Abstract
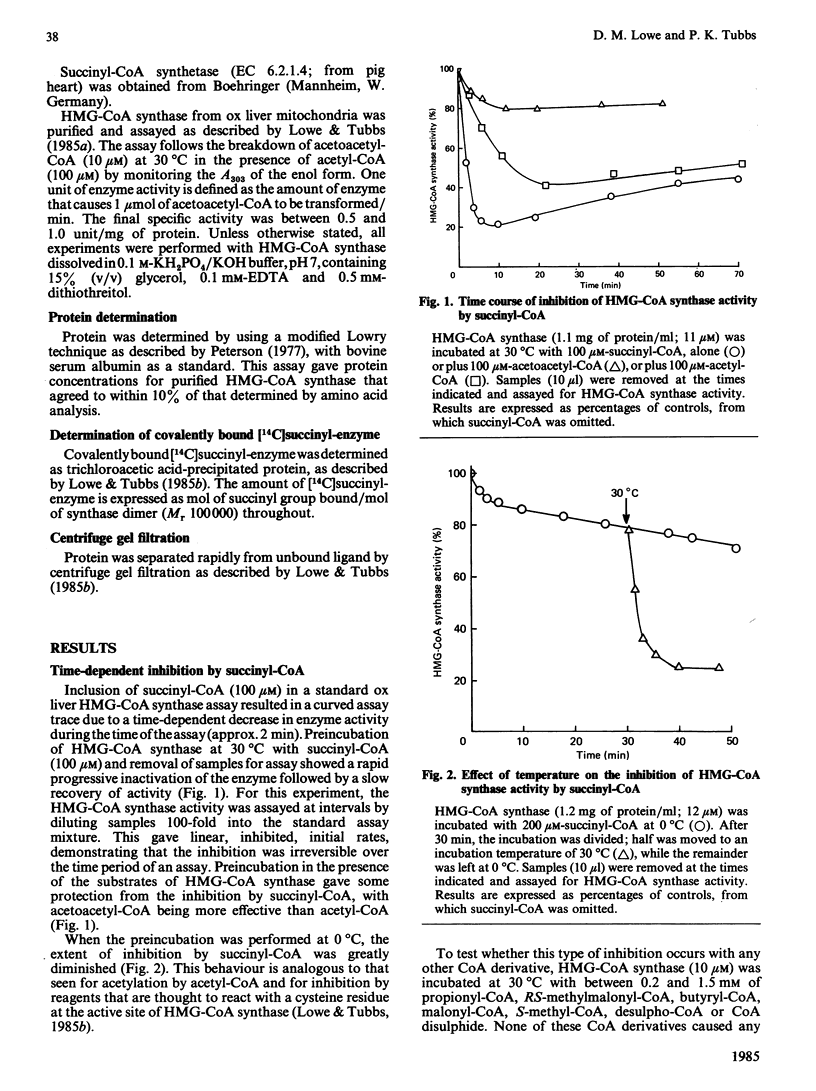
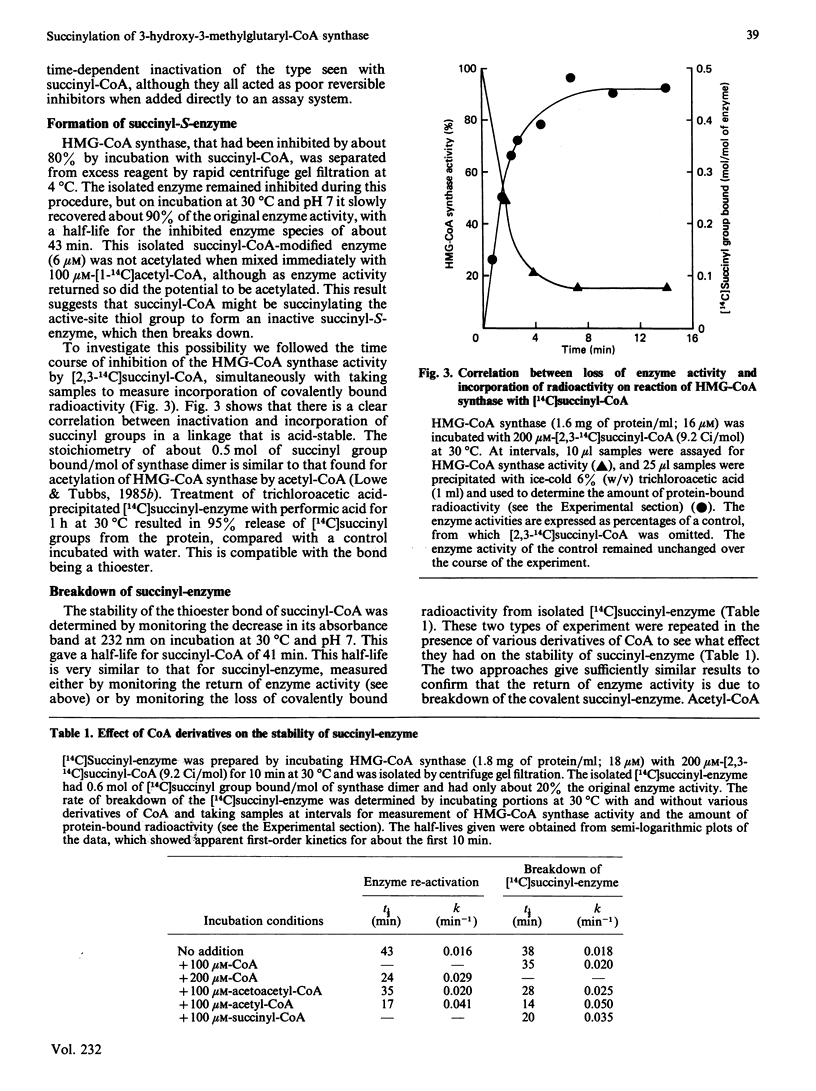
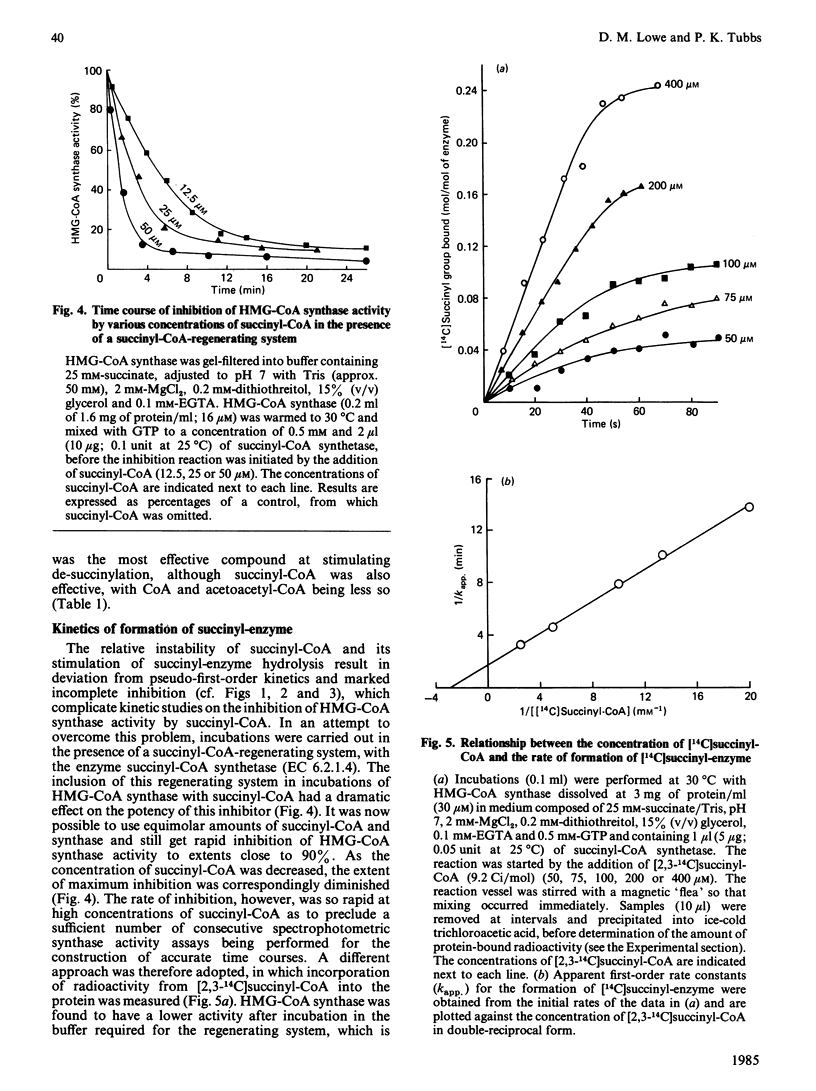
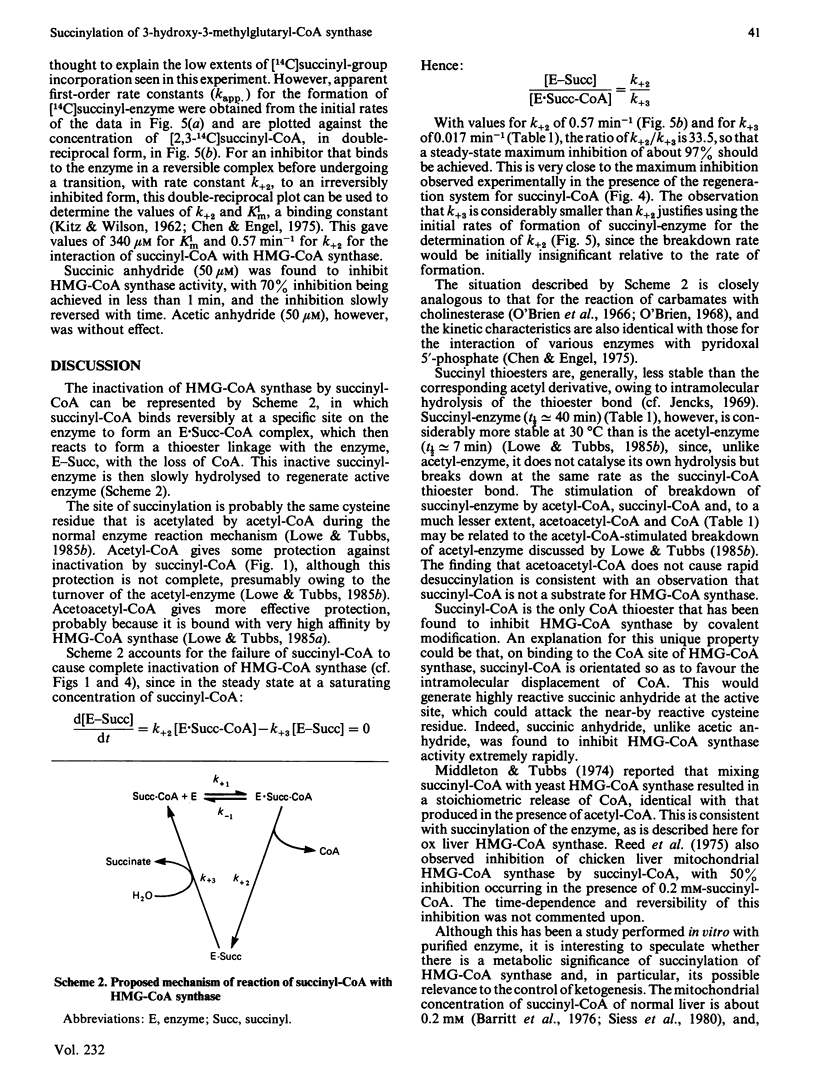
Succinyl-CoA (3-carboxypropionyl-CoA) inactivates ox liver mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase (EC 4.1.3.5) in a time-dependent manner, which is partially prevented by the presence of substrates of the enzyme. The inactivation is due to the enzyme catalysing its own succinylation. Complete inactivation corresponds to about 0.5 mol of succinyl group bound/mol of enzyme dimer. The succinyl-enzyme linkage appears to be a thioester bond and is probably formed with the active-site cysteine residue that is normally acetylated by acetyl-CoA. Succinyl-CoA binds to 3-hydroxy-3-methylglutaryl-CoA synthase with a binding constant of 340 microM and succinylation occurs with a rate constant of 0.57 min-1. Succinyl-enzyme breaks down with a half-life of about 40 min (k = 0.017 min-1) at 30 degrees C and pH 7 and is destabilized by the presence of acetyl-CoA and succinyl-CoA. A control mechanism is postulated in which flux through the 3-hydroxy-3-methylglutaryl-CoA cycle of ketogenesis is regulated according to the extent of succinylation of 3-hydroxy-3-methylglutaryl-CoA synthase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chase J. F., Middleton B., Tubbs P. K. A coenzyme A analogue, desulpho-coA; preparation and effects on various enzymes. Biochem Biophys Res Commun. 1966 Apr 19;23(2):208–213. doi: 10.1016/0006-291x(66)90529-8. [DOI] [PubMed] [Google Scholar]
- Chen S. S., Engel P. C. The equilibrium position of the reaction of bovine liver glutamate dehydrogenase with pyridoxal5'-phosphate. A demonstration that covalent modification with this reagent completely abolishes catalytic activity. Biochem J. 1975 May;147(2):351–358. doi: 10.1042/bj1470351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkey B. E., Brandt M., Williams R. J., Williamson J. R. Assay of short-chain acyl coenzyme A intermediates in tissue extracts by high-pressure liquid chromatography. Anal Biochem. 1981 Nov 15;118(1):30–41. doi: 10.1016/0003-2697(81)90152-4. [DOI] [PubMed] [Google Scholar]
- Dashti N., Ontko J. A. Rate-limiting function of 3-hydroxy-3-methylglutaryl-coenzyme A synthase in ketogenesis. Biochem Med. 1979 Dec;22(3):365–374. doi: 10.1016/0006-2944(79)90024-3. [DOI] [PubMed] [Google Scholar]
- KITZ R., WILSON I. B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962 Oct;237:3245–3249. [PubMed] [Google Scholar]
- Keller U., Chiasson J. L., Liljenquist J. E., Cherrington A. D., Jennings A. S., Crofford O. S. The roles of insulin, glucagon, and free fatty acids in the regulation of ketogenesis in dogs. Diabetes. 1977 Nov;26(11):1040–1051. doi: 10.2337/diab.26.11.1040. [DOI] [PubMed] [Google Scholar]
- Lowe D. M., Tubbs P. K. 3-Hydroxy-3-methylglutaryl-coenzyme A synthase from ox liver. Properties of its acetyl derivative. Biochem J. 1985 Apr 15;227(2):601–607. doi: 10.1042/bj2270601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. M., Tubbs P. K. 3-Hydroxy-3-methylglutaryl-coenzyme A synthase from ox liver. Purification, molecular and catalytic properties. Biochem J. 1985 Apr 15;227(2):591–599. doi: 10.1042/bj2270591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- McGarry J., Wright P. H., Foster D. W. Hormonal control of ketogenesis. Rapid activation of hepatic ketogenic capacity in fed rats by anti-insulin serum and glucagon. J Clin Invest. 1975 Jun;55(6):1202–1209. doi: 10.1172/JCI108038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton B., Tubbs P. K. An enzyme-bound intermediate in the biosynthesis of 3-hydroxy-3-methylglutaryl-coenzyme A. Biochem J. 1974 Jan;137(1):15–23. doi: 10.1042/bj1370015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miziorko H. M., Lane M. D. 3-Hydroxy-3-methylgutaryl-CoA synthase. Participation of acetyl-S-enzyme and enzyme-S-hydroxymethylgutaryl-SCoA intermediates in the reaction. J Biol Chem. 1977 Feb 25;252(4):1414–1420. [PubMed] [Google Scholar]
- O'Brien R. D., Hilton B. D., Gilmour L. The reaction of carbamates with cholinesterase. Mol Pharmacol. 1966 Nov;2(6):593–605. [PubMed] [Google Scholar]
- O'Brien R. D. Kinetics of the carbamylation of cholinesterase. Mol Pharmacol. 1968 Mar;4(2):121–130. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Reed W. D., Clinkenbeard D., Lane M. D. Molecular and catalytic properties of mitochondrial (ketogenic) 3-hydroxy-3-methylglutaryl coenzyme A synthase of liver. J Biol Chem. 1975 Apr 25;250(8):3117–3123. [PubMed] [Google Scholar]
- Siess E. A., Brocks D. G., Wieland O. H. Distribution of metabolites between the cytosolic and mitochondrial compartments of hepatocytes isolated from fed rats. Hoppe Seylers Z Physiol Chem. 1978 Jul;359(7):785–798. doi: 10.1515/bchm2.1978.359.2.785. [DOI] [PubMed] [Google Scholar]
- Siess E. A., Fahimi F. M., Wieland O. H. Decrease by glucagon in hepatic succinyl-CoA. Biochem Biophys Res Commun. 1980 Jul 16;95(1):205–211. doi: 10.1016/0006-291x(80)90725-1. [DOI] [PubMed] [Google Scholar]



