Abstract
Polyanionic antisense oligonucleotides hold great promise as RNA targeting drugs but issues with bioavailability hinder their development. Uncharged phosphorus-based backbones are promising alternatives but robust methods to produce them are limited. We report the synthesis and properties of oligonucleotides containing charge-neutral LNA alkyl phosphothiotriester backbones combined with 2′-O-methyl phosphorothioate nucleotides for therapeutic applications. The nature of the triester alkyl group dictates the success of solid-phase synthesis; tertiary alkyl groups are lost during the P(III) oxidation step, whereas primary alkyl groups are partially cleaved during deprotection. In contrast, oligonucleotides containing secondary phosphothiotriester linkages are stable, and large numbers of triesters can be incorporated. The modified oligonucleotides have excellent duplex stability with complementary RNA and exhibit strong nuclease resistance. To expand synthetic flexibility, oligonucleotides containing multiple internal alkynyl phosphothiotriesters can be conjugated to lipids, carbohydrates, or small molecules through CuAAC click chemistry. Oligonucleotides containing LNA-THP phosphothiotriesters exhibit high levels of pre-mRNA splice switching in eukaryotic cells.
Introduction
Antisense oligonucleotides are modified short single stranded synthetic nucleic acids that alter gene or protein expression via interactions with cellular RNAs.1−5 They function principally through mRNA splicing modulation6,7 and RNase H-mediated mRNA degradation.8 Double stranded siRNAs are also highly effective in gene silencing.9,10 Modified oligonucleotides hold promise for treating cancer,11 neuromuscular and genetic disorders,12 with the recent clinical approval of Inclisiran (Leqivo)13 for the relatively common condition primary hypercholesterolemia sparking intense interest in the field. Advantages over small molecule drugs include simple and logical design, strong and predictable RNA binding, and exquisite target specificity.14 Unmodified oligonucleotides are unsuitable for therapeutic purposes as they are rapidly digested by enzymes in cells. Hence, modifications must be introduced to enhance nuclease stability as well as improve pharmacokinetics, cellular uptake and reduce off-target effects.15 Sugar-modified nucleic acids including 2′-O-Me,16 2′-O-(2-methoxyethyl),17 LNA,18 cEt19 and 2′-fluoro20 have been developed to provide nuclease resistance. The widely used phosphorothioate (PS) backbone21 improves cell uptake and enhances stability to nucleases but slightly reduces RNA target affinity, which can be restored by 2′-sugar substituents.
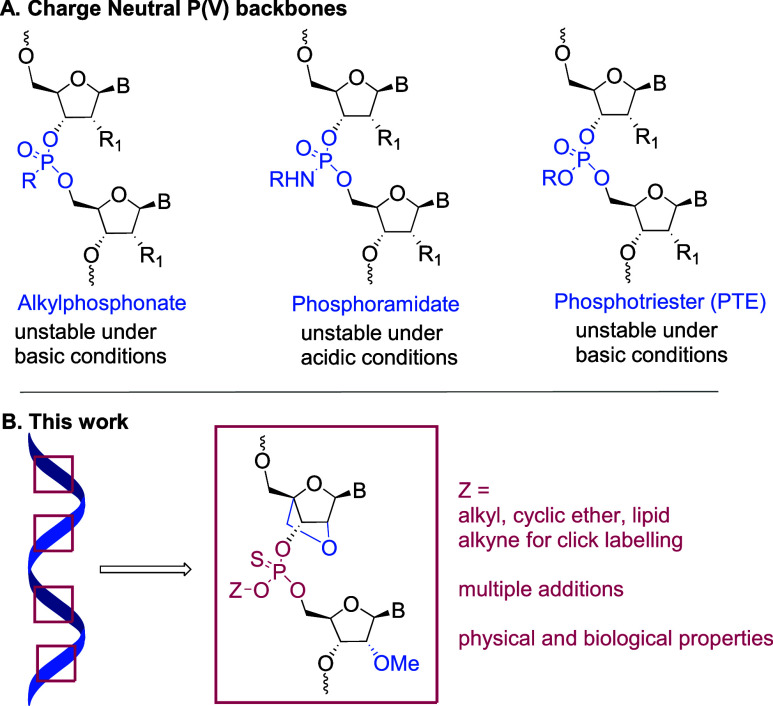
Reducing the net anionic charge of the oligonucleotide backbone has been explored in attempts to increase nuclease resistance, cell uptake and improve pharmacokinetics. This can be achieved by introducing charge-neutral internucleotide linkages.22−24 Charge-neutral phosphorodiamidate morpholino oligonucleotides (PMOs)25 are used to treat Duchenne Muscular Dystrophy (DMD), a genetic disease affecting 1 in 5000 boys globally, characterized by progressive muscle breakdown and with an average lifespan in the mid–late twenties. No cure exists but several PMO exon skipping oligonucleotide therapies have received FDA approval to treat genetic variants of this disease: Eteplirsen (exon 51),26,27 Golodirsen (exon 53),28 and Casimersen (exon 45).29 This provides the impetus to develop new charge-neutral oligonucleotide backbones, particularly as those currently in the clinic have limited efficacy. Some charge-neutral backbones can be challenging to synthesize; for example if the modified backbone is introduced as a dinucleotide (as is common), 16 dinucleotide phosphoramidites are required to enable synthesis of any required sequence.30,31 In contrast, phosphorus-based charge-neutral backbones can be introduced into oligonucleotides as phosphoramidites on solid-phase via just four modified phosphoramidite monomers.32 These “P-backbones” are of three main types: alkylphosphonate,33,34 phosphoramidate,35,36 and phosphotriester37 (PTE) (Figure 1A). Despite their favorable physical and biological properties, these backbones are reported to be unstable under the standard acidic or basic conditions used in solid-phase oligonucleotide synthesis and deprotection.38
Figure 1.
(A) Charge-neutral P-backbones. R, R1= various substituents. (B) Current study.
Previous studies on phosphotriester oligonucleotides include their use as biomarkers39,40 and as biodegradable pro-drugs.24,41,42 Our own interest is based on the ability to vary the alkyl group to improve chemical, physical, and particularly therapeutic properties including cell uptake, and especially exon-skipping activity. Phosphotriester dinucleotides were first synthesized over 50 years ago.43 However, due to reported instability, the equivalent oligonucleotides are challenging to synthesize.44 This has been addressed by changing protecting group strategies44−46 and recently a method was developed for synthesizing hydrophobic and cationic PTE oligonucleotides.37 The alkyl-PTE oligonucleotides that have been studied so far include isopropyl,47 neo-pentyl,48 phenyl,45 dodecyl49 and cleavable disulfide.41 Stearyl,50 phenylethyl,51 isopropyl and tetrahydrofuranyl PTE oligonucleotides have all shown improved activity in mice.24 In addition, short 8-mer oligo dA8 and dT8 lipophilic phosphothiotriester oligonucleotides have been proposed as cellular delivery agents for PNA and PMO DNA analogues.52
Results and Discussion
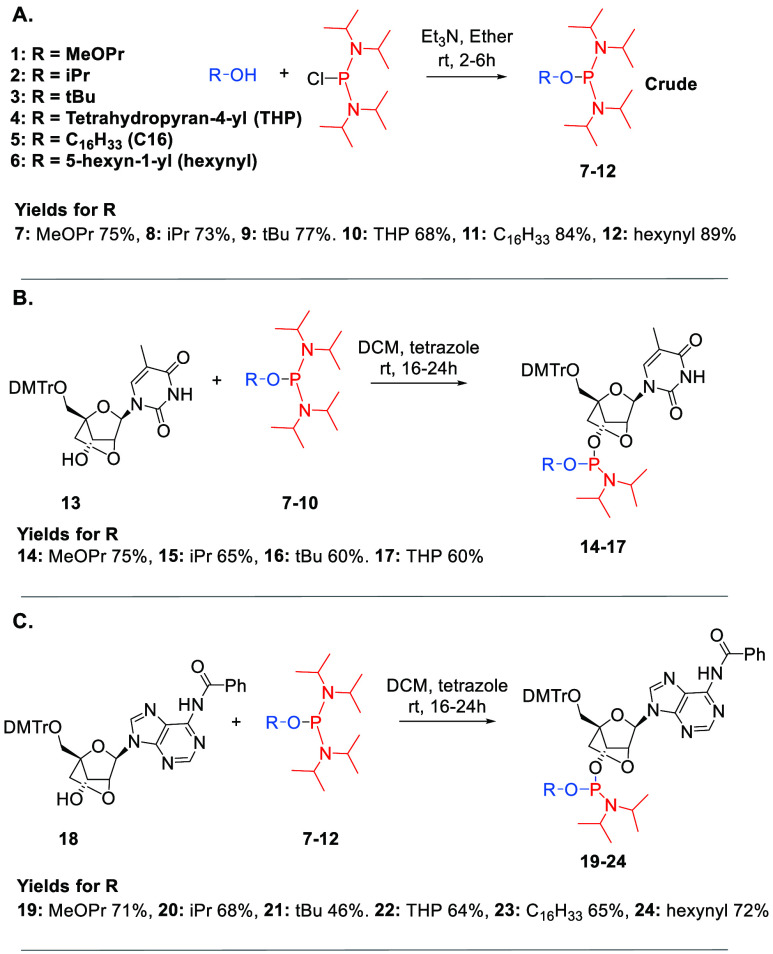
Here, we report the synthesis of mixed-sequence therapeutically relevant oligonucleotides containing charge-neutral phosphothiotriester (PTTE) and phosphotriester (PTE) backbones with locked nucleic acid (LNA) sugars, using monomers that are fully compatible with standard solid-phase assembly. Following our methods, we have introduced more than 50% of charge-neutral PTTE linkages, and we are not limited to this percentage. The synthesis of both PTE and PTTE oligonucleotides requires the preparation of nucleoside 3′-phosphoramidites in which the chosen alkyl group replaces the 2-cyanoethyl moiety of standard phosphoramidites. To prepare the required monomers, alcohols 1–6 were reacted with bis(diisopropylamino)chlorophosphine to give the phosphorodiamidite reagents 7–12 (Scheme 1).53−55 Next, commercially available 5′-O-DMTr-protected locked nucleoside 13 was reacted with 7–10 in the presence of tetrazole to afford the thymidine LNA phosphoramidites 14–17. Similarly, locked nucleoside 18 was reacted with 7–12 to give LNA A-phosphoroamidites 19–24. Phosphoramidite monomers 14–17 and 19–24 along with 2′-O-methyl derivatives of N6-benzoyl-A, N2-isobutyryl-G, N4-acetyl-C and U (Supporting Information Figure S1) were used for the synthesis of a wide range of oligonucleotides on the 1 μmole scale using EDITH (3-ethoxy-1,2,4-dithiazole-5-one) as sulfurizing reagent (ON1–ON41, ONOX1, ONOX4, Table 1). Coupling efficiencies of the triester monomers measured by liberated DMT cations were high (Supporting Information 7.0). All oligonucleotides were cleaved from the solid support and deprotected with a 1:1 mixture of THF-ethylene diamine (EDA), purified by HPLC and analyzed by UPLC-MS (Supporting Information 2.0). The chosen oligonucleotide sequence is designed to correct an aberrant luciferase mRNA splice site to give a luminescent readout of exon skipping in model HeLa cells (Table 1).56
Scheme 1. (A) Synthesis of Phosphoramidite Reagent. (B) Synthesis of Modified Thymidine Phosphoramidite Monomers 14–17. (C) Synthesis of Modified Adenosine Phosphoramidite Monomers 19–24. DMTr = 4,4′-Dimthoxytrityl.
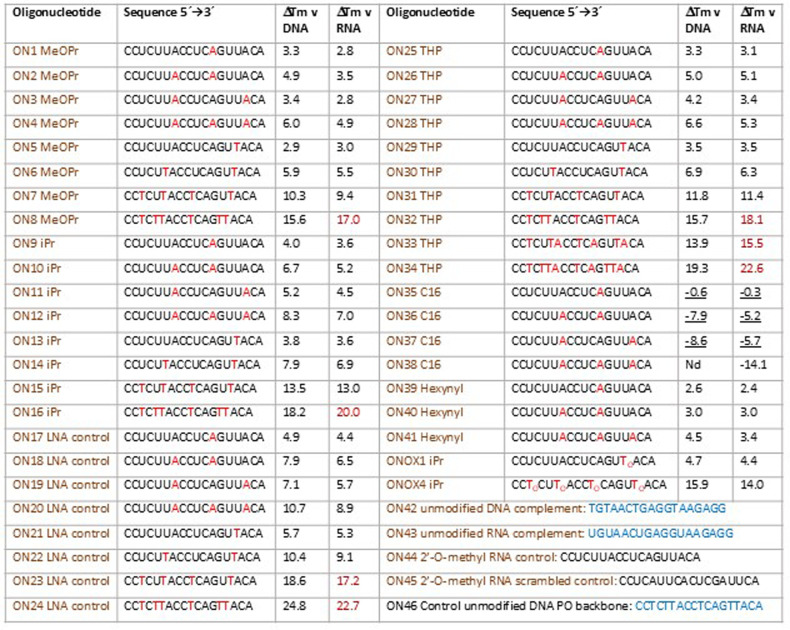
Table 1. Oligonucleotides Used and Duplex Melting dataa.
Nucleotides in black have 2′-O-Me ribose sugars and phosphorothioate internucleoside linkages. Nucleotides in red are locked nucleic acid phosphothiotriesters (except ON17–ON24 which have phosphorothioate internucleoside linkages), the red ‘o’ indicates phosphotriester linkage instead of phosphothiotriester. ΔTm = difference in duplex melting temperature of ON1–ON41 against DNA and RNA compared to control ON44 (2′-O-methyl phosphorothioate). Tm of control vs DNA = 48.5 °C, Tm of control vs RNA = 61.3 °C. Melting temperatures were recorded in 10 mM Na-phosphate buffer, pH = 7.0. The melting buffer for complementary DNA contained additional 100 mM NaCl and the melting buffer for complementary RNA contained additional 25 mm NaCl. Tm values used for the ΔTm calculations are an average of three experiments with an error of ±0.25 °C. Comprehensive melting temperature data is in supporting information 4.0, Tables T6–T17. In some cases, the Tm against complementary RNA was too high to determine so additional Tm data was obtained in 10 mM Na-phosphate buffer, pH = 7.0 with no additional NaCl. These Tm values were adjusted using the following tool: http://biotools.nubic.northwestern.edu/OligoCalc.html. These ΔTm values are in red. See Supporting Information4.8 for melting curves without additional NaCl.
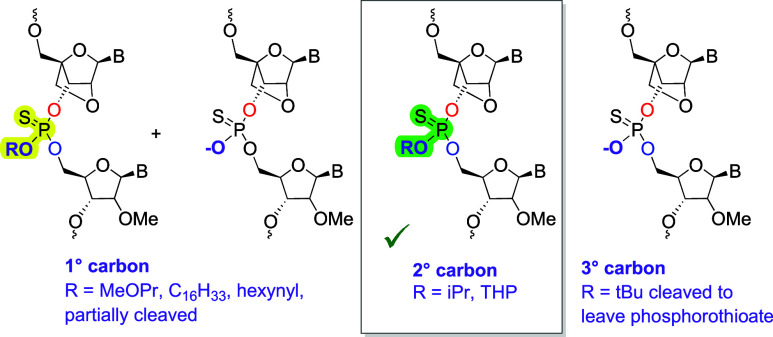
The primary methoxypropyl alkyl groups in ON1–ON8 were partially cleaved during deprotection with EDA-THF (Figure 2). Approximately 15% of undesired phosphodiester backbone oligonucleotide was obtained in the synthesis of ON1 and ON5 which contain a single addition of the MeOPr-A and MeOPr-T monomers respectively (Supporting Information Figures S5 and S21). Oligonucleotides with more than three methoxypropyl modifications (ON7, ON8) were difficult to purify and lower yields were obtained (Supporting Information Table T2). The same instability problem was encountered with ammonia deprotection of these oligonucleotides, even at room temperature (Supporting Information 2.1, 2.6). Formation of phosphodiester side products was not significant for ON9–ON16 which contain from one to six isopropyl phosphothiotriesters, and good yields were obtained (Supporting Information Table T2). However, for oligonucleotides ON17–ON24, the t-butyl groups were cleaved from the triesters during solid-phase synthesis to give phosphorothioates, i.e. no PTTE linkages were found (Figure 2 and Supporting Information Tables T3 and T4). Cleavage of the t-butyl group was independent of the capping and detritylation steps and probably occurred during the oxidation or sulfurization step (Table T4 and Figures S66–S69). However, we cannot exclude loss of t-butyl during oligonucleotide deprotection. Potential mechanisms explaining the instability of primary and tertiary phosphothiotriester backbones are shown in the Figure S3. In contrast to the instability of 1o and 3° triesters, oligonucleotides ON25–ON32 containing 2° tetrahydropyranyl phosphothiotriesters (THP) were stable, and yields were similar to those of the isopropyl PTTE oligonucleotides (Supporting Information Table S2). We were able to successfully synthesize ON33 and ON34, containing seven and nine THP triesters, respectively. In the latter case this reduces the negative charge in the oligonucleotide by more than 50%. Subsequently, we synthesized ON35–ON38 containing one to three additions of the lipophilic C16-alkyl group which could influence cell uptake. As found for other primary alcohols, a mixture of phosphothiodiester and PTTE was obtained. Finally, we synthesized the hexynyl-functionalized PTTE oligonucleotides ON39–ON41 for subsequent click labeling. In future we plan to use a secondary alcohol/alkyne to facilitate the incorporation of large numbers of alkynes. In summary, the nature of the alkyl group dictates synthesis efficiency of oligonucleotides with LNA-PTTE backbones, secondary alcohols being the best. Moreover, we were able to synthesize the oligonucleotides ONOX1 and ONOX4 which have one and four isopropyl phosphotriester linkages respectively (Table T5 and Figures S125–S128). To summarize, the stability of the LNA-secondary alkyl triesters is good in both phosphothiotriester and phosphotriester formats.
Figure 2.
Synthesis of PTTE oligonucleotides using modified locked nucleic acid phosphoramidite monomers. Only secondary alkyl groups give efficient oligonucleotide synthesis. Details of possible side reactions leading to loss of triester alkyl groups are in the Supporting Information Figure S3.
Small alkyl groups in the PTE backbone such as methyl, ethyl, isopropyl and tetrahydropyran-4-yl are reported to destabilize duplexes, causing a 1–4 °C reduction in melting temperature (Tm) relative to the unmodified oligonucleotide.24 In one case, however, a slight increase in duplex stability has been observed.45 Our strategy of combining LNA sugars with triester linkages ensured increased duplex stability against both complementary DNA and RNA in addition to the required chemical stability. A reduction in Tm was only observed for the C16 lipid chain linked oligonucleotides ON35–ON38. Stability is highest per modification for ON17–ON24 but this is due to loss of the alkyl group from the triesters, leaving the LNA-phosphorothioate diester backbone linkages. This fortuitously provided LNA control oligonucleotides for the exon-skipping cell studies described below.
Overall, these results indicate that LNA-phosphothiotriester linkages are less duplex-stabilizing than LNA-phosphodiester linkages (compare ON17 with ON1, ON20 with ON4, ON24 with ON8). The duplex destabilization caused by the alkyl groups on the PTTE backbone follows the order iPr < THP ∼ MeOPr < hexynyl < C16 (Table 1 and Supporting Information 4.0). In summary, adjacent LNA sugars increase the duplex stability of phosphothiotriester oligonucleotides compared to those with deoxyribose sugars, but the stabilizing effect is not quite as extreme as for LNA-phosphorothioate diesters. We also performed UV melting studies of ON34 and ON44 with complementary DNA at different salt concentrations. The results indicate that the melting temperature of ON34 has slightly lower salt dependence compared to the control ON44 due to the smaller number of negative charges in the backbone (Supporting Information 4.7).
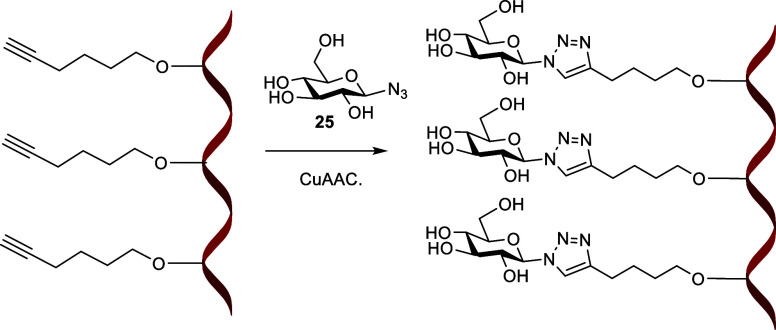
Hexynyl phosphothiotriester oligonucleotides ON39–ON41 were functionalized with glucose azide by click chemistry to generate ON47–ON49 corresponding to mono-, di- and trivalent glucose conjugates respectively (Scheme 2, Supporting Information 3.0). This represents a useful and versatile method of adding multiple internal reporter groups or other labels or cell-targeting moieties into the backbone of oligonucleotides.57
Scheme 2. CuAAC Post-Labelling of Hexynyl 18-Mer ON41 with Glucose Azide (25).
Conditions: CuSO4, sodium ascorbate, H2O, DMSO, tris(3-hydroxypropyl-triazolylmethyl)amine (THPTA) 24 h at room temperature.
Circular dichroism studies show that LNA-phosphothiotriesters have minimal effects on duplex structure, even when oligonucleotides contain large numbers of triesters. The CD spectra of oligonucleotides ON8 (6 x MeOPr), ON16 (6 x iPr), ON32 (6 x THP), ON33 (7 x THP), ON34 (9x THP), ON38 (3 x C16) and ON41 (3 x hexynyl) hybridized to complementary RNA are almost perfectly aligned with the control ON44/RNA duplex (Figure 3). The highest structural deviation is observed for ON38 which has the three lipophilic C16 alkyl groups. This could be due to intra- or intermolecular hydrophobic interactions between lipids, or changes in hydration of the duplex induced by the lipids. Either or both effects might also explain the lower duplex stability of these oligonucleotides. The PTTE backbones also cause minimal structural deviation of duplexes with complementary DNA (Supporting Information 5.0).
Figure 3.
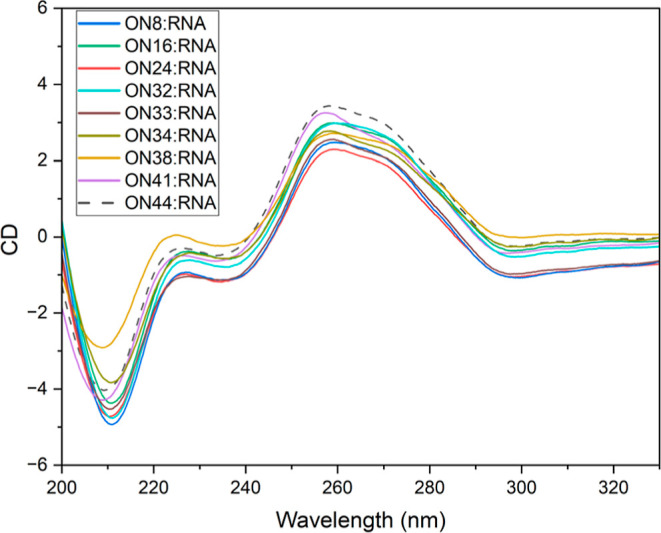
CD spectra of oligonucleotide-RNA duplexes. Y-axis is ellipticity θ, (10–3 deg.cm2/dmol).
Stability of ASOs against nuclease enzymes in vivo is essential. To evaluate this, oligonucleotides ON4 (MeOPr), ON15 (iPr), ON31 (THP), ON35 (C16) and ON41 (hexynyl), representing all alcohol variants studied, were incubated with nuclease S1 from Aspergillus oryzae. The unmodified control ON46 was converted to mononucleotides within 1 h whereas oligonucleotides carrying a hydrophobic PTTE linkage remained fully intact after 2 days (Supporting Information 6.0). Moreover, we synthesized a series of oligonucleotides ONS5–ONS8 based on (dT)12 in which one nucleotide is modified with LNA-PTE or LNA-PTTE (Supporting Information 6.1, Supporting Information Table T18 and Supporting Information Figures S177–S185). Enzymatic digestion of these oligonucleotides by exonuclease I (Escherichia coli) is blocked by the alkyl LNA PTTE and LNA PTE linkages, while phosphodiester linkages are completely digested by the enzyme within 1 hour. These experiments confirm that LNA-phosphotriester linkages are enzymatically stable, even in the absence of phosphorothioate groups. This offers the possibility of using oligonucleotides with reduced phosphorothioate content in vivo, thus potentially mediating interactions of therapeutic oligonucleotides with serum proteins and paraspeckle proteins such as P54nrb, which are reported to be undesirable.58
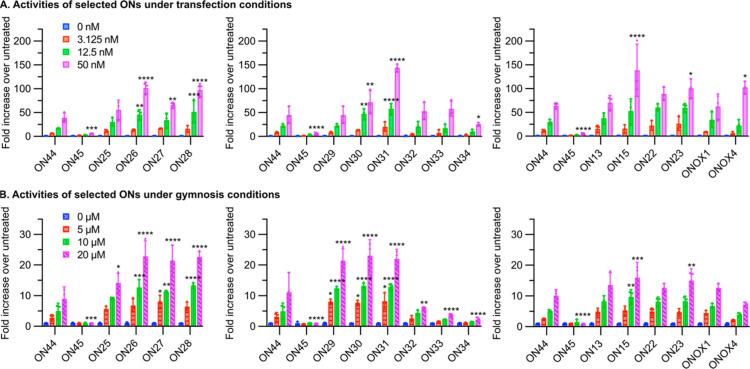
In a preliminary study of the therapeutic potential of the LNA-phosphothiotriester backbone in the modulation of splicing, a series of 18-mer splice-switching oligonucleotides (SSOs) containing LNA-THP PTTE linkages (ON25 to ON34 in Table 1) were evaluated in a luciferase exon-skipping cell assay (Figure 4).56 These oligonucleotides contain between one and nine phosphothiotriesters and were designed to determine the relationship between activity and the number of LNA-PTTE linkages. We also evaluated oligonucleotides with varying LNA-phosphorothioate content (i.e. without triesters) (ON22 and ON23). Finally, we compared the activity of oligonucleotides containing LNA-iPr-PTE and LNA-iPr-PTTE linkages (ON13 vs ONOX1 and ON15 vs ONOX4). Our primary control oligonucleotide throughout was 2′-O-methyl phosphorothioate ON44. Exon-skipping activity was determined following both transfection and gymnosis in order to determine whether the origin of any increased performance was due to improved uptake into the cells or enhanced exon-skipping efficiency.
Figure 4.
Activities of selected ONs in HeLa pLuc/705 cells. (A) ONs were transfected into HeLa pLuc/705 cells at the indicated concentrations using Lipofectamine 2000, and luciferase activity was measured 48 h later. (B) ONs were applied to HeLa pLuc/705 cells at the indicated concentrations in the absence of a transfection reagent, and luciferase activity was measured 72 h later. In all cases, luminescence was normalized to total protein quantity and untreated cells. Data are means ± standard deviations for three biological replicates (n = 3), where each biological replicate was performed in technical triplicate. Statistics are two-way analysis of variance (ANOVA) with Dunnett’s multiple comparisons test against ON44, α = 0.05: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001. None of the oligonucleotides in this study displayed any significant toxicity as judged by cell growth (Supporting Information Figure S228).
Under transfection conditions, ON26, ON27, and ON28, which contain two, two, and three LNA-THP PTTE linkages respectively at A-nucleotides, showed improved activity relative to the control ON44. The mean improvements were between 1.7 and 3.0-fold depending on oligonucleotide concentration. Similarly, ON30 and ON31, which have two and four LNA-THP PTTE linkages at T-nucleotides, showed between 1.6 and 3.2-fold improved activity relative to ON44. Additionally, ON15, with four iPr-PTTE linkages at T-nucleotides, showed 2.2-fold increased activity at 50 nM relative to ON44.
Under gymnosis conditions, ON25, ON26, ON27, and ON28, which have one, two, two, and three LNA-THP PTTE linkages respectively at A-nucleotides, all showed enhanced activity relative to ON44. The mean improvements ranged from 1.6 to 2.6-fold at 20 μM. ON29, ON30, and ON31, which have one, two, and four LNA-THP PTTE linkages at T-nucleotides, showed similar levels of improved activity relative to ON44. ON15, which has four iPr PTTE linkages at T-nucleotides also showed improved activity at both 10 and 20 μM, where the mean improvements were 1.9 and 3.2-fold, respectively. In contrast, oligonucleotides with greater numbers of LNA-neutral linkages were less active: ON32, which has six LNA-THP PTTE linkages, and ON33 and ON34, which have seven and nine LNA-THP PTTEs respectively, showed greatly reduced activities.
Comparing the activities of ON15 (4 x iPr), ON23 (4 x LNA phosphorothioate control), ON31 (4 x THP-PTTE) and ONOX4 (4 x iPr-PTE) under gymnosis conditions at seven concentrations on a single plate indicates that the LNA phosphothiotriester and phosphotriester modifications, as well as the LNA phosphorothioate control, all have similar activity (Figure 5). Importantly, both ON15 and ONOX4 which contain four LNA-iPr PTTE and PTE respectively, and therefore differ in the number of sulfur atoms in the oligonucleotide backbone, have comparable splice-switching activities. This is in line with their similar duplex stabilities with complementary RNA (Table 1) and the stability of the LNA-PTE linkage to enzymatic digestion discussed above. The use of LNA-PTE linkages in therapeutic oligonucleotides could facilitate alternative delivery mechanisms that do not depend on binding of phosphorothioates to serum proteins.59 Moderating the number of phosphorothioates has been suggested as a method to prevent excessive oligonucleotide-protein binding in vivo,58 and reducing PS content has been shown to improve the toxicity profile and acute tolerability of ASOs in vivo.60
Figure 5.
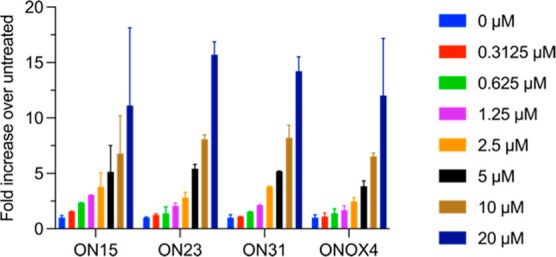
Seven-point dose response of selected ONs in HeLa pLuc/705 cells. ONs were applied to HeLa pLuc/705 cells at the indicated concentrations in the absence of a transfection reagent, and luciferase activity was measured 72 h later. In all cases, luminescence was normalized to total protein quantity and untreated cells. Data are means ± standard deviations for two biological replicates (n = 2), where each biological replicate was performed in technical triplicate.
Our study shows that oligonucleotides which have moderately but not excessively high melting temperatures relative to the control ON44 have improved splice-switching activities. Release of the oligonucleotide from the spliced-out intron will allow interactions with more pre-mRNA molecules, and this could explain why oligonucleotides with large numbers of LNA sugars (i.e., with high Tms) have decreased splice-switching activities. On the other hand, if the oligonucleotide/RNA duplex is too unstable it will not block aberrant splicing, so “low-Tm” oligonucleotides will be inactive. In this study the optimum Tm against complementary RNA is ∼8 °C above that of the control ON44 (Supporting Information Figure S229). Other mechanisms/factors may be involved and these need to be investigated in future.
Conclusions
To conclude, we have synthesized oligonucleotides containing multiple charge-neutral LNA-phosphothiotriester linkages by straightforward solid phase phosphoramidite methods, inserting methoxypropyl, isopropyl, THP, C16 lipid, and hexynyl into the PTTE backbone. We show that it is critically important to select appropriate alkyl functionalities, with secondary alkyl groups being the most suitable. To demonstrate the efficiency of our methodology we have introduced nine charge-neutral THP-PTTE linkages into a modified 18-mer oligonucleotide, reducing overall negative charge by more than 50%. The LNA-PTTE backbones stabilize duplexes with complementary DNA or RNA and do not distort their structures. With in vivo applications in mind, 2′-O-methyl phosphorothioate oligonucleotides containing LNA-PTTEs and LNA-PTEs are stable in the presence of nuclease enzymes. Our approach is applicable to the incorporation of multiple alkynes across the oligonucleotide backbone, enabling conjugation with azide derivatives, demonstrated here by glucose. This post-labeling click strategy has potential for the incorporation of other carbohydrate and peptide-based cell-receptor ligands.61−63 We used final stage intermediates of LNA phosphoramidites as starting materials to make the modified LNA phosphoramidites. This is a strength of our approach, as such intermediates are available from companies that produce special phosphoramidite monomers for the synthesis of therapeutic oligonucleotides. It also means that similar work could readily be carried out on other therapeutically relevant nucleoside phosphoramidite precursors including protected 2′-O-alkyl and 2′-fluoro nucleosides which are manufactured on an industrial scale. Importantly the nucleobase protecting groups on these nucleosides are compatible with the deprotection conditions used for PTTE triester oligonucleotides bearing secondary alcohols. Initial cell studies show a large increase in exon-skipping activity for oligonucleotides containing between two and four LNA-THP phosphothiotriester modifications whereas oligonucleotides with greater numbers of LNA-phosphothiotriesters were much less active. The fact that increased activity was observed in both transfection and gymnotic delivery suggests improved steric blocking of the enzymatic cleavage that is required for mRNA splicing. Toxicity has been an obstacle to the use of LNA oligonucleotides clinically.64,65 It will therefore be important to study the toxicological and pharmacokinetic properties of oligonucleotides containing LNA-phospho(thio)triesters in depth, as they might have more favorable toxicological properties than the equivalent well-studied LNA-diesters. Protein-binding, cell and animal studies will be carried out to investigate this.
As is the case for all clinically approved oligonucleotides containing phosphorothioates, our LNA-phospho(thio)triesters are diastereomeric mixtures at phosphorus. Sterically pure phosphorothioates can be synthesized by P(V) and P(III) chemistry but have not yet reached the clinic.66,67 Synthetic efforts to produce chirally pure LNA-PTTE/PTE oligonucleotides are worth considering, and might lead to improved properties. Finally, applications of the oligonucleotides reported here may stretch beyond therapeutics, for example into several fields such as triplexes and modified aptamers where flexibility of functionality and in vivo stability are important considerations.68
Acknowledgments
The research is funded by UK MRC grant MR/X008029/1 (TransNAT). AHES wishes to acknowledge the Royal Society research fund (RGS\R2\242523), and Royal Society of Chemistry research fund (R24-8767374007).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.4c11402.
Supplementary Tables S1–S5. Figures S1–S229. Experimental procedures, analytical data of novel compounds including 1D (1H, 13C, 31P) NMR spectra and HRMS. UPLC-ESI-MS of oligonucleotides. (PDF). UV meting data, CD curves, PAGE analysis, trityl reading, micrographs of Hela pLuc/705 cells, etc (PDF)
The authors declare no competing financial interest.
Table 1 was incomplete in the version that published on October 14, 2024. It has been updated and the revised version re-posted on October 15, 2024.
Supplementary Material
References
- Crooke S. T.; Baker B. F.; Crooke R. M.; Liang X.-h. Antisense technology: an overview and prospectus. Nat. Rev. Drug Discovery 2021, 20 (6), 427–453. 10.1038/s41573-021-00162-z. [DOI] [PubMed] [Google Scholar]
- Crooke S. T.; Witztum J. L.; Bennett C. F.; Baker B. F. RNA-targeted therapeutics. Cell Metab. 2018, 27 (4), 714–739. 10.1016/j.cmet.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Bennett C. F. Therapeutic Antisense Oligonucleotides Are Coming of Age. Annu. Rev. Med. 2019, 70 (1), 307–321. 10.1146/annurev-med-041217-010829. [DOI] [PubMed] [Google Scholar]
- Smith C. E.; Zain R. Therapeutic Oligonucleotides: State of the Art. Annu. Rev. Pharmacol. Toxicol. 2019, 59 (1), 605–630. 10.1146/annurev-pharmtox-010818-021050. [DOI] [PubMed] [Google Scholar]
- Uhlmann E.; Peyman A. Antisense oligonucleotides: a new therapeutic principle. Chem. Rev. 1990, 90 (4), 543–584. 10.1021/cr00102a001. [DOI] [Google Scholar]
- Lu S. X.; De Neef E.; Thomas J. D.; Sabio E.; Rousseau B.; Gigoux M.; Knorr D. A.; Greenbaum B.; Elhanati Y.; Hogg S. J.; et al. Pharmacologic modulation of RNA splicing enhances anti-tumor immunity. Cell 2021, 184 (15), 4032–4047. 10.1016/j.cell.2021.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens M. A.; Hastings M. L. Splice-switching antisense oligonucleotides as therapeutic drugs. Nucleic Acids Res. 2016, 44 (14), 6549–6563. 10.1093/nar/gkw533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X. H.; Sun H.; Nichols J. G.; Crooke S. T. RNase H1-Dependent Antisense Oligonucleotides Are Robustly Active in Directing RNA Cleavage in Both the Cytoplasm and the Nucleus. Mol. Ther. 2017, 25 (9), 2075–2092. 10.1016/j.ymthe.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H.; Mao Q.; Paulson H. L.; Davidson B. L. siRNA-mediated gene silencing in vitro and in vivo. Nat. Biotechnol. 2002, 20 (10), 1006–1010. 10.1038/nbt739. [DOI] [PubMed] [Google Scholar]
- Tang Q.; Khvorova A. RNAi-based drug design: considerations and future directions. Nat. Rev. Drug Discovery 2024, 23 (5), 341–364. 10.1038/s41573-024-00912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H.; Veedu R. N.; Diermeier S. D. Recent Advances in Oligonucleotide Therapeutics in Oncology. Int. J. Mol. Sci. 2021, 22 (7), 3295. 10.3390/ijms22073295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlini A.; Goyenvalle A.; Muntoni F. RNA-targeted drugs for neuromuscular diseases. Science 2021, 371 (6524), 29–31. 10.1126/science.aba4515. [DOI] [PubMed] [Google Scholar]
- Ray K. K.; Wright R. S.; Kallend D.; Koenig W.; Leiter L. A.; Raal F. J.; Bisch J. A.; Richardson T.; Jaros M.; Wijngaard P. L. J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382 (16), 1507–1519. 10.1056/NEJMoa1912387. [DOI] [PubMed] [Google Scholar]
- Egli M.; Manoharan M. Chemistry, structure and function of approved oligonucleotide therapeutics. Nucleic Acids Res. 2023, 51 (6), 2529–2573. 10.1093/nar/gkad067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano R. L.; Ming X.; Carver K.; Laing B. Cellular uptake and intracellular trafficking of oligonucleotides: implications for oligonucleotide pharmacology. Nucleic Acid Ther. 2014, 24 (2), 101–113. 10.1089/nat.2013.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo B. H.; Bochkareva E.; Bochkarev A.; Mou T. C.; Gray D. M. 2’-O-methyl-modified phosphorothioate antisense oligonucleotides have reduced non-specific effects in vitro. Nucleic Acids Res. 2004, 32 (6), 2008–2016. 10.1093/nar/gkh516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. C.; Hall J. The MOE Modification of RNA: Origins and Widescale Impact on the Oligonucleotide Therapeutics Field. Helv. Chim. Acta 2023, 106 (3), e202200169 10.1002/hlca.202200169. [DOI] [Google Scholar]
- Singh S. K.; Koshkin A.; Wengel J.; Nielsen P. LNA (locked nucleic acids): synthesis and high-affinity nucleic acid recognition. Chem. Commun. 1998, (4), 455–456. 10.1039/a708608c. [DOI] [Google Scholar]
- Pallan P. S.; Allerson C. R.; Berdeja A.; Seth P. P.; Swayze E. E.; Prakash T. P.; Egli M. Structure and nuclease resistance of 2 ’,4 ’-constrained 2 ’-O-methoxyethyl (cMOE) and 2 ’-O-ethyl (cEt) modified DNAs. Chem. Commun. 2012, 48 (66), 8195–8197. 10.1039/c2cc32286b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallan P. S.; Greene E. M.; Jicman P. A.; Pandey R. K.; Manoharan M.; Rozners E.; Egli M. Unexpected origins of the enhanced pairing affinity of 2′-fluoro-modified RNA. Nucleic Acids Res. 2010, 39 (8), 3482–3495. 10.1093/nar/gkq1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F. P. Phosphorothioates, Essential Components of Therapeutic Oligonucleotides. Nucleic Acid Ther. 2014, 24 (6), 374–387. 10.1089/nat.2014.0506. [DOI] [PubMed] [Google Scholar]
- Baker Y. R.; Thorpe C.; Chen J.; Poller L. M.; Cox L.; Kumar P.; Lim W. F.; Lie L.; McClorey G.; Epple S.; et al. An LNA-amide modification that enhances the cell uptake and activity of phosphorothioate exon-skipping oligonucleotides. Nat. Commun. 2022, 13 (1), 4036. 10.1038/s41467-022-31636-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurusaki T.; Sato K.; Imai H.; Hirai K.; Takahashi D.; Wada T. Convergent synthesis of phosphorodiamidate morpholino oligonucleotides (PMOs) by the H-phosphonate approach. Sci. Rep. 2023, 13 (1), 12576. 10.1038/s41598-023-38698-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez G.; Migawa M. T.; Wan W. B.; Low A.; Tanowitz M.; Swayze E. E.; Seth P. P. Evaluation of Phosphorus and Non-Phosphorus Neutral Oligonucleotide Backbones for Enhancing Therapeutic Index of Gapmer Antisense Oligonucleotides. Nucleic Acid Ther. 2022, 32 (1), 40–50. 10.1089/nat.2021.0064. [DOI] [PubMed] [Google Scholar]
- Maksudov F.; Kliuchnikov E.; Pierson D.; Ujwal M. L.; Marx K. A.; Chanda A.; Barsegov V. Therapeutic phosphorodiamidate morpholino oligonucleotides: Physical properties, solution structures, and folding thermodynamics. Mol. Ther.--Nucleic Acids 2023, 31, 631–647. 10.1016/j.omtn.2023.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K. R.; Maruyama R.; Yokota T. Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Des., Dev. Ther. 2017, 11, 533–545. 10.2147/DDDT.S97635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshmi R. R.; Yokota T. Viltolarsen for the treatment of Duchenne muscular dystrophy. Drugs Today 2019, 55 (10), 627. 10.1358/dot.2019.55.10.3045038. [DOI] [PubMed] [Google Scholar]
- Anwar S.; Yokota T. Golodirsen for Duchenne muscular dystrophy. Drugs Today 2020, 56 (8), 491–504. 10.1358/dot.2020.56.8.3159186. [DOI] [PubMed] [Google Scholar]
- Shirley M. Casimersen: First Approval. Drugs 2021, 81 (7), 875–879. 10.1007/s40265-021-01512-2. [DOI] [PubMed] [Google Scholar]
- Kotikam V.; Rozners E. Amide-Modified RNA: Using Protein Backbone to Modulate Function of Short Interfering RNAs. Acc. Chem. Res. 2020, 53 (9), 1782–1790. 10.1021/acs.accounts.0c00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutisya D.; Hardcastle T.; Cheruiyot S. K.; Pallan P. S.; Kennedy S. D.; Egli M.; Kelley M. L.; Smith A. V. B.; Rozners E. Amide linkages mimic phosphates in RNA interactions with proteins and are well tolerated in the guide strand of short interfering RNAs. Nucleic Acids Res. 2017, 45 (14), 8142–8155. 10.1093/nar/gkx558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P.; Caruthers M. H. DNA Analogues Modified at the Nonlinking Positions of Phosphorus. Acc. Chem. Res. 2020, 53 (10), 2152–2166. 10.1021/acs.accounts.0c00078. [DOI] [PubMed] [Google Scholar]
- Krishna H.; Caruthers M. H. Alkynyl Phosphonate DNA: A Versatile “Click”able Backbone for DNA-Based Biological Applications. J. Am. Chem. Soc. 2012, 134 (28), 11618–11631. 10.1021/ja3026714. [DOI] [PubMed] [Google Scholar]
- Shoji Y.; Akhtar S.; Periasamy A.; Herman B.; Juliano R. L. Mechansism of cellular uptake of modified oligodeoxynucleotides containing methylphosphonate linkages. Nucleic Acids Res. 1991, 19 (20), 5543–5550. 10.1093/nar/19.20.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen R. A.; Märcher A.; Pedersen K. N.; Gothelf K. V. Insertion of Chemical Handles into the Backbone of DNA during Solid-Phase Synthesis by Oxidative Coupling of Amines to Phosphites. Angew. Chem., Int. Ed. 2023, 62 (26), e202305373 10.1002/anie.202305373. [DOI] [PubMed] [Google Scholar]
- Bannwarth W. Solid-Phase Synthesis of Oligodeoxynucleotides containing phosphoramidate internucleotide linkages and their specific chemical cleavage. Helv. Chim. Acta 1988, 71 (6), 1517–1527. 10.1002/hlca.19880710618. [DOI] [Google Scholar]
- Monfregola L.; Caruthers M. H. Solid-Phase Synthesis, Hybridizing Ability, Uptake, and Nuclease Resistant Profiles of Position-Selective Cationic and Hydrophobic Phosphotriester Oligonucleotides. J. Org. Chem. 2015, 80 (18), 9147–9158. 10.1021/acs.joc.5b01512. [DOI] [PubMed] [Google Scholar]
- Ota K.; Nagao K.; Hata D.; Sugiyama H.; Segawa Y.; Tokunoh R.; Seki T.; Miyamoto N.; Sasaki Y.; Ohmiya H. Synthesis of tertiary alkylphosphonate oligonucleotides through light-driven radical-polar crossover reactions. Nat. Commun. 2023, 14 (1), 6856. 10.1038/s41467-023-42639-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. D.; Le Pla R. C.; Farmer P. B. Phosphotriester adducts (PTEs): DNA’s overlooked lesion. Mutagenesis 2010, 25 (1), 3–16. 10.1093/mutage/gep038. [DOI] [PubMed] [Google Scholar]
- Bian K.; Delaney J. C.; Zhou X.; Li D. Biological Evaluation of DNA Biomarkers in a Chemically Defined and Site-Specific Manner. Toxics 2019, 7 (2), 36. 10.3390/toxics7020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto N.; Hayashi J.; Funaki R.; Wada S.-i.; Wada F.; Harada-Shiba M.; Urata H. Prodrug-Type Phosphotriester Oligonucleotides with Linear Disulfide Promoieties Responsive to Reducing Environment. ChemBioChem 2023, 24 (24), e202300526 10.1002/cbic.202300526. [DOI] [PubMed] [Google Scholar]
- Saneyoshi H.; Iketani K.; Kondo K.; Saneyoshi T.; Okamoto I.; Ono A. Synthesis and Characterization of Cell-Permeable Oligonucleotides Bearing Reduction-Activated Protecting Groups on the Internucleotide Linkages. Bioconjugate Chem. 2016, 27 (9), 2149–2156. 10.1021/acs.bioconjchem.6b00368. [DOI] [PubMed] [Google Scholar]
- Miller P. S.; Fang K. N.; Kondo N. S.; Ts’o P. O. P. Conformation and interaction of dinucleoside mono- and diphosphates. V. Syntheses and properties of adenine and thymine nucleoside alkyl phosphotriesters, the neutral analogs of dinucleoside monophosphates. J. Am. Chem. Soc. 1971, 93 (24), 6657–6665. 10.1021/ja00753a054. [DOI] [PubMed] [Google Scholar]
- Kuijpers W. H.; Huskens J.; Koole L. H.; van Boeckel C. A. Synthesis of well-defined phosphate-methylated DNA fragments: the application of potassium carbonate in methanol as deprotecting reagent. Nucleic Acids Res. 1990, 18 (17), 5197–5205. 10.1093/nar/18.17.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y.; Hirose M.; Hayakawa M.; Noyori R. General Synthesis and Binding Affinity of Position-Selective Phosphonodiester- and Phosphotriester-Incorporated Oligodeoxyribonucleotides. J. Org. Chem. 1995, 60 (4), 925–930. 10.1021/jo00109a024. [DOI] [Google Scholar]
- Meade B. R.; Gogoi K.; Hamil A. S.; Palm-Apergi C.; Berg A. v. d.; Hagopian J. C.; Springer A. D.; Eguchi A.; Kacsinta A. D.; Dowdy C. F.; et al. Efficient delivery of RNAi prodrugs containing reversible charge-neutralizing phosphotriester backbone modifications. Nat. Biotechnol. 2014, 32 (12), 1256–1261. 10.1038/nbt.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stec W. J.; Zon G.; Gallo K. A.; Byrd R. A.; Uznanski B.; Guga P. Synthesis and absolute configuration of P-chiral -isopropyl oligonucleotide triesters. Tetrahedron Lett. 1985, 26 (18), 2191–2194. 10.1016/S0040-4039(00)98959-7. [DOI] [Google Scholar]
- Lancelot G.; Guesnet J. L.; Asseline U.; Thuong N. T. NMR studies of complex formation between the modified oligonucleotide d(T*TCTGT) covalently linked to an acridine derivative and its complementary sequence d(GCACAGAA). Biochemistry 1988, 27 (4), 1265–1273. 10.1021/bi00404a029. [DOI] [PubMed] [Google Scholar]
- Dohno C.; Shibata T.; Okazaki M.; Makishi S.; Nakatani K. Amphiphilic DNA Duplex Stabilized by a Hydrophobic Zipper. Eur. J. Org Chem. 2012, 2012 (27), 5317–5323. 10.1002/ejoc.201200540. [DOI] [Google Scholar]
- Hammill M. L.; Tsubaki K.; Salim L.; Varley A. J.; Giorgees I.; Kitamura M.; Okauchi T.; Desaulniers J.-P. SiRNAs with Neutral Phosphate Triester Hydrocarbon Tails Exhibit Carrier-Free Gene-Silencing Activity. ACS Med. Chem. Lett. 2022, 13 (4), 695–700. 10.1021/acsmedchemlett.2c00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubaki K.; Hammill M. L.; Varley A. J.; Kitamura M.; Okauchi T.; Desaulniers J.-P. Synthesis and Evaluation of Neutral Phosphate Triester Backbone-Modified siRNAs. ACS Med. Chem. Lett. 2020, 11 (7), 1457–1462. 10.1021/acsmedchemlett.0c00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain H. V.; Verthelyi D.; Beaucage S. L. Amphipathic trans-acting phosphorothioate DNA elements mediate the delivery of uncharged nucleic acid sequences in mammalian cells. RSC Adv. 2015, 5 (80), 65245–65254. 10.1039/C5RA12038A. [DOI] [Google Scholar]
- Roiban G.-D.; Mehler G.; Reetz M. T. Palladium-Catalysed Amination of Aryl- and Heteroaryl Halides Using tert-Butyl Tetraisopropylphosphorodiamidite as an Easily Accessible and Air-Stable Ligand. Eur. J. Org Chem. 2014, 2014 (10), 2070–2076. 10.1002/ejoc.201301789. [DOI] [Google Scholar]
- Reddy P. G.; Chun B.-K.; Zhang H.-R.; Rachakonda S.; Ross B. S.; Sofia M. J. Stereoselective Synthesis of PSI-352938: A β-d-2′-Deoxy-2′-α-fluoro-2′-β-C-methyl-3′,5′-cyclic Phosphate Nucleotide Prodrug for the Treatment of HCV. J. Org. Chem. 2011, 76 (10), 3782–3790. 10.1021/jo200060f. [DOI] [PubMed] [Google Scholar]
- Tanabe K.; Ando Y.; Nishimoto S.-i. Reversible modification of oligodeoxynucleotides: click reaction at phosphate group and alkali treatment. Tetrahedron Lett. 2011, 52 (52), 7135–7137. 10.1016/j.tetlet.2011.10.109. [DOI] [Google Scholar]
- Kang S. H.; Cho M. J.; Kole R. Up-Regulation of Luciferase Gene Expression with Antisense Oligonucleotides: Implications and Applications in Functional Assay Development. Biochemistry 1998, 37 (18), 6235–6239. 10.1021/bi980300h. [DOI] [PubMed] [Google Scholar]
- Fantoni N. Z.; El-Sagheer A. H.; Brown T. A Hitchhiker’s Guide to Click-Chemistry with Nucleic Acids. Chem. Rev. 2021, 121 (12), 7122–7154. 10.1021/acs.chemrev.0c00928. [DOI] [PubMed] [Google Scholar]
- Migawa M. T.; Shen W.; Wan W. B.; Vasquez G.; Oestergaard M. E.; Low A.; De Hoyos C. L.; Gupta R.; Murray S.; Tanowitz M.; et al. Site-specific replacement of phosphorothioate with alkyl phosphonate linkages enhances the therapeutic profile of gapmer ASOs by modulating interactions with cellular proteins. Nucleic Acids Res. 2019, 47 (11), 5465–5479. 10.1093/nar/gkz247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qassem S.; Breier D.; Naidu G. S.; Hazan-Halevy I.; Peer D. Unlocking the therapeutic potential of locked nucleic acids through lipid nanoparticle delivery. Mol. Ther.--Nucleic Acids 2024, 35 (2), 102224. 10.1016/j.omtn.2024.102224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazami M. P.; Rembetsy-Brown J. M.; Wang F.; Krishnamurthy P. M.; Weiss A.; Marosfoi M.; King R. M.; Motwani M.; Gray-Edwards H.; Fitzgerald K. A.; et al. Quantifying and Mitigating Motor Phenotypes Induced by Antisense Oligonucleotides in the Central Nervous System. 2021, bioRxiv:2021.02.14.431096. [DOI] [PubMed] [Google Scholar]
- Sanhueza C. A.; Baksh M. M.; Thuma B.; Roy M. D.; Dutta S.; Préville C.; Chrunyk B. A.; Beaumont K.; Dullea R.; Ammirati M.; et al. Efficient Liver Targeting by Polyvalent Display of a Compact Ligand for the Asialoglycoprotein Receptor. J. Am. Chem. Soc. 2017, 139 (9), 3528–3536. 10.1021/jacs.6b12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. Preclinical and Clinical Advances of GalNAc-Decorated Nucleic Acid Therapeutics. Mol. Ther.--Nucleic Acids 2017, 6, 116–132. 10.1016/j.omtn.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erazo-Oliveras A.; Muthukrishnan N.; Baker R.; Wang T.-Y.; Pellois J.-P. Improving the Endosomal Escape of Cell-Penetrating Peptides and Their Cargos: Strategies and Challenges. Pharmaceuticals 2012, 5 (11), 1177–1209. 10.3390/ph5111177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayze E. E.; Siwkowski A. M.; Wancewicz E. V.; Migawa M. T.; Wyrzykiewicz T. K.; Hung G.; Monia B. P.; Bennett C. F. Antisense oligonucleotides containing locked nucleic acid improve potency but cause significant hepatotoxicity in animals. Nucleic Acids Res. 2007, 35 (2), 687–700. 10.1093/nar/gkl1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuya T.; Hori S.-i.; Watanabe A.; Nakajima M.; Gahara Y.; Rokushima M.; Yanagimoto T.; Kugimiya A. Ribonuclease H1-dependent hepatotoxicity caused by locked nucleic acid-modified gapmer antisense oligonucleotides. Sci. Rep. 2016, 6 (1), 30377. 10.1038/srep30377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knouse K. W.; deGruyter J. N.; Schmidt M. A.; Zheng B.; Vantourout J. C.; Kingston C.; Mercer S. E.; Mcdonald I. M.; Olson R. E.; Zhu Y.; et al. Unlocking P(V): Reagents for chiral phosphorothioate synthesis. Science 2018, 361 (6408), 1234–1238. 10.1126/science.aau3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto N.; Butler D. C. D.; Svrzikapa N.; Mohapatra S.; Zlatev I.; Sah D. W. Y.; Meena; Standley S. M.; Lu G.; Apponi L. H.; et al. Control of phosphorothioate stereochemistry substantially increases the efficacy of antisense oligonucleotides. Nat. Biotechnol. 2017, 35 (9), 845–851. 10.1038/nbt.3948. [DOI] [PubMed] [Google Scholar]
- Ni S.; Zhuo Z.; Pan Y.; Yu Y.; Li F.; Liu J.; Wang L.; Wu X.; Li D.; Wan Y.; et al. Recent Progress in Aptamer Discoveries and Modifications for Therapeutic Applications. ACS Appl. Mater. Interfaces 2021, 13 (8), 9500–9519. 10.1021/acsami.0c05750. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.