1531:58–64. [DOI] [PubMed] [Google Scholar]
- 27.Liu H, Li L, Hao Y et al. Disrupted white matter integrity in heroin dependence: A controlled study utilizing diffusion tensor imaging. Am J Drug Alcohol Abuse 2008;34:562–575. [DOI] [PubMed] [Google Scholar]
- 28.Qiu Y, Jiang G, Su H et al. Progressive white matter microstructure damage in male chronic heroin dependent individuals: A DTI and TBSS study. PLoS One 2013;8:e63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt A, Walter M, Gerber H et al. Inferior frontal cortex modulation with an acute dose of heroin during cognitive control. Neuropsychopharmacology 2013;38:2231–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Li W, Li Q, Yang W, Zhu J, Wang W. White matter impairment in heroin addicts undergoing methadone maintenance treatment and prolonged abstinence: A preliminary DTI study. Neurosci Lett 2011;494:49–53. [DOI] [PubMed] [Google Scholar]
- 31.Chen BT, Yau HJ, Hatch C et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 2013;496:359–362. [DOI] [PubMed] [Google Scholar]
- 32.Diana M, Raij T, Melis M, Nummenmaa A, Leggio L, Bonci A. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nat Rev Neurosci 2017;18:685–693. [DOI] [PubMed] [Google Scholar]
- 33.Ekhtiari H, Tavakoli H, Addolorato G et al. Transcranial electrical and magnetic stimulation (tES and TMS) for addiction medicine: A consensus paper on the present state of the science and the road ahead. Neurosci Biobehav Rev 2019;104:118–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Enokibara M, Trevizol A, Shiozawa P, Cordeiro Q. Establishing an effective TMS protocol for craving in substance addiction: Is it possible? Am J Addict 2016;25:28–30. [DOI] [PubMed] [Google Scholar]
- 35.Pogarell O, Koch W, Popperl G et al. Striatal dopamine release after prefrontal repetitive transcranial magnetic stimulation in major depression: Preliminary results of a dynamic [123I] IBZM SPECT study. J Psychiatr Res 2006;40:307–314. [DOI] [PubMed] [Google Scholar]
- 36.Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci 2001;21:RC157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One 2009;4:e6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, Luscher C. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature 2014;509:459–464. [DOI] [PubMed] [Google Scholar]
- 39.Fecteau S, Agosta S, Hone-Blanchet A et al. Modulation of smoking and decision-making behaviors with transcranial direct current stimulation in tobacco smokers: A preliminary study. Drug Alcohol Depend 2014;140:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fecteau S, Fregni F, Boggio PS, Camprodon JA, Pascual-Leone A. Neuromodulation of decision-making in the addictive brain. Subst Use Misuse 2010;45:1766–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouellet J, McGirr A, Van den Eynde F, Jollant F, Lepage M, Berlim MT. Enhancing decision-making and cognitive impulse control with transcranial direct current stimulation (tDCS) applied over the orbitofrontal cortex (OFC): A randomized and sham-controlled exploratory study. J Psychiatr Res 2015;69:27–34. [DOI] [PubMed] [Google Scholar]
- 42.Soltaninejad Z, Nejati V, Ekhtiari H. Effect of anodal and cathodal transcranial direct current stimulation on DLPFC on modulation of inhibitory control in ADHD. J Atten Disord 2019;23:325–332. [DOI] [PubMed] [Google Scholar]
- 43.Perera T, George MS, Grammer G, Janicak PG, Pascual-Leone A, Wirecki TS. The clinical TMS society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul 2016;9:336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Andrade DC, Mhalla A, Adam F, Texeira MJ, Bouhassira D. Neuropharmaco-logical basis of rTMS-induced analgesia: The role of endogenous opioids. Pain 2011;152:320–326. [DOI] [PubMed] [Google Scholar]
- 45.Taylor JJ, Borckardt JJ, George MS. Endogenous opioids mediate left dorsolateral prefrontal cortex rTMS-induced analgesia. Pain 2012;153:1219–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lamusuo S, Hirvonen J, Lindholm P et al. Neurotransmitters behind pain relief with transcranial magnetic stimulation—positron emission tomography evidence for release of endogenous opioids. Eur J Pain 2017;21:1505–1515. [DOI] [PubMed] [Google Scholar]
- 47.Taylor JJ, Borckardt JJ, Canterberry M et al. Naloxone-reversible modulation of pain circuitry by left prefrontal rTMS. Neuropsychopharmacology 2013;38:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DosSantos MF, Oliveira AT, Ferreira NR, Carvalho ACP, Rosado de Castro PH. The contribution of endogenous modulatory systems to TMS- and tDCS-induced analgesia: Evidence from PET studies. Pain Res Manag 2018;2018:2368386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bikson M, Brunoni AR, Charvet LE et al. Rigor and reproducibility in research with transcranial electrical stimulation: An NIMH-sponsored workshop. Brain Stimul 2018;11:465–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nitsche MA, Cohen LG, Wassermann EM et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul 2008;1:206–223. [DOI] [PubMed] [Google Scholar]
- 51.Woods AJ, Antal A, Bikson M et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol 2016;127:1031–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antal A, Brepohl N, Poreisz C, Boros K, Csifcsak G, Paulus W. Transcranial direct current stimulation over somatosensory cortex decreases experimentally induced acute pain perception. Clin J Pain 2008;24:56–63. [DOI] [PubMed] [Google Scholar]
- 53.Mariano TY, Burgess FW, Bowker M et al. Transcranial direct current stimulation for affective symptoms and functioning in chronic low back pain: A pilot double-blinded, randomized, placebo-controlled trial. Pain Med 2018;20:1166–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fregni F, Boggio PS, Nitsche MA, Marcolin MA, Rigonatti SP, Pascual-Leone A. Treatment of major depression with transcranial direct current stimulation. Bipolar Disord 2006;8:203–204. [DOI] [PubMed] [Google Scholar]
- 55.Coles AS, Kozak K, George TP. A review of brain stimulation methods to treat substance use disorders. Am J Addict 2018;27:71–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Philip NS, Nelson BG, Frohlich F, Lim KO, Widge AS, Carpenter LL. Low-intensity transcranial current stimulation in psychiatry. Am J Psychiatry 2017;174:628–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tavakoli AV, Yun K. Transcranial alternating current stimulation (tACS) mechanisms and protocols. Front Cell Neurosci 2017;11:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Coo IF, Marin JC, Silberstein SD et al. Differential efficacy of non-invasive vagus nerve stimulation for the acute treatment of episodic and chronic cluster headache: A meta-analysis. Cephalalgia 2019;39:967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burger AM, Van der Does W, Thayer JF, Brosschot JF, Verkuil B. Transcutaneous vagus nerve stimulation reduces spontaneous but not induced negative thought intrusions in high worriers. Biol Psychol 2019;142:80–89. [DOI] [PubMed] [Google Scholar]
- 60.Mondal B, Choudhury S, Simon B, Baker MR, Kumar H. Noninvasive vagus nerve stimulation improves gait and reduces freezing of gait in Parkinson’s disease. Mov Disord 2019;34:917–918. [DOI] [PubMed] [Google Scholar]
- 61.Morris R, Yarnall AJ, Hunter H, Taylor JP, Baker MR, Rochester L. Noninvasive vagus nerve stimulation to target gait impairment in Parkinson’s disease. Mov Disord 2019;34:918–919. [DOI] [PubMed] [Google Scholar]
- 62.Aaronson ST, Conway CR. Vagus nerve stimulation: Changing the paradigm for chronic severe depression? Psychiatr Clin North Am 2018;41:409–418. [DOI] [PubMed] [Google Scholar]
- 63.Conway CR, Xiong W. The mechanism of action of vagus nerve stimulation in treatment-resistant depression: Current conceptualizations. Psychiatr Clin North Am 2018;41:395–407. [DOI] [PubMed] [Google Scholar]
- 64.Nemeroff CB, Mayberg HS, Krahl SE et al. VNS therapy in treatment-resistant depression: Clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology 2006;31:1345–1355. [DOI] [PubMed] [Google Scholar]
- 65.Noble LJ, Souza RR, McIntyre CK. Vagus nerve stimulation as a tool for enhancing extinction in exposure-based therapies. Psychopharmacology (Berl) 2019; 236:355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fatseas M, Denis C, Massida Z, Verger M, Franques-Reneric P, Auriacombe M. Cue-induced reactivity, cortisol response and substance use outcome in treated heroin dependent individuals. Biol Psychiatry 2011;70:720–727. [DOI] [PubMed] [Google Scholar]
- 67.McHugh RK, Fitzmaurice GM, Carroll KM et al. Assessing craving and its relationship to subsequent prescription opioid use among treatment-seeking prescription opioid dependent patients. Drug Alcohol Depend 2014;145:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jansen JM, Daams JG, Koeter MW, Veltman DJ, van den Brink W, Goudriaan AE. Effects of non-invasive neurostimulation on craving: A meta-analysis. Neurosci Biobehav Rev 2013;37:2472–2480. [DOI] [PubMed] [Google Scholar]
- 69.Maiti R, Mishra BR, Hota D. Effect of high-frequency transcranial magnetic stimulation on craving in substance use disorder: A meta-analysis. J Neuropsychiatry Clin Neurosci 2017;29:160–171. [DOI] [PubMed] [Google Scholar]
- 70.Boggio PS, Sultani N, Fecteau S et al. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: A double-blind, sham-controlled study. Drug Alcohol Depend 2008;92:55–60. [DOI] [PubMed] [Google Scholar]
- 71.Ceccanti M, Inghilleri M, Attilia ML, Raccah R, Fiore M, Zangen A. Deep TMS on alcoholics: Effects on cortisolemia and dopamine pathway modulation. A pilot study. Can J Physiol Pharmacol 2015;93:283–290. [DOI] [PubMed] [Google Scholar]
- 72.Girardi P, Rapinesi C, Chiarotti F et al. Add-on deep transcranial magnetic stimulation (dTMS) in patients with dysthymic disorder comorbid with alcohol use disorder: A comparison with standard treatment. World J Biol Psychiatry 2015;16:66–73. [DOI] [PubMed] [Google Scholar]
- 73.Herremans SC, Baeken C, Vanderbruggen N et al. No influence of one right-sided prefrontal HF-rTMS session on alcohol craving in recently detoxified alcohol-dependent patients: Results of a naturalistic study. Drug Alcohol Depend 2012;120:209–213. [DOI] [PubMed] [Google Scholar]
- 74.Herremans SC, Vanderhasselt MA, De Raedt R, Baeken C. Reduced intra-individual reaction time variability during a Go-NoGo task in detoxified alcohol-dependent patients after one right-sided dorsolateral prefrontal HF-rTMS session. Alcohol Alcohol 2013;48:552–557. [DOI] [PubMed] [Google Scholar]
- 75.Hoppner J, Broese T, Wendler L, Berger C, Thome J. Repetitive transcranial magnetic stimulation (rTMS) for treatment of alcohol dependence. World J Biol Psychiatry 2011;12:57–62. [DOI] [PubMed] [Google Scholar]
- 76.Mishra BR, Nizamie SH, Das B, Praharaj SK. Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: A sham-controlled study. Addiction 2010;105:49–55. [DOI] [PubMed] [Google Scholar]
- 77.Nakamura-Palacios EM, de Almeida Benevides MC, da Penha Zago-Gomes M et al. Auditory event-related potentials (P3) and cognitive changes induced by frontal direct current stimulation in alcoholics according to Lesch alcoholism typology. Int J Neuropsychopharmacol 2012;15:601–616. [DOI] [PubMed] [Google Scholar]
- 78.Amiaz R, Levy D, Vainiger D, Grunhaus L, Zangen A. Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction 2009;104:653–660. [DOI] [PubMed] [Google Scholar]
- 79.Boggio PS, Liguori P, Sultani N, Rezende L, Fecteau S, Fregni F. Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neurosci Lett 2009;463:82–86. [DOI] [PubMed] [Google Scholar]
- 80.Dinur-Klein L, Dannon P, Hadar A et al. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: A prospective, randomized controlled trial. Biol Psychiatry 2014;76:742–749. [DOI] [PubMed] [Google Scholar]
- 81.Fregni F, Liguori P, Fecteau S, Nitsche MA, Pascual-Leone A, Boggio PS. Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: A randomized, sham-controlled study. J Clin Psychiatry 2008;69:32–40. [DOI] [PubMed] [Google Scholar]
- 82.Johann M, Wiegand R, Kharraz A et al. Transcranial magnetic stimulation for nicotine dependence. Psychiatr Prax 2003;30:S129–S131. [PubMed] [Google Scholar]
- 83.Li X, Hartwell KJ, Owens M et al. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol Psychiatry 2013;73:714–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pripfl J, Tomova L, Riecansky I, Lamm C. Transcranial magnetic stimulation of the left dorsolateral prefrontal cortex decreases cue-induced nicotine craving and EEG delta power. Brain Stimul 2014;7:226–233. [DOI] [PubMed] [Google Scholar]
- 85.Wing VC, Bacher I, Wu BS, Daskalakis ZJ, George TP. High frequency repetitive transcranial magnetic stimulation reduces tobacco craving in schizophrenia. Schizophr Res 2012;139:264–266. [DOI] [PubMed] [Google Scholar]
- 86.Boggio PS, Zaghi S, Villani AB, Fecteau S, Pascual-Leone A, Fregni F. Modulation of risk-taking in marijuana users by transcranial direct current stimulation (tDCS) of the dorsolateral prefrontal cortex (DLPFC). Drug Alcohol Depend 2010;112: 220–225. [DOI] [PubMed] [Google Scholar]
- 87.Moher D, Shamseer L, Clarke M et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Higgins JP, Altman DG, Gotzsche PC et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miranda A, Taca A. Neuromodulation with percutaneous electrical nerve field stimulation is associated with reduction in signs and symptoms of opioid withdrawal: A multisite, retrospective assessment. Am J Drug Alcohol Abuse 2018;44:56–63. [DOI] [PubMed] [Google Scholar]
- 90.Sahlem GL, Breedlove J, Taylor JJ et al. Dorsolateral prefrontal cortex transcranial magnetic stimulation as a tool to decrease pain and craving in opiate dependent individuals: A pilot study of feasibility and effect size. Brain Stimul 2017;10:482. [Google Scholar]
- 91.Shen Y, Cao X, Shan C, Dai W, Yuan TF. Heroin addiction impairs human cortical plasticity. Biol Psychiatry 2017;81:e49–e50. [DOI] [PubMed] [Google Scholar]
- 92.Shen Y, Cao XY, Tan T et al. 10-Hz repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex reduces heroin cue craving in long-term addicts. Biol Psychiatry 2016;80:E13–E14. [DOI] [PubMed] [Google Scholar]
- 93.Wang YJ, Shen Y, Cao XY et al. Transcranial direct current stimulation of the frontal-parietal-temporal area attenuates cue-induced craving for heroin. J Psychiatr Res 2016;79:1–3. [DOI] [PubMed] [Google Scholar]
- 94.Back SE, Gros DF, McCauley JL et al. Laboratory-induced cue reactivity among individuals with prescription opioid dependence. Addict Behav 2014;39:1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nieuwenhuis S, Forstmann BU, Wagenmakers EJ. Erroneous analyses of interactions in neuroscience: A problem of significance. Nat Neurosci 2011;14:1105–1107. [DOI] [PubMed] [Google Scholar]
- 96.Ward HB, Barnett BS, Suzuki J. Rapid transition from methadone to buprenorphine using naltrexone-induced withdrawal: A case report. Subst Abus 2019;40:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kosten TR, Baxter LE. Review article: Effective management of opioid withdrawal symptoms: A gateway to opioid dependence treatment. Am J Addict 2019;28:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sampaio-Junior B, Tortella G, Borrione L et al. Efficacy and safety of transcranial direct current stimulation as an add-on treatment for bipolar depression: A randomized clinical trial. JAMA Psychiat 2018;75:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hanlon CA, Dowdle L, Lench D et al. Modulating cue-reactivity with continuous theta burst stimulation to the frontal pole: A novel target with transdiagnostic relevance. Brain Stimul 2019;12:527. [Google Scholar]
- 100.Hanlon CA, Dowdle LT, Gibson NB et al. Cortical substrates of cue-reactivity in multiple substance dependent populations: Transdiagnostic relevance of the medial prefrontal cortex. Transl Psychiatry 2018;8:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brady RO Jr, Gonsalvez I, Lee I et al. Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia. Am J Psychiatry 2019;176:512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murphy SM, McCollister KE, Leff JA et al. Cost-effectiveness of buprenorphine-naloxone versus extended-release naltrexone to prevent opioid relapse. Ann Intern Med 2019;170:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ronquest NA, Willson TM, Montejano LB, Nadipelli VR, Wollschlaeger BA. Relationship between buprenorphine adherence and relapse, health care utilization and costs in privately and publicly insured patients with opioid use disorder. Subst Abuse Rehabil 2018;9:59–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Courtney KE, Schacht JP, Hutchison K, Roche DJ, Ray LA. Neural substrates of cue reactivity: Association with treatment outcomes and relapse. Addict Biol 2016;21:3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


