Abstract
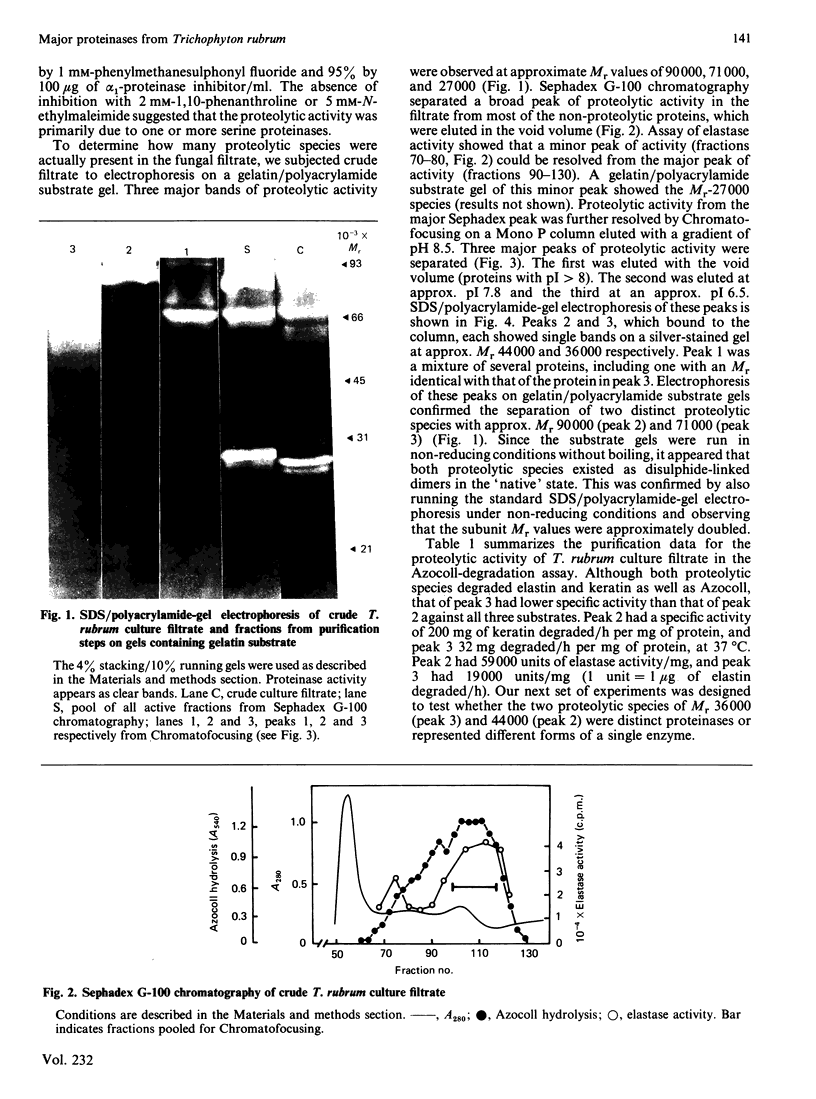
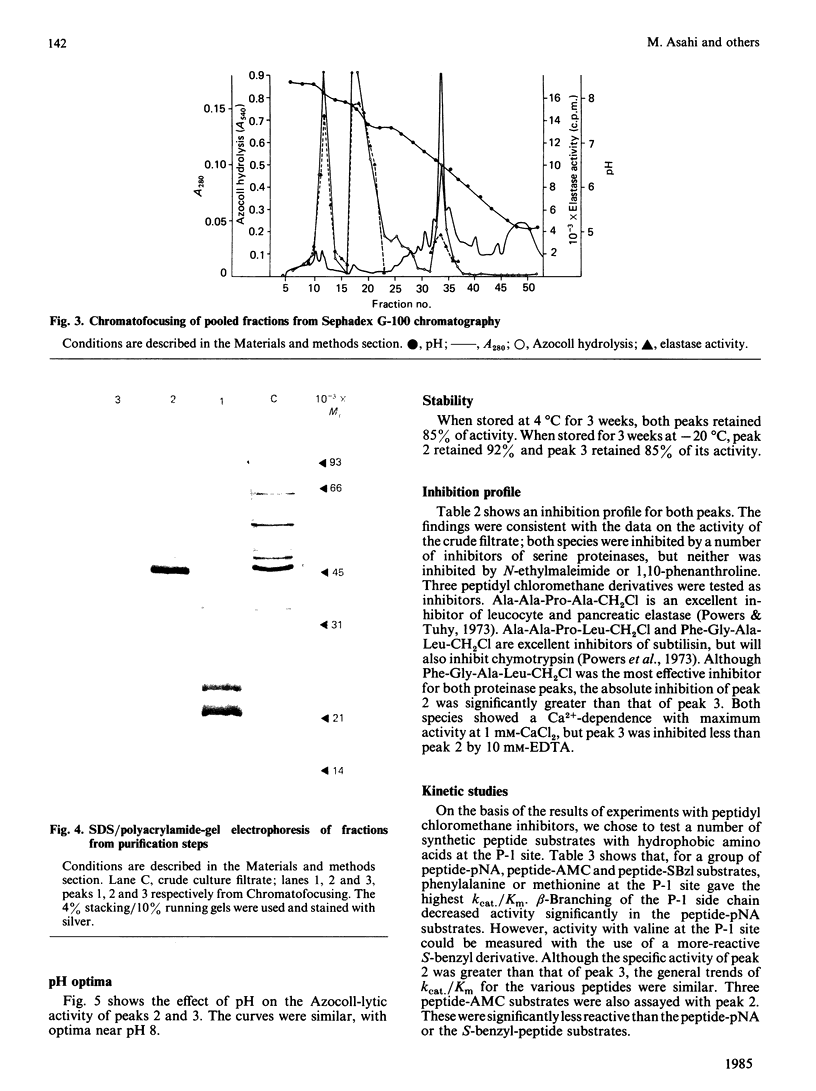
Two extracellular proteinases that probably play a central role in the metabolism and pathogenesis of the most common dermatophyte of man, Trichophyton rubrum, were purified to homogeneity. Size-exclusion chromatography and Chromatofocusing were used to purify the major proteinases 42-fold from crude fungal culture filtrate. The major enzyme has pI 7.8 and subunit Mr 44 000, but forms a dimer of Mr approx. 90 000 in the absence of reducing agents. A second enzyme with pI 6.5 and subunit Mr 36 000, was also purified. It is very similar in substrate specificity to the major enzyme but has lower specific activity, and may be an autoproteolysis product. The major proteinase has pH optimum 8, a Ca2+-dependence maximum of 1 mM, and was inhibited by serine-proteinase inhibitors, especially tetrapeptidyl chloromethane derivatives with hydrophobic residues at the P-1 site. Kinetic studies also showed that tetrapeptides containing aromatic or hydrophobic residues at P-1 were the best substrates. A kcat./Km of 27 000 M-1 X S-1 was calculated for the peptide 3-carboxypropionyl-Ala-Ala-Pro-Phe-p-nitroanilide. The enzyme has significant activity against keratin, elastin and denatured type I collagen (Azocoll).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CHATTAWAY F. W., ELLIS D. A., BARLOW A. J. PEPTIDASES OF DERMATOPHYTES. J Invest Dermatol. 1963 Jul;41:31–37. [PubMed] [Google Scholar]
- Castillo M. J., Nakajima K., Zimmerman M., Powers J. C. Sensitive substrates for human leukocyte and porcine pancreatic elastase: a study of the merits of various chromophoric and fluorogenic leaving groups in assays for serine proteases. Anal Biochem. 1979 Oct 15;99(1):53–64. doi: 10.1016/0003-2697(79)90043-5. [DOI] [PubMed] [Google Scholar]
- Day W. C., Toncic P., Stratman S. L., Leeman U., Harmon S. R. Isolation and properties of an extracellular protease of Trichophyton granulosum. Biochim Biophys Acta. 1968 Nov 19;167(3):597–606. doi: 10.1016/0005-2744(68)90050-8. [DOI] [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- Gisslow M. T., McBride B. C. A rapid sensitive collagenase assay. Anal Biochem. 1975 Sep;68(1):70–78. doi: 10.1016/0003-2697(75)90680-6. [DOI] [PubMed] [Google Scholar]
- Grassetti D. R., Murray J. F., Jr Determination of sulfhydryl groups with 2,2'- or 4,4'-dithiodipyridine. Arch Biochem Biophys. 1967 Mar;119(1):41–49. doi: 10.1016/0003-9861(67)90426-2. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McKerrow J. H., Pino-Heiss S., Lindquist R., Werb Z. Purification and characterization of an elastinolytic proteinase secreted by cercariae of Schistosoma mansoni. J Biol Chem. 1985 Mar 25;260(6):3703–3707. [PubMed] [Google Scholar]
- Meevootisom V., Niederpruem D. J. Control of exocellular proteases in dermatophytes and especially Trichophyton rubrum. Sabouraudia. 1979 Jun;17(2):91–106. doi: 10.1080/00362177985380141. [DOI] [PubMed] [Google Scholar]
- Mikhail G. R. Trichophyton rubrum granuloma. Int J Dermatol. 1970 Jan-Mar;9(1):41–46. doi: 10.1111/j.1365-4362.1970.tb04726.x. [DOI] [PubMed] [Google Scholar]
- Murozuka T., Fukuyama K., Epstein W. L. Immunochemical comparison of histidine-rich protein in keratohyalin granules and cornified cells. Biochim Biophys Acta. 1979 Aug 28;579(2):334–345. doi: 10.1016/0005-2795(79)90061-8. [DOI] [PubMed] [Google Scholar]
- Powers J. C., Lively M. O., 3rd, Tippett J. T. Inhibition of subtilisin BPN' with peptide chloromethyl ketones. Biochim Biophys Acta. 1977 Jan 11;480(1):246–261. doi: 10.1016/0005-2744(77)90338-2. [DOI] [PubMed] [Google Scholar]
- Powers J. C., Tuhy P. M. Active-site specific inhibitors of elastase. Biochemistry. 1973 Nov 6;12(23):4767–4774. doi: 10.1021/bi00747a032. [DOI] [PubMed] [Google Scholar]
- Rippon J. W., Varadi D. P. The elastases of pathogenic fungi and actinomycetes. J Invest Dermatol. 1968 Jan;50(1):54–58. doi: 10.1038/jid.1968.8. [DOI] [PubMed] [Google Scholar]
- Takiuchi I., Higuchi D., Sei Y., Koga M. Immunological studies of an extra-cellular keratinase. J Dermatol. 1983 Aug;10(4):327–330. doi: 10.1111/j.1346-8138.1983.tb01146.x. [DOI] [PubMed] [Google Scholar]
- Tzeng S., McKerrow J. H., Fukuyama K., Jeong K., Epstein W. L. Degradation of purified skin keratin by a proteinase secreted from Schistosoma mansoni cercariae. J Parasitol. 1983 Oct;69(5):992–994. [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yu R. J., Harmon S. R., Wachter P. E., Blank F. Amino acid composition and specificity of a keratinase of Trichophyton mentagrophytes. Arch Biochem Biophys. 1969 Dec;135(1):363–370. doi: 10.1016/0003-9861(69)90551-7. [DOI] [PubMed] [Google Scholar]