Abstract
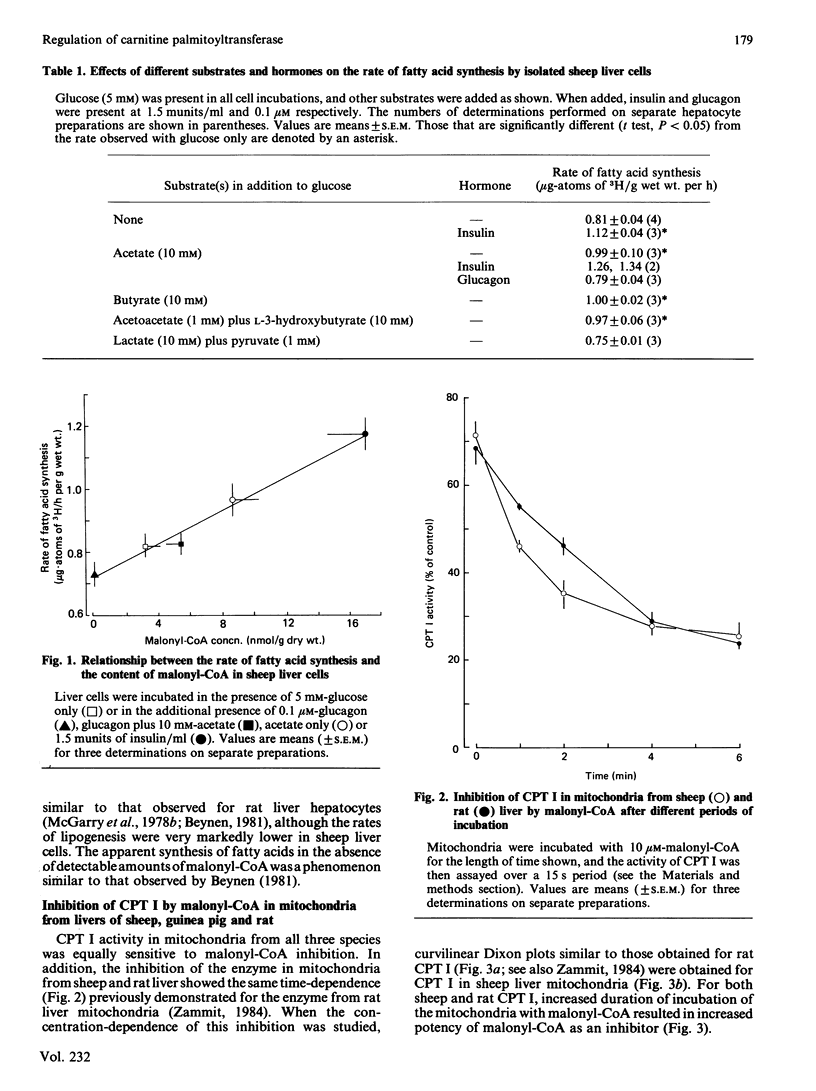
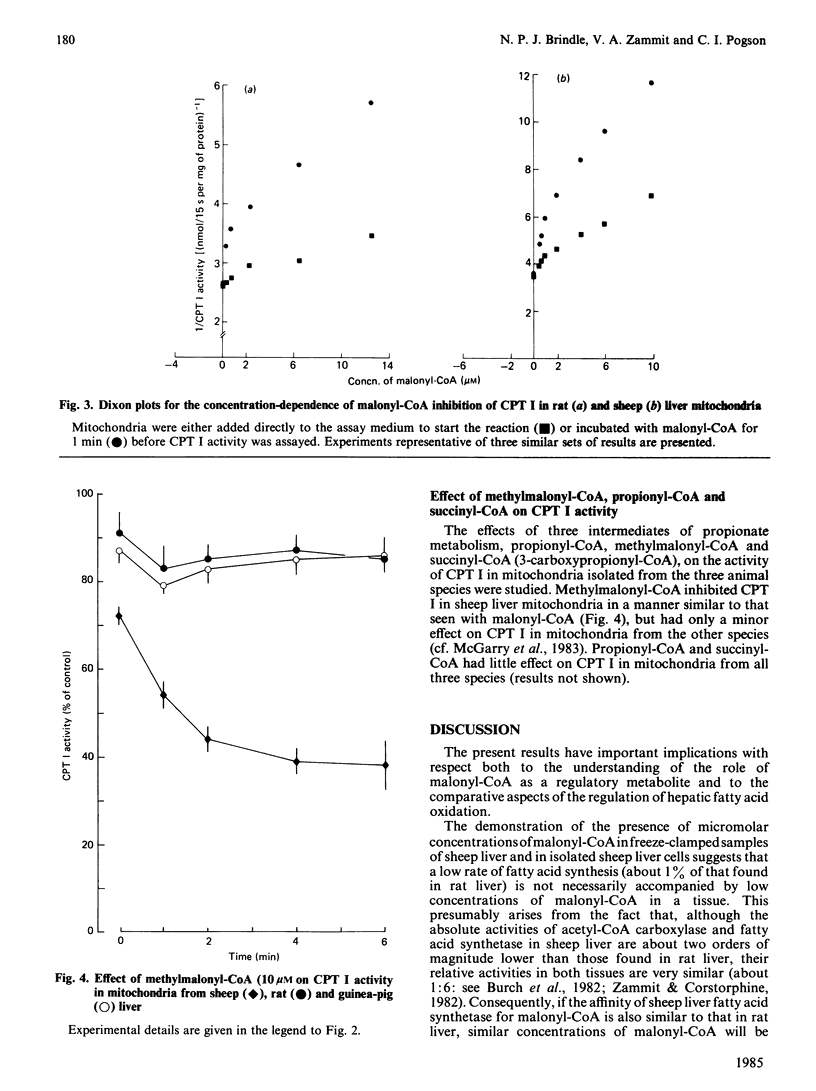
The characteristics of inhibition of carnitine palmitoyltransferase (CPT) I by malonyl-CoA were studied for the enzyme in mitochondria isolated from sheep liver, a tissue with a very low rate of fatty acid synthesis. Malonyl-CoA was as potent in inhibiting the sheep liver enzyme as in inhibiting the enzyme in rat liver mitochondria. CPT I in guinea-pig liver mitochondria was also similarly inhibited. The inhibition showed the same time-dependent characteristics previously established for the rat liver enzyme. Methylmalonyl-CoA was as effective an inhibitor of CPT I as malonyl-CoA in sheep liver mitochondria, but did not affect CPT I activity in mitochondria from rat or guinea-pig liver. The concentrations of malonyl-CoA required to inhibit CPT I in sheep liver mitochondria in vitro were similar to those found in freeze-clamped sheep liver samples (about 7 nmol of malonyl-CoA/g wet wt.). In sheep liver cells the content of malonyl-CoA was only one-tenth of that observed in vivo when glucose only was added to the incubation medium. Inclusion of acetate and/or insulin increased the malonyl-CoA content about 10-fold, to values similar to those observed in vivo. The rate of fatty acid synthesis in sheep liver cells was about 1% of that observed in rat liver, but was correlated with the concentrations of malonyl-CoA in the cells under various incubation conditions. These observations are discussed in relation to (i) the regulatory role of malonyl-CoA in tissues that have a low capacity for fatty acid synthesis, and (ii) the utilization by sheep liver of propionate as a gluconeogenic precursor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGMAN E. N., KON K. ACETOACETATE TURNOVER AND OXIDATION RATES IN OVINE PREGNANCY KETOSIS. Am J Physiol. 1964 Feb;206:449–452. doi: 10.1152/ajplegacy.1964.206.2.449. [DOI] [PubMed] [Google Scholar]
- Baird G. D. Aspects of ruminant intermediary metabolism in relation to ketosis. Biochem Soc Trans. 1977;5(3):819–827. doi: 10.1042/bst0050819. [DOI] [PubMed] [Google Scholar]
- Brunengraber H., Boutry M., Lowenstein J. M. Fatty acid and 3- -hydroxysterol synthesis in the perfused rat liver. Including measurements on the production of lactate, pyruvate, -hydroxy-butyrate, and acetoacetate by the fed liver. J Biol Chem. 1973 Apr 25;248(8):2656–2669. [PubMed] [Google Scholar]
- Buckner J. S., Kolattukudy P. E. Lipid biosynthesis in sebaceous glands: regulation of the synthesis of n- and branched fatty acids by malonyl-coenzyme A decarboxylase. Biochemistry. 1975 Apr 22;14(8):1768–1773. doi: 10.1021/bi00679a032. [DOI] [PubMed] [Google Scholar]
- Burch L., Scaife J. R., Galbraith H. Effect of anabolic steroids on lipogenic and lipolytic enzymes in sheep tissues. Horm Metab Res. 1982 Jan;14(1):52–53. doi: 10.1055/s-2007-1018919. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Guynn R. W., Veloso D., Veech R. L. The concentration of malonyl-coenzyme A and the control of fatty acid synthesis in vivo. J Biol Chem. 1972 Nov 25;247(22):7325–7331. [PubMed] [Google Scholar]
- Ingle D. L., Bauman D. E., Garrigus U. S. Lipogenesis in the ruminant: in vivo site of fatty acid synthesis in sheep. J Nutr. 1972 May;102(5):617–623. doi: 10.1093/jn/102.5.617. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liepa G. U., Beitz D. C., Linder J. R. Fatty acid synthesis in ruminating and nonruminating goats. J Nutr. 1978 Nov;108(11):1733–1739. doi: 10.1093/jn/108.11.1733. [DOI] [PubMed] [Google Scholar]
- Lomax M. A., Donaldson I. A., Pogson C. I. The control of fatty acid metabolism in liver cells from fed and starved sheep. Biochem J. 1983 Aug 15;214(2):553–560. doi: 10.1042/bj2140553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Mannaerts G. P., Foster D. W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977 Jul;60(1):265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Mannaerts G. P., Foster D. W. Characteristics of fatty acid oxidation in rat liver homogenates and the inhibitory effect of malonyl-CoA. Biochim Biophys Acta. 1978 Sep 28;530(3):305–313. doi: 10.1016/0005-2760(78)90150-9. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Mills S. E., Long C. S., Foster D. W. Observations on the affinity for carnitine, and malonyl-CoA sensitivity, of carnitine palmitoyltransferase I in animal and human tissues. Demonstration of the presence of malonyl-CoA in non-hepatic tissues of the rat. Biochem J. 1983 Jul 15;214(1):21–28. doi: 10.1042/bj2140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Takabayashi Y., Foster D. W. The role of malonyl-coa in the coordination of fatty acid synthesis and oxidation in isolated rat hepatocytes. J Biol Chem. 1978 Nov 25;253(22):8294–8300. [PubMed] [Google Scholar]
- Mills S. E., Foster D. W., McGarry J. D. Interaction of malonyl-CoA and related compounds with mitochondria from different rat tissues. Relationship between ligand binding and inhibition of carnitine palmitoyltransferase I. Biochem J. 1983 Jul 15;214(1):83–91. doi: 10.1042/bj2140083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M. S., Hanson R. W. Lipogenesis in developing guinea pig liver. Mech Ageing Dev. 1974 Mar;3(1):65–73. doi: 10.1016/0047-6374(74)90006-2. [DOI] [PubMed] [Google Scholar]
- Robinson I. N., Zammit V. A. Sensitivity of carnitine acyltransferase I to malonly-CoA inhibition in isolated rat liver mitochondria is quantitatively related to hepatic malonyl-CoA concentration in vivo. Biochem J. 1982 Jul 15;206(1):177–179. doi: 10.1042/bj2060177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Carnitine palmitoyltransferase and carnitine octanoyltransferase activities in liver, kidney cortex, adipocyte, lactating mammary gland, skeletal muscle and heart. FEBS Lett. 1981 Jul 6;129(2):229–232. doi: 10.1016/0014-5793(81)80171-8. [DOI] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Wolff J. E., Bergman E. N. Gluconeogenesis from plasma amino acids in fed sheep. Am J Physiol. 1972 Aug;223(2):455–460. doi: 10.1152/ajplegacy.1972.223.2.455. [DOI] [PubMed] [Google Scholar]
- Zammit V. A., Corstorphine C. G. Changes in the proportion of acetyl-CoA carboxylase in the active form in rat liver. Effect of starvation, lactation and weaning. Biochem J. 1982 Jun 15;204(3):757–764. doi: 10.1042/bj2040757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Regulation of hepatic fatty acid metabolism. The activities of mitochondrial and microsomal acyl-CoA:sn-glycerol 3-phosphate O-acyltransferase and the concentrations of malonyl-CoA, non-esterified and esterified carnitine, glycerol 3-phosphate, ketone bodies and long-chain acyl-CoA esters in livers of fed or starved pregnant, lactating and weaned rats. Biochem J. 1981 Jul 15;198(1):75–83. doi: 10.1042/bj1980075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Regulation of hepatic fatty acid oxidation and ketogenesis. Proc Nutr Soc. 1983 Jun;42(2):289–302. doi: 10.1079/pns19830033. [DOI] [PubMed] [Google Scholar]
- Zammit V. A. The effect of glucagon treatment and starvation of virgin and lactating rats on the rates of oxidation of octanoyl-L-carnitine and octanoate by isolated liver mitochondria. Biochem J. 1980 Aug 15;190(2):293–300. doi: 10.1042/bj1900293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Time-dependence of inhibition of carnitine palmitoyltransferase I by malonyl-CoA in mitochondria isolated from livers of fed or starved rats. Evidence for transition of the enzyme between states of low and high affinity for malonyl-CoA. Biochem J. 1984 Mar 1;218(2):379–386. doi: 10.1042/bj2180379. [DOI] [PMC free article] [PubMed] [Google Scholar]


