Abstract
Background
It is unclear how rising obesity among people with HIV (PWH) impacts their risk of type 2 diabetes mellitus (diabetes). We examined associations between HIV, prevalent diabetes and adiposity among South African PWH and their peers without HIV (PWOH).
Methods
HIV status was ascertained by antibody testing. Diabetes was defined as current use of oral hypoglycemics, insulin, and/or HbA1c ≥6.5%. Adiposity was measured by body mass index (BMI), waist circumference and waist-to-height ratio. Their associations were examined using sex-stratified multivariable fractional polynomial generalized linear models, reporting adjusted prevalence and prevalence ratios (adjPR).
Results
The mean age among 1,254 PWH and 4,381 PWOH was 41 years (95%CI 28, 56). The prevalence of diabetes among males was similar between PWH [11.3% (7.1, 15.5)] and PWOH [9.8% (8.5, 11.1); p=0.740]. By contrast, diabetes prevalence was higher among female PWOH [15.7% (14.4, 17.0)] than female PWH [10.5 (8.3, 12.8)%; adjPR: 0.67 (0.51, 0.82); p<0.001]. This difference was accentuated with obesity but reversed with leanness. At BMI ≥25 kg/m2, female PWH had lower diabetes prevalence [adjPR: 0.58 (0.41, 0.76); p<0.001] than female PHIV. In contrast, at BMI <18 kg/m2, female PWH had higher prevalence [adjPR: 1.72 (−1.53, 4.96); p=0.756] than female PWOH.
Conclusion
We found sex-specific differences in the relationship between adiposity and diabetes prevalence by HIV serostatus in South Africa. Notably, females living with obesity and HIV had lower prevalence of diabetes than females living with obesity and without HIV, which may have particular implications for diabetes prevention programs in the region.
Keywords: HIV/ART, diabetes, sex/gender, obesity, South Africa
Introduction
The anticipated pandemic of HIV-related cardiometabolic disorders in sub-Saharan Africa (SSA) has not yet become evident, 20 years into the era of widely accessible antiretroviral treatment (ART).1–3 In high-income countries (HICs), treated HIV is a recognized risk factor for numerous cardiovascular and metabolic disorders, such as heart failure, stroke, metabolic syndrome, obesity, hypertension, and type 2 diabetes mellitus (diabetes), among others.4,4–7 People with HIV infection (PWH) in HICs, for example, have greater risk of both prevalent8–10 and incident11–13 diabetes than comparable persons without HIV (PWOH). It was expected that a similar upsurge will occur in SSA as ART uptake increases.14,15,
However in SSA, PWH who are on ART, including older-generation thymidine analogues and protease inhibitors, appear to have comparable16,17 if not better18–20 glucose metabolism profiles than the general population. While this may be attributed to insufficient research in SSA, especially the dearth of longitudinal studies with incidence measures, 21 physiological adaptations in PWH in SSA that potentially protect against cardiometabolic disease, including diabetes, have not been considered.
In the recent landmark REPREIVE trial (2023) among PWH, major adverse cardiovascular events were noted to be more common in HICs (10.7 per 1,000 person-years) than in SSA (1.8 events per 1,000 person-years). 22 Relatedly, Brenan et. al., (2023) using country-wide data from 373,889 patients tested for diabetes through South Africa’s National Health Laboratory Service, found PWH were less likely to have diabetes (adjusted relative risk: 0.73; 95% CI: 0.71–0.74) than PWOH. 20 If indeed ART-experienced PWH in SSA, unlike their counterparts in HICs, are spared from excess cardiometabolic disease risk, particularly diabetes, it is imperative to understand the underlying mechanisms. Given the rising diabetes rates in the general population23,24 such insights may inform novel therapeutic and risk stratification approaches in SSA and HICs.
Obesity is integral to diabetes pathogenesis, with over 80% of individuals with diabetes in the general population in HICs living with overweight or obesity as defined by a body mass index (BMI) ≥25 kg/m2.25,26 Among the metabolic consequences of obesity are adipose tissue inflammation, disordered adipokine signaling, mitochondrial dysfunction, dyslipidemia including increased circulating free fatty acids (FFAs), and ectopic fat deposition.26,27 These in turn drive β-cell dysfunction as well as insulin resistance,27,28 the two gateways to impaired glucose tolerance and diabetes. 29 On the other hand, HIV itself and the side effects of ART, particularly for older generations of therapy, may directly and indirectly cause similar metabolic derangements.30,31 With more modern regimens, weight gain following ART initiation also often surges beyond “return to health” into the deleterious categories of excess adiposity.32,33 This includes central obesity, with or without lipodystrophy, both significant determinants of prevalent and new-onset diabetes. 32 It is the nexus of these factors, i.e., HIV, ART metabolic side effects, excess weight gain and an obesogenic environment, that is believed to drive excess diabetes risk in persons PWH in HICs11–13 whereas its occurrence in SSA,18,19,34 is yet to be mirrored in an excess burden of diabetes in the population of PWH.
Understanding the interplay of obesity, HIV, and ART’s metabolic effects in SSA is essential to elucidate the unique factors influencing diabetes prevalence in this region. We used data from a national survey in South Africa to examine whether HIV is associated with diabetes prevalence, and if such associations vary with obesity.
Methods
We followed the guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) in the conduct and reporting of our analyses. 35
Study population and analytic sample
The present study is a secondary analysis of the 2016 South Africa Demographic Survey (SADHS), which is fully described elsewhere. 36 The 2016 SADHS is a cross-sectional survey of the health status, and its determinants, of noninstitutionalized South Africans. Sampling followed a stratified two-stage design with primary sampling units drawn from urban, rural, and farming areas. The division of South Africa into urban, rural and farming areas accounts for its historical race-based spatial socio-economic development. Data were collected between June and November 2016 using questionnaires, physical examination, and laboratory testing of blood. Only participants aged at least 20 years, with no upper limit on age, with complete information on HIV testing, anthropometry, and HbA1c were eligible for inclusion in the present analysis. We used ≥20 years (versus lower) age as cut-off to reduce the likelihood of including undiagnosed type 1 diabetes in our analytic sample.
Study measures
Glucose metabolism
Finger prick blood was tested for glycated hemoglobin (HbA1c) using the Roche Tina-quant® II immunoturbidimetric assay on a blood chemistry analyzer (Hitachi 912 Analyzer, Hitachi, Tokyo, Japan). In addition to HbA1c as a continuous study endpoint, we defined diabetes as any current use of clinician-prescribed oral hypoglycemic medicines and/or insulin and/or HbA1c ≥6.5%. 37 A second definition of diabetes, HbA1c ≥6.5% in a subsample (age ≥40 years old) without self-reported current use of oral hypoglycemic medicines or insulin, was used for the sensitivity analysis.
HIV serostatus
HIV serostatus was ascertained from dry blood spots using an HIV antibody testing algorithm with three different enzyme-linked immunosorbent assays (ELISA). Concordant negative results on the ELISA 1 (Genscreen HIV 1/2 Combi Assay, Bio-Rad, France) and ELISA 2 (E411 Cobas HIV 1/2 Combi Assay, Roche, Switzerland) were recorded as negative. Discordant ELISA 1 and ELISA 2 results were repeated, and if still discordant, were classified as indeterminant. Concordant positive results on the ELISA 1 and ELISA 2 were classified as positive if a third confirmatory test (Geenius™ HIV1/2 confirmatory rapid test, Bio-Rad, France) was positive, and inconclusive if the confirmatory test was negative or indeterminate. 36
Body mass index and waist circumference
Weight and height were measured using portable digital scales (Seca 878 dr, Seca, Hamburg, Germany) and stadiometers (Seca 213 dr, Seca, Hamburg, Germany), respectively, while waist circumference (WC) was measured using non-stretch tape (Seca 203 dr, Seca, Hamburg, Germany) at the level mid-way between the lowest rib margin and the iliac crest with the subject standing erect and at the end of gentle expiration. Body mass index (BMI) estimated from weight (in kilograms)/height (in meters) 2 was classified as underweight (<18.5kg/m2), normal (18.5–24.9kg/m2), overweight (25.0–29.9kg/m2), and obese (≥30.0kg/m2). Waist circumference was categorized as elevated if ≥94cm for males and ≥80cm for females, or normal if <94cm for males and <80cm for females. 38 Lastly, we defined waist-to-height ratio (WtHR) (waist circumference/height) ≥0.5 as elevated. 39
Sociodemographic and other covariates
Sex was self-reported as male or female. Participants described their race/ethnicity as any one of Black, White, Colored (for mixed race), or Indian/Asian, while their place of residence was classified as either urban or rural. Residence in farming areas was considered rural. Socioeconomic position was indicated by the household wealth index. The latter is a composite measure of a household’s cumulative living standard calculated using principal components analysis 40 and based on ownership of selected household assets such as television, radio, refrigerator, and vehicle; materials used for housing construction; and access to sanitation facilities and clean water. Cigarette use daily or on some days of the week was categorized as current smoking versus no cigarette use which was categorized as nonsmoking. Current drinkers were defined as those reporting alcohol consumption in the last 12 months; whereas non-drinkers were those reporting no alcohol consumption in the last 12 months. We reported blood pressure (BP) as the average of the last two of three automated (Omron 1300, Omron Healthcare, Bannockburn, IL, USA) resting state BP readings, and hypertension as any of systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg or self-reported use of prescribed antihypertensive medication. Comorbid cardiovascular disease (CVD), chronic respiratory disease and past tuberculosis (TB) were also noted. These were defined based on self-reported clinician-led diagnoses of any of heart failure, stroke, or coronary artery disease for CVD; asthma, chronic bronchitis or chronic obstructive pulmonary disease chronic respiratory disease for chronic respiratory disease; and any completed drug treatment for TB.
Ethics review
The South African Medical Research Council’s Research Ethics Committee and the institutional review board of ICF approved the 2016 SADHS and all its data collection procedures, and written informed consent was obtained prior to participation. As a secondary data analysis, our study did not require any ethics review or approval. These data are publicly available in a de-identified form.
Statistical analysis
We performed a complete case analysis given negligible missingness (<2%) in HIV serostatus, HbA1c, sex, BMI, WtHR and WC data. Our outcome of interest was glucose metabolism which we modelled as prevalent diabetes (dichotomous endpoint) and mean HbA1c (continuous endpoint). Because our primary goal was to understand the complex relationships between diabetes, HbA1c and anthropometric indices between PWH and PWOH, and not to estimate population level parameters, we did not apply inverse probability sampling weights to our analysis. We described participants` characteristics according to HIV serostatus. Raincloud plots were used to visualize the distribution of HIV- and sex-stratified HbA1c, with mean differences examined by linear regression. Next, sex-stratified multivariable fractional polynomial (MFP) generalized linear models were used to explore HIV-specific associations between HbA1c and each of (continuous) BMI, WtHR and WC. MFP modeling provides flexible parameterization to reveal non-linear associations, 41 and was thus suited to our hypothesis of complex relationships between HbA1c and obesity indices.
We also modeled the association between diabetes and (separately) categorical BMI, WtHR and WC using similar MFP generalized linear models stratified by sex and including HIV as a predictor. Diabetes prevalence for each obesity category was derived from postestimation margins from these models. The corresponding PWH versus PWOH prevalence differences (PD) and prevalence ratios (PR) of diabetes across obesity categories were estimated from the postestimation margins and multiple linear combinations (for PD) or non-linear combinations (for PR) using Stata 18’s mlincom and nlcom commands, respectively. These analyses were labelled “primary MFP models”. MFP models initially included age, race, area of residence, household wealth index, smoking and alcohol drinking status, and history of TB drug treatment for TB use as covariates. Only covariates that were statistically significant at p-value <0.05 were retained in each final model (Supplemental Table 1).
Sensitivity analyses
Sensitivity analyses re-examined the sex-stratified association between HIV and diabetes, and reported overall adjusted prevalence differences and prevalence ratios. The first approach used generalized linear models after propensity score (PS) matching on the same covariates as were used in primary analyses. These analyses were labelled “sensitivity, PS matched models”. The second approach used MFP generalized linear models adjusting for the same covariates as were used in the primary analyses. These analyses were labelled “sensitivity, MFP models”. However, this time diabetes was defined as HbA1c ≥6.5% and analysis was restricted to a subsample of adults ≥40 years old not using oral hypoglycemic medicines or insulin. The PS matching approach assessed how sensitive our findings were to differences in data analysis methods and their attendant assumptions. With a higher cut-off age (≥40 years), the prevalence of undiagnosed type 1 diabetes and thus subsequent confounding is likely further reduced, as is excluding treated diabetes which may indicate differential access to healthcare.
Analyses were conducted using R, version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria), and Stata version 18.0 (StataCorp, College Station, TX, USA). All probability values were 2-sided, with p-values <0.05 considered indicative of statistical significance.
Results
Sample characteristics
The analytic sample consisted of 5,635 total participants, of whom 4,381 (77.7%) were PWOH and 1,254 (22.3%) were PWH (Table 1). Mean age (95%CI) was 42 years (27, 59) for PWOH and 38 years (31, 47) for PWH. PWH were more frequently females (73.7% vs. 60.9%) and of Black race (97.8% vs. 84.4%) than PWOH. While rates of urban/rural residence were comparable, PWH came from poorer households than PWOH. Smoking and alcohol consumption were similar between the two groups, as were the anthropometric measures. Overall, over half of participants had overweight/obesity (58.5%) according to BMI, and central obesity (52.6%) according to waist circumference. Comorbidities were also otherwise equally prevalent between the two groups except for diabetes (which is discussed below), treated TB, which was nearly threefold more common in PWH (14.7%) than PWOH (4.8%), and hypertension which was less common among PWH (46.6%) than PWOH (53.3%).
Table 1.
Sample characteristics by HIV serostatus among adults aged ≥20 years old: unweighted South Africa Demographic Health Survey, 2016.
| Characteristic | Total | HIV Serostatus | |
|---|---|---|---|
| PWOH | PWH | ||
| Number | 5,635 | 4,381 | 1,254 |
| Sociodemographic | |||
| Male sex | 2,041 (36.2%) | 1,711 (39.1%) | 330 (26.3%) |
| Age (years) | 41.0 (28.0, 56.0) | 42.0 (27.0, 59.0) | 38.0 (31.0, 47.0) |
| Self-reported race | |||
| Black | 4,939 (87.7%) | 3,712 (84.8%) | 1,227 (97.8%) |
| White | 222 (3.9%) | 221 (5.0%) | 1 (0.1%) |
| Colored | 423 (7.5%) | 398 (9.1%) | 25 (2.0%) |
| Asian/Indian | 48 (0.9%) | 47 (1.1%) | 1 (0.1%) |
| Urban residence | 2,906 (51.6%) | 2,272 (51.9%) | 634 (50.6%) |
| Rural | 2,729 (48.4%) | 2,109 (48.1%) | 620 (49.4%) |
| Household asset index | 1.4 (−5.7, 6.9) | 2.1 (−4.8, 7.5) | −0.7 (−8.6, 4.7) |
| Behavioral | |||
| Current smokers | 1,032 (18.3%) | 843 (19.2%) | 189 (15.1%) |
| Non-smokers | 4,603 (81.7%) | 3,538 (80.8%) | 1,065 (84.9%) |
| Current drinkers | 1,802 (32.0%) | 1,450 (33.1%) | 352 (28.1%) |
| Non-drinkers | 3,833 (68.0%) | 2,931 (66.9%) | 902 (71.9%) |
| Anthropometric | |||
| Overweight/obesity | 3,243 (58.5%) | 2,544 (59.0%) | 699 (56.6%) |
| Overweight | 1,457 (26.3%) | 1,117 (25.9%) | 340 (27.6%) |
| Obesity | 1,786 (32.2%) | 1,427 (33.1%) | 359 (29.1%) |
| Elevated waist circumference | 2,939 (53.3%) | 2,286 (53.5%) | 653 (52.6%) |
| Elevated waist-to-height ratio | 3,371 (61.2%) | 2,642 (61.9%) | 735 (59.3%) |
| BMI (kg/m2) | 27.2 (6.4) | 27.8 (7.2) | 27.1 (6.7) |
| Waist circumference (cm) | 87.5 (14.8) | 87.7 (14.7) | 85.4 (13.0) |
| Waist-to-height ratio | 0.54 (0.10) | 0.54 (0.10) | 0.53 (0.09) |
| Cardiometabolic | |||
| Prevalent diabetes mellitus | 805 (14.4%) | 693 (15.9%) | 112 (9.0%) |
| HbA1c ≥6.5% | 769 (13.6%) | 662 (15.1%) | 107 (8.5%) |
| HbA1c (%) | 6.2 (6.2, 6.3) | 6.3 (6.2, 6.3) | 6.1 (6.0, 6.1) |
| Prevalent hypertension | 2801 (51.8%) | 2,245 (53.3%) | 556 (46.6%) |
| Systolic BP (mmHg) | 132.8 (23.0) | 134.4 (23.7) | 127.5 (20.8) |
| Diastolic BP (mmHg) | 86.0 (12.7) | 86.0 (12.8) | 86.0 (12.4) |
| Self-reported comorbidities | |||
| Past drug treated tuberculosis | 392 (7.0%) | 208 (4.8%) | 184 (14.7%) |
| Cardiovascular disease | 227 (4.0%) | 183 (4.2%) | 44 (3.5%) |
| Chronic respiratory disease | 230 (4.1%) | 192 (4.4%) | 38 (3.0%) |
Values are mean (SD) or αmedian (25th, 75th percentile) or number (percent).
Elevated waist circumference if ≥94cm for males and ≥80cm for females. Elevated waist-to-height ratio if ≥0.5.
PWH = people with HIV; PWOH = people without HIV.
Association between HbA1c (continuous endpoint), HIV and obesity indices
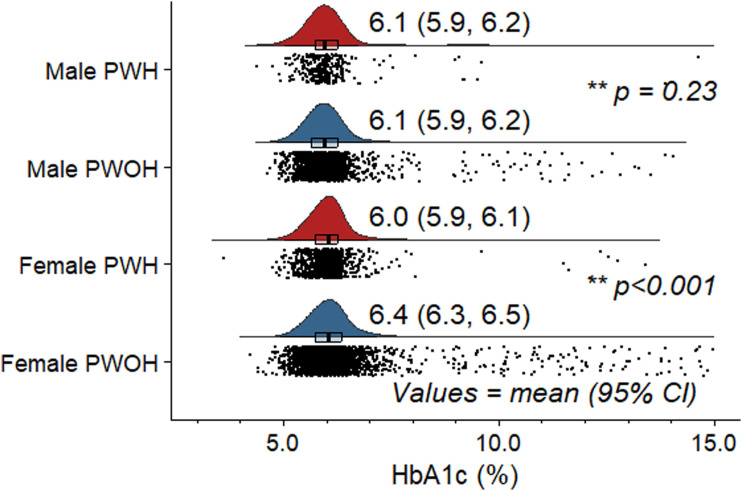
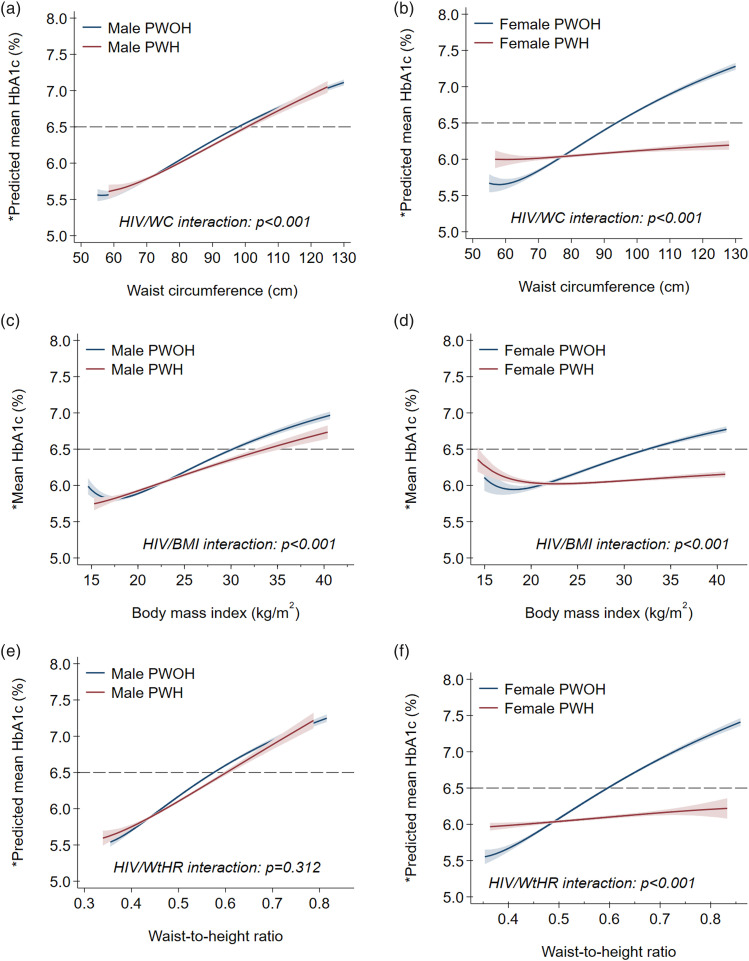
Stratified by HIV serostatus only, PWH had lower mean (95%CI) HbA1c [6.1% (6.0, 6.1)] than PWOH [6.3% (6.2, 6.3); p<0.001] (Table 1). In sex-specific comparisons, however, differences in mean HbA1c were seen only between female PWH [6.0% (5.9, 6.1)] and female PWOH [6.4% (6.3, 6.5); p<0.001], but not male PWH [6.1% (5.9, 6.2)] and male PWOH [6.1% (5.9, 6.2); p=0.234] (Figure 1). Results from primary MFP models are depicted in Supplemental Figures 2a-(f) and Figures 2a-(f), which show, respectively, unadjusted and adjusted sex stratified HIV-specific associations between HbA1c and each of BMI, WtHR and WC. Among men, unadjusted and adjusted mean HbA1c had a largely linear relation to each of BMI, WtHR and WC with no differences by HIV serostatus.
Figure 1.
** P values for sex-specific persons PWH versus persons PWOH unadjusted comparisons of mean HbA1c level. PWH = people with HIV; PWOH = people without HIV.
Figure 2.
* Predicted from multivariable fractional polynomial models adjusted for age, sex, self-reported race, area of residence, years spent in formal education, health insurance coverage, smoking status and alcohol use. PWH = people with HIV; PWOH = people without HIV.
In contrast, we noted a J-shaped relationship between (unadjusted and adjusted) mean HbA1c and each obesity index among females (Figure 2(b), (d) and (f)). At higher values of BMI (>21 kg/m2), WtHR (>0.48) and WC (>78 cm), the adjusted mean HbA1c was higher in female PWOH than female PWH. Conversely, below these thresholds, adjusted mean HbA1c was higher in female PWH than female PWOH with decreasing BMI, WtHR or WC.
Association between prevalent diabetes (dichotomous endpoint), HIV and obesity indices
Diabetes was more prevalent among PWOH (15.9%) than PWH (9.0%; p<0.001), overall (Table 1). In both unadjusted (Supplemental Table 2) and adjusted (Table 2) sex stratified (primary MFP) analyses, however, there were no significant differences in diabetes prevalence between male PWH and PWOH, both overall and by obesity categories. This contrasted sharply with females among whom PWOH [15.7% (14.4, 17.0)] had higher adjusted diabetes prevalence than PWH [10.5% (8.3, 12.8); p<0.001], corresponding to a prevalence difference of −5.2% (−7.8, −2.6) (Table 3). These HIV-related differences in diabetes prevalence among females were more pronounced with increasing obesity. For example, diabetes prevalence at BMI ≥30kg/m2 was 12.8% (9.1, 16.5) among female PWH versus 22.0% (19.9, 24.0) among female PWOH (p<0.001). That is, females with HIV and obesity had half the relative prevalence of diabetes than female PWOH [adjusted prevalence ratio (adjPR) 0.58 (0.41, 0.76); p<0.001] (Table 3). These observation were largely similar in the unadjusted analyses (Supplemental Table 3).
Table 2.
Adjusted prevalence, prevalence difference and prevalence ratio of type 2 diabetes mellitus* (dichotomous endpoint) according to waist circumference, waist-to-height ratio and body mass index categories by HIV serostatus for males ≥20 years old: unweighted South Africa Demographic Health Survey, 2016.
| Adiposity category | Adjusted α estimate (95%CI); Males | |||||
|---|---|---|---|---|---|---|
| Prevalence | Prevalence | P value | Prevalence | P value | ||
| PWOH | PWH | difference β | ratio β | |||
| Overall | 9.8 (8.5, 11.1) | 11.3 (7.1, 15.5) | 1.5 (−2.9, 5.9) | 0.660 | 1.15 (0.69, 1.61) | 0.740 |
| Waist circumference | ||||||
| Normal | 6.3 (4.8, 7.8) | 5.3 (2.3, 8.3) | −1.0 (−4.3, 2.3) | 0.547 | 0.84 (0.33, 1.35) | 0.774 |
| Elevated | 15.7 (12.6, 18.7) | 21.4 (11.7, 31.0) | 5.7 (−4.4, 15.8) | 0.269 | 1.37 (0.69, 2.4) | 0.516 |
| Waist-to-height ratio | ||||||
| Normal | 4.8 (3.1, 6.5) | 4.4 (1.2, 7.7) | −0.4 (−4.1, 3.3) | 0.839 | 0.92 (0.17, 1.67) | 0.899 |
| Elevated | 13.2 (11.0, 15.4) | 13.7 (8.0, 19.4) | 0.5 (−5.7, 6.6) | 0.868 | 1.04 (0.57, 1.50) | 0.932 |
| Body mass index | ||||||
| Underweight | 2.6 (−0.3, 5.6) | 5.6 (−2.3, 13.5) | 2.9 (−5.4, 11.3) | 0.689 | 2.11 (−1.66, 5.89) | 0.515 |
| Normal | 7.4 (5.4, 9.3) | 5.2 (1.7, 8.8) | −2.1 (−6.2, 1.9) | 0.295 | 0.71 (0.19, 1.22) | 0.284 |
| Overweight | 11.6 (9.0, 14.2) | 11.6 (4.4, 18.8) | 0.0 (−7.6, 7.6) | 0.998 | 1.00 (0.34, 1.65) | 0.714 |
| Obese | 15.7 (12.0, 19.5) | 26.8 (8.4, 45.2) | 11.1 (−7.7, 29.9) | 0.249 | 1.70 (0.46, 2.95) | 0.769 |
αVariables and their transformation for which adjustment was made using multivariable fractional polynomial models are outlined in Supplemental Table 1.
βPWOH are the reference group.
Diabetes = HbA1c ≥6.5% and/or current use of oral hypoglycemic medicines and/or insulin.
Elevated waist circumference if ≥94cm (for males). Elevated waist-to-height ratio if ≥0.5. Body mass index categories: underweight: <18.5 kg/m2; normal 18.5-24.9 kg/m2; overweight 25-29.9 kg/m2; obese ≥30 kg/m2.
PWH = people with HIV; PWOH = people without HIV.
Table 3.
Adjusted prevalence, prevalence difference and prevalence ratio of type 2 diabetes mellitus* (dichotomous endpoint) according to waist circumference, waist-to-height ratio and body mass index categories by HIV serostatus for females ≥20 years old: unweighted South Africa Demographic Health Survey, 2016.
| Adiposity category | Adjusted α estimate (95%CI); Females | |||||
|---|---|---|---|---|---|---|
| Prevalence | Prevalence | P value | Prevalence | P value | ||
| PWOH | PWH | difference β | ratio β | |||
| Overall | 15.7 (14.4, 17.0) | 10.5 (8.3, 12.8) | −5.2 (−7.8, −2.6) | <0.001 | 0.67 (0.51, 0.82) | <0.001 |
| Waist circumference | ||||||
| Normal | 5.1 (3.3, 7.0) | 6.2 (2.9, 9.4) | 1.0 (−2.7, 4.8) | 0.595 | 1.20 (0.43, 1.97) | 0.551 |
| Elevated | 18.5 (17.0, 20.1) | 11.7 (9.0, 14.4) | −6.9 (−10.0, −3.7) | <0.001 | 0.63 (0.48, 0.78) | <0.001 |
| Waist-to-height ratio | ||||||
| Normal | 6.4 (4.1, 8.7) | 8.3 (4.5, 12.1) | 1.9 (−2.3, 6.3) | 0.241 | 1.30 (0.55, 2.05) | 0.241 |
| Elevated | 19.5 (18.0, 21.1) | 12.1 (9.3, 14.8) | −7.5 (−10.7, −4.3) | <0.001 | 0.62 (0.46, 0.77) | <0.001 |
| Body mass index | ||||||
| Underweight | 4.6 (−1.4, 10.5) | 7.8 (−2.9, 18.5) | 3.3 (−8.9, 15.4) | 0.602 | 1.72 (−1.53, 4.96) | 0.756 |
| Normal | 7.2 (5.1, 9.3) | 7.1 (3.4, 10.8) | −0.1 (−4.4, 4.1) | 0.956 | 0.98 (0.39, 1.57) | 0.944 |
| Overweight | 11.6 (9.4, 13.8) | 9.3 (5.3, 13.2) | −2.3 (−6.8, 2.2) | 0.312 | 0.80 (0.43, 1.17) | 0.585 |
| Obese | 22.0 (19.9, 24.0) | 12.8 (9.1, 16.5) | −9.2 (−13.4, −4.9) | <0.001 | 0.58 (0.41, 0.76) | <0.001 |
αVariables and their transformation for which adjustment was made using multivariable fractional polynomial models are outlined in Supplemental Table 1.
βPWOH uninfected are the reference group.
Diabetes = HbA1c ≥6.5% and/or current use of oral hypoglycemic medicines and/or insulin.
Elevated waist circumference if ≥80cm (for females). Elevated waist-to-height ratio if ≥0.5. Body mass index categories: underweight: <18.5 kg/m2; normal 18.5-24.9 kg/m2; overweight 25-29.9 kg/m2; obese ≥30 kg/m2.
PWH = people with HIV; PWOH = people without HIV.
Sociodemographic and behavioral determinants of HbA1c and prevalent diabetes
Among males, age, smoking status and household wealth index emerged from the primary MFP models as significantly associated with mean HbA1c and diabetes prevalence (Supplemental Table 1). Whereas age and household wealth index were positively correlated with diabetes prevalence, current smoking (versus none) was inversely associated. On the other hand, only age and alcohol use emerged as significant determinants of diabetes prevalence among females in addition to HIV serostatus. Current drinking status (versus non-drinking) was negatively correlated with diabetes prevalence.
Sensitivity analyses
Female PWOH [19.9% (17.8, 22.0)] had greater diabetes prevalence than female PWH [12.5% (8.9, 16.0); p<0.011] in sensitivity MFP analyses using a higher inclusion age (≥40 vs. ≥20 years old) and a diabetes definition based solely on HbA1c (Table 4). That is, the relative prevalence of diabetes among female PWH ≥40 years old was nearly two-thirds that of female PWOH [adjPR 0.62 (0.43, 0.82); p<0.001] (Table 3). However, diabetes prevalence was similar between male (≥40 years old) PWOH [13.3% (10.9, 15.8)] and male PWH [8.0% (3.6, 12.5); p=0.414]. Sensitivity PS matching (Supplemental Figure 1) produced results that were consistent with both MFP-based primary and sensitivity analyses (Table 3). For example, the adjPR for diabetes among females was 0.67 (0.51, 0.82) for the primary MFP model, 0.62 (0.43, 0.82) for sensitivity MFP model and 0.63 (0.45, 0.81) for the sensitivity PS matched model.
Table 4.
Sensitivity analysis of adjusted prevalence, prevalence difference and prevalence ratio of type 2 diabetes mellitus (dichotomous endpoint) according to HIV serostatus and sex: unweighted South Africa Demographic Health Survey, 2016.
| Analytic Approach* | Adjusted α estimate (95%CI) | |||||
|---|---|---|---|---|---|---|
| Prevalence | Prevalence | P value | Prevalence | P value | ||
| PWOH | PWH | difference β | ratio β | |||
| Males: Diabetes | ||||||
| Primary, MFP model | 9.8 (8.5, 11.1) | 11.3 (7.1, 15.5) | 1.5 (−2.9, 5.9) | 0.660 | 1.15 (0.69, 1.61) | 0.740 |
| Sensitivity, MFP model | 13.3 (10.9, 15.8) | 8.0 (3.6, 12.5) | −5.1 (−10.4, 2,1) | 0.414 | 0.60 (0.34, 1.08) | 0.089 |
| Sensitivity, PS matching | 8.3 (5.3, 11.4) | 7.7 (4.7, 10.7) | −0.6 (−0.4, 3.6) | 0.768 | 0.92 (0.43, 1.41) | 0.768 |
| Females: Diabetes | ||||||
| Primary, MFP model | 15.7 (14.4, 17.0) | 10.5 (8.3, 12.8) | −5.2 (−7.8, −2.6) | <0.001 | 0.67 (0.51, 0.82) | <0.001 |
| Sensitivity, MFP model | 19.9 (17.8, 22.0) | 12.5 (8.9, 16.0) | −7.5 (−11.7, −3.3) | 0.003 | 0.62 (0.43, 0.82) | <0.001 |
| Sensitivity, PS matching | 12.8 (10.6, 14.9) | 8.1 (6.3, 9.9) | −4.7 (−7.5, −18.5) | 0.001 | 0.63 (0.45, 0.81) | 0.001 |
αVariables for which adjustment was made are outlined in Supplemental Table 1.
βPWOH are the reference group.
Primary, MFP model: diabetes = HbA1c ≥6.5% and/or current use of oral hypoglycemic medicines and/or insulin among adults ≥20 years old; using multivariable fractional polynomial generalized linear models (GLM).
Sensitivity, MFP model: diabetes = HbA1c ≥6.5% and taking neither oral hypoglycemic medicines nor insulin among adults ≥40 years old; using multivariable fractional polynomial GLM.
Sensitivity, PS matching: diabetes = HbA1c ≥6.5% and/or current use of oral hypoglycemic medicines and/or insulin among adults ≥20 years old; using propensity score matched GLM.
PWH = people with HIV; PWOH = people without HIV.
Discussion
In a large national sample of South African adults we found no HIV-related differences in prevalent diabetes or HbA1c distribution among males. In contrast, female PWH had a lower prevalence of diabetes and lower mean HbA1c than peers without HIV. Notably, these differences were greatest among those living with obesity. Our results raise important questions. Whether PWH in SSA have increased risk of diabetes remains to be ascertained, at least in the South African context. Among women living with HIV and obesity in particular, the comparatively reduced prevalence of diabetes suggests possible protective adaptations, physiological or otherwise. Future work in South Africa and other HIV-endemic SSA settings should be directed towards better mechanistic understanding of sex-specific adipose tissue biology in HIV as well as map its implications for diabetes risk, clinical phenotypes, management and prognosis.
Excess fat accumulation in visceral depots is a major cause of diabetes25,27,42 via worsening of insulin sensitivity25,28 and/or loss of β-cell function and islet mass.27,28 Current understanding, albeit gained from clinical and epidemiological studies30,31,43 in HICs, is that HIV/ART and obesity synergistically drive the excess diabetes risk in both males and females living with HIV compared to PWOH.10–12 HIV/ART have been demonstrated to induce deleterious qualitative, quantitative and distributive changes in adipose tissue.43,44 Mechanistically, HIV infects adipose tissue resident CD4+ T cells and macrophages. 43 ART causes mitochondrial toxicity, oxidative stress and altered gene expression in adipocytes. 45 This is in addition to causing weight gain. The resulting and frequently excessive adipose tissue is characterized by, among other disorders, increased insulin resistance. 43 Our findings appear, however, to contradict this paradigm. Male PWH had similar diabetes prevalence to male PWOH at all levels of adiposity, whereas diabetes prevalence in female PWH was reduced with obesity but accentuated with leanness.
There are several potential explanations for our findings. First, BMI, WC and WtHR are inadequate indices of fat accumulation. BMI poorly discriminates between lean and fat mass. WC and WtHR, on the other hand, more accurately measure central obesity although they cannot distinguish between abdominal subcutaneous (SAT) and visceral adipose tissue (VAT) depots. Neither can they measure ectopic sites like hepatic, pancreatic, and omental adipose tissue. 44 Different fat depots and ectopic sites have different dysmetabolic potential. 44 Relatedly, HIV/ART are known to influence the partitioning and distribution of adipose tissue.44,46,47 Thus, similar BMI, WC or WtHR between PWH and PWOH may not reflect similar diabetes risk as the underlying adipose tissue composition and distribution can vary substantially. In South Africa, for example, Goedecke et al., (2013) 46 used dual energy X-ray absorptiometry (DXA) and found that despite similar BMI, ART-experienced female PWH had greater VAT and lesser leg fat than ART-naive female PWH. Our hypothesis is, therefore, that increasing BMI, WC and WtHR in our cohort corresponded to increasing fat accumulation albeit in less diabetogenic depots and/or ectopic sites in female but not male PWH compared to PWOH.
This hypothesis may assume greater relevance in light of growing evidence associating newer generation integrase inhibitors (INSTI) with weight gain and obesity, and the subsequent concerns about diabetes risk.48–50 Of note, INST-related weight gain disproportionally affects women and those of Black ethnicity. Their uptake is also increasing across SSA since their recommendation by WHO as preferred first line ART. Despite substantial weight gain, the incidence of diabetes has been noted to be very low following INSTI initiation in SSA.48–50 However, the relevant literature from SSA is presently very sparse and contradictory. INSTI became publicly available in South Africa since 2019 whereas data collection for our study took place in 2016. It, therefore, remains to be determined how excess adipose tissue gained from INSTI use is partitioned and impacts diabetes risk.
It also remains to be established how, if, HIV/adiposity interactions shape clinical phenotype(s) of diabetes in SSA. The presence of diabetes phenotypes in the general population in SSA that may not fit the conventional classification of (type II) diabetes has already been noted.23,51 For example, the majority of patients with diabetes in the general population in SSA are young, lean, and have β-cell secretory dysfunction more than insulin resistance compared to those in HICs.23,51 Our finding of worse glucose profiles in female PWH compared to female PWOH at lower adiposity levels may speak to this pathophysiological heterogeneity and, in turn, the phenotypic diversity of diabetes in SSA. Exploration and subsequent identification of distinct HIV-related diabetes phenotypes, if they exist, is therefore critical to informing optimal therapeutic approaches and preventive strategies among SSA`s 26 million PWH. 52 Future work in South Africa should combine imaging-based assessment of adipose tissue depots and ectopic sites; detailed assessments of glycemic and insulin indices using, for example, 2 hour multiple-sampled oral glucose oral testing; together with adipose tissue sampling and molecular characterization.
Strengths and limitations
Our participants were drawn from across South Africa`s racial/ethnic, demographic, socioeconomic and geospatial groups. However, we did not apply sampling weights to our analyses and thus our results are not population-level estimates. Similarly, the cross-sectional design forestalls causal inferences and warrants caution in interpretation of these results. While the large sample size permitted examination of important subgroups, our lack of data on CD4+ counts, ART history and other HIV clinical measures was another limitation. PWH on ART versus those not on ART, for example, are likely to differ in terms of cardiometabolic and inflammation profiles in ways that may impact our findings. We also cannot determine the contribution, if any, of primary care access to the observed effects. Likewise, our tools to assess body size as a marker of adiposity were comparatively blunt.
Our definition of diabetes is another major limitation. Oral glucose tolerance testing is the diagnostic gold standard. Data from South Africa 53 and elsewhere54,55 show that the HbA1c threshold of 6.5% has low sensitivity compared to lower thresholds, e.g., 5.8%, for diagnosing diabetes among PWH. Notwithstanding, we used the ≥6.5% threshold in keeping with current South African clinical guidelines. 37 On the other hand, the limitation of single HbA1c measurement in community-based diabetes screening are highlighted by the observation that a screening threshold of HbA1c of >16.6% is needed to ensure a 90% positive predictive value. 56 Lastly, the flexibility and data-driven approach 41 of MFP modeling were particularly suited to our goal of teasing out the complex relationships between adiposity and glucose metabolism indices, HIV status and sex. We are not aware of any previous reports that have described the non-linear relationships observed in our study. These may have been missed hitherto due to reliance on parametric modeling.
Conclusion
In a large sample of people with and without HIV in South Africa, we found glycemic profiles were similar between people with and without HIV. These data call into question whether PWH in the South African context are at increased risk of diabetes. In fact, women living with HIV and obesity in particular had comparatively lower risk of diabetes than women living with obesity without HIV. The mechanisms, physiological or otherwise, conferring this potential protection from diabetes, particularly at greater obesity, must be identified. Future work in South Africa and other HIV-endemic SSA settings should be directed towards better mechanistic understanding of sex-specific adipose tissue biology in HIV, especially with increasing INSTI use and the associated higher odds of weight gain and obesity.
Supplemental Material
Supplemental Material for Associations of HIV and prevalent type 2 diabetes mellitus in the context of obesity in South Africa by Itai M Magodoro, Alison C Castle, Ndumiso Tshuma, Julia Goedecke, Ronel Sewpaul, Justen Manasa, Jennifer Manne-Goehler, Ntobeko Ntusi, Moffat Nyirenda, and Mark Siedner in Journal of Multimorbidity and Comorbidity
Author contributions: IMM, ACC, MJS – conceptualization; IMM - data curation; IMM - formal analysis; IMM, ACC, NT, JHG, RS, JMG, NABN, MJN, MJS – investigation; IMM, ACC, NT, JHG, RS, JMG, NABN, MJN, MJS – methodology; IMM - project administration; validation, IMM - visualization, IMM -writing – original draft; and IMM, ACC, NT, JHG, RS, JMG, NABN, MJN, MJS - writing – review & editing. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: ACC is supported by career development grants from the Fogarty International Center (D43 TW010543) and the National Institute of Allergy and Infectious Diseases (T32 AI007433) of the National Institutes of Health. MJS is supported by the National Institutes of Health (K24 HL166024). NABN gratefully acknowledges funding from the National Research Foundation, South African Medical Research Council, US National Institutes of Health, Medical Research Council (UK), and the Lily and Ernst Hausmann Trust.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Itai M Magodoro https://orcid.org/0000-0002-3879-6126
Data availability statement
All the data reported in this analysis are freely accessible from the Demographic Health Survey (DHS) Program. https://dhsprogram.com/
References
- 1.Bloomfield GS, et al. HIV and non-communicable cardiovascular and pulmonary diseases in low-and middle-income countries in the ART era: what we know and best directions for future research. J. Acquir. Immune Defic. Syndr 1999. 67, S40 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narayan KV, et al. HIV and noncommunicable disease comorbidities in the era of antiretroviral therapy: a vital agenda for research in low-and middle-income country settings. JAIDS J. Acquir. Immune Defic. Syndr 67, S2–S7 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Ntusi NAB, Ntsekhe M. Human immunodeficiency virus‐associated heart failure in sub-Saharan Africa: evolution in the epidemiology, pathophysiology, and clinical manifestations in the antiretroviral era. ESC Heart Fail. 3, 158–167 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah AS, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation 138, 1100–1112 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freiberg MS, et al. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging Cohort Study. JAMA Cardiol. 2, 536–546 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalra S, Agrawal N. Diabetes and HIV: current understanding and future perspectives. Curr. Diab. Rep 13, 419–427 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Benjamin LA, et al. HIV infection and stroke: current perspectives and future directions. Lancet Neurol. 11, 878–890 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galli L, et al. Risk of type 2 diabetes among HIV-infected and healthy subjects in Italy. Eur. J. Epidemiol 27, 657–665 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Hanttu A, et al. Prevalence of obesity and disturbances in glucose homeostasis in HIV-infected subjects and general population–missed diagnoses of diabetes? HIV Med. 22, 244–253 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez-Romieu AC, Garg S, Rosenberg ES, et al. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009–2010. BMJ Open Diabetes Res Care 5, e000304 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samad F, et al. Incidence of diabetes mellitus and factors associated with its development in HIV-positive patients over the age of 50. BMJ Open Diabetes Res Care 5, e000457 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasmussen LD, et al. Risk of diabetes mellitus in persons with and without HIV: a Danish nationwide population-based cohort study. (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nansseu JR, Bigna JJ, Kaze AD, et al. Incidence and risk factors for prediabetes and diabetes mellitus among HIV-infected adults on antiretroviral therapy: a systematic review and meta-analysis. Epidemiology 29, 431–441 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Joint United Nations Programme on HIV/AIDS . Chronic care of HIV and noncommunicable diseases: how to leverage the HIV experience. (2011). [Google Scholar]
- 15.Smit M, et al. The growing burden of noncommunicable disease among persons living with HIV in Zimbabwe. AIDS Lond. Engl 32, 773 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeremiah K, et al. Diabetes prevalence by HbA1c and oral glucose tolerance test among HIV-infected and uninfected Tanzanian adults. PloS One 15, e0230723 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magodoro IM, et al. Association between HIV and Prevalent Hypertension and Diabetes Mellitus in South Africa: Analysis of a Nationally Representative Cross-Sectional Survey. Int. J. Infect. Dis 121, 217–225 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prioreschi A, et al. Incidence and prevalence of type 2 diabetes mellitus with HIV infection in Africa: a systematic review and meta-analysis. BMJ Open 7, e013953 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peer N, et al. Prevalence and influences of diabetes and prediabetes among adults living with HIV in Africa: a systematic review and meta-analysis. J. Int. AIDS Soc 26, e26059 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brennan AT, et al. Gaps in the type 2 diabetes care cascade: a national perspective using South Africa’s National Health Laboratory Service (NHLS) database. BMC Health Serv. Res 23, 1452 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okello S, et al. Prevention of cardiovascular disease among people living with HIV in sub-Saharan Africa. Prog. Cardiovasc. Dis 63, 149–159 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grinspoon SK, et al. Pitavastatin to Prevent Cardiovascular Disease in HIV Infection. N. Engl. J. Med 389, 687–699 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kibirige D, et al. Understanding the manifestation of diabetes in sub Saharan Africa to inform therapeutic approaches and preventive strategies: a narrative review. Clin. Diabetes Endocrinol. 5, 1–8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogurtsova K, et al. IDF diabetes Atlas: Global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res. Clin. Pract 183, 109118 (2022). [DOI] [PubMed] [Google Scholar]
- 25.Johnson AMF., Olefsky J. M. The Origins and Drivers of Insulin Resistance. Cell 152, 673–684 (2013). [DOI] [PubMed] [Google Scholar]
- 26.de Mello AH, Costa AB, Engel JDG, et al. Mitochondrial dysfunction in obesity. Life Sci. 192, 26–32 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Inaishi J., Saisho Y. Beta-cell mass in obesity and type 2 diabetes, and its relation to pancreas fat: a mini-review. Nutrients 12, 3846 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 46, 3–19 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell 152, 673–684 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Willig AL, Overton ET. Metabolic complications and glucose metabolism in HIV infection: a review of the evidence. Curr. HIV/AIDS Rep 13, 289–296 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hruz PW. Molecular mechanisms for insulin resistance in treated HIV-infection. Best Pract. Res. Clin. Endocrinol. Metab 25, 459–468 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S, Samaras K. The impact of weight gain during HIV treatment on risk of pre-diabetes, diabetes mellitus, cardiovascular disease, and mortality. Front. Endocrinol 9, 705 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dulloo AG, Jacquet J, Seydoux J, et al. The thrifty ‘catch-up fat’phenotype: its impact on insulin sensitivity during growth trajectories to obesity and metabolic syndrome. Int. J. Obes 30, S23–S35 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Moyo-Chilufya M, et al. The burden of non-communicable diseases among people living with HIV in Sub-Saharan Africa: a systematic review and meta-analysis. Eclinicalmedicine 65, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Von Elm E, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. The Lancet 370, 1453–1457 (2007). [DOI] [PubMed] [Google Scholar]
- 36.National Department of Health (NDoH) , Statistics South Africa (Stats SA), South African Medical Research Council (SAMRC), and ICF. South Africa Demographic and Health Survey 2016 Key Findings. (2018). [Google Scholar]
- 37.Amod A. The society for endocrinology, metabolism and diabetes of South Africa type 2 diabetes guidelines expert committee. The 2017 SEMDSA guideline for the management of type 2 diabetes guideline committee. J. Endocrinol. Metab. Diabetes South Afr. 21, (2017). [Google Scholar]
- 38.Matsha T, et al. Derivation and validation of a waist circumference optimal cutoff for diagnosing metabolic syndrome in a South African mixed ancestry population. Int. J. Cardiol 168, 2954–2955 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Ware L, et al. Evaluation of waist-to-height ratio to predict 5 year cardiometabolic risk in sub-Saharan African adults. Nutr. Metab. Cardiovasc. Dis 24, 900–907 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Rutstein SO. Steps to constructing the new DHS Wealth Index. Rockv. MD ICF Int 542, (2015). [Google Scholar]
- 41.Binder H, Sauerbrei W, Royston P. Comparison between splines and fractional polynomials for multivariable model building with continuous covariates: a simulation study with continuous response. Stat. Med 32, 2262–2277 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Artasensi A, Mazzolari A, Pedretti A, et al. Obesity and Type 2 Diabetes: Adiposopathy as a Triggering Factor and Therapeutic Options. Molecules 28, 3094 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lagathu C, et al. Basic science and pathogenesis of ageing with HIV: potential mechanisms and biomarkers. AIDS 31, S105–S119 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Lake JE. The Fat of the Matter: Obesity and Visceral Adiposity in Treated HIV Infection. Curr. HIV/AIDS Rep 14, 211–219 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shlay JC, et al. Long-Term Body Composition and Metabolic Changes in Antiretroviral Naive Persons Randomized to Protease Inhibitor-, Nonnucleoside Reverse Transcriptase Inhibitor-, or Protease Inhibitor Plus Nonnucleoside Reverse Transcriptase Inhibitor-Based Strategy. JAIDS J. Acquir. Immune Defic. Syndr 44, 506–517 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Goedecke JH, et al. Effect of Different Antiretroviral Drug Regimens on Body Fat Distribution of HIV-Infected South African Women. AIDS Res. Hum. Retroviruses 29, 557–563 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Abrahams Z, Levitt N, Lesosky M, et al. Changes in Body Fat Distribution on Dual-Energy X-Ray Absorptiometry in Black South Africans Starting First-Line Antiretroviral Therapy. AIDS Patient Care STDs 30, 455–462 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Brennan AT, et al. Change in body weight and risk of hypertension after switching from efavirenz to dolutegravir in adults living with HIV: evidence from routine care in Johannesburg, South Africa. eClinicalMedicine 57, 101836 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tovar Sanchez T, et al. Risks of metabolic syndrome in the ADVANCE and NAMSAL trials. Front. Reprod. Health 5, 1133556 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Namara D, et al. The risk of hyperglycemia associated with use of dolutegravir among adults living with HIV in Kampala, Uganda: A case-control study. Int. J. STD AIDS 33, 1158–1164 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goedecke JH, Mendham AE. Pathophysiology of type 2 diabetes in sub-Saharan Africans. Diabetologia 65, 1967–1980 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Confronting Inequalities: Global AIDS Update 2021. https://www.unaids.org/sites/default/files/media_asset/2021-global-aids-update_en.pdf (2021).
- 53.Nguyen KA, et al. Glycated haemoglobin threshold for dysglycaemia screening, and application to metabolic syndrome diagnosis in HIV-infected Africans. PLOS ONE 14, e0211483 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eckhardt BJ, Holzman RS, Kwan CK, et al. Glycated Hemoglobin A1c as Screening for Diabetes Mellitus in HIV-Infected Individuals. AIDS Patient Care STDs 26, 197–201 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slama L, et al. Inaccuracy of haemoglobin A1c among HIV-infected men: effects of CD4 cell count, antiretroviral therapies and haematological parameters. J. Antimicrob. Chemother 69, 3360–3367 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olivier S, et al. Pitfalls of Single Measurement Screening for Diabetes and Hypertension in Community-Based Settings. Glob. Heart 16, 79 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Associations of HIV and prevalent type 2 diabetes mellitus in the context of obesity in South Africa by Itai M Magodoro, Alison C Castle, Ndumiso Tshuma, Julia Goedecke, Ronel Sewpaul, Justen Manasa, Jennifer Manne-Goehler, Ntobeko Ntusi, Moffat Nyirenda, and Mark Siedner in Journal of Multimorbidity and Comorbidity
Data Availability Statement
All the data reported in this analysis are freely accessible from the Demographic Health Survey (DHS) Program. https://dhsprogram.com/