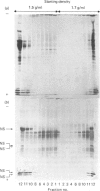
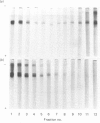
Abstract
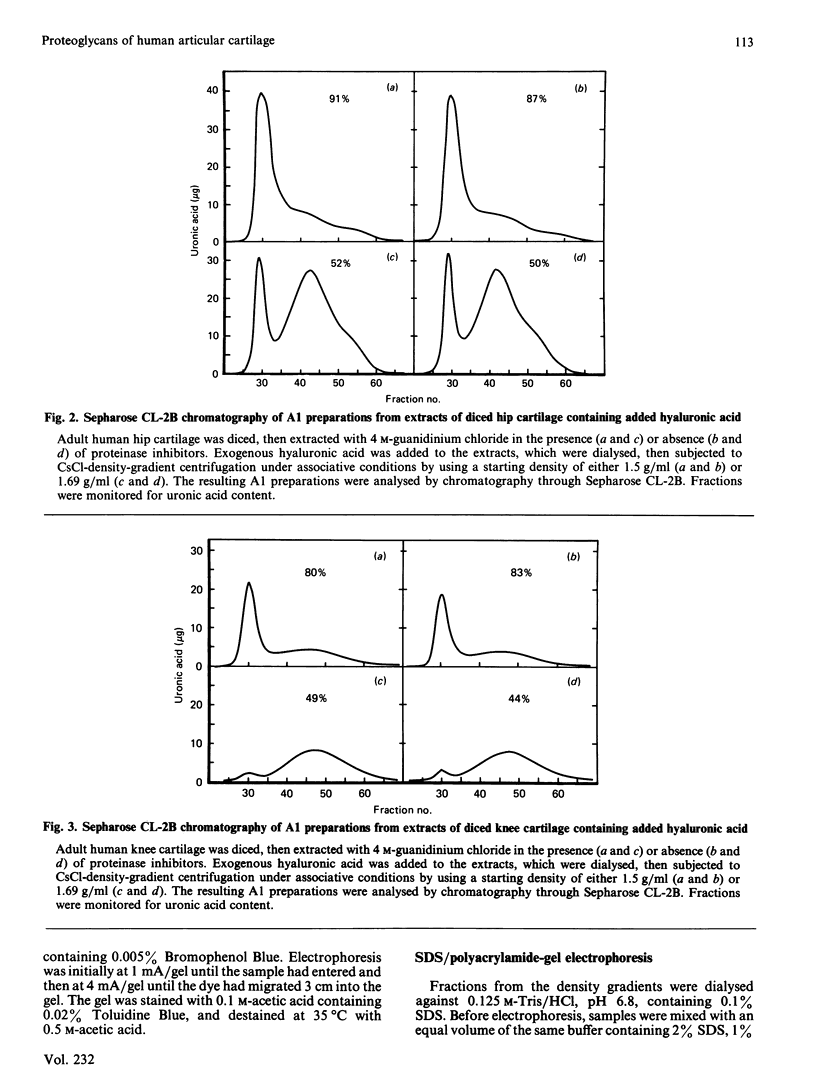
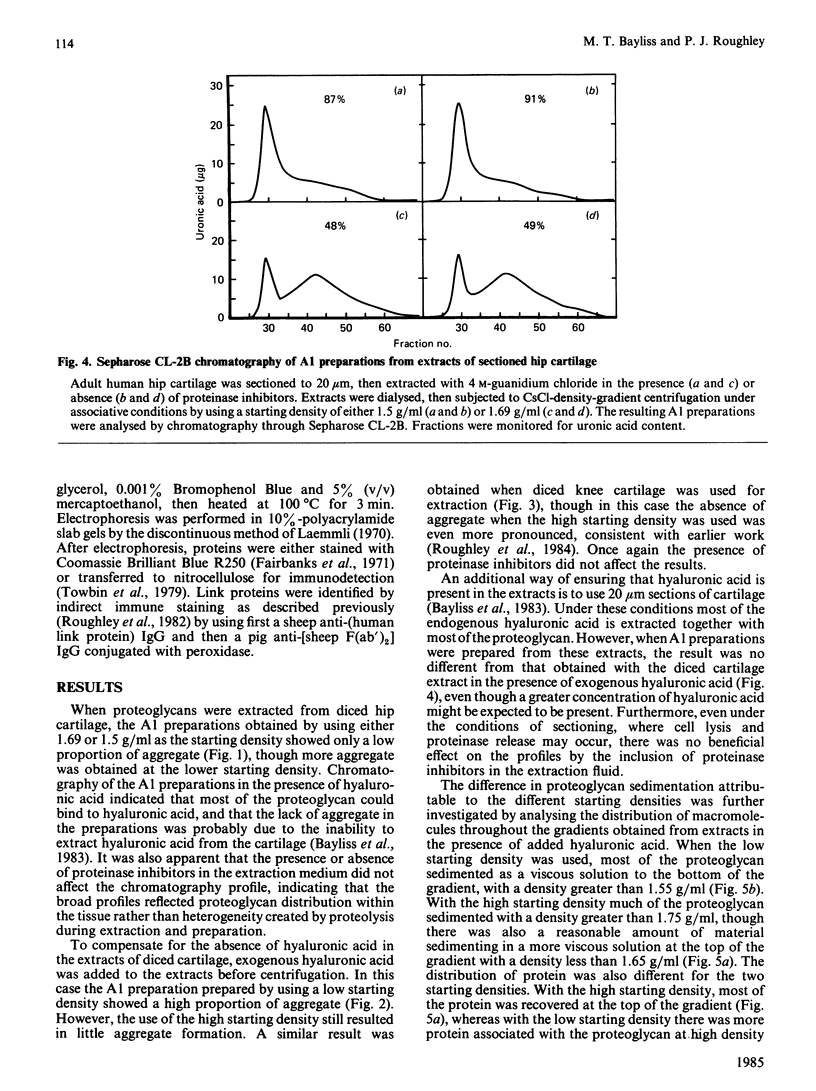
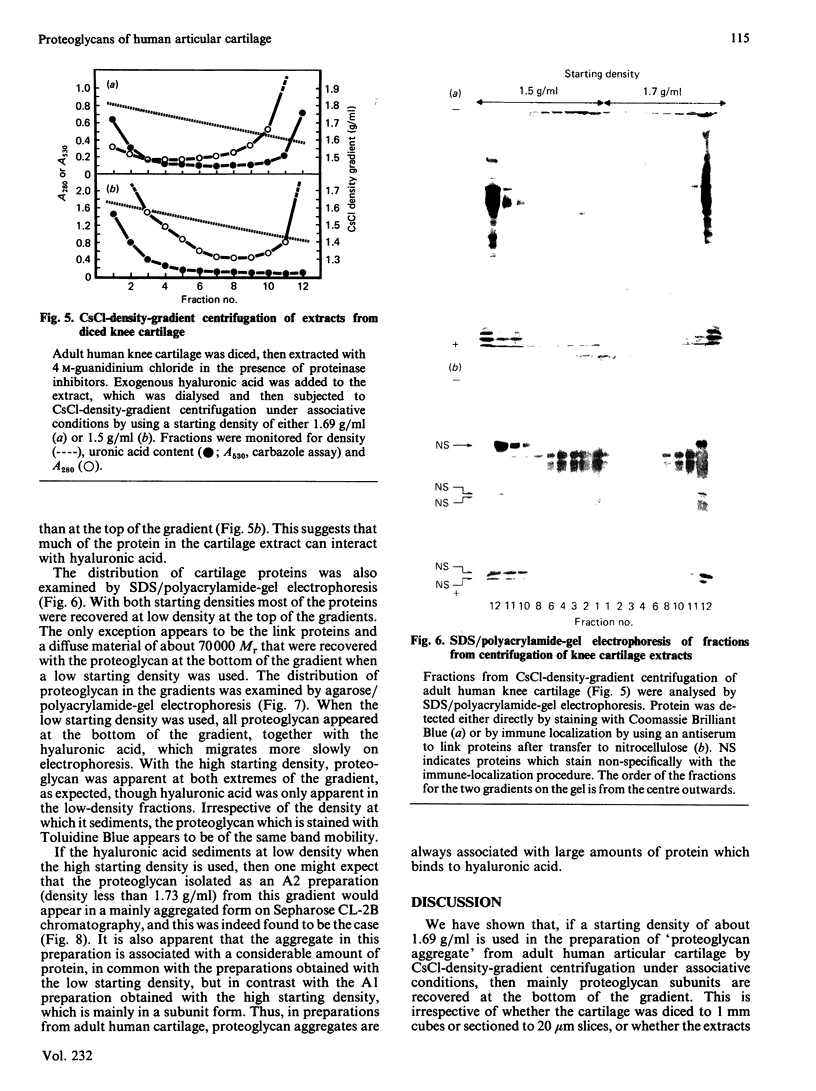
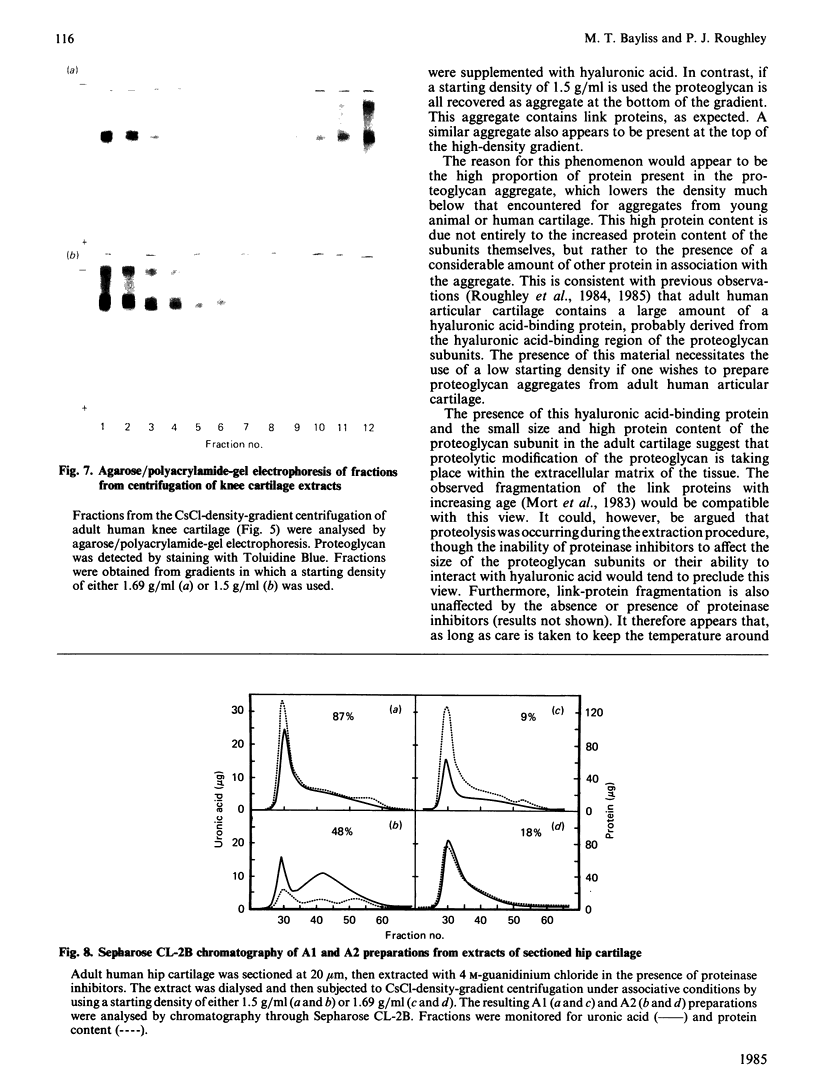
Proteoglycan was extracted from adult human articular cartilage from both the knee and the hip, and A1 preparations were prepared by CsCl-density-gradient centrifugation at starting densities of 1.69 and 1.5 g/ml. Irrespective of whether the cartilage was diced to 1 mm cubes or sectioned to 20 micron slices there was always a lower proportion of both protein and proteoglycan aggregate in the A1 preparation prepared at 1.69 g/ml. Furthermore, the addition of exogenous hyaluronic acid to the extracts before centrifugation did not improve the yield of aggregate at 1.69 g/ml. These results were not affected by the presence of proteinase inhibitors in the extraction medium. It appears that adult human articular cartilage contains a high proportion of low-density proteoglycan subunits and hyaluronic acid-binding proteins that make most of the re-formed proteoglycan aggregates of a lower density than is usually encountered with younger human and mammalian hyaline cartilages.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bayliss M. T., Ali S. Y. Age-related changes in the composition and structure of human articular-cartilage proteoglycans. Biochem J. 1978 Dec 15;176(3):683–693. doi: 10.1042/bj1760683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss M. T., Venn M., Maroudas A., Ali S. Y. Structure of proteoglycans from different layers of human articular cartilage. Biochem J. 1983 Feb 1;209(2):387–400. doi: 10.1042/bj2090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson I. R., Roughley P. J. A comparative study of the proteoglycan of growth cartilage of normal and rachitic chicks. Biochem J. 1978 Jun 1;171(3):675–682. doi: 10.1042/bj1710675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Franzén A., Björnsson S., Heinegård D. Cartilage proteoglycan aggregate formation. Role of link protein. Biochem J. 1981 Sep 1;197(3):669–674. doi: 10.1042/bj1970669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Ewins R. J., Muir H. Cartilage proteoglycans. Structure and heterogeneity of the protein core and the effects of specific protein modifications on the binding to hyaluronate. Biochem J. 1976 Jul 1;157(1):127–143. doi: 10.1042/bj1570127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E. The role of link-protein in the structure of cartilage proteoglycan aggregates. Biochem J. 1979 Jan 1;177(1):237–247. doi: 10.1042/bj1770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C. Interaction of cartilage proteoglycans with hyaluronic acid. J Supramol Struct. 1977;7(1):101–120. doi: 10.1002/jss.400070110. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Kempson G. E., Tuke M. A., Dingle J. T., Barrett A. J., Horsfield P. H. The effects of proteolytic enzymes on the mechanical properties of adult human articular cartilage. Biochim Biophys Acta. 1976 May 28;428(3):741–760. doi: 10.1016/0304-4165(76)90205-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mason R. M., Mayes R. W. Extraction of cartilage protein-polysaccharides with inorganic salt solutions. Biochem J. 1973 Mar;131(3):535–540. doi: 10.1042/bj1310535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt C. A., Muir H. Gel electrophoresis of proteoglycans and glycosaminoglycans on large-pore composite polyacrylamide-agarose gels. Anal Biochem. 1971 Dec;44(2):612–622. doi: 10.1016/0003-2697(71)90250-8. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Poole A. R., Roughley P. J. Age-related changes in the structure of proteoglycan link proteins present in normal human articular cartilage. Biochem J. 1983 Jul 15;214(1):269–272. doi: 10.1042/bj2140269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema T. R., Jr, Hascall V. C., Dziewiatkowski D. D. Isolation and characterization of proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1975 Aug 10;250(15):6151–6159. [PubMed] [Google Scholar]
- Poole A. R., Pidoux I., Reiner A., Rosenberg L. An immunoelectron microscope study of the organization of proteoglycan monomer, link protein, and collagen in the matrix of articular cartilage. J Cell Biol. 1982 Jun;93(3):921–937. doi: 10.1083/jcb.93.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley P. J., Poole A. R., Mort J. S. The heterogeneity of link proteins isolated from human articular cartilage proteoglycan aggregates. J Biol Chem. 1982 Oct 25;257(20):11908–11914. [PubMed] [Google Scholar]
- Roughley P. J., White R. J., Poole A. R. Identification of a hyaluronic acid-binding protein that interferes with the preparation of high-buoyant-density proteoglycan aggregates from adult human articular cartilage. Biochem J. 1985 Oct 1;231(1):129–138. doi: 10.1042/bj2310129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley P. J., White R. J., Poole A. R., Mort J. S. The inability to prepare high-buoyant-density proteoglycan aggregates from extracts of normal adult human articular cartilage. Biochem J. 1984 Aug 1;221(3):637–644. doi: 10.1042/bj2210637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdera S. W., Hascall V. C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969 Jan 10;244(1):77–87. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]