Abstract
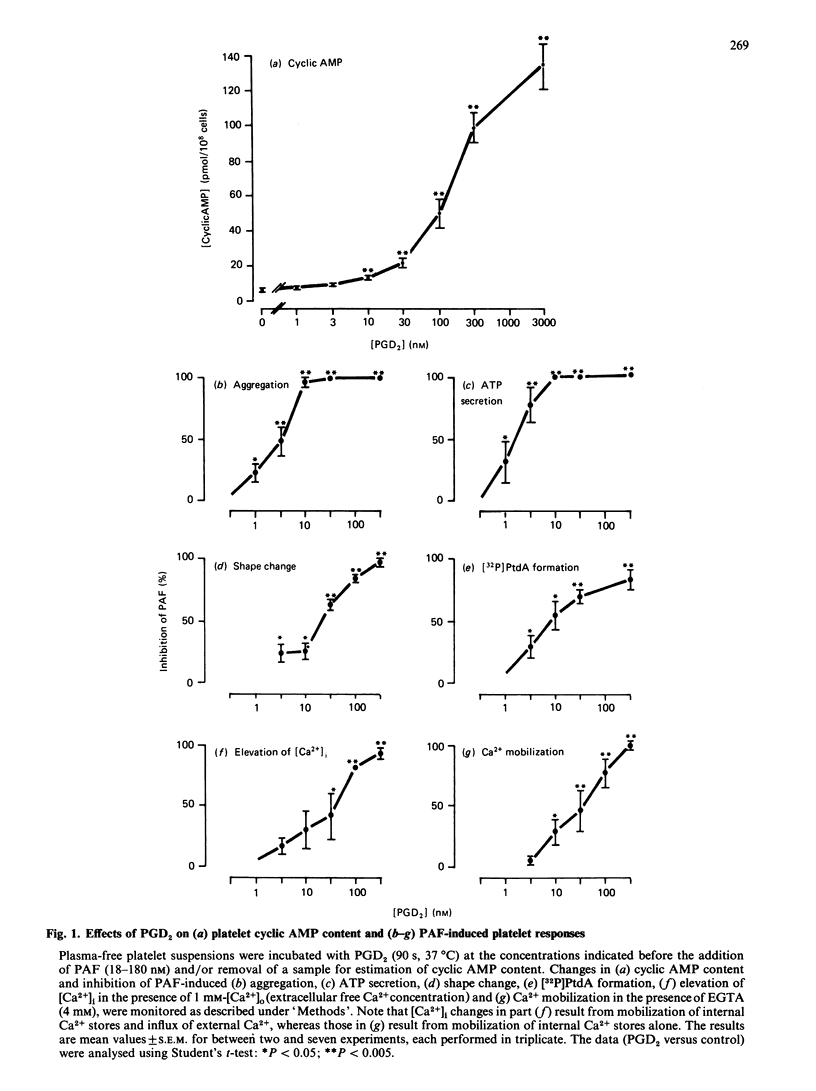
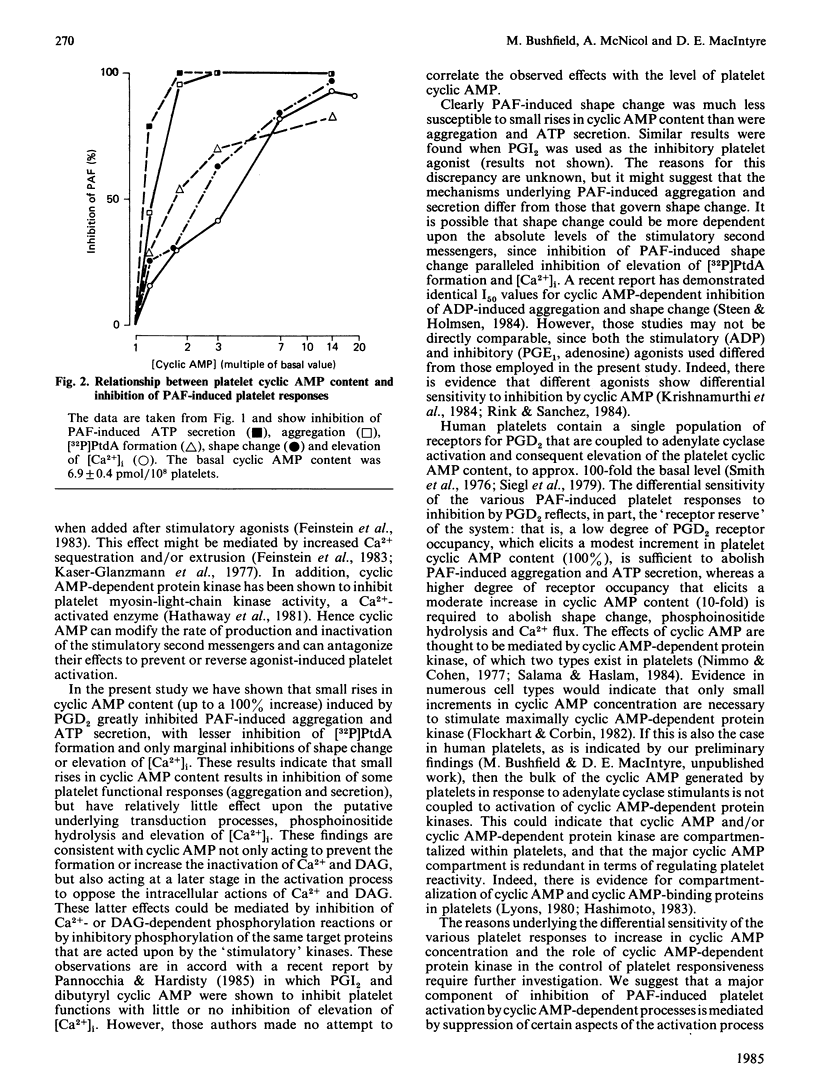
It has been proposed that cyclic AMP inhibits platelet reactivity: by preventing agonist-induced phosphoinositide hydrolysis and the resultant formation of 1,2-diacylglycerol and elevation of cytosolic free Ca2+ concentration [( Ca2+]i); by promoting Ca2+ sequestration and/or extrusion; and by suppressing reactions stimulated by (1,2-diacylglycerol-dependent) protein kinase C and/or Ca2+-calmodulin-dependent protein kinase. We used the adenylate cyclase stimulant prostaglandin D2 to compare the sensitivity to cyclic AMP of the transduction processes (phosphoinositide hydrolysis and elevation of [Ca2+]i) and functional responses (shape change, aggregation and ATP secretion) that are initiated after agonist-receptor combination on human platelets. Prostaglandin D2 elicited a concentration-dependent elevation of platelet cyclic AMP content and inhibited platelet-activating-factor(PAF)-induced ATP secretion [I50 (concn. causing 50% inhibition) approximately 2 nM], aggregation (I50 approximately 3 nM), shape change (I50 approximately 30 nM), elevation of [Ca2+]i (I50 approximately 30 nM) and phosphoinositide hydrolysis (I50 approximately 10 nM). A 2-fold increase in cyclic AMP content resulted in abolition of PAF-induced aggregation and ATP secretion, whereas maximal inhibition of shape change, phosphoinositide hydrolysis and elevation of [Ca2+]i required a greater than 10-fold elevation of the cyclic AMP content. This differential sensitivity of the various responses to inhibition by cyclic AMP suggests that the mechanisms underlying PAF-induced aggregation and ATP secretion differ from those underlying shape change. Thus a major component of the cyclic AMP-dependent inhibition of PAF-induced platelet aggregation and ATP secretion is mediated by suppression of certain components of the activation process that occur distal to the formation of DAG or elevation of [Ca2+]i.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker G., Harper J. F., Terasaki W. L., Moylan R. D. Radioimmunoassay of cyclic AMP and cyclic GMP. Adv Cyclic Nucleotide Res. 1979;10:1–33. [PubMed] [Google Scholar]
- Feinstein M. B., Egan J. J., Sha'afi R. I., White J. The cytoplasmic concentration of free calcium in platelets is controlled by stimulators of cyclic AMP production (PGD2, PGE1, forskolin). Biochem Biophys Res Commun. 1983 Jun 15;113(2):598–604. doi: 10.1016/0006-291x(83)91768-0. [DOI] [PubMed] [Google Scholar]
- Flockhart D. A., Corbin J. D. Regulatory mechanisms in the control of protein kinases. CRC Crit Rev Biochem. 1982 Feb;12(2):133–186. doi: 10.3109/10409238209108705. [DOI] [PubMed] [Google Scholar]
- Fouque F., Vargaftig B. B. Triggering by Paf-acether and adrenaline of cyclo-oxygenase-independent platelet aggregation. Br J Pharmacol. 1984 Nov;83(3):625–633. doi: 10.1111/j.1476-5381.1984.tb16216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. E., Say A. K., Haslam R. J. Subcellular distribution of the different platelet proteins phosphorylated on exposure of intact platelets to ionophore A23187 or to prostaglandin E1. Possible role of a membrane phosphopolypeptide in the regulation of calcium-ion transport. Biochem J. 1979 Dec 15;184(3):651–661. doi: 10.1042/bj1840651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Sanchez A., Rink T. J. Stimulus-response coupling in human platelets. Changes evoked by platelet-activating factor in cytoplasmic free calcium monitored with the fluorescent calcium indicator quin2. Biochem J. 1984 Mar 15;218(3):819–827. doi: 10.1042/bj2180819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S. Functional pool of cyclic adenosine 3',5'-monophosphate in rabbit platelets. Thromb Haemost. 1983 Feb 28;49(1):8–12. [PubMed] [Google Scholar]
- Hathaway D. R., Eaton C. R., Adelstein R. S. Regulation of human platelet myosin light chain kinase by the catalytic subunit of cyclic AMP-dependent protein kinase. Nature. 1981 May 21;291(5812):252–256. doi: 10.1038/291252a0. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Dangelmaier C. A., Rongved S. Tight coupling of thrombin-induced acid hydrolase secretion and phosphatidate synthesis to receptor occupancy in human platelets. Biochem J. 1984 Aug 15;222(1):157–167. doi: 10.1042/bj2220157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthi S., Westwick J., Kakkar V. V. Regulation of human platelet activation--analysis of cyclooxygenase and cyclic AMP-dependent pathways. Biochem Pharmacol. 1984 Oct 1;33(19):3025–3035. doi: 10.1016/0006-2952(84)90604-x. [DOI] [PubMed] [Google Scholar]
- Käser-Glanzmann R., Jakäbovä M., George J. N., Lüscher E. F. Stimulation of calcium uptake in platelet membrane vesicles by adenosine 3',5'-cyclic monophosphate and protein kinase. Biochim Biophys Acta. 1977 May 2;466(3):429–440. doi: 10.1016/0005-2736(77)90336-4. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G. Prostacyclin inhibition of phosphatidic acid synthesis in human platelets is not mediated by protein kinase C. Biochem Biophys Res Commun. 1984 Apr 16;120(1):37–44. doi: 10.1016/0006-291x(84)91410-4. [DOI] [PubMed] [Google Scholar]
- Lyons R. M. Compartmentalization of adenosine 3':5'-monophosphate-binding proteins in human platelets. Thromb Res. 1980 Aug 1;19(3):317–332. doi: 10.1016/0049-3848(80)90260-1. [DOI] [PubMed] [Google Scholar]
- MacIntyre D. E., McNicol A., Drummond A. H. Tumour-promoting phorbol esters inhibit agonist-induced phosphatidate formation and Ca2+ flux in human platelets. FEBS Lett. 1985 Jan 28;180(2):160–164. doi: 10.1016/0014-5793(85)81063-2. [DOI] [PubMed] [Google Scholar]
- MacIntyre D. E., Pollock W. K. Platelet-activating factor stimulates phosphatidylinositol turnover in human platelets. Biochem J. 1983 May 15;212(2):433–437. doi: 10.1042/bj2120433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo H. G., Cohen P. Hormonal control of protein phosphorylation. Adv Cyclic Nucleotide Res. 1977;8:145–266. [PubMed] [Google Scholar]
- O'Rourke F. A., Halenda S. P., Zavoico G. B., Feinstein M. B. Inositol 1,4,5-trisphosphate releases Ca2+ from a Ca2+-transporting membrane vesicle fraction derived from human platelets. J Biol Chem. 1985 Jan 25;260(2):956–962. [PubMed] [Google Scholar]
- Pannocchia A., Hardisty R. M. Cyclic AMP inhibits platelet activation independently of its effect on cytosolic free calcium. Biochem Biophys Res Commun. 1985 Feb 28;127(1):339–345. doi: 10.1016/s0006-291x(85)80164-9. [DOI] [PubMed] [Google Scholar]
- Pollock W. K., Armstrong R. A., Brydon L. J., Jones R. L., MacIntyre D. E. Thromboxane-induced phosphatidate formation in human platelets. Relationship to receptor occupancy and to changes in cytosolic free calcium. Biochem J. 1984 May 1;219(3):833–842. doi: 10.1042/bj2190833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Sanchez A. Effects of prostaglandin I2 and forskolin on the secretion from platelets evoked at basal concentrations of cytoplasmic free calcium by thrombin, collagen, phorbol ester and exogenous diacylglycerol. Biochem J. 1984 Sep 15;222(3):833–836. doi: 10.1042/bj2220833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama S. E., Haslam R. J. Characterization of the protein kinase activities of human platelet supernatant and particulate fractions. Biochem J. 1984 Mar 1;218(2):285–294. doi: 10.1042/bj2180285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano K., Takai Y., Yamanishi J., Nishizuka Y. A role of calcium-activated phospholipid-dependent protein kinase in human platelet activation. Comparison of thrombin and collagen actions. J Biol Chem. 1983 Feb 10;258(3):2010–2013. [PubMed] [Google Scholar]
- Siegl A. M., Smith J. B., Silver M. J. Specific binding sites for prostaglandin D2 on human platelets. Biochem Biophys Res Commun. 1979 Sep 12;90(1):291–296. doi: 10.1016/0006-291x(79)91623-1. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Ingerman C. M., Silver M. J. Formation of prostagland in D2 during endoperoxide-induced platelet aggregation. Thromb Res. 1976 Oct;9(4):413–418. doi: 10.1016/0049-3848(76)90141-9. [DOI] [PubMed] [Google Scholar]
- Steen V. M., Holmsen H. Inhibition of ADP-induced responses in human platelets by agents elevating the cyclic AMP level: comparison of aggregation and shape change. Thromb Haemost. 1984 Dec 29;52(3):333–335. [PubMed] [Google Scholar]
- Yavin E., Zutra A. Separation and analysis of 32P-labeled phospholipids by a simple and rapid thin-layer chromatographic procedure and its application to cultured neuroblastoma cells. Anal Biochem. 1977 Jun;80(2):430–437. doi: 10.1016/0003-2697(77)90665-0. [DOI] [PubMed] [Google Scholar]


