Abstract
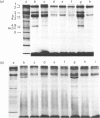
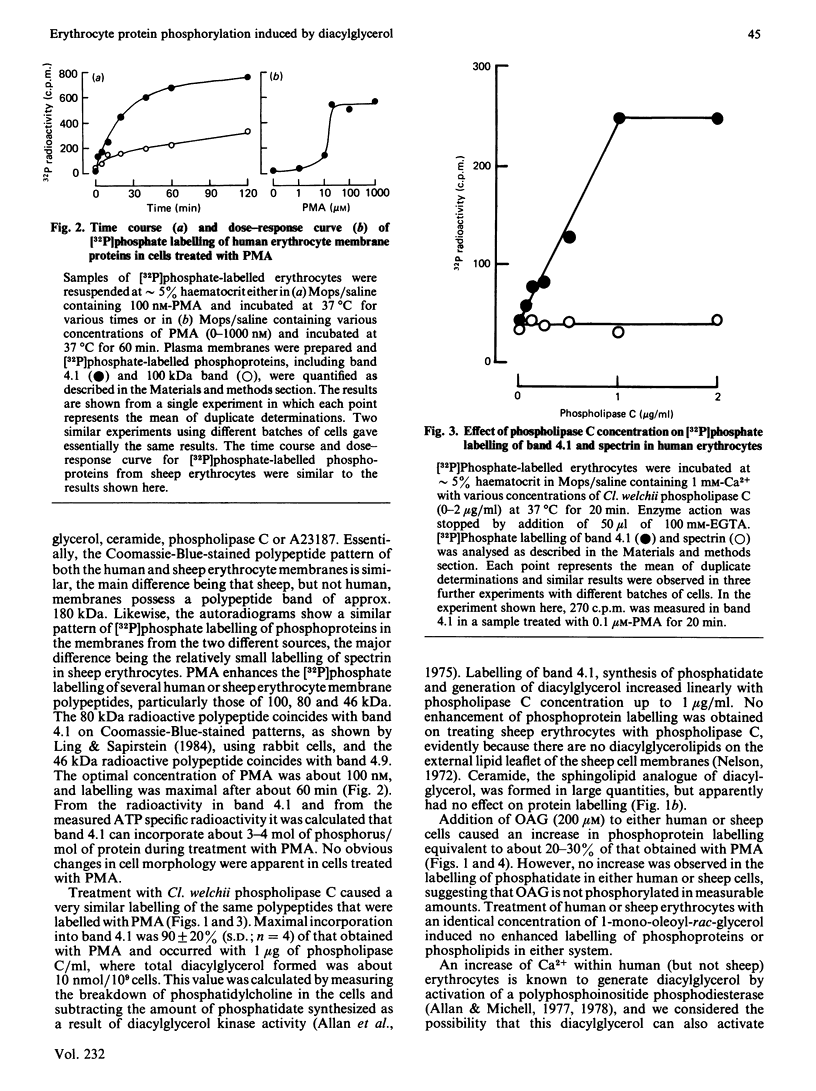
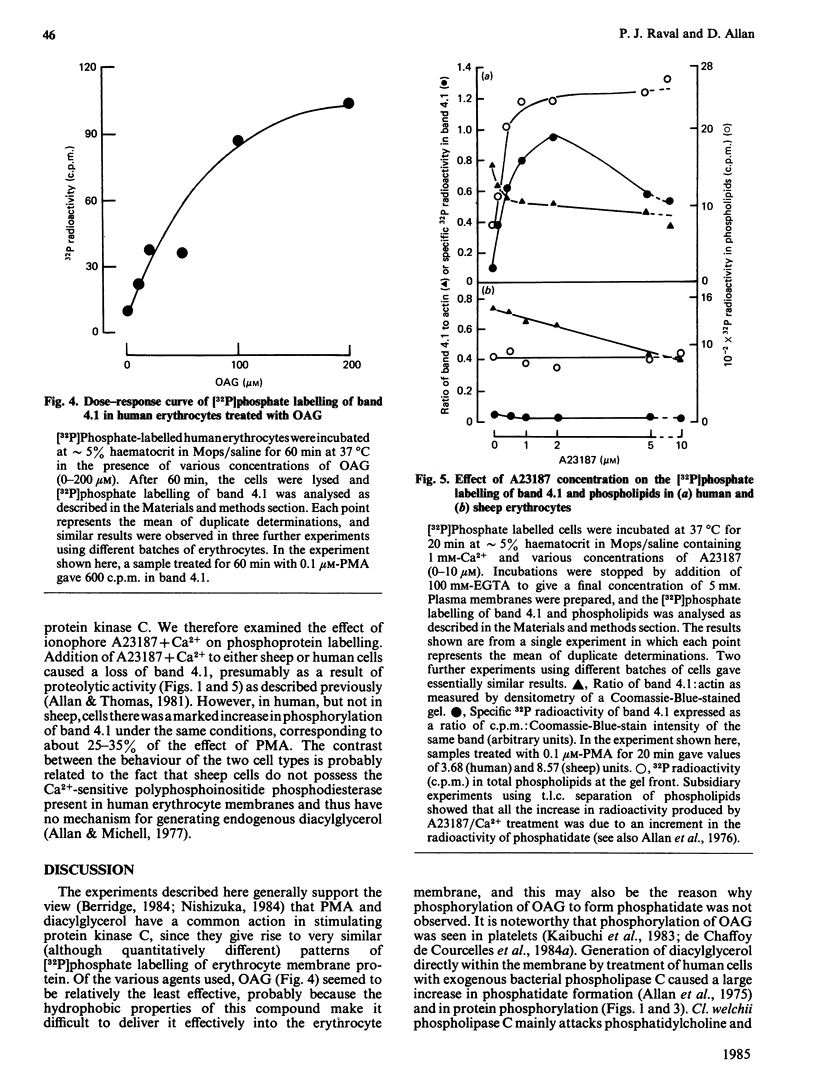
Treatment of human or sheep erythrocytes with PMA (phorbol myristate acetate) enhanced [32P]phosphate labelling of membrane polypeptides of approx. 100, 80 and 46 kDa. The 80 kDa and 46 kDa polypeptides coincided with bands 4.1 and 4.9 respectively on Coomassie-Blue-stained gels. Similar but smaller effects were obtained by treating human cells with 1-oleoyl-2-acetyl-rac-glycerol (OAG), exogenous bacterial phospholipase C or ionophore A23187 + Ca2+, each of which treatments would be expected to raise the concentration of membrane diacylglycerol. In contrast, sheep cells, which do not increase their content of diacylglycerol when treated with phospholipase C or A23187 + Ca2+, only showed enhanced phosphorylation with OAG. Neither human nor sheep cells showed any enhanced [32P]phosphate labelling of phosphoproteins when treated with 1-mono-oleoyl-rac-glycerol. It is concluded that diacylglycerol from a variety of sources can activate erythrocyte protein kinase C, but that the most effective diacylglycerol is that derived from endogenous polyphosphoinositides. In contrast with bacterial phospholipase C and A23187, which stimulate synthesis of phosphatidate by increasing the cell-membrane content of diacylglycerol in human erythrocytes, PMA, OAG or 1-mono-oleoyl-rac-glycerol caused no change in phospholipid metabolism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Low M. G., Finean J. B., Michell R. H. Changes in lipid metabolism and cell morphology following attack by phospholipase C (Clostridium perfringens) on red cells or lymphocytes. Biochim Biophys Acta. 1975 Dec 1;413(2):309–316. doi: 10.1016/0005-2736(75)90116-9. [DOI] [PubMed] [Google Scholar]
- Allan D., Michell R. H. A calcium-activated polyphosphoinositide phosphodiesterase in the plasma membrane of human and rabbit erythrocytes. Biochim Biophys Acta. 1978 Apr 4;508(2):277–286. doi: 10.1016/0005-2736(78)90330-9. [DOI] [PubMed] [Google Scholar]
- Allan D., Michell R. H. Calcium ion-dependent diacylglycerol accumulation in erythrocytes is associated with microvesiculation but not with efflux of potassium ions. Biochem J. 1977 Sep 15;166(3):495–499. doi: 10.1042/bj1660495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan D., Thomas P. Ca2+-induced biochemical changes in human erythrocytes and their relation to microvesiculation. Biochem J. 1981 Sep 15;198(3):433–440. doi: 10.1042/bj1980433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan D., Thomas P., Limbrick A. R. The isolation and characterization of 60 nm vesicles ('nanovesicles') produced during ionophore A23187-induced budding of human erythrocytes. Biochem J. 1980 Jun 15;188(3):881–887. doi: 10.1042/bj1880881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan D., Watts R., Michell R. H. Production of 1,2-diacylglycerol and phosphatidate in human erythrocytes treated with calcium ions and ionophore A23187. Biochem J. 1976 May 15;156(2):225–232. doi: 10.1042/bj1560225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson F. S., Murphy R. C. Isocratic separation of some purine nucleotide, nucleoside, and base metabolites from biological extracts by high-performance liquid chromatography. J Chromatogr. 1976 Jun 23;121(2):251–262. doi: 10.1016/s0021-9673(00)85021-9. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Barrowman M. M., Gomperts B. D. Breakdown and synthesis of polyphosphoinositides in fMetLeuPhe-stimulated neutrophils. FEBS Lett. 1985 Feb 25;181(2):259–263. doi: 10.1016/0014-5793(85)80271-4. [DOI] [PubMed] [Google Scholar]
- Ganong B. R., Bell R. M. Transmembrane movement of phosphatidylglycerol and diacylglycerol sulfhydryl analogues. Biochemistry. 1984 Oct 9;23(21):4977–4983. doi: 10.1021/bi00316a023. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Goodman S. R., Shiffer K. The spectrin membrane skeleton of normal and abnormal human erythrocytes: a review. Am J Physiol. 1983 Mar;244(3):C121–C141. doi: 10.1152/ajpcell.1983.244.3.C121. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Sawamura M., Hoshijima M., Fujikura T., Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983 Jun 10;258(11):6701–6704. [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Minakuchi R., Inohara S., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase from rat brain. Subcellular distribution, purification, and properties. J Biol Chem. 1982 Nov 25;257(22):13341–13348. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ling E., Sapirstein V. Phorbol ester stimulates the phosphorylation of rabbit erythrocyte band 4.1. Biochem Biophys Res Commun. 1984 Apr 16;120(1):291–298. doi: 10.1016/0006-291x(84)91447-5. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. V., Metcalfe J. C., Hesketh T. R., Smith G. A., Moore J. P. Mitogens increase phosphorylation of phosphoinositides in thymocytes. 1984 Nov 29-Dec 5Nature. 312(5993):462–465. doi: 10.1038/312462a0. [DOI] [PubMed] [Google Scholar]
- Thomas P., Limbrick A. R., Allan D. Limited breakdown of cytoskeletal proteins by an endogenous protease controls Ca2+-induced membrane fusion events in chicken erythrocytes. Biochim Biophys Acta. 1983 May 5;730(2):351–358. doi: 10.1016/0005-2736(83)90352-8. [DOI] [PubMed] [Google Scholar]
- de Chaffoy de Courcelles D., Roevens P., Van Belle H. 1-Oleoyl-2-acetyl-glycerol (OAG) stimulates the formation of phosphatidylinositol 4-phosphate in intact human platelets. Biochem Biophys Res Commun. 1984 Sep 17;123(2):589–595. doi: 10.1016/0006-291x(84)90270-5. [DOI] [PubMed] [Google Scholar]
- de Chaffoy de Courcelles D., Roevens P., van Belle H. 12-O-Tetradecanoylphorbol 13-acetate stimulates inositol lipid phosphorylation in intact human platelets. FEBS Lett. 1984 Aug 6;173(2):389–393. doi: 10.1016/0014-5793(84)80811-x. [DOI] [PubMed] [Google Scholar]