Abstract
Background:
Independent use of artificial intelligence with computer-aided detection (CADe) and Endocuff Vision (ECV) has demonstrated enhanced adenoma detection rates (ADRs).
Objective:
Our pilot study aimed to define the necessary participant number for future randomized controlled trials (RCTs) by comparing the ADR of combined CADe + ECV against CADe alone and standard colonoscopy.
Design:
This single-center pilot study retrospectively analyzed a prospectively maintained database, where patients underwent screening colonoscopies sequentially by standard method, CADe alone, and then CADe + ECV.
Method:
The allocation of the technique depended on the study period. Patients were randomly selected from the cohort to form three groups of 30 patients, with stratification based on factors influencing the ADR. The primary endpoint was the ADR.
Results:
From April to June 2021, 244 patients underwent screening colonoscopy. 198 were eligible, and after randomization, 90 patients were included across three groups (colonoscopy n = 30, CADe n = 30, CADe + ECV = 30). The ADR was higher in the CADe + ECV group compared to the CADe and colonoscopy groups: 60% versus 40%, and 30%, respectively (p = 0.03). The number of polyps ⩽3 mm detected was greater in the CADe + ECV group (n = 23) versus CADe (n = 7) and colonoscopy (n = 12) groups, respectively (p = 0.03). CADe + ECV identified more polyps in the cecum/right colon (n = 26) compared to CADe (n = 18) and colonoscopy (n = 12) groups (p = 0.04), and in the left colon/sigmoid (n = 14) compared to CADe (n = 5) and colonoscopy (n = 2) (p = 0.02).
Conclusion:
These findings underscore the synergic potential of combining CADe with ECV to enhance ADR and enable us to perform sample size calculations for future RCTs.
Registration:
Clinical Trials number: NCT05080088. Registration 06/06/2021.
Keywords: adenoma detection rate, artificial intelligence, colonoscopy, colorectal cancer screening, innovation
Plain language summary
Improving polyp detection during colonoscopy: comparing three techniques
Colorectal cancer is a leading cause of cancer-related deaths, and early detection of adenomas (precancerous polyps) during colonoscopy is crucial in preventing this disease. Our study aimed to evaluate the effectiveness of three different colonoscopy techniques in detecting adenomas: standard colonoscopy, colonoscopy with computer-aided detection (CADe), and colonoscopy combining CADe with Endocuff Vision (ECV). We conducted a pilot study with 90 patients, divided into three groups of 30 each. One group underwent standard colonoscopy, another had colonoscopy with CADe, and the third group experienced colonoscopy with both CADe and ECV. Our results showed that the combined CADe + ECV technique detected the highest number of adenomas, significantly outperforming both standard colonoscopy and CADe alone. Specifically, 60% of patients in the CADe + ECV group had adenomas detected, compared to 40% in the CADe group and 30% in the standard colonoscopy group. This study highlights the potential benefits of using advanced technologies like CADe and ECV together to improve adenoma detection rates during colonoscopy, ultimately aiding in better prevention of colorectal cancer. Future larger-scale studies are needed to confirm these findings and refine the use of these technologies in clinical practice.
Background
Colorectal cancer is the most common digestive cancer globally and stands as the second leading cause of cancer death for both sexes combined. 1 In France, colorectal cancer screening has contributed to a decrease in deaths from this cause. 2 During the screening, detection, and resection of precancerous lesions are critical in reducing the burden of colorectal cancer in terms of morbidity and mortality. In this context, the adenoma detection rate (ADR) is a major factor, as it has been directly correlated with the risk of interval cancer. 3 Therefore, the improvement of adenoma detection and resection has become a key quality goal in colonoscopy for colorectal cancer prevention.
The gradual integration of new technologies to increase the ADR is progressively becoming a standard of care in practice. Over the past decade, two innovative devices have been developed and adopted to enhance the ADR in screening efforts: first, endoscopic caps, known as mucosa-exposure devices, have demonstrated their utility by enhancing visualization in the colon by unfolding colonic haustrations, thereby proving to be effective and user-friendly tools for improved observation. And second, artificial intelligence (AI) has become a crucial tool in digestive endoscopy, aiding gastroenterologists in real-time identification of precancerous lesions during screening colonoscopies. 4 Utilizing a deep learning architecture based on endoscopic models,5,6 several computer-aided detection (CADe) devices have been developed, including GI GENIUS™ (Medtronic, MN, USA). This specific AI system has proven effective in self-detecting colonic adenomas, enhancing procedural speed with a sensitivity of 99.7%, a false positive rate of 1%, and an average reaction time of 1.27 s. 7
Both mucosa-exposure devices such as Endocuff Vision [ECV]® (Olympus, Tokyo, Japan) and AI such as GI GENIUS™ using CADe have shown respective increases in ADR by 10% 8 and 11% 4 compared to colonoscopy alone. This advancement in detection with these aids is primarily attributed to the increased detection of diminutive polyps, in the right colon (ECV and CADe) and the left colon (ECV). 8
Recent findings have demonstrated that the combination of CADe and ECV improve the ADR by approximately 5%. 9 However, at the time our study began, data on this specific combination was scarce. To fill this knowledge gap, we conducted an exploratory pilot study at our center to evaluate the effect size of the CADe + ECV combination versus AI alone. The aim was to determine the necessary sample size for a future randomized controlled trial (RCT). This report presents the findings of our pilot study, primarily focused on comparing the effects of CADe + ECV versus CADe alone and standard colonoscopy.
Methods
Study design
This preliminary, single-center study utilized a prospectively maintained database (open cohort of 244 patients), encompassing consecutive patients who underwent one of three procedures: combined ECV and CADe, CADe alone, and standard colonoscopy.
The analysis reported in this article was conducted retrospectively based on a subset formed from the acquired data.
Patients
The patients who participated in the study were over 18 and eligible for outpatient care. Patients were recruited from the Endoscopy Unit in our center, where they presented for routine colorectal cancer screening. Eligibility for patient participation was determined based on one or more of the following clinical indications: a positive fecal immunochemical test with a threshold of 30 microg Hb/g of stool (19), presence of risk factors for colorectal cancer such as a personal or family history of colonic adenomas, a family history of colorectal cancer, or the manifestation of rectal bleeding.
Patients were not included if they had a history of inflammatory diseases of the digestive tract or colorectal cancer, or if there were contraindications to polypectomy. Exclusion also extended to patients with previous unsuccessful colonoscopies, those suspected of having polyposis syndrome or with a known diagnosis of familial polyposis. Additionally, patients were excluded if their colonoscopy was incomplete due to the impossibility of cecal intubation, or if they recorded a Boston bowel preparation score below 6 during the procedure.
Patients followed the standard care pathway. They were selected to be included in the cohort after a decision to proceed with a colonoscopy during consultations in the gastroenterology department. The inclusion visit took place occur within a window of 30 days (±30 days) from the day the endoscopy procedure is scheduled. After obtaining consent on the day of the colonoscopy, the investigator included the patient in the cohort. The choice of colonoscopy method was made based on the patient’s inclusion period in the study. Patients underwent a standard colonoscopy in April 2021, a colonoscopy with CADe alone in May 2021, and a colonoscopy with CADe + ECV in June 2021.
Baseline demographic characteristics such as sex, age, body mass index, use of antiplatelet therapy, use of anticoagulant therapy, the indication for colonoscopy, and presence of one or more several risk factors for colorectal cancer, as well as the type of preparation, were recorded prior to performing the colonoscopy. The operator’s experience, categorized as either greater than or less than 1000 colonoscopies performed, was documented. Any complications occurring during the procedure were also recorded.
Constitution of the groups
In the course of standard clinical care, patients undergoing colorectal cancer screening via colonoscopy were allocated to one of three distinct screening techniques as part of their routine care: (i) standard colonoscopy, (ii) colonoscopy enhanced with CADe only, and (iii) colonoscopy augmented by both CADe and an Endoscopic Cap Visualization (ECV) mucosa-exposure device. Patient data were systematically collected in real-time, contributing to an open cohort for ongoing analysis. For the purposes of this exploratory study, we retrospectively selected a subset of 90 patients (30 per screening technique) using a computer-generated random selection process. This selection was aimed at balancing the groups according to the indications for the procedure. To ensure balanced study groups, patients included in the analysis were randomly selected for each of the three groups. The randomization was stratified by indication, sex, and age group (<50 years old; 50–70 years old; >70 years old) to maintain equivalence across these variables.
Colonoscopy preparation
The colonic preparation was either PEG type or a combination of citric acid, magnesium oxide, and sodium picosulfate based on the operator’s choice. This preparation was administered the day before the endoscopic examination, following standard procedures.
Patients were advised to adhere to a residue-free diet for 3 days prior to the examination (a guidance sheet was provided to the patient), irrespective of the preparation type chosen. The quality of the colonic preparation was assessed during the procedure using the Boston classification system, 10 with scores ranging from 0 to 3 for each explored colonic segment.
Colonoscopy exam
Colonoscopies were performed following standard outpatient protocols. The GI GENIUS™ (v2.0.1, Medtronic) software was installed on the endoscopy unit. A video and auditory signal was emitted each time the system detects a polyp, and the endoscopist then verified each detected polyp. The cap used in this study was the Endocuff Vision [ECV]® (Olympus). 11 All colonoscopies were done with high definition scopes from Olympus, series 190 (Olympus).
For patients randomized into the ECV + CADe group, the ECV cap was attached to the distal end of the colonoscope before its insertion, once the patient was sedated. The patient was positioned in the left lateral decubitus position. The colonoscope was advanced to the cecum and appendix orifice.
The procedure duration was recorded, starting with the insertion of the colonoscope and ending upon the endoscope’s withdrawal from the patient. However, in case of polyp resection, the timing was temporarily paused during the procedures. The size of the polyps was estimated by juxtaposing them with an open biopsy forceps, which have a diameter of 7 mm. These polyps were then categorized following the Paris classification system. 12
The polyps collected were analyzed by an expert pathologist and classified according to the Vienna classification. 13 No pathologist knew the patient’s group during the colonoscopy.
Statistical analysis
The ColoDetect database was created using ReDCap© 11.3.1-022 Vanderbilt University. The SAS® Enterprise Guide software version 9.4 (SAS Institute, Cary, NC, USA) was employed for data cleaning and analysis in the study. Results were presented as mean ± standard deviation for quantitative variables, and for qualitative variables, frequencies and associated percentages were reported. This is a descriptive study, and no imputation method for missing data was planned. A statistical significance threshold of 5% (alpha level) was used for all tests. Given the pilot and exploratory nature of this study, and in order to have a representative sample in each of the groups set up and studied, a minimum number of 30 patients per group was set. No justification for this number has been made a priori, and it is precisely the data from this study that will enable us to best estimate the size of an expected effect.
Analysis of the primary endpoint
The primary outcome measure was the ADR. It was calculated from histological analysis, based on the 30 colonoscopies in each of the three groups. ADR was defined as the ratio of the number of colonoscopies that detected at least one histologically confirmed colonic adenoma to the total number of colonoscopies performed in each group. These rates were compared using a mean comparison test.
Analysis of secondary endpoints
The secondary outcome measures included the average adenoma rate per colonoscopy, the rate of adenomas based on their size (using 5 and 3 mm as size thresholds), histology according to the Vienna classification, and morphology according to the Paris classification. Other secondary measures included the total colonoscopy time, the cecal intubation rate, and immediate per- and postoperative adverse events (bleeding, perforation).
Patients were followed up at 24 h after the procedure to assess adverse events or delayed complications. There was no follow-up in the medium- and long-term.
These various quantitative secondary outcome measures were compared among the three groups using an analysis of variance. Qualitative outcome measures were compared among the three groups using a chi-square test or Fisher’s exact test if the conditions for using the chi-square test were not met.
Ethical consideration
All procedures conducted in this study involving patients were carried out after obtaining approval from the local ethics committee (IRB number: Nîmes CHU Hospital IRB 21.0042/21.06.06). Written consent was obtained from each patient participating in the study.
Furthermore, we did not employ a methodology where patients serve as their own controls as it does not seem ethically appropriate to subject the same patient to two colonoscopies within the same time interval.
Our study was submitted and registered with the Clinical Trials registry (NCT05080088 and date of registration 06/06/2021). All authors had access to the study data and reviewed and approved the final manuscript.
Results
Patient’s characteristics
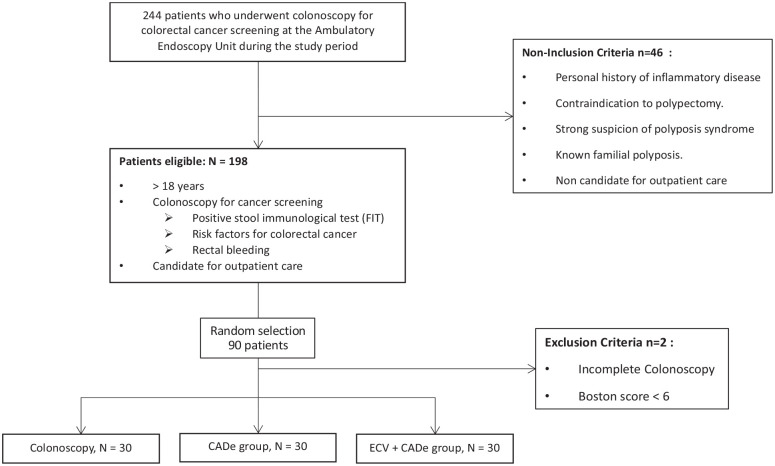
From April to June 2021, 244 colonoscopies were performed in the endoscopy unit, 198 patients were eligible to be included in the study (69 colonoscopy control group, 62 ECV and 67 CADe + ECV). We included 90 patients who met the previously defined criteria and underwent a screening colonoscopy: 30 patients in the colonoscopy control group, 30 patients in the CADe-assisted colonoscopy, and 30 patients in the CADe-assisted colonoscopy coupled with ECV group (Figure 1).
Figure 1.
Flow-chart.
The populations of the three groups were comparable, with similar general characteristics. There was no gender predominance, with an average age of 62 ± 11 years. There was no significant difference in the use of anticoagulant (p = 0.22) or antiaggregant treatment (p = 0.63). The characteristics of each group were compared in Table 1.
Table 1.
Baseline patient characteristics and screening colonoscopy data by intervention group (analysis per patient).
| Groups | Colonoscopy (n = 30) | CADe (n = 30) | CADe + ECV (n = 30) | Total (n = 90) | p |
|---|---|---|---|---|---|
| Gender, n (%) | 0.46 | ||||
| Female | 14 (47%) | 17 (57%) | 19 (63%) | 50 (56%) | |
| Male | 16 (53%) | 13 (43%) | 11 (37%) | 40 (46%) | |
| Age (mean, SD), years | 60.8 ± 8.1 | 63 ± 12 | 62 ± 11 | 62 ± 11 | 0.86 |
| BMI (kg/m2) | 25.5 ± 3.3 | 26.2 ± 4.3 | 25.1 ± 3.9 | 25.6 ± 3.9 | 0.72 |
| Antiaggregant treatment, n | 0.63 | ||||
| Aspirin | 4 | 7 | 4 | 15 | |
| Clopidogrel | 0 | 1 | 1 | 2 | |
| Ticagrelor | 0 | 0 | 0 | 0 | |
| No antiaggregant treatment | 26 | 23 | 26 | 75 | |
| Anticoagulant treatment, n | 0 | 6 | 1 | 7 | 0.22 |
| No anticoagulant treatment | 30 | 24 | 29 | 83 | |
| Indication, n (%) | 0.29 | ||||
| FIT+ | 7 (23) | 11 (37) | 9 (30) | 27 (30) | |
| Personal/family history of adenoma | 12 (40) | 8 (26) | 14 (47) | 34 (38) | |
| Family history of cancer | 10 (33) | 10 (33) | 4 (13) | 24 (26) | |
| GI symptoms | 1 (3) | 1 (3) | 3 (10) | 5 (5) | |
| Mean exam time (IQR), m:s | 14:38 ± 4:29 | 20:55 ± 9:21 | 16:18 ± 4:45 | 17:34 ± 7:10 | <0.0001 |
| Mean insertion time (IQR), m:s | 6:9 ± 3:4 | 11:05 ± 9:07 | 7:23 ±3:21 | 8:25 ± 5:09 | <0.0001 |
| Mean withdrawal time (IQR), m:s | 7:46 ± 2:29 | 10:19 ± 3:41 | 9:13 ± 3:10 | 9:10 ± 3:03 | <0.0001 |
| Mean Boston score | 8.4 ± 0.9 | 8.1 ± 1 | 8.3 ± 0.9 | 8.1 ± 1.03 | 0.9 |
| Complications, n (%) | 0.13 | ||||
| Postresection bleedings | 2 (2) | 2 (2) | 7 (8) | 11 (12) | |
| Incomplete colonoscopy | 0 (0) | 1 (1) | 1 (1) | 2 (2) | |
| No complications | 28 (31) | 27 (30) | 22 (24) | 77 (85) |
CADe, computer-aided detection; FIT, fecal immunochemical test; IQR, interquartile range; SD, standard deviation.
Primary endpoint: ADR
The ADR was significantly higher in the CADe + ECV coupled group: 60% ± 5, compared to 40% ± 5 in the CADe-assisted colonoscopy group and 30% ± 5 in the colonoscopy group (p = 0.037) (Figure 2).
Figure 2.
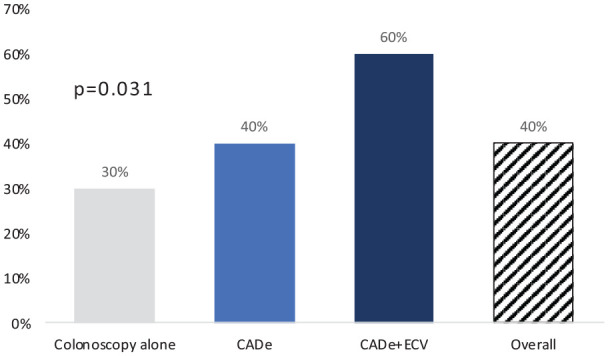
Adenoma detection rate according to colonoscopy group.
A total of 122 polyps were detected in 53 colonoscopies, including 68 adenomas (58%), 13 sessile-serrated adenomas (SSAs) (11%), 36 (29.5%) nonneoplastic polyps and 5 (4.1%) nonrecovered polyps. Among the 90 colonoscopies performed, 39 endoscopic examinations identified at least one histologically neoplastic lesion: 18 colonoscopies in the CADe + ECV group, 12 in the CADe group, and 9 in the colonoscopy alone group.
Secondary endpoints
Endoscopic procedure
There is no difference in the distribution of colonoscopy indications among the groups (p = 0.29). There is also no difference in the quality of colonic preparation, with a mean Boston score of 8/9 (Boston 8.1 ± 1.03, p = 0.9). None of the patients were excluded due to poor colonic preparation (Boston score less than 7/9). Twenty-one (23.3%) colonoscopies were performed by junior operators, while 69 (76,7%) were performed by more experienced endoscopists.
The durations of colonoscopies were significantly different. We observe a significantly longer insertion and withdrawal time in the CADe group (mean total of time of 20 min and 55 s ± 9:21 in the CADe group vs 14 min 38 s ± 4:29 min in the colonoscopy-only group and 16 min and 18 s ± 4:45 in the coupled group, p < 0.001).
Average adenoma per colonoscopy
There was no significant difference in the average number of adenomas detected per colonoscopy: 2 ± 2.3 adenomas in the coupled group versus 0.8 ± 1.4 in the colonoscopy-only group and 1.4 ± 2 in the CADe group, p = 0.09 (Figure 3).
Figure 3.
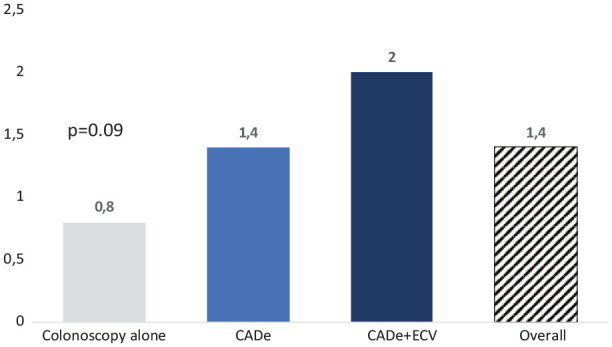
Mean adenoma rate according to colonoscopy group.
Distribution and characteristics of polyps
Regarding the size of the polyps, the proportion of lesions less than 5 mm was 37/59 (62.7%) in the CADe + ECV group, 17/25 (68%) in the colonoscopy alone group and 18/38 (47.4%) in the CADe group, p = 0.45. The average polyp size was comparable for all groups, with a mean of 6.8 mm ± 6.4 mm (p = 0.51). The study demonstrated a difference in the detection of polyps smaller than 3 mm across the three groups. In the standard colonoscopy group, 12 out of 25 polyps (48%) were less than 3 mm in size. In the CADe group, 7 out of 38 polyps (18.4%) measured less than 3 mm. Meanwhile, in the CADe + ECV group, 23 out of 59 polyps (39%) were under 3 mm (p = 0.03).
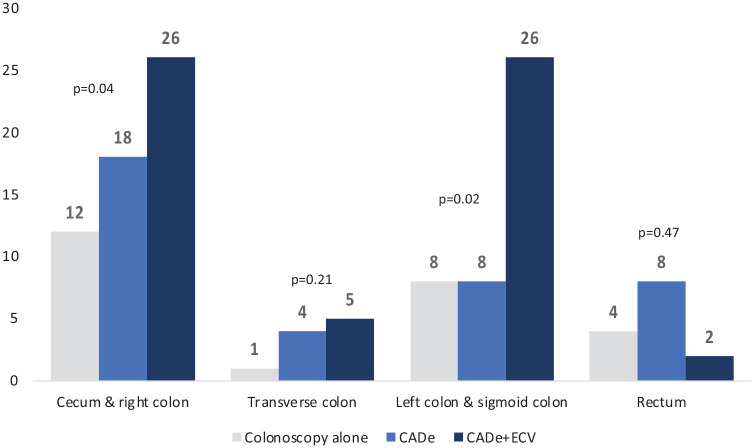
In the cecum and right colon, the number of polyps detected was significantly higher in the CADe + ECV group with 26 polyps, compared to 18 and 12 polyps detected in the CADe and colonoscopy alone groups, respectively (p = 0.04). Similarly, the left colon and sigmoid colon demonstrated a notable increase in polyp detection with 26 polyps identified in the CADe + ECV group versus 8 in both the CADe and colonoscopy alone groups (p = 0.02) (Figure 4).
Figure 4.
Comparison of the number of colonic polyps detected by segment according to colonoscopy group.
No significant differences were observed in the secondary criteria for adenoma distribution based on the Paris morphology classification (p = 0.1), location (p = 0.08), and Vienna histology (p = 0.07) across the three groups. Predominantly sessile and flat polyps, classified as Paris 0-Is and Paris 0-IIa, were identified in various locations, most frequently in the right colon. No invasive cancers were detected. However, regarding SSAs specifically, the CADe + ECV approach allowed for a more effective detection, identifying 10 SSAs, compared to two detected with colonoscopy alone and one with CADe (p = 0.02). Within the CADe group, two large spreading tumor-type adenomatous lesions were noted; however, these were neither biopsied nor resected during the colonoscopy. A total of three polyps were not retrieved, with two in the coupled group and one in the CADe group. The histological characteristics of the polyps are detailed in Table 2.
Table 2.
Secondary criteria—distribution of colonic adenomas by size, morphology, location, and histology (analysis per polyp), n (%).
| Groups | Colonoscopy (n = 25) | CADe (n = 38) | CADe + ECV (n = 59) | Total (n = 122) | p |
|---|---|---|---|---|---|
| Size category | |||||
| Size (mean, SD) | 6.24 ± 6.6 | 7.8 ± 7 | 6.33 ± 6 | 6.8 ± 6.4 | 0.51 |
| Threshold size 3 mm | 0.03 | ||||
| ⩽3 mm | 12 (48) | 7 (18.4) | 23 (39) | 42 (34) | |
| >3 mm | 13 (52) | 31 (81.6) | 36 (61) | 80 (66) | |
| Threshold size 5 mm | 0.45 | ||||
| ⩽5 mm | 17 (68) | 18 (47.4) | 37 (62.7) | 72 (59) | |
| [6–9] mm | 5 (20) | 13 (34.2) | 16 (27.1) | 34 (28) | |
| ⩾10 mm | 3 (12) | 7 (18.4) | 6 (10.2) | 16 (13) | |
| Morphology category according to Paris | 0.1 | ||||
| Polypoid a | 14 (56) | 11 (28.9) | 34 (57.6) | 59 (48.4) | |
| 0-Ip | 3 (12) | 1 (2.6) | 8(13.6) | 12 (9.8) | |
| 0-Is | 11 (44) | 10 (25.6) | 26 (44.1) | 47 (38.5) | |
| Nonpolypoid b | 11 (44) | 27 (71.1) | 25 (42.3) | 63 (52.6) | |
| 0-IIa | 8 (32) | 23 (60.5) | 21 (35.6) | 52 (42.6) | |
| 0-IIb | 3 (2) | 3 (7.9) | 4 (6.8) | 10 (8.2) | |
| 0-IIc | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| 0-III | 0 (0) | 1 (2.6) | 0 (0) | 1 (0.8) | |
| Localization category | |||||
| Cecum/right colon | 12 (48) | 18 (47) | 26 (44) | 56 (46) | 0.04 |
| Transverse colon | 1 (4) | 4 (11) | 5 (8) | 10 (8) | 0.21 |
| Left colon/sigmoid colon | 8 (32) | 8 (21) | 26 (44) | 42 (34) | 0.02 |
| Rectum | 4 (16) | 8 (21) | 2 (3) | 14 (11) | 0.47 |
| Pathologic classification | 0.07 | ||||
| Neoplastic lesions | 18 (72) | 22 (57.9) | 41 (69.5) | 81 (66.4) | |
| Adenoma, low-grade dysplasia | 15 (60) | 21 (55.3) | 30 (50.8) | 66 (54.1) | |
| Adenoma, high-grade dysplasia | 1 (4) | 0 (0) | 0 (0) | 1 (0.8) | |
| Carcinoma | 0 (0) | 0 (0) | 1 (1.7) | 1 (0.8) | |
| Sessile serrated adenoma | 2 (8) | 1 (2.6) | 10(16.9) | 13 (10.7) | |
| Nonadenomatous polyps c | 7 (28) | 13 (34.2) | 16 (27.1) | 36 (29.5) | |
| Hyperplastic polyps | 3 (12) | 10(26.3) | 5 (8.5) | 18 (14.8) | |
| Nonspecific | 4 (16) | 3 (7.9) | 11 (18.6) | 18 (14.8) | |
| Nonrecovered polyps d | 0 (0) | 3 (7.9) | 2 (3.4) | 5 (4.1) | |
| Sessile serrated adenoma vs other polyps | 2 (8) vs 23 (92) | 1 (2.6) vs 34 (97.4) | 10 (16.9) vs 47 (83.1) | 13 (10.7) vs 104 (89.3) | 0.02 |
CADe, computer-aided detection; ECV, Endocuff Vision; SD, standard deviation.
Polypoid: polyps Paris 0-Ip et 0-Is.
Nonpolypoid: polyps 0-IIa, 0-IIb, 0-IIc et 0-III.
Resection of nonadenomatous polyps: aspecific histology, hyperplastic polyps, inflammatory polyps.
Unrecovered: polyps not found/large spreading tumor not biopsied or resected.
Complications and adverse events
We observed no significant differences in immediate periprocedural and postprocedural complications (Table 1) among the patients. The primary complication encountered was postpolypectomy bleeding, which was either spontaneously controlled or managed using endoscopic hemostatic techniques during the procedure. Furthermore, no complications were reported during the 24-h postcolonoscopy follow-up conducted by the endoscopy nurse, and no reinterventions were necessary. Notably, there were no instances of colonic perforation in this cohort of 90 patients.
Discussion
In this single-center preliminary study assessing the impact of various tools on the detection of neoplastic lesions during colonoscopy, we observed in our cohort of 90 patients that the combination of CADe + ECV showed a significant increase in ADR. Specifically, there was a 30% increase in ADR compared to colonoscopy alone, and a 20% increase compared to CADe-assisted colonoscopy.
Our study is an exploratory investigation aimed at extrapolating the necessary sample size for a future randomized clinical trial. Data analysis was conducted post hoc; however, a strength of our study lies in the fact that the database was prospectively maintained, and we sought to minimize biases by allocating patients to each group through stratified randomization. This allocation was based on factors that influence the ADR to render the groups as comparable as possible within this retrospective design. Our findings are largely in line with previous literature: we observed an increased ADR with the addition of CADe and CADe + ECV, particularly in the detection of polyps smaller than 3mm and those located in the left colon and sigmoid. This aligns with data from previous RCTs.8,14 Specifically, in our study, the CADe + ECV group identified over three times as many polyps in the left colon and sigmoid compared to the CADe and colonoscopy alone groups (26 vs 8 vs 8, respectively, p = 002). For polyps smaller than 3 mm, the CADe + ECV combination detected 2–3 times more polyps than the colonoscopy alone and CADe groups (n = 23 vs 12 vs 7, respectively, p = 0.03). The increase in ADR with the addition of ECV to CADe may be attributed to a synergistic effect of these technologies, particularly evident in the enhanced exposure of the mucosal surface to CADe through ECV. This reveals mucosal folds that could conceal small polyps, an effect more pronounced in the left colon and sigmoid where folds are more abundant and the colon’s caliber is narrower, making mucosal exposure without ECV more challenging. 8 Therefore, our series corroborates the trend of improved detection of small polyps with CADe and suggests that ECV maintains a detection advantage in the left colon.
The small sample size of our study necessitates cautious interpretation. We noted a 10% increase in ADR for colonoscopy with CADe compared to colonoscopy alone, consistent with literature showing an 11.4% ADR improvement with CADe (25.4% vs 36.6%). 4 The 20% ADR rise in our CADe + ECV group is notably high, diverging from a recent RCT that found only a 5.6% increase with ECV addition to CADe. 9 Additionally, we found that the insertion time for colonoscopy was longer in the CADe group than in the colonoscopy alone group (+4 min 56 s) and the CADe + ECV group (+3 min 42 s). While it has been shown that ECV-assisted colonoscopy can reduce insertion time for caecal intubation, 8 the significance of the difference observed here, particularly the markedly shorter insertion time in the colonoscopy alone group, can be largely attributed to one extremely prolonged procedure in the CADe + ECV group. This likely had a significant impact on the overall insertion time in this smaller group of 30 patients. Such a difference might not have been as pronounced in larger groups.
Some results in our study should be interpreted considering potential confounding factors: the ADR in the CADe + ECV group might be overestimated due to longer examination times. It has been well demonstrated that an increase in withdrawal time linearly enhances the ADR, 15 and, in our study, the mean withdrawal time was 9.28 min in the CADe + ECV group, which is longer than reported in literature without the association of the two devices, where withdrawal times range from 6.2 to 7.0 min for CADe alone15,16 and approximately 7–7.77 min for colonoscopy with ECV alone.8,17 The increased withdrawal time in the CADe + ECV group could be attributed to the small size of this group. However, based on our experience, we interpret this increase more because of extended examination time due to CADe alerts prolonging withdrawal duration. We hypothesize that the number of AI-generated alerts is higher in the CADe + ECV group, especially in the left colon, though we lack data to confirm this.
Conclusion
Our preliminary data suggest an additive effect of the CADe + ECV combination in enhancing the ADR. However, subsequent larger randomized studies will be necessary to confirm these findings and to adjust the values observed in this current study. Our study provides a solid foundation for calculating the sample size for a RCT comparing the combination of CADe + ECV to CADe alone. However, as the standard of care involving AI use is evolving, the design of this RCT should now encompass three groups: CADe alone, ECV alone, and the combination of CADe + ECV. This RCT is currently underway (COLODETECT 2) and has been registered on ClinicalTrials.gov (NCT number: NCT05594576).
Acknowledgments
The authors wish to thank Sabrina Nicolas and Nelly Pourteau for their assistance with the logistical organization and administrative management of this research project.
Footnotes
ORCID iDs: Ludovic Caillo  https://orcid.org/0000-0002-4110-1866
https://orcid.org/0000-0002-4110-1866
Antoine Debourdeau  https://orcid.org/0000-0003-3785-6948
https://orcid.org/0000-0003-3785-6948
Contributor Information
Ludovic Caillo, Department of Gastroenterology, Carémeau Hospital, University Hospital of Nîmes, Place du Professeur Robert Debre, Nîmes, Gard 30029, France.
Clément Delliot, Department of Gastroenterology, University Hospital of Nîmes, Nîmes, France.
Thierry Chevallier, Department of Biostatistics, Epidemiology, Public Health and Methodological innovation (BESPIM), University Hospital of Nîmes, Nîmes, France, University Montpellier 1, Montpellier, France UMR 1302, Institute Desbrest of Epidemiology and Public Health, INSERM, University of Montpellier, Montpellier, France.
Jean-Francois Bourgaux, Department of Gastroenterology, University Hospital of Nîmes, Nîmes, France.
Ardavan Prost, Department of Gastroenterology, University Hospital of Nîmes, Nîmes, France.
Bénédicte Brunaud-Gagniard, Department of Gastroenterology, University Hospital of Nîmes, Nîmes, France.
Valérie Phoutthasang, Department of Gastroenterology, University Hospital of Nîmes, Nîmes, France.
Clémentine Clerc, Department of Gastroenterology, University Hospital of Nîmes, Nîmes, France.
Thomas Borderie, Department of Gastroenterology, University Hospital of Nîmes, Nîmes, France.
Jules Daniel, Department of Gastroenterology, University Hospital of Nîmes, Nîmes, France.
Philippe Pouderoux, Department of Gastroenterology, University Hospital of Nîmes, Nîmes, France.
Antoine Debourdeau, Department of Gastroenterology, University Hospital of Nîmes, Nîmes, France.
Declarations
Ethics approval and consent to participate: All procedures conducted in this study involving patients were carried out after obtaining approval from the local ethics committee (IRB number: Nîmes CHU Hospital IRB 21.0042/21.06.06). Written consent was obtained from each patient participating in the study.
Consent for publication: Not applicable.
Author contributions: Ludovic Caillo: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Clément Delliot: Conceptualization; Data curation; Writing – original draft.
Thierry Chevallier: Conceptualization; Data curation; Investigation; Methodology; Software; Supervision; Validation; Visualization; Writing – review & editing.
Jean-Francois Bourgaux: Data curation; Funding acquisition; Project administration; Writing – review & editing.
Ardavan Prost: Investigation.
Bénédicte Brunaud-Gagniard: Investigation.
Valérie Phoutthasang: Investigation.
Clémentine Clerc: Investigation.
Thomas Borderie: Investigation.
Jules Daniel: Investigation.
Philippe Pouderoux: Investigation; Methodology.
Antoine Debourdeau: Formal analysis; Investigation; Methodology; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the Universitary Hospital of Nîmes.
The authors declare that there is no conflict of interest.
Availability of data and materials: Data are available upon reasonable request from the corresponding author.
Guarantor of the article: Clément Delliot/Ludovic Caillo
Appendix
Abbreviations
ADR adenoma detection rate
AI artificial intelligence
CADe computer-aided detection
ECV Endocuff Vision
FIT fecal immunochemical test
PEG polyethylene glycol
RCT randomized controlled trial
SSA sessile serrated adenoma
References
- 1. Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med 1993; 328: 1365–1371. [DOI] [PubMed] [Google Scholar]
- 2. Le cancer colorectal—Les cancers les plus fréquents, https://www.e-cancer.fr/Professionnels-de-sante/Les-chiffres-du-cancer-en-France/Epidemiologie-des-cancers/Les-cancers-les-plus-frequents/Cancer-colorectal (2024).
- 3. Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med 2010; 362: 1795–1803. [DOI] [PubMed] [Google Scholar]
- 4. Hassan C, Spadaccini M, Iannone A, et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc 2021; 93: 77–85.e6. [DOI] [PubMed] [Google Scholar]
- 5. Wang P, Xiao X, Brown JRG, et al. Development and validation of a deep-learning algorithm for the detection of polyps during colonoscopy. Nat Biomed Eng 2018; 2: 741–748. [DOI] [PubMed] [Google Scholar]
- 6. Repici A, Badalamenti M, Maselli R, et al. Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Gastroenterology 2020; 159: 512–520.e7. [DOI] [PubMed] [Google Scholar]
- 7. Hassan C, Wallace MB, Sharma P, et al. New artificial intelligence system: first validation study versus experienced endoscopists for colorectal polyp detection. Gut 2020; 69: 799. [DOI] [PubMed] [Google Scholar]
- 8. Karsenti D, Tharsis G, Perrot B, et al. Adenoma detection by Endocuff-assisted versus standard colonoscopy in routine practice: a cluster-randomised crossover trial. Gut 2020; 69: 2159–2164. [DOI] [PubMed] [Google Scholar]
- 9. Spadaccini M, Hassan C, Rondonotti E, et al. Combination of mucosa-exposure device and computer-aided detection for adenoma detection during colonoscopy: a randomized trial. Gastroenterology 2023; 165: 244–251.e3. [DOI] [PubMed] [Google Scholar]
- 10. Lai EJ, Calderwood AH, Doros G, et al. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc 2009; 69: 620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marsano J, Johnson S, Yan S, et al. Comparison of colon adenoma detection rates using cap-assisted and Endocuff-assisted colonoscopy: a randomized controlled trial. Endosc Int Open 2019; 07: E1585–E1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Workshop P in the P. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon November 30 to December 1, 2002. Gastrointest Endosc 2003; 58: S3–S43. [DOI] [PubMed] [Google Scholar]
- 13. Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000; 47: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hassan C, Senore C, Manes G, et al. Diagnostic yield and miss rate of EndoRings in an organized colorectal cancer screening program: the SMART (Study Methodology for ADR-Related Technology) trial. Gastrointest Endosc 2019; 89: 583–590.e1. [DOI] [PubMed] [Google Scholar]
- 15. Wang P, Berzin TM, Brown JRG, et al. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomised controlled study. Gut 2019; 68: 1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Doorn S, van der Vlugt M, Depla A, et al. Adenoma detection with Endocuff colonoscopy versus conventional colonoscopy: a multicentre randomised controlled trial. Gut 2017; 66: 438. [DOI] [PubMed] [Google Scholar]
- 17. Floer M, Tschaikowski L, Schepke M, et al. Standard versus Endocuff versus cap-assisted colonoscopy for adenoma detection: a randomised controlled clinical trial. United European Gastroenterol J 2021; 9: 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]