Abstract
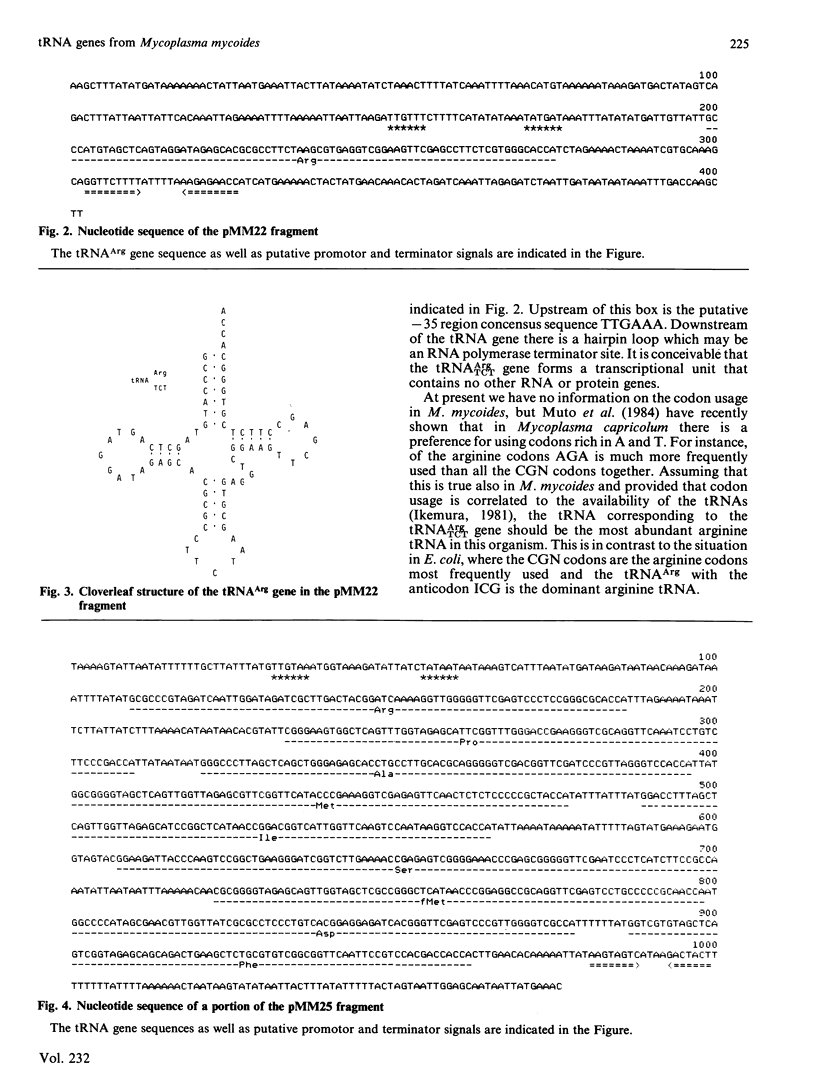
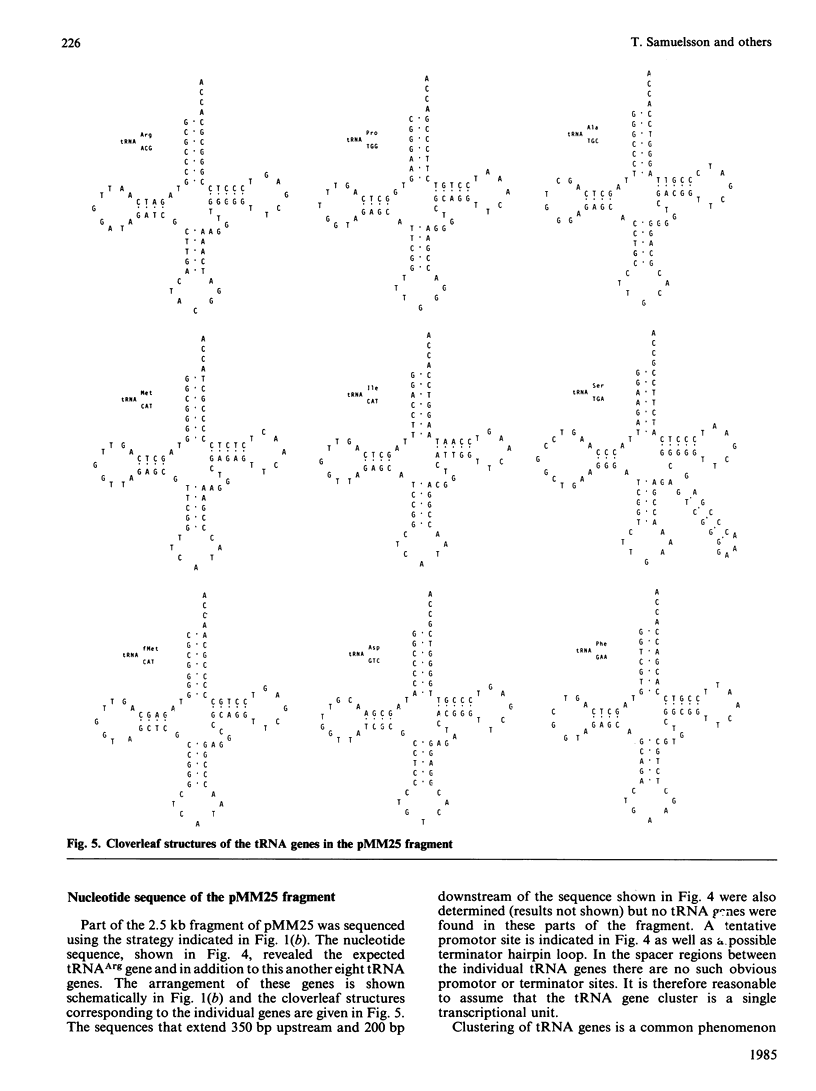
As part of an investigation of the tRNA genes of Mycoplasma mycoides, two HindIII fragments of mycoplasma DNA comprising 0.4 and 2.5 kilobases (kb), respectively, were cloned in pBR322 and their nucleotide sequences determined. Only one tRNA gene was found in the 0.4 kb fragment, the gene for tRNAArg with the anticodon TCT, while the 2.5 kb fragment contained nine different tRNA genes arranged in a cluster which presumably constitutes a transcriptional unit. The clustered tRNA genes, with their respective anticodons, were as follows: Arg (ACG), Pro (TGG), Ala (TGC), Met (CAT), Ile (CAT), Ser (TGA), fMet (CAT), Asp (GTC), and Phe (GAA).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Bischoff R., Graeser E., McLaughlin L. W. tRNA separation by high-performance liquid chromatography using an aggregate of ODS-Hypersil and trioctylmethylammonium chloride. J Chromatogr. 1983 Mar 4;257(2):305–315. doi: 10.1016/s0021-9673(01)88186-3. [DOI] [PubMed] [Google Scholar]
- Bonitz S. G., Berlani R., Coruzzi G., Li M., Macino G., Nobrega F. G., Nobrega M. P., Thalenfeld B. E., Tzagoloff A. Codon recognition rules in yeast mitochondria. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3167–3170. doi: 10.1073/pnas.77.6.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- England T. E., Bruce A. G., Uhlenbeck O. C. Specific labeling of 3' termini of RNA with T4 RNA ligase. Methods Enzymol. 1980;65(1):65–74. doi: 10.1016/s0076-6879(80)65011-3. [DOI] [PubMed] [Google Scholar]
- Fukada K., Abelson J. DNA sequence of a T4 transfer RNA gene cluster. J Mol Biol. 1980 May 25;139(3):377–391. doi: 10.1016/0022-2836(80)90136-9. [DOI] [PubMed] [Google Scholar]
- Gillam I., Blew D., Warrington R. C., von Tigerstrom M., Tener G. M. A general procedure for the isolation of specific transfer ribonucleic acids. Biochemistry. 1968 Oct;7(10):3459–3468. doi: 10.1021/bi00850a022. [DOI] [PubMed] [Google Scholar]
- Gillam I., Millward S., Blew D., von Tigerstrom M., Wimmer E., Tener G. M. The separation of soluble ribonucleic acids on benzoylated diethylaminoethylcellulose. Biochemistry. 1967 Oct;6(10):3043–3056. doi: 10.1021/bi00862a011. [DOI] [PubMed] [Google Scholar]
- Green C. J., Vold B. S. Sequence analysis of a cluster of twenty-one tRNA genes in Bacillus subtilis. Nucleic Acids Res. 1983 Aug 25;11(16):5763–5774. doi: 10.1093/nar/11.16.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Heckman J. E., Sarnoff J., Alzner-DeWeerd B., Yin S., RajBhandary U. L. Novel features in the genetic code and codon reading patterns in Neurospora crassa mitochondria based on sequences of six mitochondrial tRNAs. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3159–3163. doi: 10.1073/pnas.77.6.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes. J Mol Biol. 1981 Feb 15;146(1):1–21. doi: 10.1016/0022-2836(81)90363-6. [DOI] [PubMed] [Google Scholar]
- Kashdan M. A., Dudock B. S. The gene for a spinach chloroplast isoleucine tRNA has a methionine anticodon. J Biol Chem. 1982 Oct 10;257(19):11191–11194. [PubMed] [Google Scholar]
- Kilpatrick M. W., Walker R. T. The nucleotide sequence of glycine tRNA from Mycoplasma mycoides sp. capri. Nucleic Acids Res. 1980 Jun 25;8(12):2783–2786. doi: 10.1093/nar/8.12.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball M. E., Szeto K. S., Soll D. The nucleotide sequence of phenylalanine tRNA from Mycoplasma sp. (Kid). Nucleic Acids Res. 1974 Dec;1(12):1721–1732. [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Watanabe S., Harada F., Nishimura S. Primary structure of AUA-specific isoleucine transfer ribonucleic acid from Escherichia coli. Biochemistry. 1980 May 13;19(10):2085–2089. doi: 10.1021/bi00551a013. [DOI] [PubMed] [Google Scholar]
- Lustig F., Elias P., Axberg T., Samuelsson T., Tittawella I., Lagerkvist U. Codon reading and translational error. Reading of the glutamine and lysine codons during protein synthesis in vitro. J Biol Chem. 1981 Mar 25;256(6):2635–2643. [PubMed] [Google Scholar]
- Maniloff J. Evolution of wall-less prokaryotes. Annu Rev Microbiol. 1983;37:477–499. doi: 10.1146/annurev.mi.37.100183.002401. [DOI] [PubMed] [Google Scholar]
- Mitra S. K., Lustig F., Akesson B., Axberg T., Elias P., Lagerkvist U. Relative efficiency of anticodons in reading the valine codons during protein synthesis in vitro. J Biol Chem. 1979 Jul 25;254(14):6397–6401. [PubMed] [Google Scholar]
- Mitra S. K., Lustig F., Akesson B., Lagerkvist U. Codon-acticodon recognition in the valine codon family. J Biol Chem. 1977 Jan 25;252(2):471–478. [PubMed] [Google Scholar]
- Muto A., Kawauchi Y., Yamao F., Osawa S. Preferential use of A- and U-rich codons for Mycoplasma capricolum ribosomal proteins S8 and L6. Nucleic Acids Res. 1984 Nov 12;12(21):8209–8217. doi: 10.1093/nar/12.21.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. The mycoplasmas. Microbiol Rev. 1978 Jun;42(2):414–470. doi: 10.1128/mr.42.2.414-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M. J., Simmons J., Walker R. T., Weisburg W. G., Woese C. R., Tanner R. S., Robinson I. M., Stahl D. A., Olsen G., Leach R. H. Construction of the mycoplasma evolutionary tree from 5S rRNA sequence data. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1160–1164. doi: 10.1073/pnas.82.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M. J., Steinmetz A. A., Walker R. T. A Spiroplasma tRNA gene cluster. Isr J Med Sci. 1984 Sep;20(9):768–772. [PubMed] [Google Scholar]
- Samuelsson T., Axberg T., Borén T., Lagerkvist U. Unconventional reading of the glycine codons. J Biol Chem. 1983 Nov 10;258(21):13178–13184. [PubMed] [Google Scholar]
- Samuelsson T., Elias P., Lustig F., Axberg T., Fölsch G., Akesson B., Lagerkvist U. Aberrations of the classic codon reading scheme during protein synthesis in vitro. J Biol Chem. 1980 May 25;255(10):4583–4588. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. T., RajBhandary U. L. The nucleotide sequence of formylmethionine tRNA from Mycoplasma mycoides sp. capri. Nucleic Acids Res. 1978 Jan;5(1):57–70. doi: 10.1093/nar/5.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrousek E. F., Narasimhan N., Hansen J. N. Two large clusters with thirty-seven transfer RNA genes adjacent to ribosomal RNA gene sets in Bacillus subtilis. Sequence and organization of trrnD and trrnE gene clusters. J Biol Chem. 1984 Mar 25;259(6):3694–3702. [PubMed] [Google Scholar]


