Abstract
Alcohol abuse remains one of the primary preventable sources of mortality in the United States. Model species can be used to evaluate behavioral and other biological changes associated with alcohol and to identify novel treatments. This report describes methods for evaluating the behavioral effects of ethanol (EtOH) in crayfish. Crayfish (Procambarus clarkii) were immersed in ethanol concentrations ranging from 0.1 to 1.0 molar, for 10–30 min. Studies evaluated hemolymph alcohol concentration, locomotion in an open field and anxiety-like behavior using a Light/Dark transfer approach. EtOH immersion produced dose-dependent increases in hemolymph EtOH (up to 249 mg/dL) and reductions in open field locomotion that depended on EtOH concentration or exposure duration. Untreated crayfish exhibit avoidance of the open parts of the locomotor arena and a preference for a covered portion. Acute EtOH immersion decreased time spent in the covered portion of the Light/Dark arena, consistent with a decrease in anxiety-like behavior. Daily EtOH immersion for 5 days did not alter locomotor responses, however, activity was increased 3 days after the repeated EtOH regimen. Overall, this study shows that this inexpensive, easily maintained species can be used for behavioral pharmacological experiments designed to assess the acute and repeated effects of EtOH.
Keywords: alcohol, locomotor, crustacean, anxiety, ethanol, withdrawal, toxicity
About 14.4 million adults have an Alcohol Use Disorder (AUD) and some 88,000 Americans die from alcohol-related causes annually, making it the third leading preventable cause of death (https://www.niaaa.nih.gov/sites/default/files/AlcoholFactsAndStats.pdf). It continues to be a pressing concern to learn more about the biological impact of alcohol exposure, so as to generate novel avenues for remediation. A recent review of animal models of substance use disorder calls in part for expansion of behavioral models suitable for substance abuse investigations (Smith, 2020), including invertebrate models. The crayfish, a Genus of numerous species, is a potential animal model which offers significant advantages in terms of cost over more common small vertebrate species such as rats or mice. Species such as the red swamp crayfish (Procambarus clarkii) can be housed in fresh water, at normal room temperature, in small (10–30 gallon) home aquarium tanks. If desired, they have robust reproduction in captivity with scores to hundreds of offspring born from a single egg clutch.
A limited number of papers have shown that crayfish can be used to assess the effects of ethanol on neurotransmission (Swierzbinski et al., 2017) and behavioral effects of other abused drugs, including locomotor responses to intravenous cocaine, morphine and methamphetamine (Imeh-Nathaniel et al., 2017) and the intravenous self-administration of amphetamine (Datta et al., 2018). Furthermore, crayfish are a popular electrophysiology model for introductory neuroscience laboratory classes (Cooper et al.; Ewing & Medler, 2020; Land et al., 2001). Acute serotonin, but not dopamine, injection causes an anxiety-like response in crayfish using a light/dark plus-maze-like assay (Fossat et al., 2015), indicating potential utility as a model of affective disorders.
This study was designed to determine if the crayfish is a suitable model for behavioral studies of the effects of ethanol. Swierzbinski and colleagues showed that if juvenile crayfish are immersed in ethanol (1 M concentration) for 20–40 min they exhibit significant behavioral changes, although the behavior was not well described in that paper. Analysis of the video recordings focused on ataxia (“supine position”) and rapid tail-flips which were only associated with high levels of intoxication. Viewing of the supplementary movie suggests this was potentially a toxic level of intoxication (Swierzbinski et al., 2017). This latter possibility was not determined since the primary goal involved euthanizing the subjects for electrophysiological recording. Therefore, one primary goal of this study was to determine if quantitative alterations of spontaneous locomotor behavior are produced by doses of EtOH below the toxic thresh-old. To determine if ethanol alters locomotor behavior in juvenile crayfish in a dose dependent manner, we used immersion time and EtOH concentration to alter the dose. The open field arena was chosen to investigate locomotor behavior, with a logic similar to that of the open field studies used in rodent models. In short, crayfish are prey species and tend to avoid staying in open water for long. Crayfish will use tunnels or huts provided in the home aquaria for extended periods of time and will defend them against cohoused conspecifics, if present. Thus, the tendency to avoid the center of an open field arena exhibited by rodents was predicted to obtain in the crayfish. In addition, the Light/Dark transfer test in rodents predicts that crayfish would also prefer to spend more time under a cover, when provided. Light/Dark transfer models in rodents typically involve light and dark painted or illuminated regions of an arena with open tops in both cases for observation. Pilot studies attempting to adjust the luminance by placing light or dark material under the transparent bins were unsuccessful at generating differential behavior, thus the more naturalistic full-cover was adopted for this study. While this limited full assessment of behavior (e.g., distance traveled, speed, time immobile/mobile) to the open portion, it permitted assessment of time spent under the cover, versus other zones of the arena, and any transition events (entries), including those between the open and closed portions.
Ethanol was predicted to alter activity of the crayfish, as it does in other laboratory animal models. Bättig first described the effects of ethanol on open field behavior in the rat (Bättig, 1969) and subsequent work showed that 0.4 g/kg, EtOH, i.p., increased ambulatory activity and decreased immobility in an open field in rats (Cappeliez & White, 1981). It was found that 2 g/kg, p.o. increased activity in rats, whereas 4 g/kg was reported to decrease activity (Prunell et al., 1987). Similarly, 2 g/kg EtOH, i.p., decreased motor activity in 20-, 40- or 60-day-old rats (Lamble & Rydberg, 1982) and 0.75 g/kg, i.p. depressed horizonal activity in rats (June et al., 1989). Thus, it was predicted that lower exposure levels would be associated with increased activity and higher exposure levels with decreased activity in the crayfish.
Ethanol was also predicted to decrease anxiety-like behavior in the crayfish. It has been shown that EtOH (1 g/kg, i.p.) in male rats resulted in decreased latency to emerge from the dark portion on a Light/Dark transfer test, as well as increased distance traveled in, and entries into, the light portion of the arena (Sharko et al., 2016). Ethanol in adolescent rats also increased distance traveled and time spent in the open area of an open field, but decreased time spent in the bright portion of a Light/Dark transfer apparatus (Acevedo et al., 2014). Anxiolytic-like effects of EtOH in the Light/Dark test also have been reported for mice (Belzung et al., 1988; Gilmore et al., 1991). Interestingly, a recent report found that adding the selective serotonin reuptake inhibitor citalopram to the housing water decreased the latency of crayfish to emerge from a sheltered location and increased the amount of time spent exploring a food- or conspecific-associated region of an artificial stream environment (Reisinger et al., 2021). This enhances confidence in the ability to assess decreases in anxiety-like behavior in the crayfish.
Method
Subjects
Crayfish (Procambarus clarkii) used in five experimental groups [Cohort 1 (N = 15; 9 female / 6 male), Cohort 2 (N = 11; 7 female / 4 male), Cohort 3 (N = 13; 3 female / 10 male), Cohort 4 (N = 18; 7 female / 11 male), Cohort 5 (N = 29; 12 female / 17 male)] for behavioral studies were either hatched in the laboratory or obtained from a commercial supplier (Carolina Biological Supply). Additional individuals hatched in the laboratory were used for lethality studies including a group < 2.5 cm long (N= 40) and a group ~2.5–4.5 cm long (N = 30). The parents of laboratory bred individuals were obtained from a local pet store. Animals were hatched into a 10 or 20 gallon communal tank and then eventually separated, once reaching ~5 cm in length, into groups (N = 3–5) of similarly sized individuals in separated regions (1/3–1/2 of tank size) of a tank. The tanks were maintained in open laboratory space with natural light and indoor florescent lighting; all experiments were conducted during the daylight hours. Tanks were equipped with a thin layer of aquarium gravel, 2–4 huts and hideouts, free floating anacharis plants and a continuously running filter. Animals were fed every third day with a rotating variety of foodstuffs including anacharis, frozen krill, shrimp pellets and fish food flakes. During the course of any repeated-measures studies, nail polish markings on the carapace were used to identify individuals.
Behavioral Assessment
Locomotor behavior was measured in aquatic open field arenas (see Fig. 1A). These consisted of 56 cm L x 43 cm W x 15 cm D clear plastic bins placed on a light surface and filled to a 11.5-cm depth with water. Dark cardboard shrouds extended approximately 35 cm above the walls of each arena on all four sides. Locomotor activity was recorded videographically using webcams (Logitech Model C270) mounted approximately 1 meter above the arena. Analysis of the behavioral sessions was conducted off-line using ANY-maze behavior tracking software (Stoelting). Initially the tracking was configured for Center versus the Periphery, with a 34 cm X 18 cm defined Center area, as schematized in Figure 1B.
Figure 1. A) A Crayfish Pictured in the Arena. Schematic of B) the Open Area and C) the Arena Configured for a Light/Dark Experiment with Approximately 1/3 of the Area Covered.
Note. The dotted lines did not appear but approximate where the zones were drawn for the video tracking/ scoring.
Ethanol Immersion Exposure
Crayfish were exposed to immersion treatment conditions in 16 cm L X 16 cm W X 10 cm H plastic bins filled with 1 L of water by immersion, following the methods described by Swierzbinski and colleagues (Swierzbinski et al., 2017). Immersion conditions included normal aquarium water or aquarium water adulterated with different concentrations of EtOH (0.1, 0.5, 1.0 Molar [M]) as detailed for specific experiments below. Following immersion, individuals were briefly rinsed in clean water and then were placed in the Center Zone of the arena and the video recording interval was initiated.
Experiment 1: Duration of Exposure to 1.0 M EtOH
The first study was a baseline recording (no treatment) in the arena for 30 min to habituate the animals to the apparatus prior to evaluating the ethanol (EtOH) conditions in a counterbalanced order. For the evaluation of exposure duration, Cohort 1 crayfish (~5–7 cm; ~11 weeks of age) were immersed in water for 20 min, or in 1 M EtOH for 10, 20 or 30 min and then evaluated in the open field for 30 min. The 30-min maximum interval and the EtOH concentration was selected from a prior paper (Swierzbinski et al., 2017). Treatment conditions were counterbalanced across individuals and conducted no more frequently than every 3–4 days per individual. Two female animals did not complete the study due to mortality during molting, thus N = 13 for the analysis.
Experiment 2: EtOH Concentration
For the evaluation of concentration, the same individual crayfish (N = 7 female, N = 6 male) were immersed for 30 min in water, 0.1 M, 0.5 M or 1M EtOH and then recorded in the open field for 30 min. Treatment conditions were counterbalanced across individuals and conducted no more frequently than every 3–4 days per individual. Two of the male animals did not complete the study due to mortality during molting.
Experiment 3: Light/Dark Transfer
Individuals remaining from the same group (N = 6; 4 female / 2 male) of crayfish used in Experiment 2, now adult and with prior EtOH and arena experience, were used to pilot the Light/Dark arena approach and to test the hypothesis that crayfish would spend time preferentially under a cover, when provided. For this purpose one end of the arena was covered ~1/3 with an opaque lid (see Fig. 1C for schematic). The cover was placed in a fixed location for all individuals in this study, that is, at the rear relative to where the experimenter faced the arena and placed/removed the animals. Animals were evaluated in a single 30-min session, without any immersion treatment prior to the session. The open area corresponding to Center for the video tracking was now 21.5 cm X 18 cm and there was a new Transition Zone defined, covering an 8.5 cm L rectangle extending from the cover across the width of the arena. The inclusion of the Transition Zone was designed to capture the extension of visible parts of the animal from under the cover when the animal was not fully emerged, based on pilot studies. (Those pilot studies also confirmed that the animals do not treat the “edge” defined by the cover across the arena as if it were another wall, that is, they do not move along it as they do the walls.) The video tracking zone mapping used for the Light/Dark arena also was used to reanalyze locomotor behavior when the arena was completely open for comparison with the behavior when the cover was in place.
Experiment 4: Effects of EtOH Immersion on Light/Dark Transfer
The effects of acute EtOH immersion on behavior in the Light/Dark arena were assessed in two cohorts of behaviorally and treatment-naïve juveniles. Each individual began with one baseline recording session (no prior treatment) for habituation to the arena. Experimental sessions were run no more frequently than twice per week (3–4-day interval) and the cover placement over front versus rear sections of the arena was balanced across individuals for these studies.
Cohort 2 (N = 11; 7 Female / 4 Male) were ~5–7 cm in length and naïve to the arena and EtOH immersion prior to this study. All animals first received one baseline (i.e., no immersion) session in the Light/Dark arena and then were exposed to water or EtOH (0.05, 0.1 or 1.0 M) for 30 min, with the exposure conditions conducted in a counter-balanced order. Out of the total sample, two females and one male completed only one to two conditions each due to molting and/or mortality and were omitted from the analysis.
Cohort 3 (N = 13; 3 Female / 10 Male) were ~5–7 cm in length and naïve to the arena and EtOH immersion prior to this study. All animals first received one baseline (i.e., no immersion) session in the Light/Dark arena and then were exposed to water or EtOH (0.1, 0.5 or 1.0 M) for 30 mins, with the exposure conditions conducted in a counter-balanced order.
Experiment 5: Lethality of EtOH Immersion in Juvenile Crayfish
Crayfish were immersed in 0.1, 0.5 and 1.0 M EtOH for 30 min and then placed in normal aquarium water in a 20 cm X 20 cm plastic bin for observation. Two age groups (< 2.5 cm / 5–6 weeks of age; ~2.5–4.5 cm / ~9 weeks) were assessed at each concentration with a sample size of 10 individuals per cohort. One cohort was run for most conditions save that two cohorts were run for the 2.5–4.5 cm 0.5 M and the 2.5cm / 1.0 M conditions. Lethality was recorded 15 min into immersion, at the end of immersion, and at 30-min intervals up to 2 hr after the beginning of immersion. All of the individuals below 5 cm were euthanized at the conclusion of this experiment. Any individuals who were observed to be killed by a conspecific or that molted during the assessment interval were removed from the analysis. This involved one < 2.5 cm individual for 0.5 M and 1.0 M EtOH immersion, and two 2.5–4.5 cm individuals for 0.5 M EtOH immersion. Survival data for ~5–7 cm / ~11–12 weeks individuals is derived from the initial behavioral studies (i.e., those described in the main report) and reflects only those individuals exposed for 30 min to the respective EtOH concentration as their first-ever EtOH condition. This included 10 individuals at 1.0 M EtOH immersion, 2 individuals at 0.5 M EtOH immersion and 3 individuals at 0.1 M EtOH immersion.
Experiment 6: Effects of Repeated immersion on Light/Dark Transfer
For this experiment, Cohort 5 crayfish (> 7 cm) were immersed for 30 min each day for 5 sequential days. Animals of > 7cm were selected because the immobilization observed after 1 M EtOH immersion in Experiment 4 was substantial and minimal in Experiments 1 and 2. This, combined with results of Experiment 5, suggested the size/age of the animals were critical. Locomotor testing in the Light/ Dark Transfer configuration was conducted after the first (N = 18 EtOH; N = 11 Water), third (N = 12 EtOH; N = 11 Water) and fifth (N = 16 EtOH; N = 11 Water) days. Locomotor testing was also evaluated on Day 8 after 30 min of water immersion (both groups).
Experiment 7: Hemolymph EtOH Concentration
Hemolymph samples were collected from the pericardial sinus of adult crayfish in EDTA tubes following exposure to 0.1 and 1.0 M EtOH for 30 min. Samples were centrifuged for 10 min at 5,000 G and the supernatant was collected, frozen (−80 °C), and stored for later assessment. Ethanol concentrations were thereafter determined by GC-MS (Agilent 7820A GC coupled to a 7697A headspace sampler, Agilent Technologies, Santa Clara, CA, USA). Crayfish were euthanized immediately after hemolymph sample collections.
Data Analysis
ANY-maze software was used to score behavior in the Center versus the surrounding Periphery zone for the Open Field Arena (Fig. 1B), and in the Center, Periphery, Covered and Transition zones for the Light/Dark Arena (Fig. 1C). For the Open Field arena assessments, the dependent measures included Distance Traveled, Percent of the Time spent Immobile, Speed, Center Entries, and Time spent in Center versus Periphery of the arena. Treatment of missing data (e.g., due to an animal failing to enter the peripheral zone at all, failing to ever stop moving in a zone, or being unavailable for a given treatment condition) was handled by using mixed-effects analysis. One-way ANOVA (or mixed-effects) was used for overall Distance Traveled, Center Entries and Center Time with the single factor of Immersion Condition. Two-way ANOVA (or mixed-effects) was used with factors of Zone and Immersion Condition to analyze Distance Traveled by zone, Speed and Percent of the Time spent Immobile. Initial analysis considered the entire sample and limited follow-up analysis divided the sample into male and female individuals to assess any possible sex differences. For the Light/Dark arena validation, the dependent measures include Distance Traveled, Speed of travel, Percent Time spent, and Time spent Immobile, by zone.
In the repeated immersion study, the dependent measures for the Light/Dark arena included Distance Traveled, Speed of travel, Time spent in a given zone and the number of Entries into a given zone. The first analysis contrasted the treatment groups on Day 1 and Day 8, thus there was a repeated measures factor of Zone and a between-subjects factor of Immersion condition (Water vs. EtOH). The data then were analyzed within-group by treatment Day (D1-D8) because the first hypothesis under investigation involved tolerance with repeated administration (D1 to D5 as the critical test) and any effect of discontinuation (i.e., D8 compared with D1 and D5). A D3 test was included for most of the study because the initial cohort (N = 6) exhibited no difference between D1 and D5 and thus there was a concern a biphasic trend was being missed. Thus, the design included repeated measures factors for Zone and assessment Day, and again, a mixed-effects analysis was used to account for missing subjects in a given treatment condition or behavioral measure. Any significant effects were followed with post-hoc analysis using Tukey correction for all multilevel, and Sidak correction for any two-level, comparisons. Prism 9 for Windows (v. 9.1.1; GraphPad Software, Inc, San Diego CA) was used for all analyses.
Results
Baseline Behavior
The Procambarus clarkii juvenile crayfish exhibit significant locomotor behavior under baseline conditions when placed in the brightly illuminated open field, traveling an average of 23 (SEM 2.9) meters in 60 min (Fig. 2A). The distribution of the animals’ time and activity in the Center versus the Periphery under baseline conditions showed a distinct preference for the Periphery. They moved faster (2–3 x) when in the center (Fig. 3A) while spending less time (~90 out of the 1800-s session; Fig. 3D) and moving less (~10%) total distance (Figs. 2B, C) in the Center Zone. These relative patterns were consistent across all treatment conditions in the EtOH experiments, as well.
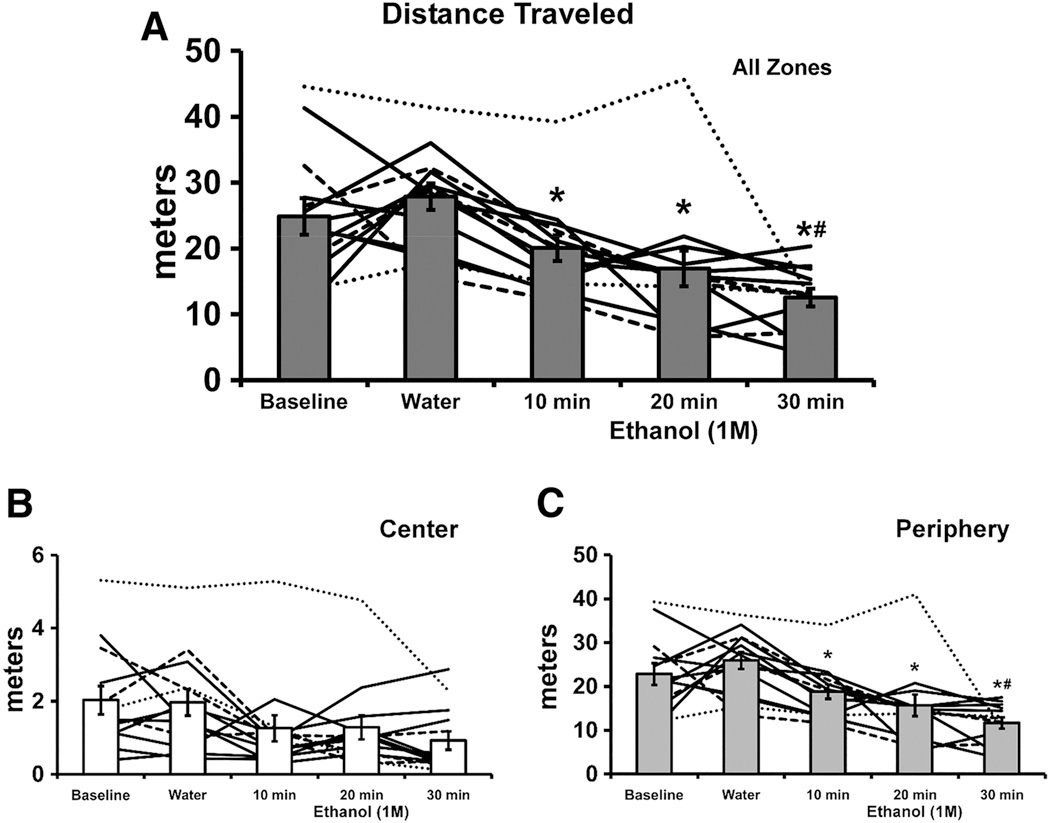
Figure 2. Mean (N = 13; ±SEM) Distance Traveled by Crayfish in the Open Arena over 30 Min Is Depicted with the Bars.
Note. The lines depict individual subject data. There is a 10-fold difference in the scale for Center and Periphery. A significant difference from Water is indicated with * and a difference from 10 min with #.
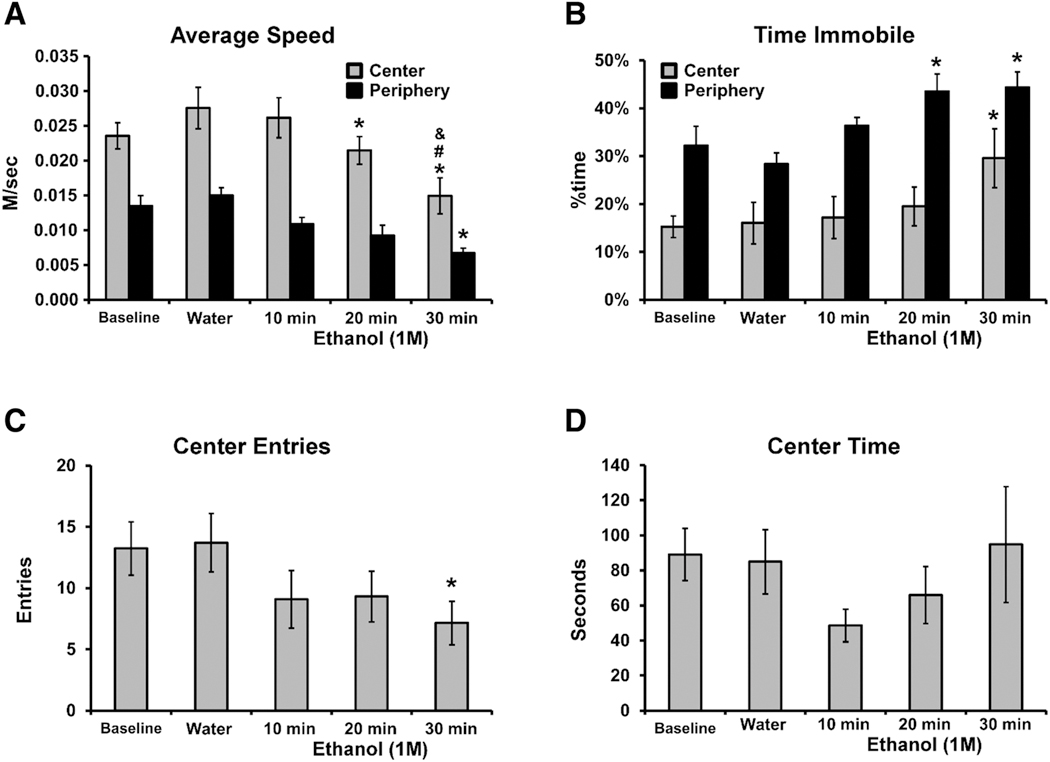
Figure 3. Mean (N = 13; ±SEM) Speed, Time Spent Immobile, Center Entries and Time Spent on the Center of the Open Arena over 30 Min.
Note. A significant difference from Water is indicated with * and a difference from 10 min with # and a difference from 20 min with &.
Effects of Ethanol Exposure Duration
The effects of ethanol on locomotor behavior were immersion-interval dependent in a monotonic relationship for most activity measures. Following immersion in EtOH (1.0 M) for 30 min., crayfish traveled approximately half of the distance traveled after water immersion, with an approximately similar magnitude of reduction in both Center and Peripheral zones (Fig. 2). The statistical analysis confirmed significant effects of EtOH immersion on Distance Traveled overall [F (2.817, 33.80) = 13.40; p < .0001; Fig. 2A] and Center Entries [F (2.769, 33.23) = 4.84; p < .01; Fig. 3C]. Crayfish traveled significantly more distance in the Periphery, compared with the Center, as confirmed in the analysis [significant effects of Zone, F (1, 12) = 199.9; p < .0001; Immersion Condition, F (4, 48) = 13.42; p < .0001; and the interaction, F (4, 48) = 11.68; p < .0001; Fig. 2B, C]. The post-hoc test confirmed significant reductions in peripheral distance traveled, relative to water immersion, for all three EtOH durations and also after 30 min compared with 10 min of EtOH immersion. In addition, significantly less distance was traveled in the Center versus the Periphery after each of the immersion conditions. The crayfish moved faster in the Center compared with the Periphery [significant effects of Zone, F (1, 12) = 107.7; p < .0001; Immersion Condition, F (4, 48) = 8.93; p < .0001; interaction, n. s.; Fig. 3A]. The post-hoc test confirmed that movement was faster in the Center following each of the Immersion conditions, and that significantly slower movement was observed in the Center after 20- or 30-min immersion, and in the Periphery after 30 min of immersion, relative to water immersion. Slower movement in the Center was also confirmed after 30 min of immersion compared with 10 or 20 min. The crayfish spent a larger proportion of their time immobile when in the Periphery compared to when in the Center (significant effects of Zone, F (1, 12) = 48.97; p < .0001; Immersion Condition, F (4, 48) = 5.09; p < .005; interaction, n.s.; Fig. 3B). The post-hoc test confirmed that less time was spent immobile in the Center across all Immersion conditions, and that significantly more time was spent immobile in the Center after the 30-min. immersion, and in the Periphery after 20 or 30 min of immersion, relative to water immersion.
Effects of Ethanol Concentration
In the second experiment, the effects of ethanol depended on the concentration, again in a monotonic relationship for most activity measures, including overall Distance Traveled [F (2.335, 23.35) = 10.65; p < .0005; Fig. 4]. There was no significant effect of immersion condition on time spent in the Center (Fig. 5D) or Center entries (p < .06; Fig. 5C). Crayfish traveled significantly more distance in the Periphery, compared with the Center, of the arena and this distance was altered by EtOH immersion [significant effects of Zone, F (1, 10) = 96.04; p < .0001; Immersion Condition, F (3, 30) = 10.64; p < .0001; and the interaction, F (3, 30) = 9.98; p = .0001; Fig. 4B, C]. The post-hoc test confirmed a significant reduction in peripheral distance traveled after 1.0 M EtOH immersion, relative to all other immersion conditions. There was also a significant difference in between the 0.1 M and 0.5 M EtOH immersion conditions. In addition, the post-hoc test confirmed that significantly less distance was traveled in the Center versus the Periphery for each of the immersion conditions.
Figure 4. Mean (N = 11; ±SEM) Distance Traveled by Crayfish in A) All of the Open Arena, or in the B) Center and C) Peripheral Zones, over 30 Min Is Depicted with the Bars.
Note. The lines depict individual subject data. There is a 10-fold difference in the scale for Center and Periphery. A significant difference from Water is indicated with *, a difference from 0.1 M immersion with # and from 0.5 immersion with &.
Figure 5. Mean (N = 11; ±SEM) A) Speed of Travel, B) Time Spent Immobile, C) Entries into the Center Zone and D) Time Spent in the Center Zone, in the Open Arena over 30 Min.
Note. A significant difference from Water is indicated with *, a difference from 0.1 M immersion with # and a difference from 0.1 M immersion with &.
The crayfish moved faster in the Center compared with the Periphery [significant effects of Zone, F (1, 10) = 104.2; p < .0001; Immersion Condition, F (3, 30) = 11.44; p < .0001; and of the interaction, F (3, 30) = 5.30; p < .005; Fig. 5A]. The post-hoc test confirmed that movement was faster in the Center for each of the Immersion conditions, and that significantly slower movement was observed in the Center after 1.0 M EtOH immersion compared with all other conditions. Movement in the Center was also faster after the 0.1 M EtOH immersion compared with all other immersion conditions. Movement speed was slower in the Periphery after 1.0 M EtOH immersion compared with water or 0.1 M EtOH immersion. The crayfish spent a larger proportion of their time immobile when in the Periphery compared to when in the Center (significant effects of Zone, F (1, 10) = 51.08; p < .0001; Immersion Condition, F (3, 30) = 3.289; p < .05; interaction, n.s.; Fig. 5B). The post-hoc test confirmed that less time was spent immobile in the Center after each of the Immersion conditions, and that significantly more time was spent immobile in the Periphery after 1.0 M EtOH immersion compared with 0.1 M EtOH immersion.
Hemolymph Ethanol Levels
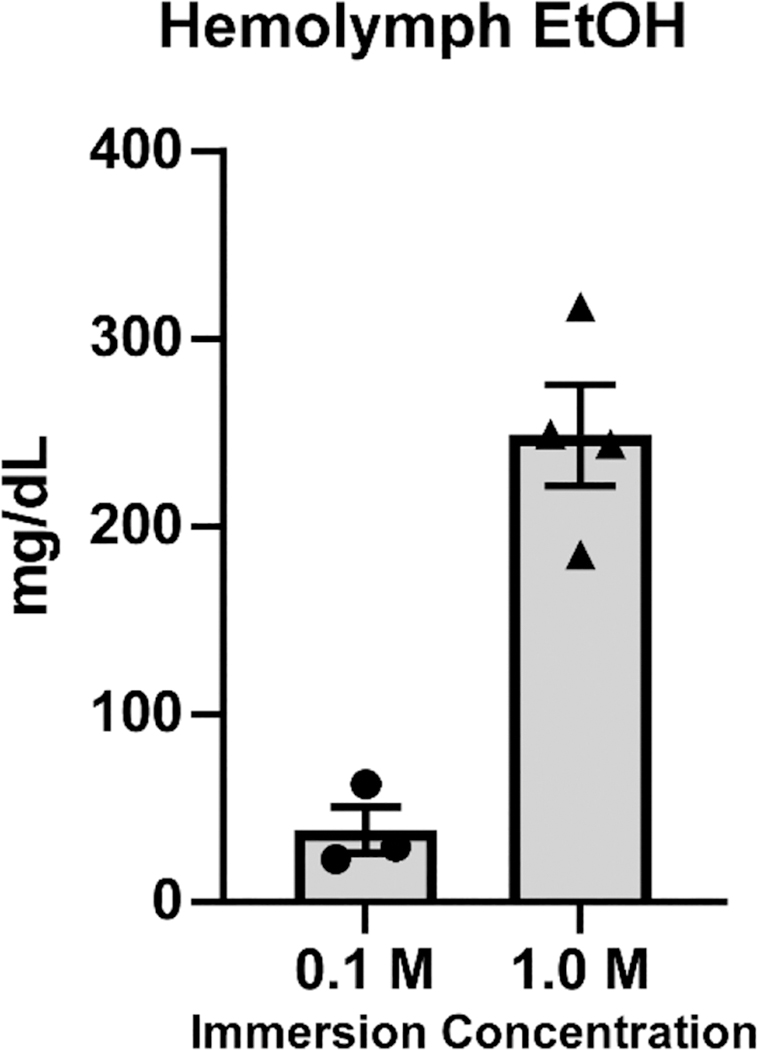
Hemolymph was collected from crayfish following 30-min immersion in EtOH 0.1 M (N = 3) and 1.0 M (N = 4) baths. Two additional untreated crayfish samples were obtained as controls (and had 0 EtOH). The blood hemolymph levels (EtOH mg/dL) are depicted in Figure 6 and show a concentration-dependent increase in circulating EtOH (Unpaired t-test: t = 6.273, df = 5, p < .005).
Figure 6. Mean (±SEM) Concentration of EtOH in the Hemolymph of Crayfish Following 30-Min Immersion in 0.1 M (N = 3) or 1.0 M (N = 4) EtOH.
Light/Dark Transfer Arena
Under baseline (no immersion) conditions the remaining adult crayfish (N = 6; 2 male) spent the majority of their time (mean 67%; range 43%−85%) under the cover in the Light/Dark arena. Their behavior was significantly affected by Zone when in the uncovered regions, as the analysis confirmed significant effects on Distance Traveled (F (1.016, 5.078) = 14.40; p < .05; Fig. 7A) and Speed (F (1.306, 6.532) = 13.98; p < .01; Fig. 7B), but not Time spent Immobile (p = .74; Fig. 7C). The post-hoc tests confirmed that they traveled the most distance in the Periphery (Fig. 7A) and moved significantly faster in the Center Zone compared with Periphery or Transition Zones (Fig. 7B). The patterns of behavior in the open part of the arena were therefore similar to the patterns when the arena was entirely uncovered.
Figure 7. Mean (N = 6; 4 Female; ±SEM). Mean A) Distance Traveled, B) Speed of Locomotion and C) Time Immobile across the Zones.
Note. A significant difference from the center zone is indicated with &, and from all other zones with #.
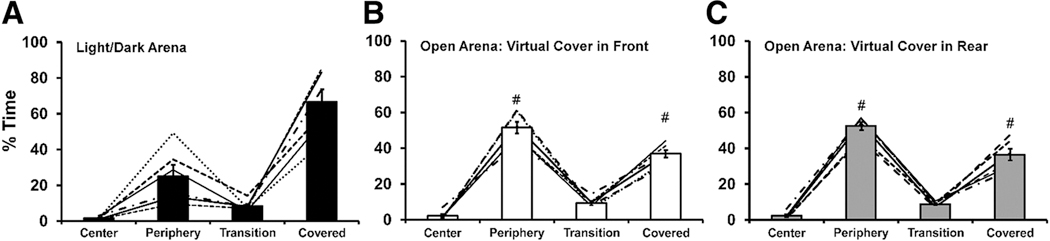
An additional analysis was done to compare the percent time spent under the cover when placed over the back third for this Light/Dark configuration, compared with the distribution of time when in the Open Field arena configuration. The open arena baseline recordings (from Experiment 1) for the sample of six animals were rescored with the ANY-maze template used for the covered run. These videos were first scored with the virtual “cover” at the rear and then were scored again with the location of the virtual cover switched to the front. The first important observation is that crayfish spent differing amounts of time in different zones (Fig. 8), as confirmed with a main effect of Zone [F (3, 15) = 154.0; p < .0001] on the percent of time spent. The post-hoc test confirmed that Percent Time differed between all zones except Center and Transition when the cover was in place. An identical result was confirmed for the virtual cover rescoring when the cover was in the rear. A similar result was also confirmed for when the virtual cover was in the front, except that Percent Time in Periphery did not differ from Percent Time under the (virtual) Cover. Importantly, the animals spent more time in the Covered Zone when there was an actual cover in place. This was confirmed with a significant interaction of Zone with Scoring template [F (6, 30) = 12.24; p < .0001]. The post-hoc test further confirmed that Percent Time in the Periphery and in the Covered portion of the area differed significantly between the actual Light/Dark condition and each of the virtual cover analysis conditions. Thus, the cover caused a significant increase in the amount of time spent in that part of the arena. There was nothing unusual about the six animals used for the Light/Dark pilot studies in terms of distribution of behavior in the open arena. Using the full sample (N = 14; 6 male) which had been run in the baseline condition in the first Open Arena experiment and rescoring the data with both front and back virtual covers, the same pattern of time distribution was confirmed (see Supplemental Fig. S5). Moreover, there was no difference between the sexes and no consistent preference for the front or rear of the arena (again, the cover is a virtual scoring template and did not exist on the arena during this recording session). Therefore, the hypothesis that crayfish would preferentially avoid the open areas when provided with a cover was supported.
Figure 8. A) Mean (N = 6; 4 Female; ±SEM) Percent of Time Spent in Four Zones of the Light/Dark Arena during Baseline Assessment Is Indicated with the Bars; Individual Data Are Depicted with the Lines. The Mean (±SEM) Percent of Time Spent in Areas of the Open Arena Corresponding to Light/Dark Zones during Baseline Assessment Is Presented for When the Virtual Cover Was Scored in the Front (B) or Rear (C).
Note. A significant difference between scoring runs is indicated with #.
Effects of EtOH Immersion on Light/Dark Transfer
The Cohort 2 group of crayfish, experimentally naïve prior to this study, were evaluated for activity in the Light/Dark arena after EtOH immersion. Due to what appeared to be a potentially stimulatory effect of 0.1 M immersion for 30 min in the Open Field study (Fig. 5A), the immersion conditions were Water or EtOH (0.05 M, 0.1 M or 1.0 M) for 30 min prior to individual sessions. The group was N = 10 total but two individuals only completed one to two conditions each and were omitted from the analysis. The behavior of this group was altered after the 1.0 M EtOH condition but not in the lower concentration conditions (Fig. 9). Significant reductions in speed were observed in all three uncovered zones, and reduction in distance traveled in the Periphery zone. This was associated with a large increase in time spent in the Center zone and a reduction in time spent under the Cover. In essence the animals were immobilized for much of the session and did not move from where they were placed in the Center to start the test. The statistical analyses confirmed significant effects of Zone, Immersion Condition and/or the interaction for Speed [Zone: F (2, 14) = 41.08, p < .0001; Immersion: F (3, 21) = 36.34, p < .0001; Interaction: F (6, 33) = 3.92, p < .005], Time [Zone: F (3, 114) = 45.00, p < .0001; Immersion: n.s.; Interaction: F (9, 114) = 14.93, p < .0001], Distance [Zone: F (2, 14) = 48.93, p < .0001; Immersion: F (3, 21) = 6.30, p < .005; Interaction: F (6, 42) = 8.86, p < .0001] and Entries [F (3, 24) = 11.24, p < .0001; Immersion: n.s.; Interaction: n.s.].
Figure 9. Mean (±SEM) Light/Dark Performance for Cohort 3: The Effect of 0.05–1.0 M EtOH Immersion.
Note. A significant difference from all other immersion conditions, within zone, is indicated with #, and from the water and 0.1 EtOH immersion with $.
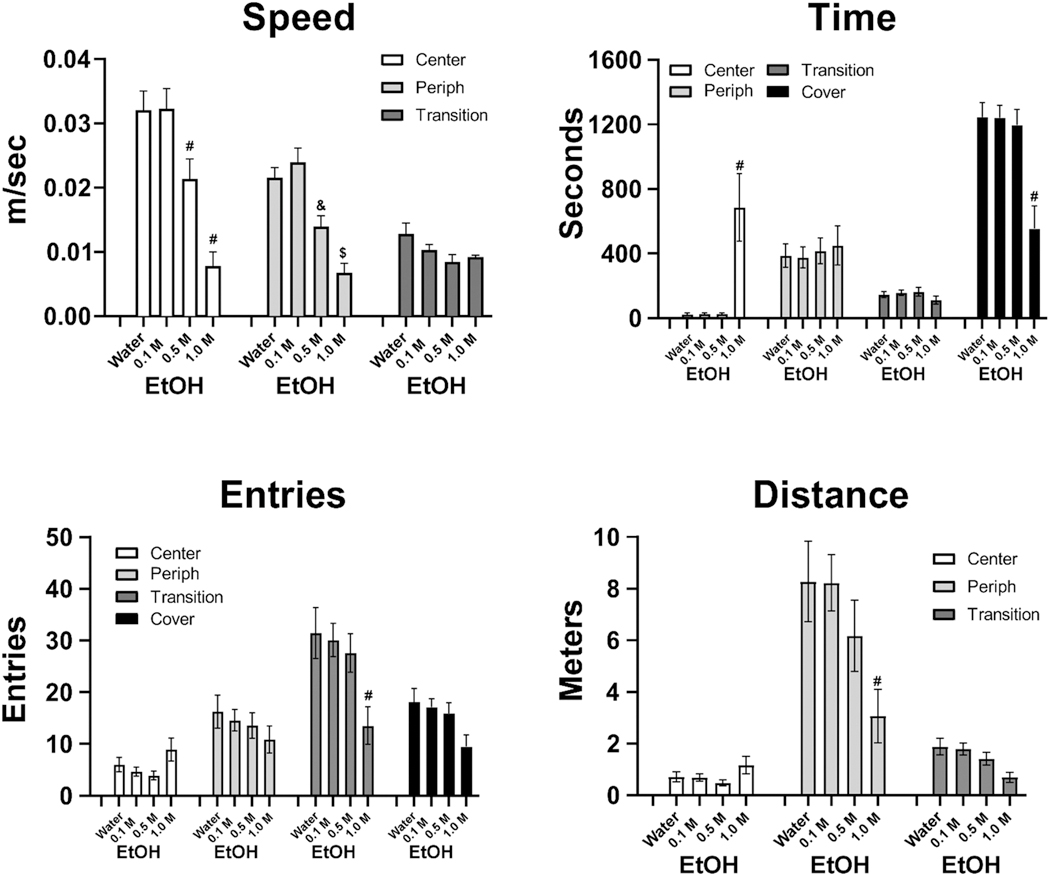
A total of 13 individuals (3 female) from Cohort 4 were evaluated for activity in the Light/Dark arena after immersion with a slightly different set of EtOH concentrations, i.e. after 30 min of immersion in Water or EtOH (0.1, 0.5 or 1.0 M). Individuals were lost to the study (generally due to molting) prior to completing water and 0.1 M (N = 1), 0.1 and 0.5 M (N = 1) and prior to completing 0.5 M (N = 1). Thus, sample size for analysis cells ranged from N = 11 to N = 13 and a mixed-effects analysis was selected to accommodate missing cells. EtOH immersion produced dose-dependent effects on movement Speed, Time in Zone, Zone entries and Distance traveled, as shown in Figure 10.
Figure 10. The Effect of 0.1–1.0 M EtOH Immersion on Locomotor Behavior in Light/Dark Cohort 4.
Note. A significant difference from all other immersion conditions, within zone, is indicated with #, from the water and 01 M EtOH immersion with $ and from the 0.1 M EtOH immersion with &.
The analyses confirmed significant effects of Zone, Immersion Condition and/or the interaction for Speed [Zone: F (2, 24) = 40.88, p < .0001; Immersion: F (3, 36) = 19.78, p < .0001; Interaction: F (6, 38) = 5.023, p < .001], Time [Zone: F (3, 172) = 83.92, p < .0001; Immersion: n.s.; Interaction: F (9, 172) = 9.479, p < .0001], Distance [Zone: F (2, 24) = 66.68, p < .0001; Immersion: F (3, 36) = 3.570, p < .05; Interaction: F (6, 57) = 5.083, p < .0005] and Entries [Zone: F (3, 36) = 95.00, p < .0001; Immersion: n.s.; Interaction: F (9, 88) = 6.846, p < .0001].
To further explicate the time course of effects for the Distance and Speed measures, collapsed across zone, patterns were analyzed by 5-min bins within the session (Fig. 11). The mixed-effect analyses confirmed significant effects of Time Bin, Immersion Condition and/or the interaction for Distance [Time Bin: F (5, 60) = 13.34, p < .0001; Immersion: F (3, 36) = 3.574, p < .05; Interaction: F (15, 150) = 4.229, p < .001] and for Speed [Time Bin: F (5, 60) = 15.68, p < .0001; Immersion: F (3, 36) = 3.651, p < .05; Interaction: F (15, 150) = 3.952, p < .001]. The post-hoc test confirmed that crayfish traveled significantly less distance and moved significantly more slowly in the first 5 min after EtOH 0.5 or 1.0 M immersion compared with water or 0.1 M immersion and in the 10-min Bin after EtOH 1.0 M immersion compared with water or 0.1 M immersion.
Figure 11. The Effect of 0.1–1.0 M EtOH Immersion on Distance and the Speed (across all Visible Zones) Is Presented for each 5 Min Bin within the 30-Min Session to Show the Time-Course of Effects.
Note. A significant difference from the water and 0.1 M EtOH immersion conditions is indicated with $.
Lethality of EtOH Immersion in Juvenile Crayfish
The effects of 30-min immersion in 1.0 M EtOH were more severe in Cohorts 2 and 3 compared with Cohort 1. This was potentially because the first cohort was initially exposed to various durations to 1.0 M and thus only a subset of individuals experienced the 30-min exposure first. Therefore, it was possible that a degree of tolerance affected the estimate of the effects. Alternately, it may have been the case that by chance a slightly larger size subset experienced the 30-min/1.0 M exposure first in Cohort 1, relative to the distributions in Cohorts 2 and 3. To determine potential size/age sensitivities, groups of juveniles of smaller size ranges were exposed to 30 min of 0.1, 0.5 M and 1.0 M EtOH to assess lethality. The 0.1 M EtOH resulted in no mortality of the < 2.5 cm crayfish (thus this concentration was not assessed in the 2.5–4.5 cm size range), however some mortality was observed after 0.5 and 1.0 M immersion (Fig. 12). This appeared to be dose dependent, particularly in the 2.5–4.5 cm size range. All Cohort 1 individuals in the ~5–7 cm range used for behavioral experiments survived the initial immersion, regardless of concentration.
Figure 12. Survival after 30-Min Immersion in EtOH (0.1, 0.5, 1.0 M) for A) Juveniles under 2.5 Cm, B) Juveniles between 2.5 and 4.5 Cm and C) Juveniles of 5–7 Cm.
Note. The 0.1 M EtOH immersion condition was omitted for the 2.5–4.5cm group. One (N = 10) cohort of < 2.5cm animals was only observed for 90 min, the second (N = 9 cohort was observed for 120 min; thus the final point reflects the percentage survival out of 9.
Effects of Repeated EtOH on Light/Dark Arena Activity
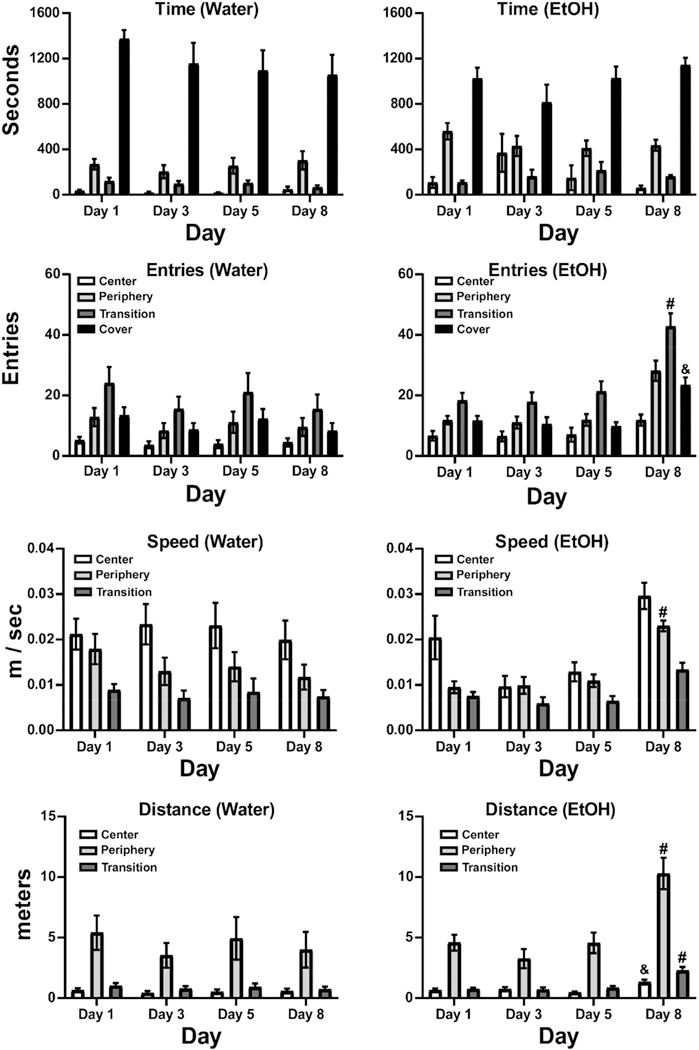
For the group (N = 18, 7 female, for Day 1, N = 12, 4 female, on Day 3 and N = 16, 3 female, on Days 5 and 8) of crayfish treated with 5 sequential days of EtOH (1.0 M) immersion for 30 min and then assessed after water immersion 3 days later, the EtOH significantly altered behavior in the Light/Dark arena (Fig. 13). First, the analysis of activity on Day 1 compared with the Water (N= 11, 5 female) immersion group, confirmed that there was a significant effect of Zone (F (1.327, 47.78) = 130.6, p < .0001) and of the interaction of Zone with Immersion condition (F (3, 108) = 8.534, p < .0001) on Time spent. The post-hoc test confirmed that the EtOH animals spent less time under the Cover and more time in the Periphery Zone compared with the Water immersion group. No group differences were confirmed for Entries, Speed or Distance on Day 1.
Figure 13. Mean (±SEM) Time Spent, Entries, Speed of Travel and Distance Traveled by Zone for Groups Immersed in Water [N = 11 (5 Female) or EtOH [N = 18 (7 Female)] on Day 1, [N = 16 (6 Female) on Day 8] for 5 Days.
Note. A significant difference between immersion condition is indicated with *.
On Day 8, however, there was a significant effect of Zone (F (1.384, 46.15) = 102.7; p < .0001), but not any significant effect of the interaction of immersion group with zone on Time spent (p = .09). However, there were significant effects of immersion Group, and/or the interaction of Group with Zone on Entries [Group, F (1, 25) = 16.73, p < .0005; Zone, F (1.403, 35.07) = 53.92, p < .0001; Interaction, F (3, 75) = 12.26, p < .0001], Speed [Group, F (1, 25) = 8.390, p < .01; Zone, F (1.596, 39.10) = 41.45, p < .0001; Interaction, n.s.] and Distance [Group, F (1, 25) = 16.73, p < .005; Zone, F (1.403, 35.07) = 53.92, p < .0001; Interaction, F (3, 75) = 12.26, p < .001] on Day 8. The post-hoc test confirmed significant differences between immersion groups on Entries (all zones), Speed (Center and Periphery) and Distance (all zones). The within-group analyses across the four recording Days in the Water immersion group did not confirm any significant effects of Day, nor any interaction of Day with Zone, for Time spent, Entries, Speed or Distance traveled (Fig. 14).
Figure 14. Mean (±SEM) Time Spent, Entries, Speed of Travel and Distance Traveled by Zone and Day for Groups Immersed in (Left Panels) Water (N = 11, 5 Female) or (Right Panels) EtOH (N = 18, 7 Female on Day 1, N = 16, 6 Female on Day 8) for 5 Days.
Note. A significant difference from all other days, within Zone, is indicated with #, and from Day 5, within Zone, with &.
In the EtOH immersion group, there were no significant effects of Day, nor of the interaction of Day with Zone, for Time spent. However, there were significant effects confirmed for Entries [Zone: F (1.885, 32.05) = 45.09, p < .0001; Day: F (2.246, 38.18) = 15.76, p < .0001; Interaction: F (3.611, 43.74) = 6.741, p < .0005], Speed [Zone: F (1.139, 19.36) = 16.75, p < .0005; Day: F (2.087, 35.48) = 14.14, p < .0001; Interaction: n.s.] and Distance traveled [Zone: F (1.037, 17.63) = 59.51, p < .0001; Day: F (2.127, 36.16) = 18.14, p < .0001; Interaction: F (1.876, 21.89) = 11.04, p < .001]. As depicted on Figure 14, the post-hoc tests confirmed that these effects were due to increased entries, speed and distance traveled on Day 8 (after water immersion) as compared with prior days in which EtOH immersion was conducted prior to locomotor assessment.
Discussion
The experiments show that the Red Swamp Crayfish, Procambarus clarkii, is a suitable model for the evaluation of behavioral effects of ethanol (EtOH). Administration by bath immersion results in levels of EtOH in the hemolymph that approximate blood EtOH concentrations that are significant in laboratory mammals as well as in humans. Dose-dependent effects can be produced by altering the concentration of ethanol in the immersion bath or by changing the amount of time in which they are immersed in a given concentration. The EtOH immersion was shown to alter locomotor behavior in an aquatic version of an open field arena, and locomotor suppression was produced in a dose dependent manner. There was also some evidence of a locomotor stimulant effect at the 0.1 M dose. Crayfish exhibited zone preference in both the Open Field and in the partially covered, Light/Dark version of the arena in a manner consistent with avoidance of the open parts, similar to preferences expressed by laboratory rodents on similar assays. This shows consistency of the crayfish model with an anxiety-like behavior in rats or mice. Finally, immersion in EtOH produced an anxiolytic-like effect in the Light/Dark by decreasing time spent under the cover.
Open Field
Crayfish behavior in the Open field was differentially distributed by zone in a manner that showed avoidance of the Center area. That is, crayfish moved more quickly in the Center Zone and traveled about 1/10 of the distance within the Center, compared with the Peripheral Zone. They also spent approximately half as much of their time immobile in the Center, as compared with time immobile in the Periphery. There was no clear evidence of an anxiolytic or anxiogenic effect of EtOH immersion in the Open Field experiment, since there were no consistent shifts to greater preference for, or avoidance of, the Center produced. While fewer entries into the Center were made in the 30-min immersion / 1.0 M EtOH condition, the animals also spent more time immobile in the Periphery. In addition, there was a monotonic dose-related decrease in distance traveled in both Center and Peripheral zones and a decrease in the speed of movement as well. This suggests that the reduction in Center entries associated with EtOH immersion is more likely attributed to the general reduction in locomotor activity, and not with altered sensitivity to the open space. There were no sex differences observed in terms of zone, or in terms of the effects of EtOH immersion, as is outlined in the Supplemental Materials (Supplemental Figs. S1–S4). There was evidence of a small locomotor stimulant effect of 30-min immersion in 0.1 M EtOH, as was reflected in a significant increase in Center speed. This is consistent with observations in the rat in which lower doses produce locomotor stimulation while higher doses suppress activity (Prunell et al., 1987). There was, however, no indication of a more robust stimulant effect of 0.05 M immersion, see Figure 9.
Hemolymph Ethanol Concentration
The hemolymph EtOH concentrations averaged 38.4 mg/dL (0.038 HAC) after immersion in 0.1 M EtOH and 248.9 mg/dL (0.249 HAC) after immersion in 1.0 M EtOH. This corresponds well with relevant blood EtOH levels in vertebrate animals, including mice, rats (de Guglielmo et al., 2017; Dilley et al., 2018; Ginsburg et al., 2008), monkeys (Crean et al., 2011; Katner et al., 2007; Wright & Taffe, 2014) and humans (Irwin et al., 2013; Phillips et al., 2015), after exposure to EtOH by the oral route of administration. The lower end of this range is associated with a threshold for observable behavioral effects and the high end is associated with significant intoxication in vertebrates. Importantly, this is the range of blood EtOH concentrations reached in voluntary, self-administration approaches in rat and monkey models (de Guglielmo et al., 2017; Katner et al., 2004; Wright & Taffe, 2014).
Light/Dark Test
The Light/Dark transfer test results further indicated that crayfish avoid the open areas and prefer the dark or covered portion of an environment, when it is available. In the initial experiment, crayfish spent two-thirds of their time under the cover in approximately one-third of the arena (by area or by peripheral wall length) under baseline, or untreated conditions. In contrast, they spent 36% of their time in the same location when the arena was entirely open (Fig. 8). Thus, the study found that the distribution of zone time in the Open arena (Center versus Periphery) and in the Light/Dark arena (Covered versus Center or Periphery) may serve as an anxiety-like measure akin to the analogous Light/Dark tests used in rodents. The utility of the assay was further supported by the lack of change in the pattern of behavior across the four sessions for the Water control group in the repeated-immersion experiment. That is, time spent in each zone, zone entries, speed and distance traveled did not change over the four test sessions. There was, however, a consistent effect of zone across all three measures and, again, animals spent most of their time under the cover and the least amount of time in the center zone. Their speed was fastest in the center zone and slowest in the transition zone, while they traveled the most distance in the periph-ry. The fewest entries were made in the center zone as well. Importantly, this confirms the behavioral stability over repeated testing within a single group.
There was no clear evidence of an anxiolytic or anxiogenic effect of acute EtOH immersion in the Light/Dark study with Cohorts 2 and 3. Although there was less time spent under the Cover after 1.0 M EtOH immersion this was attended by significantly more time spent in the Center, significantly slower Speed and reduced Distance traveled in the periphery (Figs. 9, 10). It was confirmed by visual inspection of videos that animals were immobilized in the Center after the highest dose condition, to an extent not seen in any other condition. One possibility is that although the size range in precedent literature (Swierzbinski et al., 2017) was described as “5–7 cm”, it is possible that there are significant developmental effects across this size range. We used this definition of the target size distribution, that is, between 5 cm and 7 cm, in these cohorts. In contrast, the naïve animals used in the Cohort 1 Open field exposure duration study were mostly at the 7 cm size or slightly larger. Thus, for the Light/Dark repeated-dosing study we ensured a minimum of 7 cm size for the final investigations.
Effect of Repeated EtOH on Light/Dark Behavior
There was evidence for an anxiolytic effect of EtOH immersion in the Light/Dark experiment. The EtOH group spent significantly less time under the Cover and more time in the Peripheral zone compared with the Water control group on Day 1, as is illustrated in Figure 13. Zone Entries, Speed and Distance Traveled did not differ significantly between the groups on Day 1, ruling out explanations based on general locomotor suppression.
The crayfish did not develop tolerance (or sensitization) to EtOH when the group was exposed daily for 5 days, as no substantial change in behavior was observed between Day 1 and Day 5 of the repeated-dosing experiment. More specifically, the pattern of fewer entries into the Center Zone, more entries into the Peripheral and Covered Zones and the most entries in the Transition Zone persisted across the EtOH (Days 1, 3 and 5) sessions with no difference across the days. Likewise, crayfish traveled the most distance in the Peripheral zone and the least in the Center zone across all observation days. One potential difference associated with repeated EtOH immersion was the observation that Center speed was not higher than the speed of travel in the other zones on Day 3 and Day 5, as it was on the water day and in general across all the experiments reported herein. There were no other similar consistent differences that emerged only on Day 3 and Day 5, making this difficult to interpret other than it may reflect an anxiolytic effect of EtOH which was obscured on the Day 1 due to the novelty of the procedure. Although two outliers (0.06 and 0.082 m/sec respectively) contributed to the high Center speed average on Day 1 for the EtOH group, removal of these two values did not eliminate the pattern of increased Center speed. Results of analyses either between groups (Fig. 13) or within the EtOH treated group (Fig. 14) revealed increased zone entries, speed and distance traveled after 3 days of abstinence from repeated EtOH immersion (Day 8). Despite the repeated EtOH having no substantial differential effect on Zone Entries, Speed and Distance Traveled compared with the water group on Day 1, there was an excess of activity in the repeated EtOH group compared with the water group on Day 8, which was specific to the discontinuation from repeated EtOH. Although the neuropharmacological underpinnings of this phenomenon are as yet unknown, this highlights the utility of this model to examine short (and possibly long) term effects of abstinence from repeated EtOH exposure.
Replication and Reliability
Although the study constitutes an initial development effort, it had a set of design features that address replicability and stability of the observed effects. First, the Open Field experiment generated similar results across the baseline (first arena exposure) and the water immersion condition (which was conducted in a randomized order with the EtOH immersion in the time-of-immersion study). Second, the dose-immersion replication of the 1M 30-min immersion across the two Open Field experiments produced a similar magnitude of effect. In addition, no significant sex differences were observed in either baseline behavior, namely, in the effects of ethanol on locomotor behavior or in the distribution of behavior in the virtual cover rescoring of the baseline activity. This constitutes an important subgroup replication. Finally, the stability of the behavioral pattern of the repeated Water-immersion group (Fig. 13, Supp. Fig. S6) across four sequential assessments also confirms stability of the assay.
This study was not designed to provide developmental information on the locomotor effects of ethanol immersion, given the sequential study in the same animals (Cohort 1) and minimal attempt to define developmental stage beyond length and approximate time since hatching. Nevertheless, the effect of 1.0 M EtOH immersion for 30 min was nearly identical across the first two experiments when animals had grown from late juvenile to early adult length, and also similar to the similarly sized animals used in the repeated exposure experiments. Thus, there was apparent similarity across these ages. However, the increase in center immobilization after 1.0 M immersion in Experiment 4, and the lethality experiment, show that there may be some boundaries of developmental stage related to the effects of EtOH that will need to be more precisely defined.
Advantages of Crayfish Models
Crayfish species are widely distributed, worldwide, in natural environments (https://en.wikipedia.org/wiki/Crayfish). Subjects for use in laboratory experiments can be purchased from pet stores (as we did for some of our subjects), biological suppliers (Swierzbinski & Herberholz, 2018; Teshiba et al., 2001), or are easily fished from the wild, if locally available (Datta et al., 2018; Imeh-Nathaniel et al., 2017). This invertebrate species can be used for research within institutions that do not maintain regulatory approvals and facilities appropriate for vertebrate animal research subjects. The marbled crayfish, Procambarus virginalis, (“Marmokrebs”) is a parthogenic species; since individuals are genetic clones, and the P. virginalis genome was sequenced in 2018 (Gutekunst et al., 2018), genetic experiments might be facilitated. The fact that these species have external egg incubation might be leveraged with Cas9-mediated gene editing techniques (e.g., Chaverra-Rodriguez et al., 2018). In addition, crayfish are a popular electrophysiology model for introductory neuroscience laboratory classes (Cooper et al., 2011; Ewing & Medler, 2020; Land et al., 2001), and the present studies potentially expand the scope of behavioral and pharmacological experiments that could be adapted. More simply, crayfish are often maintained as classroom pets in primary schools in the US. This latter familiarity in the educational environment may support demonstration labs, and/or science fair projects in primary and secondary educational settings.
Alternative Approaches and Caveats
The materials necessary for these experiments are relatively inexpensive and are, for the most part, available from local pet stores, home goods, liquor / grocery and electronic stores. The most expensive component of these studies is the video tracking suite, ANY-maze, which could potentially be replaced with open source software such as ToxTrac (Rodriguez et al., 2018) or DeepLabCut (http://www.mackenziemathislab.org/deeplabcut). The methodological parameters selected for this study could be varied and other choices may influence behavior. Notably, the crayfish is more active at night and therefore patterns of behavior in open field and Light/Dark assays may differ at a different time of day, or under low-light conditions. Similarly, while the crayfish is a bottom dweller, it exists natively in a three-dimensional ecology, and future experiments might investigate vertical movements with a deeper water depth.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the efforts of Mitchell L. Turner and Rachelle N. Tran who conducted many preliminary experiments to develop and refine the methods for evaluating the behavioral effects of drugs in crayfish. We are likewise grateful to Michael Userenko for assistance with conducting some experiments and to COJT for donation of nail polish to identify animals. We are grateful to Professor Zen Faulkes, Ph.D., for critical initial advice on crustacean handling, immobilization and euthanasia. Hemolymph alcohol assessment was conducted with resources provided by the TSRI Alcohol Research Center (P60 AA006420). Author AG was supported by T32 AA007456 and by a UCSD Chancellor’s Postdoctoral Fellowship.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s website.
References
- Acevedo MB, Nizhnikov ME, Molina JC, & Pautassi RM (2014). Relationship between ethanol-induced activity and anxiolysis in the open field, elevated plus maze, light-dark box, and ethanol intake in adolescent rats. Behavioural Brain Research, 265, 203–215. 10.1016/j.bbr.2014.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bättig K. (1969). Drug effects on exploration of a combined maze and open-field system by rats. Annals of the New York Academy of Sciences, 159(3), 880–897. 10.1111/j.1749-6632.1969.tb12986.x [DOI] [PubMed] [Google Scholar]
- Belzung C, Misslin R, & Vogel E. (1988). The benzodi-azepine receptor inverse agonists beta-CCM and RO 15–3505 both reverse the anxiolytic effects of ethanol in mice. Life Sciences, 42(18), 1765–1772. 10.1016/0024-3205(88)90043-4 [DOI] [PubMed] [Google Scholar]
- Cappeliez P, & White N. (1981). Lithium induces dose-related increases and decreases in activity levels in the rat. Psychopharmacology (Berl), 73(1), 34–38. 10.1007/bf00431097 [DOI] [PubMed] [Google Scholar]
- Chaverra-Rodriguez D, Macias VM, Hughes GL, Pujhari S, Suzuki Y, Peterson DR, Kim D, McKeand S, & Rasgon JL (2018). Targeted delivery of CRISPR-Cas9 ribonucleoprotein into arthropod ovaries for heritable germline gene editing. Nature Communications, 9(1), 3008. 10.1038/s41467-018-05425-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AS, Leksrisawat B, Gilberts AB, Mercier AJ, & Cooper RL (2011). Physiological experimentation with the crayfish hindgut: A student laboratory exercise. Journal of Visualized Experiments, 47, 2324. 10.3791/2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Vandewater SA, Katner SN, Huitron-Resendiz S, & Taffe MA (2011). Chronic alcohol consumption impairs visuo-spatial associative memory in periadolescent rhesus monkeys. Drug and Alcohol Dependence, 114(1), 31–40. 10.1016/j.drugalcdep.2010.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta U, van Staaden M, & Huber R. (2018). Crayfish self-administer amphetamine in a spatially contingent task. Frontiers in Physiology, 9, 433. 10.3389/fphys.2018.00433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Cole MD, & George O. (2017). Voluntary induction and maintenance of alcohol dependence in rats using alcohol vapor self-administration. Psychopharmacology (Berl), 234(13), 2009–2018. 10.1007/s00213-017-4608-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilley JE, Nicholson ER, Fischer SM, Zimmer R, & Froehlich JC (2018). Alcohol drinking and blood alcohol concentration revisited. Alcoholism: Clinical and Experimental Research, 42(2), 260–269. 10.1111/acer.13549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing MD, & Medler S. (2020). Quantifying the effects of two local anesthetics on the crayfish stretch receptor organ: An integrated neurophysiology lab. Journal of Undergraduate Neuroscience Education, 18(2), A121–A128. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/32848520 [PMC free article] [PubMed] [Google Scholar]
- Fossat P, Bacque-Cazenave J, De Deurwaerdere P, Cattaert D, & Delbecque JP (2015). Serotonin, but not dopamine, controls the stress response and anxiety-like behavior in the crayfish Procambarus clarkii. Journal of Experimental Biology, 218(Pt 17), 2745–2752. 10.1242/jeb.120550 [DOI] [PubMed] [Google Scholar]
- Gilmore N, Cherian L, & Klemm WR (1991). Ganglioside or sialic acid attenuates ethanol-induced decrements in locomotion, nose-poke exploration, and anxiety, but not body temperature. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 15(1), 91–104. 10.1016/0278-5846(91)90044-2 [DOI] [PubMed] [Google Scholar]
- Ginsburg BC, Martinez G, Friesenhahn G, Javors M, & Lamb RJ (2008). Acute tolerance to rate-decreasing effects of single doses of ethanol. Physiology & Behavior, 94(3), 374–383. 10.1016/j.physbeh.2008.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutekunst J, Andriantsoa R, Falckenhayn C, Hanna K, Stein W, Rasamy J, & Lyko F. (2018). Clonal genome evolution and rapid invasive spread of the marbled crayfish. Nature Ecology & Evolution, 2(3), 567–573. 10.1038/s41559-018-0467-9 [DOI] [PubMed] [Google Scholar]
- Imeh-Nathaniel A, Rincon N, Orfanakos VB, Brechtel L, Wormack L, Richardson E, Huber R, & Nathaniel TI (2017). Effects of chronic cocaine, morphine and methamphetamine on the mobility, immobility and stereotyped behaviors in crayfish. Behavioural Brain Research, 332, 120–125. 10.1016/j.bbr.2017.05.069 [DOI] [PubMed] [Google Scholar]
- Irwin C, Leveritt M, Shum D, & Desbrow B. (2013). The effects of dehydration, moderate alcohol consumption, and rehydration on cognitive functions. Alcohol, 47(3), 203–213. 10.1016/j.alcohol.2012.12.016 [DOI] [PubMed] [Google Scholar]
- June HL, Johnson LT, & Lewis MJ (1989). Ro15–4513 antagonizes depression of open-field horizontal activity by ethanol in rats. Alcohol, 6(4), 335–337. 10.1016/0741-8329(89)90093-1 [DOI] [PubMed] [Google Scholar]
- Katner SN, Flynn CT, Von Huben SN, Kirsten AJ, Davis SA, Lay CC, Cole M, Roberts AJ, Fox HS, & Taffe MA (2004). Controlled and behaviorally relevant levels of oral ethanol intake in rhesus macaques using a flavorant-fade procedure. Alcoholism: Clinical and Experimental Research, 28(6), 873–883. 10.1097/01.alc.0000128895.99379.8c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katner SN, Von Huben SN, Davis SA, Lay CC, Crean RD, Roberts AJ, Fox HS, & Taffe MA (2007). Robust and stable drinking behavior following long-term oral alcohol intake in rhesus macaques. Drug and Alcohol Dependence, 91(2–3), 236–243. 10.1016/j.drugalcdep.2007.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamble R, & Rydberg U. (1982). Effects of ethanol on locomotor activity in rats of different ages. Acta Pharmacologica et Toxicologica (Copenh), 50(4), 246–250. 10.1111/j.1600-0773.1982.tb00970.x [DOI] [PubMed] [Google Scholar]
- Land BR, Wyttenbach RA, & Johnson BR (2001). Tools for physiology labs: An inexpensive high-performance amplifier and electrode for extracellular recording. Journal of Neuroscience Methods, 106(1), 47–55. 10.1016/s0165-0270(01)00328-4 [DOI] [PubMed] [Google Scholar]
- Phillips DP, Sousa AL, & Moshfegh RT (2015). Official blame for drivers with very low blood alcohol content: There is no safe combination of drinking and driving. Injury Prevention, 21(e1), e28–35. 10.1136/injuryprev-2013-040925 [DOI] [PubMed] [Google Scholar]
- Prunell M, Boada J, Feria M, & Benitez MA (1987). Antagonism of the stimulant and depressant effects of ethanol in rats by naloxone. Psychopharmacology (Berl), 92(2), 215–218. 10.1007/BF00177918 [DOI] [PubMed] [Google Scholar]
- Reisinger AJ, Reisinger LS, Richmond EK, & Rosi EJ (2021). Exposure to a common antidepressant alters crayfish behavior and has potential subsequent ecosystem impacts. Ecosphere, 12(6), 303527. 10.1002/ecs2.3527 [DOI] [Google Scholar]
- Rodriguez A, Zhang H, Klaminder J, Brodin T, Andersson PL, & Andersson M. (2018). ToxTrac: a fast and robust software for tracking organisms. Methods in Ecology and Evolution, 9(3), 460–464. https://sourceforge.net/projects/toxtrac/ [Google Scholar]
- Sharko AC, Kaigler KF, Fadel JR, & Wilson MA (2016). Ethanol-induced anxiolysis and neuronal activation in the amygdala and bed nucleus of the stria terminalis. Alcohol, 50, 19–25. 10.1016/j.alcohol.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA (2020). Nonhuman animal models of substance use disorders: Translational value and utility to basic science. Drug and Alcohol Dependence, 206, 107733. 10.1016/j.drugalcdep.2019.107733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swierzbinski ME, & Herberholz J. (2018). Effects of ethanol on sensory inputs to the medial giant interneurons of crayfish. Frontiers in Physiology, 9, 448. 10.3389/fphys.2018.00448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swierzbinski ME, Lazarchik AR, & Herberholz J. (2017). Prior social experience affects the behavioral and neural responses to acute alcohol in juvenile cray-fish. Journal of Experimental Biology, 220(Pt 8), 1516–1523. 10.1242/jeb.154419 [DOI] [PubMed] [Google Scholar]
- Teshiba T, Shamsian A, Yashar B, Yeh SR, Edwards DH, & Krasne FB (2001). Dual and opposing modulatory effects of serotonin on crayfish lateral giant escape command neurons. Journal of Neuroscience, 21(12), 4523–4529. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11404440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ Jr., & Taffe MA (2014). Chronic periadolescent alcohol consumption produces persistent cognitive deficits in rhesus macaques. Neuropharmacology, 86, 78–87. 10.1016/j.neuropharm.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.