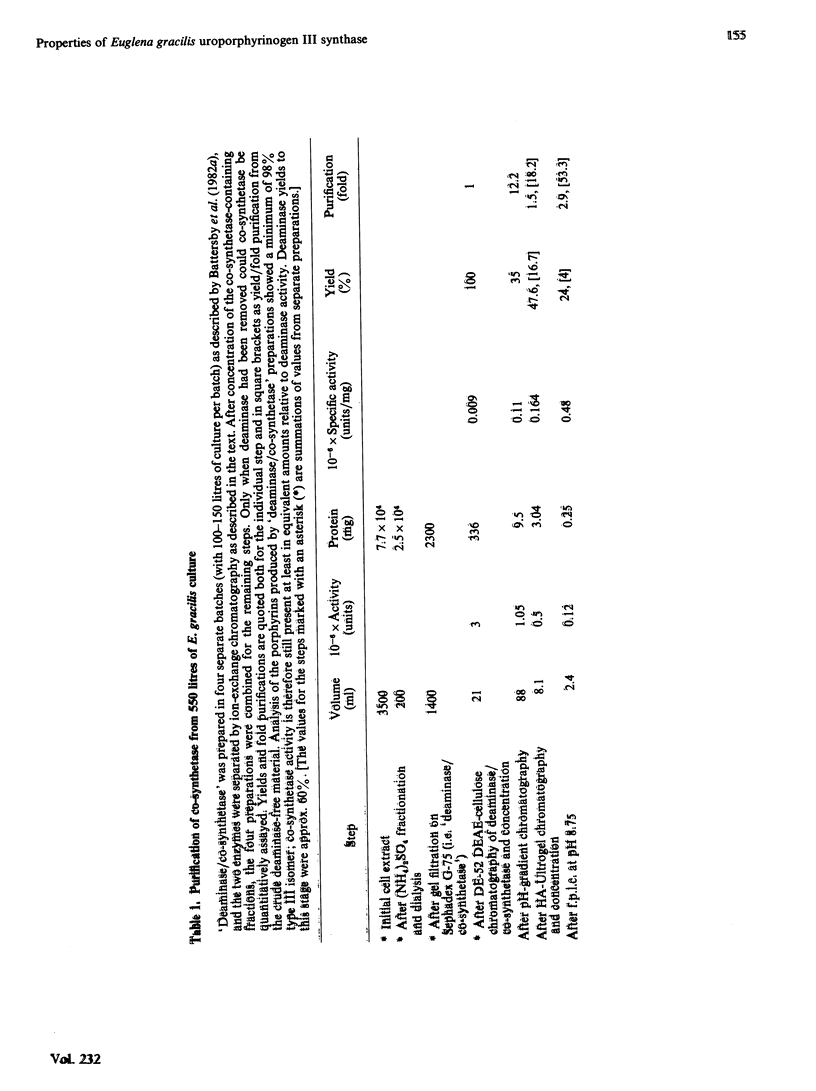
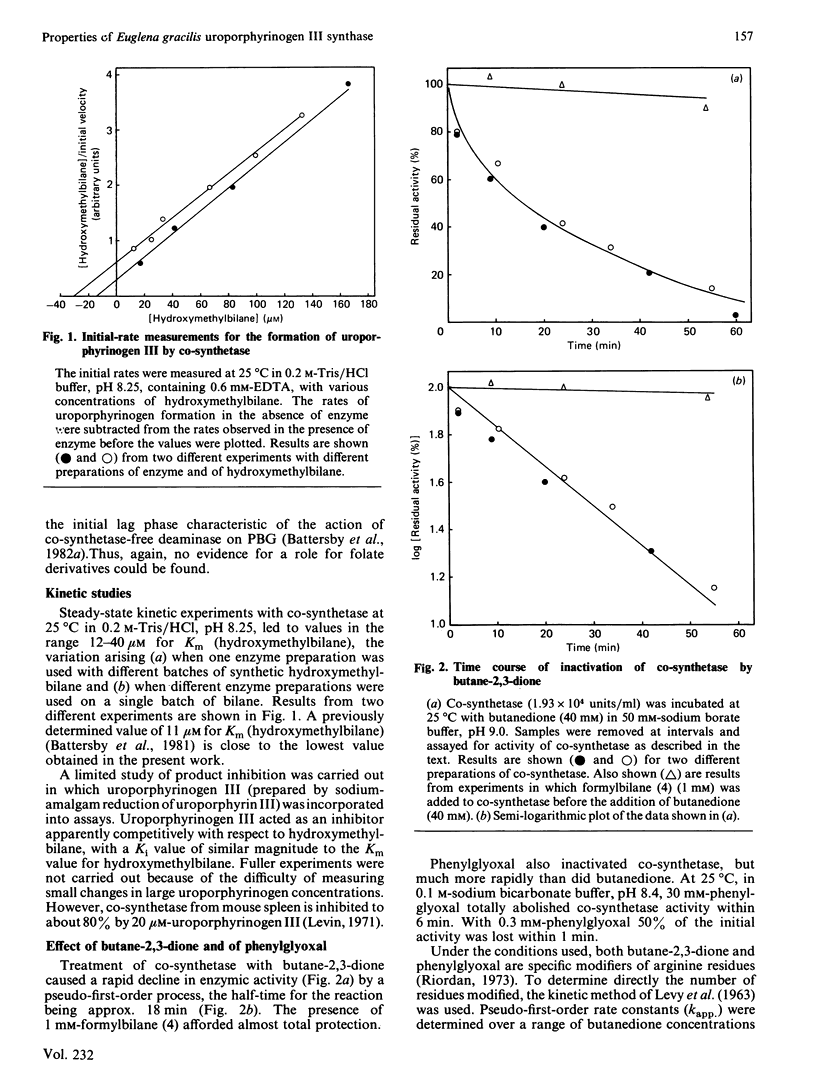
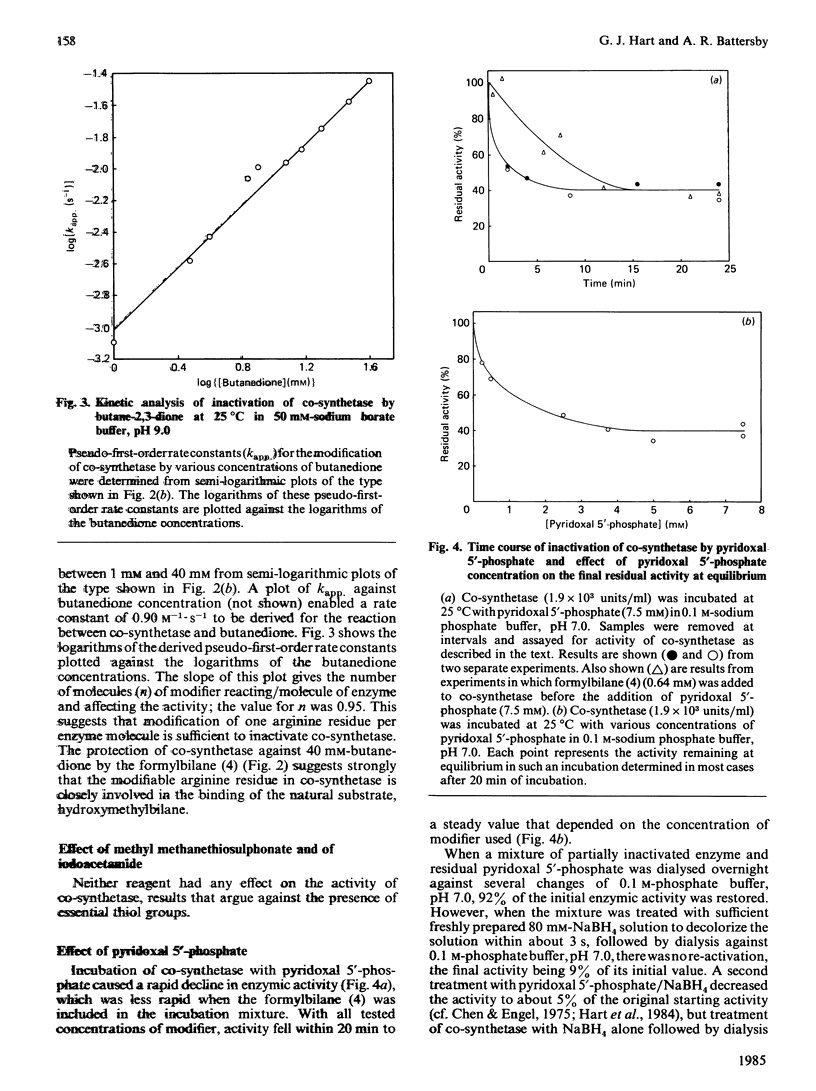
Abstract
Uroporphyrinogen III synthase (co-synthetase) purified from Euglena gracilis is a monomer of Mr 38 500 by gel-filtration studies and 31 000 by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. The pI is apparently in the range 4.8-5.1. No evidence for any cofactors was found, and folate derivatives were shown to be absent; no metal ions appear to be present in the enzyme. The Km for hydroxymethylbilane is in the range 12-40 microM, and the product, uroporphyrinogen III, is an inhibitor. Modification studies suggest that arginine residues are essential for the activity of co-synthetase; lysine residues may also be essential, but histidine, cysteine and tyrosine residues are not.
Full text
PDF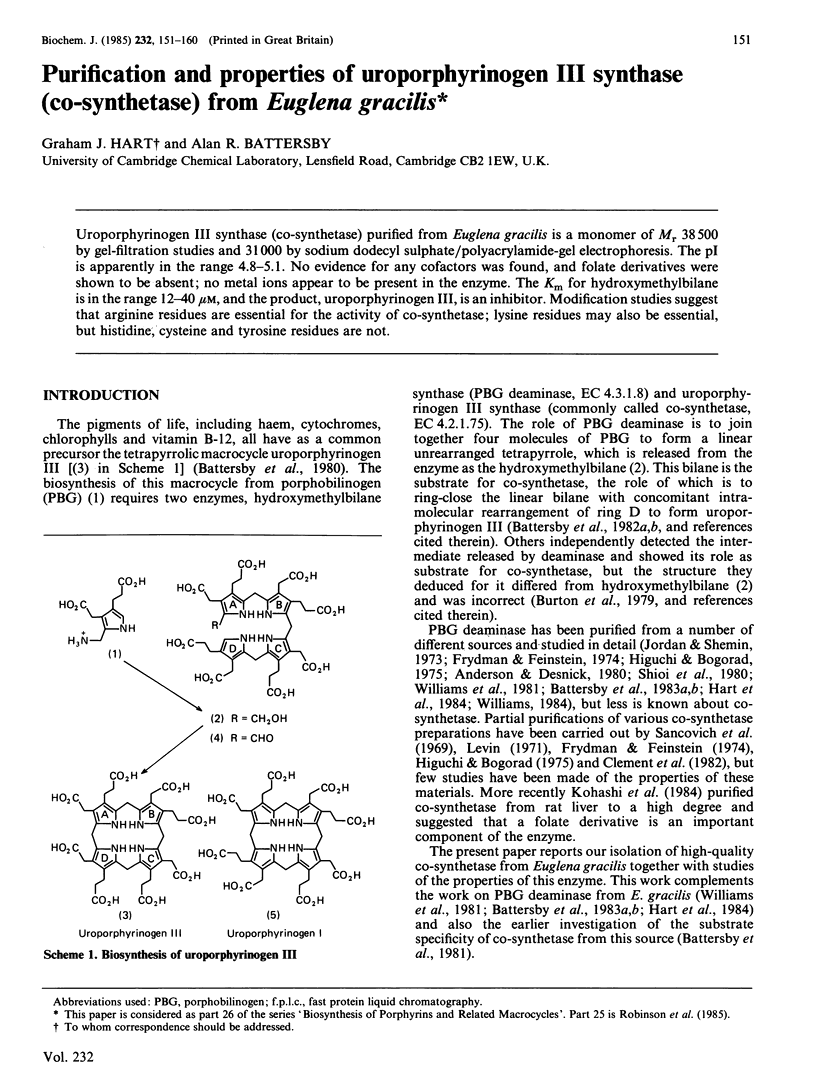






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Desnick R. J. Purification and properties of uroporphyrinogen I synthase from human erythrocytes. Identification of stable enzyme-substrate intermediates. J Biol Chem. 1980 Mar 10;255(5):1993–1999. [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battersby A. R., Fookes C. J., Matcham G. W., McDonald E. Biosynthesis of the pigments of life: formation of the macrocycle. Nature. 1980 May 1;285(5759):17–21. doi: 10.1038/285017a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CREMONA T., SINGER T. P. THE LACTIC DEHYDROGENASES OF YEAST. V. CHEMICAL PROPERTIES AND FUNCTION OF THE ZINC COMPONENT OF D-LACTIC CYTOCHROME REDUCTASE. J Biol Chem. 1964 May;239:1466–1473. [PubMed] [Google Scholar]
- Chen S. S., Engel P. C. Modification of pig M4 lactate dehydrogenase by pyridoxal 5'-phosphate. Demonstration of an essential lysine residue. Biochem J. 1975 Jul;149(1):107–113. doi: 10.1042/bj1490107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement R. P., Kohashi M., Piper W. N. Rat hepatic uroporphyrinogen III cosynthase: purification, properties, and inhibition by metal ions. Arch Biochem Biophys. 1982 Apr 1;214(2):657–667. doi: 10.1016/0003-9861(82)90071-6. [DOI] [PubMed] [Google Scholar]
- Dickenson C. J., Dickinson F. M. The role of an essential histidine residue of yeast alcohol dehydrogenase. Eur J Biochem. 1975 Apr 1;52(3):595–603. doi: 10.1111/j.1432-1033.1975.tb04031.x. [DOI] [PubMed] [Google Scholar]
- Dickinson F. M., Berrieman S. The reactions of 1,10-phenanthroline with yeast alcohol dehydrogenase. Biochem J. 1977 Oct 1;167(1):237–244. doi: 10.1042/bj1670237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydman R. B., Feinstein G. Studies on porphobilinogen deaminase and uroporphyrinogen 3 cosynthase from human erythrocytes. Biochim Biophys Acta. 1974 Jun 18;350(2):358–373. doi: 10.1016/0005-2744(74)90510-5. [DOI] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hart G. J., Leeper F. J., Battersby A. R. Modification of hydroxymethylbilane synthase (porphobilinogen deaminase) by pyridoxal 5'-phosphate. Demonstration of an essential lysine residue. Biochem J. 1984 Aug 15;222(1):93–102. doi: 10.1042/bj2220093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M., Bogorad L. The purification and properties of uroporphyrinogen I synthases and uroporphyrinogen III cosynthase. Interactions between the enzymes. Ann N Y Acad Sci. 1975 Apr 15;244:401–418. doi: 10.1111/j.1749-6632.1975.tb41545.x. [DOI] [PubMed] [Google Scholar]
- Janatova J., Fuller J. K., Hunter M. J. The heterogeneity of bovine albumin with respect to sulfhydryl and dimer content. J Biol Chem. 1968 Jul 10;243(13):3612–3622. [PubMed] [Google Scholar]
- Jordan P. M., Nordlov H., Burton G., Scott A. I. A rapid direct assay for uroporphyrinogen III cosynthetase. FEBS Lett. 1980 Jun 30;115(2):269–272. doi: 10.1016/0014-5793(80)81184-7. [DOI] [PubMed] [Google Scholar]
- Jordan P. M., Shemin D. Purification and properties of uroporphyrinogen I synthetase from Rhodopseudomonas spheroides. J Biol Chem. 1973 Feb 10;248(3):1019–1024. [PubMed] [Google Scholar]
- Kohashi M., Clement R. P., Tse J., Piper W. N. Rat hepatic uroporphyrinogen III co-synthase. Purification and evidence for a bound folate coenzyme participating in the biosynthesis of uroporphyrinogen III. Biochem J. 1984 Jun 15;220(3):755–765. doi: 10.1042/bj2200755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVY H. M., LEBER P. D., RYAN E. M. INACTIVATION OF MYOSIN BY 2,4-DINITROPHENOL AND PROTECTION BY ADENOSINE TRIPHOSPHATE AND OTHER PHOSPHATE COMPOUNDS. J Biol Chem. 1963 Nov;238:3654–3659. [PubMed] [Google Scholar]
- Levin E. Y. Enzymatic properties of uroporphyrinogen 3 cosynthetase. Biochemistry. 1971 Dec 7;10(25):4669–4675. doi: 10.1021/bi00801a012. [DOI] [PubMed] [Google Scholar]
- Miles E. W. Modification of histidyl residues in proteins by diethylpyrocarbonate. Methods Enzymol. 1977;47:431–442. doi: 10.1016/0076-6879(77)47043-5. [DOI] [PubMed] [Google Scholar]
- Riordan J. F. Functional arginyl residues in carboxypeptidase A. Modification with butanedione. Biochemistry. 1973 Sep 25;12(20):3915–3923. doi: 10.1021/bi00744a020. [DOI] [PubMed] [Google Scholar]
- Sancovich H. A., Battle A. M., Grinstein M. Porphyrin biosynthesis. VI. Separation and purification of porphobilinogen deaminase and uroporphyrinogen isomerase from cow liver. Porphobilinogenase an allosteric enzyme. Biochim Biophys Acta. 1969 Sep 30;191(1):130–143. doi: 10.1016/0005-2744(69)90322-2. [DOI] [PubMed] [Google Scholar]
- Shioi Y., Nagamine M., Kuroki M., Sasa T. Purification by affinity chromatography and properties of uroporphyrinogen I synthetase from Chlorella regularis. Biochim Biophys Acta. 1980 Dec 4;616(2):300–309. doi: 10.1016/0005-2744(80)90147-3. [DOI] [PubMed] [Google Scholar]
- Sokolovsky M., Riordan J. F., Vallee B. L. Tetranitromethane. A reagent for the nitration of tyrosyl residues in proteins. Biochemistry. 1966 Nov;5(11):3582–3589. doi: 10.1021/bi00875a029. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Williams D. C. Characterization of the multiple forms of hydroxymethylbilane synthase from rat spleen. Biochem J. 1984 Feb 1;217(3):675–683. doi: 10.1042/bj2170675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. C., Morgan G. S., McDonald E., Battersby A. R. Purification of porphobilinogen deaminase from Euglena gracilis and studies of its kinetics. Biochem J. 1981 Jan 1;193(1):301–310. doi: 10.1042/bj1930301. [DOI] [PMC free article] [PubMed] [Google Scholar]



