Abstract
Background:
This study assessed changes in actigraphy-estimated sleep and glycemic outcomes after initiating automated insulin delivery (AID).
Methods:
Ten adults with long-standing type 1 diabetes and impaired awareness of hypoglycemia (IAH) participated in an 18-month clinical trial assessing an AID intervention on hypoglycemia and counter-regulatory mechanisms. Data from eight participants (median age = 58 years) with concurrent wrist actigraph and continuous glucose monitoring (CGM) data were used in the present analyses. Actigraphs and CGM measured sleep and glycemic control at baseline (one week) and months 3, 6, 9, 12, 15, and 18 (three weeks) following AID initiation. HypoCount software integrated actigraphy with CGM data to separate wake and sleep-associated glycemic measures. Paired sample t-tests and Cohen’s d effect sizes modeled changes and their magnitude in sleep, glycemic control, IAH (Clarke score), hypoglycemia severity (HYPO score), hypoglycemia exposure (CGM), and glycemic variability (lability index [LI]; CGM coefficient-of-variation [CV]) from baseline to 18 months.
Results:
Sleep improved from baseline to 18 months (shorter sleep latency [P < .05, d = 1.74], later sleep offset [P < .05, d = 0.90], less wake after sleep onset [P < .01, d = 1.43]). Later sleep onset (d = 0.74) and sleep midpoint (d = 0.77) showed medium effect sizes. Sleep improvements were evident from 12 to 15 months after AID initiation and were preceded by improved hypoglycemia awareness (Clarke score [d = 1.18]), reduced hypoglycemia severity (HYPO score [d = 2.13]), reduced sleep-associated hypoglycemia (percent time glucose was < 54 mg/dL, < 60 mg/dL,< 70 mg/dL; d = 0.66-0.81), and reduced glucose variability (LI, d = 0.86; CV, d = 0.62).
Conclusion:
AID improved sleep initiation and maintenance. Improved awareness of hypoglycemia, reduced hypoglycemia severity, hypoglycemia exposure, and glucose variability preceded sleep improvements.
This trial is registered with ClinicalTrials.gov NCT03215914 https://clinicaltrials.gov/ct2/show/NCT03215914.
Keywords: circadian, diabetes therapeutic technologies, sleep, type 1 diabetes, hypoglycemia, insulin delivery
Introduction
Improved glycemic outcomes are a driving force for the increased use of diabetes therapeutic technology in individuals with type 1 diabetes.1-4 Recurrent exposure to hypoglycemia experienced in the context of periods of therapeutic insulin excess impairs epinephrine and autonomic symptom responses to hypoglycemia leading to defective glucose counter-regulation and impaired awareness of hypoglycemia (IAH), collectively recognized as the syndrome of hypoglycemia-associated autonomic failure (HAAF).5,6 Nocturnal hypoglycemia is implicated in the development of HAAF as sympathetic nervous system responses to hypoglycemia are further diminished during sleep 7 that itself may impair symptom and wakening responses.8,9 Technologies that mitigate nocturnal hypoglycemia are important for adults with long-standing type 1 diabetes complicated by IAH because mitigating nocturnal hypoglycemia is critical to break the cycle of HAAF that leads to life-threatening hypoglycemia.6,10,11 Improved overnight glycemic control is a reported benefit of automated insulin delivery (AID) with integrated continuous glucose monitoring (CGM).12,13 AID adjusts insulin delivery using a predictive low glucose suspension of insulin delivery for anticipated hypoglycemia and a hybrid closed-loop (HCL) increase of basal and/or bolus insulin delivery for hyperglycemia. 14 However, the glycemic improvements achieved using AID are juxtaposed with high rates of discontinuation. Up to 50% of AID users discontinue device use three to six months after initiation,15-18 and 31% of adult AID users never initiate the HCL feature. 19 Drawing from the CGM literature, only about one-third of adults with long-standing type 1 diabetes complicated by IAH continued using CGM one year later, despite glycemic improvements. 20
Myriad reasons have been identified for discontinuing AID and other diabetes technologies, including disrupted sleep.18,21-25 Unsolicited sleep disruption themes emerge in qualitative studies among adolescents and adults with type 1 diabetes suggesting the relevance of sleep disruption across the lifespan. 21 Disrupted sleep may be of particular concern for older adults with type 1 diabetes due to age-related declines in stage N3 sleep and associated increases in nocturnal arousals. 26 Disrupted sleep has also been reported by caretakers and bed partners of persons with type 1 diabetes. 27 All told, disrupted sleep may dampen enthusiasm for AID and other diabetes technology use.
Undisrupted sleep is important for normoglycemia and vice versa. Disrupted sleep (eg, nighttime awakenings) has been linked to greater glucose variability, hyperglycemia, and hypoglycemia.28-30 Difficulty returning to sleep and daytime napping are common following nocturnal hypoglycemia. 31 This body of evidence highlights the importance of targeting normoglycemia overnight to reduce sleep disruptions. Thus, it is reasonable to predict that AID will improve sleep given improved glycemic outcomes, particularly nocturnal glycemia. Yet, evidence is mixed for improvements in sleep after initiating AID.32-34 One explanation for this mixed evidence is that prolonged adjustment periods, perhaps longer than one year, may be needed for individuals with type 1 diabetes to acclimate and to trust the new technology before sleep improvements can be realized. 33 Few, if any studies have followed sleep outcomes in adults with type 1 diabetes for longer than three months after initiating AID. This study addresses these shortcomings by evaluating the impact of initiating an AID paired with CGM on actigraphy-estimated sleep over an 18-month period. We hypothesized that a prolonged adjustment period to AID, perhaps longer than one year, may be needed prior to detecting robust, significant improvements in sleep.
Methods
Study Participants
Participants were recruited between 2017 and 2020 for a parent study that determined whether intervention with AID could achieve clinically important hypoglycemia avoidance hypothesized to reverse defective glucose counter-regulation and improve hypoglycemia symptom recognition in long-standing type 1 diabetes complicated by IAH. 35 Recruitment strategies have been described previously. 13 Adults (25-70 years) were eligible if they met the following inclusion criteria: C-peptide negative type 1 diabetes diagnosed prior to 40 years of age, present for a > 10-year duration, and maintaining intensive diabetes management (multiple-dose insulin injections or continuous subcutaneous insulin infusion with > 3 times/day self-blood glucose monitoring with or without CGM and ≥3 clinic evaluations with an endocrinologist or diabetes nurse practitioner during the previous 12 months), IAH as determined by a Clarke score ≥ 4, and hypoglycemia severity (HYPO score) ≥90th percentile or both a HYPO score ≥ 75th percentile and a glycemic lability index (LI) ≥75th, confirmed hypoglycemia exposure that was determined by sensor glucose levels <60 mg/dL >5% and at least one episode of nocturnal hypoglycemia during seven days of CGM. Exclusion criteria and additional details are available at ClinicalTrials.gov (NCT03215914).
Study Procedures
Participants provided written informed consent prior to any study procedures and underwent a multistage screening process to determine eligibility for the single arm intervention study. Participants wore a blinded CGM (iPro 2) or their current CGM for eligibility determination. Retained participants were required to demonstrate tolerability and compliance with using AID (MiniMed 670G, Medtronic Diabetes, Northridge, CA; t:slimX2, Tandem Diabetes, San Diego, CA) prior to the intervention during a two-week run-in monitoring period while using a wrist actigraph (Actigraph GT3X) and the CGM without automated features. Participants meeting all eligibility criteria and confirming tolerability and compliance were trained on using the automated features of either the MiniMed 670G (n = 7) or the Tandem t-slim system (n = 1). The participant using the Tandem t-slim used the sleep mode program the majority of the nights throughout the study period. Participants transitioned to the intervention phase after one week during which the insulin pump was placed in automated mode. Participants returned for monthly visits during the first six months of the intervention and every three months thereafter (months 9, 12, 15, and 18) to review the CGM and insulin delivery data. Participants wore a wrist actigraph for at least one week during the baseline screening period and for at least two weeks every three months after initiating AID (months 3, 6, 9, 12, 15, and 18). Glucose levels were measured concurrently using a CGM system.
Data Collection and Measures
Sleep
Sleep was estimated from data collected using a well-validated wrist actigraph (Actigraph wGT3X-BT).13,36 Wrist movements were recorded at a sample rate of 30 Hz and data were downloaded using the ActiLife software (version 6.13.3). Several sleep dimensions were estimated, including duration, onset, midpoint, efficiency, and regularity. Actigraphy has been demonstrated to be robustly stable within people over time. 37
Glycemic control changes over time
Hemoglobin A1c (HbA1c) provided a two- to three-month average of pre- and postprandial glucose levels 38 and were measured at the 3, 6, 9, 12, 15, and 18-month visits from whole blood samples using high-performance liquid chromatography (Primus CLC330; Tosoh A1c 2.2 Plus).
CGM estimated interstitial glucose every ten seconds using an electrochemical subcutaneous sensor. Interstitial glucose estimates were averaged every five minutes. CGM data were used to calculate mean sensor glucose, glucose coefficient-of-variation (CV), and the percentage of time sensor glucose was below range (< 54 mg/dL,< 60 mg/dL,< 70 mg/dL), in range (70-180 mg/dL), and above range (> 180, > 250 mg/dL) using HypoCount software (version 2.0; PRECISE Center, University of Pennsylvania, Philadelphia, PA). This software enabled integration of wrist actigraphy and CGM data to objectively separate wake and sleep-associated periods based on sleep onset and offset. CGM sensor accuracy was assessed at each study visit. 39
IAH, hypoglycemia severity, and glycemic lability
IAH was assessed using the Clarke score that was derived from a reliable and valid eight-item survey. 40 This survey queries participants about the frequency of hypoglycemic episodes in the past month and year and participants symptomatic responses to hypoglycemia. 40 The Clarke score has been validated as an accurate predictor of hypoglycemia symptom recognition during hypoglycemic clamp testing. 41
The frequency and severity of clinically important hypoglycemia was assessed using hypoglycemia severity (HYPO score). The HYPO score was calculated by combining participants’ recollection of hypoglycemic episodes requiring assistance to recognize or treat the episode over the previous year with prospective data of symptoms and assistance associated with clinically important hypoglycemia (< 54 mg/dL) events from four-week blood glucose records. HYPO scores ≥ 1047 indicate severe hypoglycemic problems; HYPO scores between 423 and 1046 indicate moderate hypoglycemia problems; HYPO scores ≤ 423 indicate no hypoglycemia problems. 42
Changes in glucose over time were estimated using the LI. 42 The LI was calculated from glucose records using the formula described by Ryan et al. 42 Higher LI scores indicate less stable glucose levels with LI > 433 indicating severe glycemic lability. 42 Consistency of repeated measures of HYPO score and LI for use in longitudinal studies has previously been established. 43
Statistical Analyses
Ten participants with long-standing type 1 diabetes and IAH participated in this 18-month clinical trial. Two participants were excluded from the present analyses because they did not wear the wrist actigraph at month 18. Data from eight participants providing complete data from concurrently worn wrist actigraphs and CGM were included in the present analyses. Data are presented as means ± standard deviations (SDs) or medians and interquartile ranges (IQRs). Paired sample t-tests were used to assess changes in means for sleep metrics from baseline to 18 months within each individual. Cohen’s d effect sizes were used to estimate the magnitude of change from baseline to 18 months using the following ranges: ≥ 0.2 small, ≥ 0.5 medium, and ≥ 0.8 large. 44
Results
Participants were a median age of 57.5 (IQR = 38-63) years. Most participants were non-Hispanic (n = 7, 87.5%), White (n = 7, 87.5%), females (n = 5, 62.5%) with a median duration of type 1 diabetes of 41.0 (IQR = 24.5-47.5) years. The mean body mass index was in the healthy range (23.95 ± 1.19 kg/m2).
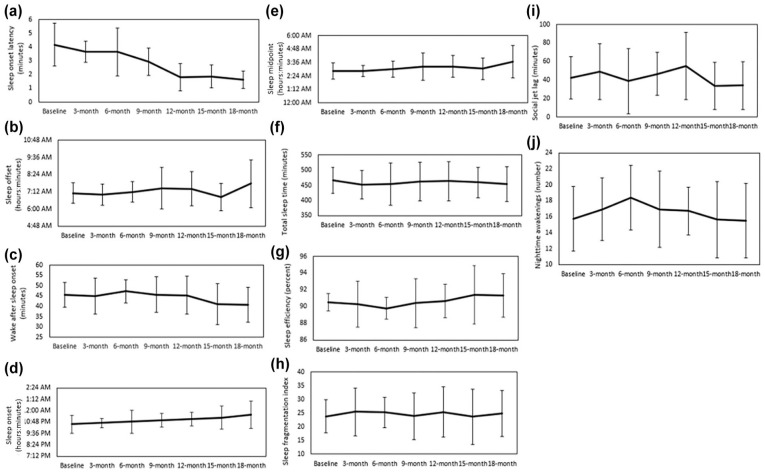
There were statistically significant changes in several actigraphy-estimated sleep characteristics from baseline to 18 months. Statistically significant shorter sleep onset latency indicated improved sleep initiation (P < .05; d = 1.74, Figure 1a) and statistically significant later sleep offset and less wake after sleep onset (WASO) indicated improved sleep maintenance (P < .05; d = 0.90, Figure 1b; P < .01; d = 1.43, Figure 1c, respectively). There was a moderate magnitude of change for the time of sleep onset and sleep midpoint, a proxy of circadian phase, indicating a shift toward later sleep times (d = 0.74, Figure 1d; d = 0.77; Figure 1e, respectively). There were no statistically significant changes in other actigraphy-estimated sleep characteristics, including total sleep time, sleep efficiency, sleep fragmentation index, and social jet lag (the difference in sleep midpoints between free and work days), 45 or in the number of nighttime awakenings from baseline to 18 months (Figure 1f-j).
Figure 1.
Changes from baseline to 18 months for actigraphy-estimated (n = 8): (a) Sleep onset latency (t = −4.93, P < .05, d = 1.74), (b) sleep offset (t = −2.58, P < .05, d = 0.90), (c) wake after sleep onset (WASO; t = 4.05, P < .01, d = 1.43), (d) sleep onset (t =2.10, d = 0.47), (e) sleep midpoint (t = 2.17, d = 0.77), (f) total sleep time (t = −0.43, d = 0.15), (g) sleep efficiency (t = 0.97, d = 0.34), (h) sleep fragmentation index (t = 0.73, d = 0.26), (i) social jet lag (t = −0.62, d = 0.22), and (j) nighttime awakenings (t = 0.13, d = 0.05). All figures show mean ± SD.
As already reported, 35 there were improvements in awareness of hypoglycemia and glucose control from baseline to 18 months. In the current study analysis, lower Clarke scores reflected improved awareness of hypoglycemia (d = 1.18). Lower HYPO scores indicated reduced hypoglycemia severity (d = 2.13), and lower LI reflected more stable glucose levels (d = 0.86). There were also several changes in glucose control from baseline to end-of-treatment that were of moderate and large effect sizes. There was a large magnitude of change for the percentage of time glucose was < 70 mg/dL during the sleep-associated period (d = 0.79). There was a moderate magnitude of change for the percentage of time glucose was in target range (d = 0.57), for the percentage of time glucose was < 70 mg/dL during the wake period (d = 0.46), for the percentage of time glucose was < 70 mg/dL during the sleep and wake period (d = 0.72), and for the CV (d = 0.65). These changes indicated less overall and in particular less sleep-associated hypoglycemia, as well as lower glucose variability. Moreover, AID led to an improved epinephrine response and partial recovery of glucose counter-regulation as evidenced by reduced peripheral glucose utilization during the hyperinsulinemic hypoglycemic clamp experiments, as well as an improved autonomic symptom response evidencing improved hypoglycemia symptom recognition. 35 There were no statistically significant changes in HbA1c, nor in mean or time-in-range sensor glucose levels from baseline to 18 months (Table 1).
Table 1.
Glycemic Characteristics at Baseline and at 18 Months After Initiating a Hybrid Closed Loop Insulin Delivery System and the Change in Values From Baseline to 18 Months (n = 8).
| Glycemic characteristic | Baseline, mean (SD) | Baseline, median (IQR) | 18 months, mean (SD) | 18 months, median (IQR) | t-test (df), baseline to 18 months | Effect size (d) a |
|---|---|---|---|---|---|---|
| HbA1c (%) | 7.05 (1.21) | 7.00 (6.40-7.25) | 7.21 (0.78) | 7.20 (6.90-7.85) | −0.44 (7) | 0.16 |
| Mean sensor glucose (mg/dL) | 147.69 (21.60) | 114.82 (129.45-165.18) | 151.64 (26.44) | 145.90 (136.69–165.58) | −0.64 (7) | 0.23 |
| Percentage of time sensor glucose was in range | ||||||
| 70-180 mg/dL | 55.89 (18.9) | 57.59 (47.95-64.67) | 65.27 (21.95) | 70.73 (51.37-83.17) | −1.60 (7) | 0.57 |
| Percentage of time sensor glucose was below and above range (daytime and nighttime) as defined by accelerometry estimated sleep-wake period | ||||||
| < 54 mg/dL | 2.38 (2.62) | 1.70 (0.32-3.81) | 0.55 (0.75) | 0.24 (0.15-0.70) | 1.79 (7) | 0.63 |
| < 60 mg/dL | 3.30 (3.13) | 2.82 (0.48-5.93) | 1.05 (1.27) | 0.41 (0.35-1.58) | 1.83 (7) | 0.65 |
| < 70 mg/dL | 6.22 (5.22) | 6.12 (1.08-11.28) | 2.26 (2.27) | 1.17 (0.87-3.55) | 2.05 (7) | 0.72 |
| > 180 mg/dL | 26.19 (13.52) | 27.22 (14.02-36.09) | 25.82 (18.08) | 22.80 (13.73-34.03) | 0.11 (7) | 0.04 |
| > 250 mg/dL | 6.02 (5.75) | 4.43 (2.00-9.13) | 5.05 (5.13) | 3.33 (0.83-9.12) | 0.67 (7) | 0.24 |
| Percentage of time sensor glucose was below and above range during the wake period as defined by accelerometry estimated sleep-wake period | ||||||
| < 54 mg/dL | 1.46 (1.13) | 1.56 (0.49-2.18) | 0.79 (1.07) | 0.35 (0.17-1.13) | 1.24 (7) | 0.44 |
| < 60 mg/dL | 2.35 (1.89) | 2.31 (0.76-3.61) | 1.48 (1.75) | 0.62 (0.14-2.47) | 1.15 (7) | 0.41 |
| < 70 mg/dL | 4.88 (4.12) | 3.52 (1.51-8.28) | 3.09 (3.11) | 1.54 (1.14-5.45) | 1.29 (7) | 0.46 |
| > 180 mg/dL | 26.73 (15.57) | 23.75 (13.57-36.91) | 29.46 (18.28) | 27.35 (14.85-42.09) | −1.09 (7) | 0.38 |
| > 250 mg/dL | 5.59 (5.18) | 4.47 (1.44-9.25) | 7.07 (7.50) | 4.21 (1.23-11.77) | −1.06 (7) | 0.37 |
| Percentage of time sensor glucose was below and above range during the sleep-associated period as defined by accelerometry estimated sleep-wake period | ||||||
| < 54 mg/dL | 4.12 (6.15) | 1.05 (0-7.03) | 0.09 (0.19) | 0 (0-0.09) | 1.85 (7) | 0.65 |
| < 60 mg/dL | 5.03 (6.2) | 2.43 (0-9.06) | 0.25 (0.55) | 0 (0-0.19) | 2.01 (7) | 0.71 |
| < 70 mg/dL | 8.41 (9.80) | 4.35 (0.38-16.04) | 0.68 (1.29) | 0.09 (0.03-0.75) | 2.22 (7) | 0.79 |
| > 180 mg/dL | 25.45 (14.06) | 26.36 (16.44-34.69) | 19.06 (19.84) | 13.13 (5.76-25.88) | 0.99 (7) | 0.35 |
| > 250 mg/dL | 6.99 (8.52) | 4.69 (0-12.49) | 3.68 (6.01) | 1.11 (0-4.97) | 0.91 (7) | 0.32 |
| Coefficient-of-variation | 0.37 (0.09) | 0.36 (0.29-0.42) | 0.31 (0.04) | 0.30 (0.28-0.33) | 1.84 (7) | 0.65 |
| Clarke score | 5.38 (1.19) | 5.00 (4.50-6.05) | 3.38 (2.39) | 4.00 (1.00-5.50) | 3.35* (7) | 1.18 |
| HYPO score b | 1288.13 (642.73) | 1300.50 (901.00-1628.00) | 94.63 (126.72) | 126.72 (0-184.00) | 6.01** (7) | 2.13 |
| Lability index | 362.48 (205.47) | 396.40 (198.00-463.30) | 178.83 (43.63) | 170.90 (141.00-215.30) | 2.43* (7) | 0.86 |
Abbreviations: SD, standard deviation; IQR, interquartile range.
Cohen’s d, *P < .05, **P < .001.
Hypoglycemia severity score.
Discussion
This study evaluated the impact of initiating an AID system on actigraphy-estimated sleep over an 18-month period in adults with long-standing type 1 diabetes complicated by IAH, a group at increased risk for severe, life-threatening hypoglycemia. Actigraphy-estimated improvements in sleep initiation and maintenance, included a shorter sleep onset latency, later sleep offset, and less WASO 18 months after AID system initiation. These changes were first evident 12 to 15 months after AID system initiation. This evidence supports the hypothesis that a prolonged adjustment period to AID may be needed prior to observing robust improvements in sleep and may explain the mixed evidence for sleep improvements following AID system initiation reported by others. 33 There were also several improvements in glycemic control, including improved awareness of hypoglycemia symptoms, less severe hypoglycemia, and more stable glucose levels. A large effect size for reduced sleep-associated hypoglycemia was also found. Glycemic improvements were already evident within the first three to six months of AID system initiation and preceded improvements in sleep.
Reduced nocturnal awakenings are expected in individuals with IAH because of even further diminished counter-regulatory hormone and symptom responses to hypoglycemia during sleep interfering with arousal by hypoglycemia symptoms; these contribute to maintenance of HAAF and its associated defects in glucose counter-regulation and hypoglycemia symptom recognition.7-9 However, reduced nocturnal awakenings in the current study occurred in the context of improved awareness of hypoglycemia, as assessed by both questionnaires and hypoglycemic clamp testing. This may be explained by the near elimination of exposure to hypoglycemia in the sleep-associated period during intervention with AID that suspends insulin delivery for predicted hypoglycemia, and perhaps as well by some protection from developing low blood glucose imparted by the modest improvements in physiologic counter-regulation, 35 although the latter was not tested during periods of sleep. Another factor that may contribute to the observed reduction in nocturnal awakenings may be the reduction in glucose variability. Brandt et al reported that reduced glucose variability was associated with nights of good sleep quality, hence suggestive of reduced nocturnal awakenings in adults with type 1 diabetes. 46
These sleep-associated hypoglycemia reductions and glycemic variability improvements would have also led to fewer nocturnal alarms (percent time < 70 mg/dL = 7.24% baseline], 1.33% [18 months]; CV = 35% [baseline], 31% [18 months], respectively). It is also possible that participants experienced a feeling of safety using the AID system and experienced less hypoglycemia fear, particularly during sleep. 47 Findings from the current study thus support that improvements in hypoglycemia avoidance, as well as in glucose variability may be needed before improvements in sleep can be observed in adults with long-standing type 1 diabetes initiating AID.
The improvements in sleep initiation and maintenance in the current study reflect improvements in sleep quality (eg, fewer sleep disruptions). These improvements in sleep onset, sleep offset, and nocturnal awakenings are in line with some,47-49 but not all33,34,50 evidence that self-reported sleep quality is improved in insulin pump and/or AID system users. A potential reason for the disparate findings is that the current study did not rely on self-reported sleep quality, which can be inaccurate, but rather actigraphy-estimated measures of sleep initiation and sleep maintenance.
Finally, there were no statistically significant changes in several actigraphy-estimated sleep dimensions, such as sleep duration and efficiency. However, it is important to note that participants in this sample had adequate total sleep time (>7 hours), a high sleep efficiency (>90.5%), and <1 hour of social jet lag at baseline (Figure 1). This suggests that there was little room or need for improvement in these dimensions of sleep for this study cohort.
Improvements in Clarke score, HYPO score, LI, and CV were found in this current study and reported previously 35 indicating improved awareness of hypoglycemia, less problematic hypoglycemia, and reduced glucose variability. We have previously reported a moderate effect size for improved IAH, reduced hypoglycemia severity and more stable glucose levels using Clarke scores, HYPO scores and LI, respectively, nine months after AID initiation. 13 The now completed study supports these earlier findings by reporting continued improvements in these measures after the nine-month time point and reaching statistically significant improvements 18 months after AID system initiation. 35 These findings are clinically relevant because IAH is a limiting factor for achieving glycemic goals for many individuals with type 1 diabetes. Improvements in awareness of hypoglycemia open the possibility for achieving glycemic goals without increasing life-threatening hypoglycemia risk.
The large effect size for reduced sleep-associated hypoglycemia 18 months after AID initiation is clinically relevant. This finding is consistent with other reports for decreases in nocturnal hypoglycemia following AID initiation,50-52 and our earlier report of reduced sleep-associated hypoglycemia nine months after AID initiation. 13 A particularly unique contribution of this current study is that the CGM data were integrated with each participant’s actigraphy-estimated sleep data to more precisely separate wake and sleep-associated periods rather than relying on a generic a priori defined nighttime period (eg, 00:00-6:00), which can be misrepresentative due to differences in sleep parameters between individuals as well as night to night variability within individuals. 53 Reducing sleep-associated hypoglycemia is clinically relevant for contributing to the reversal of HAAF because nocturnal hypoglycemia is particularly associated with severe, life-threatening hypoglycemia. 54
The strengths of this study include the 18-month longitudinal design and concurrently estimated objective sleep, glycemic control, and glucose counter-regulatory outcome measures. This study is limited by the small sample size and narrow demographic characteristics of the participants. In addition, diet and exercise can influence glucose fluctuations and these were not systematically controlled for in this study. 55 Future analyses should aim to evaluate sleep quality subjectively and the extent to which daily variations in sleep characteristics predict next-day variations in glucose characteristics and vice versa, 30 as well as the coupling between sleep-wake states with glucose metrics using approaches, such as wavelet coherence analysis. 56
Conclusion
Sleep disruptions are a common barrier for individuals with type 1 diabetes initiating new diabetes technology devices. Insulin pumps, CGMs, and mobile phones are new bed partners for individuals initiating AID therapy and may be a contributing factor for discontinuing AID therapy. The current study’s findings suggest that glycemic improvements precede sleep improvements and that prolonged adjustment periods, perhaps longer than one year, may be needed for individuals with type 1 diabetes to acclimate to the new technology before sleep improvements can be realized.
Acknowledgments
The authors thank the study participants with type 1 diabetes for their contribution, Cornelia Dalton-Bakes of the University of Pennsylvania Institute for Diabetes, Obesity & Metabolism Human Metabolism Resource for providing clinical research and regulatory coordination of the parent clinical trial, and Margaret Evangelisti of the University of Pennsylvania Center for Human Phenomic Science for providing actigraphy support. The authors acknowledge support from the Human Metabolism Resource of the Institute for Diabetes, Obesity, & Metabolism at the University of Pennsylvania. They would like to acknowledge Dr Jason Fletcher for providing additional feedback on analyzing the continuous glucose monitoring data and for his input on this manuscript.
Footnotes
Abbreviations: AID, automated insulin delivery; CGM, continuous glucose monitoring; CV, coefficient-of-variation; HAAF, hypoglycemia-associated autonomic failure; HbA1c, Hemoglobin A1c; HCL, hybrid closed-loop; HYPO, hypoglycemia severity score; IAH, impaired awareness of hypoglycemia; IQR, interquartile range; LI, Lability Index; WASO, wake after sleep onset.
Authorship Confirmation/Contribution Statement: MRR was responsible for the conceptualization, funding, and project administration of the parent study. SJ, JW, and IL were responsible for the software development and supporting algorithms for the parent study. AJP was responsible for conducting the investigation process and together with AJF was responsible for collection of data. NG and SKM were responsible for the conceptualization of the sleep analyses in this manuscript. SKM and AMM were responsible for the preparing the actigraphy data for analyses. GY and LG were responsible for the formal statistical analyses. SKM was responsible for writing the original draft. All authors reviewed the manuscript and approved the final submitted version.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH grants (R01DK117488 [to N.G.], R01DK091331 (to M.R.R.), K99NR017416 (to S.K.M.), and UL 1TR001878 (University of Pennsylvania Center for Human Phenomic Science). Other support was provided by the National Aeronautics and Space Administration (NASA) (NNX14AN49G and 80NSSC20K0243 [to N.G.]) and from the Pennsylvania Department of Health (SAP 4100079750 [to I.L.]), and from the Charles B. Humpton, Jr. Endowed Fellowship in Diabetes Research (to A.J.F.). Medtronic supplied discounted 670G insulin pumps and continuous glucose monitoring devices for the study through investigator-initiated grant NERP16-015 (to M.R.R.).
ORCID iDs: Susan Kohl Malone  https://orcid.org/0000-0001-6861-9377
https://orcid.org/0000-0001-6861-9377
Anneliese J. Flatt  https://orcid.org/0000-0003-4315-5136
https://orcid.org/0000-0003-4315-5136
Sooyong Jang  https://orcid.org/0000-0002-4136-8835
https://orcid.org/0000-0002-4136-8835
Namni Goel  https://orcid.org/0000-0003-2602-1996
https://orcid.org/0000-0003-2602-1996
References
- 1. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21:66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Silva JD, Lepore G, Battelino T, Arrieta A, Castañeda J, Grossman B, et al. Real-world performance of the MiniMed 780G system: first report of outcomes from 4120 users. Diabetes Technol Ther. 2022;24(2):113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Breton MD, Kovatchev BP. One year real-world use of the control-IQ advanced hybrid closed-loop technology. Diabetes Technol Ther. 2021;23:601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stone MP, Agrawal P, Chen X, Liu M, Shin J, Cordero TL, et al. Retrospective analysis of 3-month real-world glucose data after the MiniMed 670G system commercial launch. Diabetes Technol Ther. 2018;20(10):689-692. [DOI] [PubMed] [Google Scholar]
- 5. Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2013;369:362-372. [DOI] [PubMed] [Google Scholar]
- 6. Rickels MR. Hypoglycemia-associated autonomic failure, counterregulatory responses, and therapeutic options in type 1 diabetes. Ann N Y Acad Sci. 2019;1454(1):68-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones TW, Porter P, Sherwin RS, et al. Decreased epinephrine responses to hypoglycemia during sleep. New England Journal of Medicine. 1998;338:1657-1662. [DOI] [PubMed] [Google Scholar]
- 8. Banarer S, Cryer PE. Sleep-related hypoglycemia-associated autonomic failure in type 1 diabetes: reduced awakening from sleep during hypoglycemia. Diabetes. 2003;52(5):1195-1203. [DOI] [PubMed] [Google Scholar]
- 9. Schultes B, Jauch-Chara K, Gais S, et al. Defective awakening response to nocturnal hypoglycemia in patients with type 1 diabetes mellitus. Plos Med. 2007;4(2):e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The DCCT Research Group. Epidemiology of severe hypoglycemia in the diabetes control and complications trial. Am J Med. 1990;90:450-459. [PubMed] [Google Scholar]
- 11. Nathan DM, DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381:1707-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malone SK, Peleckis AJ, Grunin L, et al. Characterizing glycemic control and sleep in adults with long-standing type 1 diabetes and hypoglycemia unawareness initiating hybrid closed loop insulin delivery. J Diabet Res. 2021;2021:6611064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Infante M, Baidal DA, Rickels MR, et al. Dual-hormone artificial pancreas for management of type 1 diabetes: recent progress and future directions. Artif Organs. 2021;45(9):968-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berget C, Messer LH, Vigers T, Frohnert BI, Pyle L, Wadwa RP, et al. Six months of hybrid closed loop in the real-world: an evaluation of children and young adults using the 670G system. Pediatr Diabetes. 2020;21(2):310-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lal RA, Basina M, Maahs DM, Hood K, Buckingham B, Wilson DM. One year clinical experience of the first commercial hybrid closed-loop system. Diabetes Care. 2019;42(12):2190-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berget C, Akturk HK, Messer LH, et al. Real-world performance of hybrid closed loop in youth, young adults, adults and older adults with type 1 diabetes: identifying a clinical target for hybrid closed-loop use. Diabetes Obes Metab. 2021;23(9):2048-2057. [DOI] [PubMed] [Google Scholar]
- 18. Messer LH, Berget C, Vigers T, et al. Real world hybrid closed-loop discontinuation: predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes. 2020;21(2):319-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ju Z, Piarulli A, Bielick L, Marschall S, Brouillard E, Steenkamp D. Advanced diabetes technology remains underutilized in underserved populations: early hybrid closed-loop system experience at an academic safety net hospital. Diabetes Technol Ther. 2022;24(2):143-147. [DOI] [PubMed] [Google Scholar]
- 20. Flatt AJS, Little SA, Speight J, et al. Predictors of recurrent severe hypoglycemia in adults with type 1 diabetes and impaired awareness of hypoglycemia during the HypoCOMPaSS study. Diabetes Care. 2020;43(1):44-52. [DOI] [PubMed] [Google Scholar]
- 21. Carreon SA, Cao VT, Anderson BJ, Thompson DI, Marrero DG, Hilliard ME. ‘I don’t sleep through the night’: qualitative study of sleep in type 1 diabetes. Diabet Med. 2022;39(5):e14763. [DOI] [PubMed] [Google Scholar]
- 22. Messer LH, Johnson R, Driscoll KA, Jones J. Best friend or spy: a qualitative meta-synthesis on the impact of continuous glucose monitoring on life with Type 1 diabetes. Diabet Med. 2018;35:409-418. [DOI] [PubMed] [Google Scholar]
- 23. Barnard K, Crabtree V, Adolfsson P, et al. Impact of type 1 diabetes technology on family members/significant others of people with diabetes. J Diabetes Sci Technol. 2016;10(4):824-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Monzon AD, Marker AM, Noser AE, Clements MA, Patton SR. Associations between objective sleep behaviors and blood glucose variability in young children with type 1 diabetes. Ann Behav Med. 2021;55:144-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nefs G. The psychological implications of automated insulin delivery systems in type 1 diabetes care. Front Clin Diabetes Healthc. 2022;3:846162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carskadon MA, Dement WC. Normal human sleep: an overview. In: Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th ed. Amsterdam: Elsevier; 2017:15-24. [Google Scholar]
- 27. Jaser SS, Foster NC, Nelson BA, et al. Sleep in children with type 1 diabetes and their parents in the T1D Exchange. Sleep Med. 2017;39:108-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Griggs S, Strohl KP, Grey M, Barbato E, Margevicius S, Hickman RL., Jr. Circadian characteristics of the rest-activity rhythm, executive function, and glucose fluctuations in young adults with type 1 diabetes. Chronobiol Int. 2021;38(10):1477-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reutrakul S, Thakkinstian A, Anothaisintawee T, et al. Sleep characteristics in type 1 diabetes and associations with glycemic control: systematic review and meta-analysis. Sleep Med. 2016;23:26-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Griggs S, Redeker NS, Jeon S, Grey M. Daily variations in sleep and glucose in adolescents with type 1 diabetes. Pediatr Diabetes. 2020;21(8):1493-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fulcher G, Singer J, Castañeda R, et al. The psychosocial and financial impact of non-severe hypoglycemic events on people with diabetes: two international surveys. J Med Econ. 2014;17(10):751-761. [DOI] [PubMed] [Google Scholar]
- 32. Wheeler BJ, Collyns OJ, Meier RA, et al. Improved technology satisfaction and sleep quality with Medtronic MiniMed(R) Advanced Hybrid Closed-Loop delivery compared to predictive low glucose suspend in people with Type 1 Diabetes in a randomized crossover trial. Acta Diabetol. 2022;59:31-37. [DOI] [PubMed] [Google Scholar]
- 33. Cobry EC, Hamburger E, Jaser SS. Impact of the hybrid closed-loop system on sleep and quality of life in youth with type 1 diabetes and their parents. Diabetes Technol Ther. 2020;22(11):794-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bisio A, Gonder-Frederick L, McFadden R, et al. The impact of a recently approved automated insulin delivery system on glycemic, sleep, and psychosocial outcomes in older adults with type 1 diabetes: a pilot study. J Diabetes Sci Technol. 2022;16(3):663-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Flatt AJ, Peleckis AJ, Dalton-Bakes C, et al. Automated insulin delivery for hypoglycemia avoidance and glucose counterregulation in long-standing type 1 diabetes with hypoglycemia unawareness. Diabetes Technol Ther. 2023;25:302-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342-392. [DOI] [PubMed] [Google Scholar]
- 37. McHill AW, Sano A, Hilditch CJ, et al. Robust stability of melatonin circadian phase, sleep metrics, and chronotype across months in young adults living in real-world settings. J Pineal Res. 2021;70(3):e12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(suppl 1):S62-S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mastrototaro J, Shin J, Marcus A, Sulur G; STAR 1 Clinical Trial Investigators. The accuracy and efficacy of real-time continuous glucose monitoring sensor in patients with type 1 diabetes. Diabetes Technol Ther. 2008;10(5):385-390. [DOI] [PubMed] [Google Scholar]
- 40. Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM: a prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18(4):517-522. [DOI] [PubMed] [Google Scholar]
- 41. Flatt AJ, Chen E, Peleckis AJ, et al. Evaluation of clinical metrics for identifying defective physiologic responses to hypoglycemia in long-standing type 1 diabetes. Diabetes Technol Ther. 2022;24(10):737-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ryan EA, Shandro T, Green K, et al. Assessment of the severity of hypoglycemia and glycemic lability in type 1 diabetic subjects undergoing islet transplantation. Diabetes. 2004;53(4):955-962. [DOI] [PubMed] [Google Scholar]
- 43. Senior PA, Bellin MD, Alejandro R, et al. Consistency of quantitative scores of hypoglycemia severity and glycemic lability and comparison with continuous glucose monitoring system measures in long-standing type 1 diabetes. Diabetes Technol Ther. 2015;17(4):235-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, MI: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 45. Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23(1-2):497-509. [DOI] [PubMed] [Google Scholar]
- 46. Brandt R, Park M, Wroblewski K, Quinn L, Tasali E, Cinar A. Sleep quality and glycaemic variability in a real-life setting in adults with type 1 diabetes. Diabetologia. 2021;64(10):2159-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Beato-Víbora PI, Gallego-Gamero F, Lázaro-Martín L, Romero-Pérez MDM, Arroyo-Díez FJ. Prospective analysis of the impact of commercialized hybrid closed-loop system on glycemic control, glycemic variability, and patient-related outcomes in children and adults: a focus on superiority over predictive low-glucose suspend technology. Diabetes Technol Ther. 2020;22(12):912-919. [DOI] [PubMed] [Google Scholar]
- 48. Jaser SS, Ellis D. Sleep in adolescents and young adults with type 1 diabetes: associations with diabetes management and glycemic control. Health Psychol Behav Med. 2016;4:49-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pinsker JE, Müller L, Constantin A, et al. Real-world patient-reported outcomes and glycemic results with initiation of control-IQ technology. Diabetes Technol Ther. 2021;23(2):120-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chakrabarti A, Trawley S, Kubilay E, et al. Closed-loop insulin delivery effects on glycemia during sleep and sleep quality in older adults with type 1 diabetes: results from the ORACL trial. Diabetes Technol Ther. 2022;24(9):666-671. [DOI] [PubMed] [Google Scholar]
- 51. Bally L, Thabit H, Kojzar H, et al. Day-and-night glycaemic control with closed-loop insulin delivery versus conventional insulin pump therapy in free-living adults with well controlled type 1 diabetes: an open-label, randomised, crossover study. Lancet Diabetes Endocrinol. 2017;5(4):261-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ruan Y, Bally L, Thabit H, et al. Hypoglycaemia incidence and recovery during home use of hybrid closed-loop insulin delivery in adults with type 1 diabetes. Diabetes Obes Metab. 2018;20(8):2004-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dillon HR, Lichstein KL, Dautovich ND, Taylor DJ, Riedel BW, Bush AJ. Variability in self-reported normal sleep across the adult age span. J Gerontol B Psychol Sci Soc Sci. 2015;70(1):46-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tanenberg RJ, Newton CA, Drake AJ. Confirmation of hypoglycemia in the “dead-in-bed” syndrome, as captured by a retrospective continuous glucose monitoring system. Endocr Pract. 2010;16(2):244-248. [DOI] [PubMed] [Google Scholar]
- 55. Schmidt S, Christensen MB, Serifovski N, et al. Low versus high carbohydrate diet in type 1 diabetes: a 12-week randomized open-label crossover study. Diabetes Obes Metab. 2019;21(7):1680-1688. [DOI] [PubMed] [Google Scholar]
- 56. Griggs S, Barbato E, Hernandez E, et al. Glucose and unstructured physical activity coupling during sleep and wake in young adults with type 1 diabetes. Sci Rep. 2022;12:5790. [DOI] [PMC free article] [PubMed] [Google Scholar]