Abstract
Objective
After infection with SARS-CoV-2, a substantial proportion of patients develop long-lasting sequelae. These sequelae include fatigue (potentially as severe as that seen in ME/CFS cases), cognitive dysfunction, and psychiatric symptoms. Because the pathophysiology of these sequelae remains unclear, existing therapeutic concepts address the symptoms through pacing strategies, cognitive training, and psychological therapy.
Methods
Here, we present a protocol for a digital multimodal structured intervention addressing common symptoms through three intervention modules: BRAIN, BODY, and SOUL. This intervention includes an assessment conducted via a mobile “post-COVID-19 bus” near the patient's home, as well as the use of wearable devices and mobile applications to support pacing strategies and collection of data, including ecological momentary assessment.
Results
We will focus on physical component subscore of the SF36 as Quality of Life parameter as the primary outcome parameter for WATCH to take into account the holistic approach that is necessary for care of post-COVID patients
Conclusion
In the current project, we present a protocol for a holistic and multimodal structured therapeutic concept which is easily accessible, and scalable for post-COVID patients.
Keywords: Post-COVID, telemedicine < General, mobile clinic, rehabilitation < Lifestyle, fatigue
Introduction
After the first cases of acute SARS-CoV-2 infection, a substantial proportion of patients were found to experience long lasting sequelae.1,2 The WHO defines symptoms in the time window 12 weeks after acute SARS-CoV-2 infection that persist for at least 2 months and are not otherwise explainable as post-COVID-19 condition. 3 To date, more than 200 different symptoms have been reported as a consequence of post-COVID-19 condition. 4 Common symptoms include fatigue, long-lasting neuro-cognitive and psychiatric deficits, exercise intolerance, post-exertional malaise (PEM), and dyspnea. Whereas the initial waves of SARS-CoV-2 infection led to post-COVID-19 condition in approximately 6–10% of cases, 5 subsequent changes in population immunity and the prevalence of less pathogenic variants have substantially decreased the likelihood of long-term sequelae.1,6
Nonetheless, a notable proportion of patients experience persistent symptoms that may develop into myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). 7 Post-COVID-19 condition and ME/CFS can exhibit overlapping symptoms, thus leading to challenges in distinguishing between them8,9; moreover, both conditions are associated with substantial decreases in quality of life and occupational participation. Even without ME/CFS symptoms of post-COVID-19 condition, e.g., dyspnea, joint and muscle pain, concentration problems, or psychiatric symptoms can experience a severely decreased quality of life and the ability to work.10,11
Because no causative therapies have been available to date, the most effective treatment approach is holistic, symptom-focused supportive therapy including physical and exercise-based approaches to avoid PEM, address orthostatic problems, and provide regular neurocognitive training and supportive psychotherapy. Additionally, these approaches should implement appropriate means to avoid overload and post-exertional symptom exacerbation. Effective pacing strategies require knowledge of patients’ triggers of PEM episodes, to enable patients to detect and avoid these triggers on a daily basis. Such patient empowerment is not trivial, because the time between the trigger and the onset of PEM can vary from hours to several days. 12
Currently, specialized centers for treating post-COVID-19 or post-infectious diseases provide the best opportunity for interdisciplinary and complex treatment approaches. Access to this type of care for patients with post-COVID-19 condition is sometimes substantially hindered by long travel times or limited local rural care. Consequently, an important problem faced by patients with post-COVID-19 condition, particularly those with attentional deficits, concentration problems, pain, fatigue, and/or PEM, is a lack of qualified, interdisciplinary treatment centers near their place of residence. One promising tool to overcome these limitations is the use of mobile clinics operated in buses—a concept previously established for the treatment of other indications. To date, an estimated 2000 mobile clinics have operated in the US for different indications.13,14 Our group has demonstrated that providing diagnostic procedures for patients with post-COVID-19 condition by using a mobile outpatient clinic (“post-COVID-19 bus”) is feasible and accepted by patients. 15
Therefore, we have implemented a concept that combines a mobile ambulance with a multimodal telemedicine intervention, utilizing wearable devices and mobile applications to support pacing strategies and data collection, including Ecological Momentary Assessment (EMA). Patient recruitment began in November 2023 and data collection is expected to be completed by November 2025. In this manuscript, we aim to present the protocol and discuss the importance of EMA in assessing symptoms in patients with long-term COVID.
Aim
General objective
The general objective of our web-based telemedicine approach for treatment of post-COVID-19 in Thuringia (WATCH) is to implement and evaluate a multimodal telemedicine guided intervention including physical, cognitive, and psychiatric treatments (BODY, BRAIN, and SOUL modules) to enhance general quality of life and physical and mental component subdomains, and alleviate symptom burden in patients with post-COVID-19 condition.
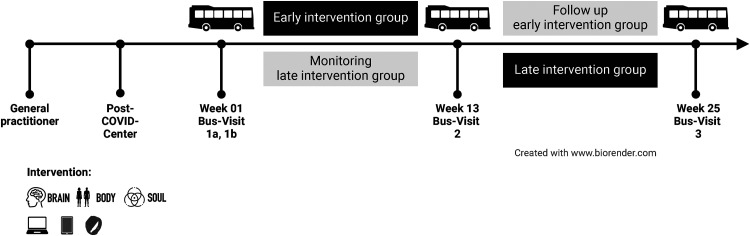
For the evaluation of the efficacy of this intervention, patients will be divided into two groups: one group receiving the intervention between weeks 1 and 12 of the project, and the other receiving the intervention between weeks 12 and 24. The concept of the waiting group is applied to compare patients receiving the intervention to patients with spontaneous course of the disease on the one hand and to offer the intervention to every participant for ethically reasons on the other hand. The group receiving the intervention at the beginning of the project will be followed for 12 weeks after their intervention to determine whether improvements in quality of life continue. This follow-up period is also used to evaluate whether participants can consistently apply pacing strategies in their daily lives.
Aim 1
To ameliorate cognitive deficits, i.e., to improve attention, concentration, and memory functions, a computerized adaptive training will be provided by the NeuroNation MED® mobile application (app) within the BRAIN module.
Aim 2
For the BODY module, we have developed a concept aimed at improving/maintaining physical fitness through pacing approaches and energy management, based on evidence-based recommendations in a four-phase model.
Aim 3
Within the SOUL module, short-term crisis intervention will be provided to enhance patients’ ability to cope with post-COVID-19 symptoms. This module also focuses on activating resources to strengthen everyday functionality and on alleviating psychopathological symptoms, particularly depression and anxiety.
Aim 4
We conduct continuous measurement of heart rate (night and day resting heart rate, and day peak heart rate), daily step count, and sleep quantity for 24 weeks, including 12 weeks of telemedicine interventions. A wearable device for continuous biological parameter measurement will be provided to all patients. With regard to using heart rate for activity guidance to prevent PEM, all patients will participate in structured webinars aimed at providing knowledge regarding PEM. In addition to the structured intervention, participants will receive guidance in managing their activity levels according to their own heart rates. Episodes of PEM will be evaluated through structured screenings at defined time points each week.
An ecological monetary assessment will be performed with questionnaires provided to patients through the Pulsatio® app, to obtain information regarding improvements in post-COVID-19 symptoms, e.g., cognitive failures in daily life, patient mood, or PEM episodes.
Project design
A mobile outpatient clinic, called the PoCo bus, will visit patients near their residences three times (in weeks 1, 13, and 25). During each visit, patients will undergo a structured evaluation in the PoCo bus, and receive an intervention manual and instructions for the use of the wearable devices and apps.
Structured evaluation in the PoCo bus
In four separate areas of the bus, consecutive, structured diagnostic examinations and instruction in the digital apps will occur. The consecutive diagnostics will include blood sampling, a 1 min sit to stand test, and handgrip measurements (ten repetitions),16,17 including analysis of subjective and objective exertion parameters; neurocognitive testing with a focus on attention, concentration and memory functions, including the Montreal Cognitive assessment 18 and the Oxford Cognitive Screen plus19,20; and lung impedance tomography. 21 Additionally, demographic and socioeconomic data, as well as data on acute SARS-CoV-2 infection, SARS-CoV-2 vaccinations, comorbidities, and post-COVID-19 symptoms, will be recorded. Wearable devices will be distributed to participants as needed, and those unfamiliar with wearable devices will be provided with instructions for their use. Participants will be guided in the use of the Pulsatio® app and the NeuroNation Med® app, which will be installed on their personal mobile devices, such as mobile phones or tablets.
All participants will be randomly assigned to receive the telemedicine intervention (computerized adaptive training provided by the NeuroNation MED® app (BRAIN), webinars, homepage, analog/digital handbook, Pulsatio-App (BODY), and video consultation (SOUL)) either in the initial 12 weeks (group 1) or the subsequent 12 weeks (group 2) of the project. The detailed design of the project is shown in Figure 1.
Figure 1.
Visualization of the project design.
Sample size
On the basis of findings from the post-COVID-19 outpatient clinic at UKJ, 10 we expect a sample size of 486 individuals to be required assuming that the standard deviation is ±9.8 points for the Short Form 36 Physical Component Subscore (SF36-PCS) used as main outcome. We aim to recruit a total of 624 individuals, to account for a potential dropout rate of 22% (138 individuals). With the planned sample size, a mean difference in 2.5 points on the SF36-PCS, which has been defined as clinically relevant, can be detected with a power of 80% at an α-level of 5%. These sample size estimates are based on a group comparison with a t-test. Although a more complex regression model will be used in the analysis, the sample size calculations can be assumed to be fairly conservative. The 2.5 points difference was chosen on the basis data of our post-COVID outpatient clinic, where patients presented with a markedly reduced SF36-PCS of 36.3 points at the first visit. With the initiation of supportive therapy, which includes pacing strategies, cognitive training and physiotherapy, an increase of 2.6 points was documented at follow-up.5,10 Further, patients who have an improvement of more than 2.5 points also report a significant improvement in in symptoms and their overall well-being. The handling of drop-outs depends on the analysis. In the main efficacy analysis, we can only consider those who have information on the baseline and the endpoint. In analyses with repeated assessments, we will allow also missing responses.
Inclusion/exclusion criteria
The inclusion criteria in the project are individuals living in the German federal state of Thuringia who are 18 years of age or older; who had a SARS-CoV-2 infection at least 84 days before, as confirmed by PCR or antigen testing; who have ongoing symptoms and diminished quality of life, as defined by an SF36-PCS score below 45 points; and who provide written informed consent. Furthermore, the participants must have a mobile phone or tablet with an internet connection to be able to use the wearable devices and receive the telemedicine intervention. The exclusion criteria includes pregnancy and breast feeding, ongoing psychiatry therapy, or other severe illnesses that would hinder either heart rate monitoring or the telemedicine intervention, e.g., severe congestive heart failure, decompensated cirrhosis, renal replacement therapy, or dementia. Furthermore, because the intervention and questionnaires will be delivered in German via a telemedicine approach, lack of German language comprehension and blindness are additional exclusion criteria.
Methods: assignment of interventions
Multimodal telemedicine intervention
All participants will receive a multimodal telemedicine intervention consisting of three modules focusing on relevant post-COVID-19 problems (BRAIN for cognitive dysfunction, BODY for fatigue and PEM, and SOUL for psychological problems).
BRAIN intervention module
The aim of the BRAIN module is to improve cognitive functioning, particularly attention, concentration, and memory functions, by using gamified, adaptive computerized training provided by the CE-certified medical product NeuroNation MED® app. Participants can entrain their cognitive performance levels at home with 30 motivating, gamified tasks. The difficulty level of the tasks, varies, e.g., by movement speed, number, and complexity of the relevant task items, and continually adapts to the individual capability level of the participant. Thus, the tasks become more challenging with increasing training success and immediately become easier if the daily performance is poor. This mobile app has already been successfully used and shown to improve cognitive performance in older healthy participants 22 and in patients with cognitive deficits due to Parkinson's disease and Huntington's disease.23,24 Adherence and progress will be recorded digitally.
Participants will receive 12-week access to the app for study purposes. The training will occur three times per week, and each training will consist of six 5-min tasks. Each individual training session will last 30 min, thus resulting in a total of 18 h of training during the intervention. All instructions will be available on the project homepage and also described in detail in the user manual.
BODY intervention module
The first aim of the BODY module is to develop self-competence in the application of effective pacing strategies for post-COVID, particularly regarding decreasing fatigue and preventing PEM occurrence. The second aim of the BODY module is to prevent worsening of symptoms, particularly fatigue and PEM onset, while participants remain as active as possible, and increase their physical and activity levels as appropriate. On the basis of pacing, in the BODY module, we have developed a concept to improve physical fitness in post-COVID-19 by using a four-phase model relying on evidence-based recommendations for pacing, PEM, heart rate, and subjective perceived exertion.25,26 Pacing in the BODY module comprises four components that provide the basis of the four-phase concept. Component 1 is associated with the energy envelope theory 27 and uses a simple energy envelope assessment based on the energy ratio between participants’ perceived expended energy and their perceived available energy resources. 28 Component 2 involves identifying factors that trigger PEM, to prevent worsening/relapse of symptoms and PEM. 29 The third component includes daily reminders of how to use pacing with the Pulsatio® app, through the engagement in pacing questionnaire.29,30 The fourth component includes recovery monitoring and exercise monitoring of the heart rate (resting heart rate, peak heart rate, steps, and sleep quantity) and rating of perceived exertion (RPE, on a scale of 6–20).
The four phases are categorized by percentage maximal heart rate, associated RPE value and corresponding activities of daily living based on metabolic equivalents. The following thresholds are defined for changing to another phase: i) phase duration of at least 3 weeks, ii) adherence to pacing and appropriateness of the energy framework, iii) absence of triggering of PEM or “crashes”, and iv) ability to perform everyday activities accordingly (see MET information in Table 1).
Table 1.
Phases, intensity, %HRmax (percentage maximal heart rate), and perceived exertion (RPE), adapted from Garber et al. 2011. 31
| Phase | Intensity (Borg scale) | %HRmax | Perceived exertion (on 6–20 RPE scale) | Metabolic equivalents (MET) |
|---|---|---|---|---|
| 1 | Not strenuous at all to very light | until 50 | 6–8/20 | <2.5 |
| 2 | Very light to light | 50–60 | 9–10/20 | 2.5–3.0 |
| 3 | Fairly light to somewhat hard | 60–75 | 11–13/20 | 3.0–5.0 |
| 4 | Very hard | 75–90 | 14–16/20 | 5.0–8.0 |
Each phase contains three components to be performed in the following order:
Breath-control exercises (3 min): The breath-control training component is the starting point of every 15-min BODY unit and includes at least one of the following aspects for a maximum of 3 min: 1-min slow wave breathing,32,33 respiratory muscle activation and relaxation, diaphragm self-mobilization, and stretching postures. 25
Heart rate-controlled physical activity management: This module consists of a 6-min interval-type activity component consisting of repeated 30-s periods of exercise and 30-s breaks (6 × [30-s exercise, 30-s break]). Intensity is controlled with the Borg scale and heart rate. For example, in phase 1, the load is controlled so that a maximum of 50% of the maximum heart rate is reached (general formula: women: 206–0.88 × age, men: 208–0.7 × age). On the Borg scale, this load corresponds to a subjective perception of exertion of 6–7/20 (Table 1).
The final component of every BODY workout is a short yoga or meditation session. Beyond learning these techniques for self-use, active relaxation is an additional focus. The video-based units are available on the project homepage.
If exercise intolerance occurs during the program, patients are advised to return to the previous phase for the next training session. Reaching phase four is not the aim of the intervention. Patients train in the BODY module three times per week for 15 min per session, thus resulting in a total of 9 h of training over the 12 weeks.
To ensure that older patients or patients with an average or lower level of physical literacy and/or medical knowledge can understand the consecutive structured program and implement it in everyday life, BODY uses the following components: Manual with weekly plan and explanations, homepage for digital content (e.g., guided breathing exercises, yoga), 2×weekly webinars (Monday 08:30, Thursday 16:30) with a recurring list of topics as well as a telephone medical consultation (if required).
SOUL intervention module
SOUL is a short psychotherapy protocol using techniques of behavioral therapy and principles of crisis intervention. This module is aimed at improving coping with sudden changes in living situation and post-COVID-19, to activate resources that strengthen everyday functioning and decrease psychopathological symptoms, particularly depression and anxiety. The SOUL module is designed to respond flexibly to the individually different, frequently complex constellations of symptom and resources. To accomplish this goal, the underlying concept is based on the BELLA crisis intervention model described by Sonneck, 34 which has been validated for the treatment of patients with (newly occurring) psychological symptoms after substantial changes in life circumstances. Demonstrated cognitive-behavioral therapy techniques are used, which are intended to convey knowledge regarding the development and handling of psychological distress, and the newly experienced changes in health and their effects on everyday life, regardless of the main psychological symptoms (depression, anxiety, post-traumatic stress symptoms, etc.). Experienced psychological psychotherapists perform the intervention via one-on-one video consultation. The sessions are scheduled to last 30 min, taking individual pacing into account. The eight sessions over the 12 weeks may be postponed depending on the participants’ resilience and ability to focus.
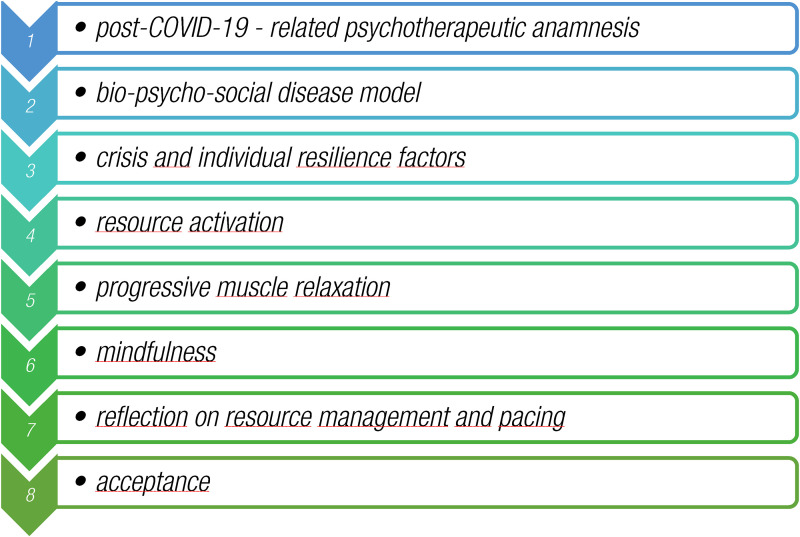
The first session covers the post-COVID-19-related psychotherapeutic anamnesis and creation of the therapeutic relationship between patients and their psychologists (Figure 2). Session two focusses on factors contributing to the ongoing struggle with psychological post-COVID-19 symptoms, according to the bio-psycho-social disease model. 35 In this session, patients are asked to record their physical, mental, and emotional distress each day, and to document their symptoms and level of exhaustion at the end of each day. The protocol is interpreted in session 7, thus providing patients with ideas regarding what causes them the most fatigue; what might recharge their energy levels; and what amount of energy they usually have available before the onset of fatigue, pain, or brain fog. The following session includes psychoeducational elements regarding crisis factors and factors of individual resilience, and builds up to the activation of already established individual resources (session 4) and to new resources, including progressive muscle relaxation, according to Jacobson (session 5) and the Kabat-Zinn mindfulness concept 36 (session 6). The seventh session involves reflection on the implementation of resources and success or problems with pacing. The final session is meant to foster acceptance of the condition and recovery.
Figure 2.
Overview of topics of the video consultations used in the SOUL module.
Patients are encouraged to freely use the acquired techniques and implement them in their daily lives after completion of the SOUL module. If patients present ongoing psychological or psychiatric distress beyond their post-COVID-19 symptoms that are otherwise not addressed by the intervention, they are provided with contact information for psychotherapists near their homes to pursue ambulatory psychotherapy.
If the therapist identifies critical symptoms, such as suicidality, ongoing substance use or acute psychotic symptoms, the case is discussed with the supervising psychiatrist of the SOUL intervention. The patient might then receive a psychiatric consultation or be advised to seek urgent psychiatric care at the closest specialist hospital. In that event, they will not be able to participate further in WATCH.
Manual, homepage and webinars
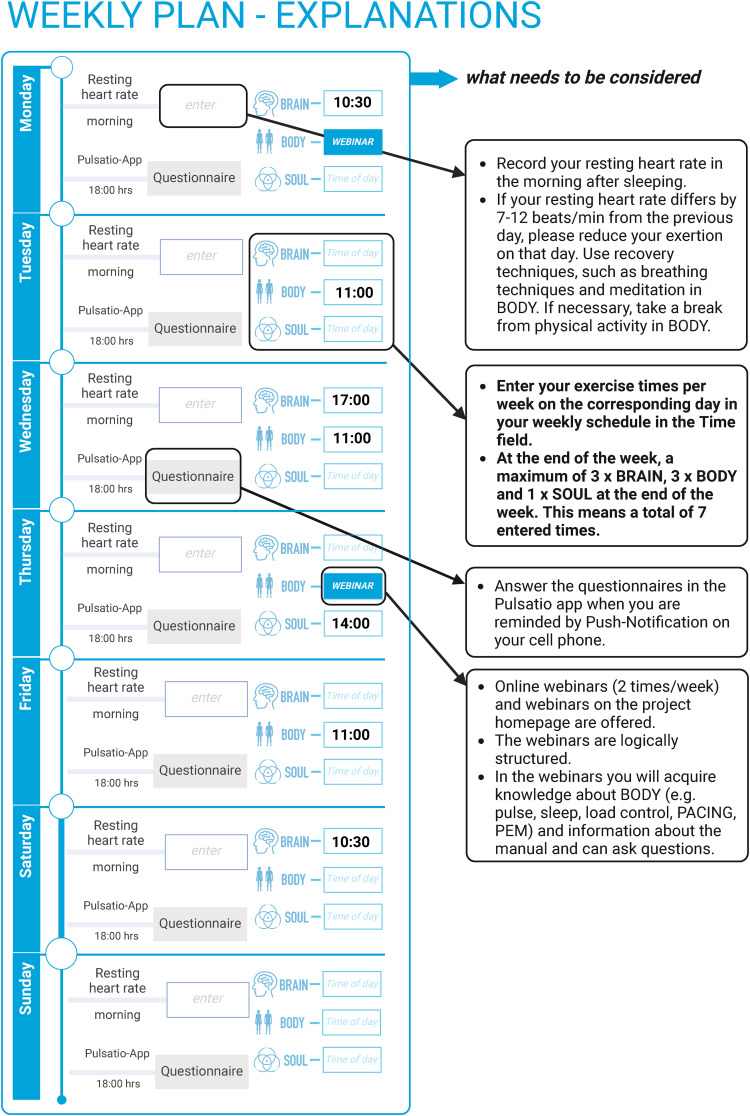
All participants receive a detailed manual with information on post-COVID-19 condition and associated symptoms, technical instructions for the telemedicine intervention, and instructions for the intervention tasks at the beginning of the telemedicine intervention. This manual serves as an advisor and project guide. It guides you step by step through the project and enables you to actively participate. Like a workbook at school, the handbook contains all the necessary information and at the same time sets tasks to be completed. The handbook consists of three parts: In the first part, it contains evidence-based “Information on post-COVID” presented in simple language. The second part summarizes the digital requirements for WATCH so that the digital offers of WATCH (NeuroNation MED app®, Pulsatio® app, fitness tracker, video consultation, webinars) can be used. The third part presents the 12-week plan and the three modules BODY, BRAIN and SOUL in more detail. For the BODY part, the 12-week plan (Figure 3) is prepared on 12 pages so that participants can follow a regular schedule week after week. The following entries are made in the weekly plan: Resting heart rate in the morning after sleeping in a sitting position, exercise times for BODY, BRAIN and SOUL. To make the scheduling feasible for patients, the manual contains 12 blank schedule pages for individual scheduling of the intervention for every participant. All intervention modules can be combined by the participants as self-training in a weekly schedule, which patients can to fit their personal exertion limits and other activities. Participants are instructed in creating such individual schedules (Table 2).
Figure 3.
Overview of the weekly schedule (excerpt from the manual).
Table 2.
Parameters of general intervention prescription for the modules in WATCH.
| Module | Duration | Frequency | Density | Total duration | Webinar |
|---|---|---|---|---|---|
| Single session | per week | Distribution/day | 12 weeks | ||
| [min] | [Sessions|min] | (Maximum two modules/day) | Number|hours | Number|hours | |
| BRAIN | 30 | 3|90 | 1 | 36|18 | 1|30 min |
| BODY | 15 | 3|45 | 1 | 36|9 | |
| SOUL | 30 | 1|30 | 1/wo | 8|4 | |
| Total | 75 | 7|165 | 2 | 80|31 |
Participants are given access to the project homepage at the beginning of the intervention, where the manual is available in digital format; additionally, video content regarding post-COVID-19 knowledge and video instructions for the intervention tasks are available. The homepage is structured analogously to the manual and contains digitally prepared content from the manual and the digital guided sequences for BODY e.g., videos on breath-control exercises (e.g., https://www.youtube.com/watch?v = Hf1djad0jbE&t = 2s), meditation and yoga (e.g., https://www.youtube.com/watch?v = CyQnVY3phc8&t = 3s). In addition, the homepage contains the recurring schedule for the webinars (dial-in data, table of contents and e-mail support) and recorded webinars on the topics. On Monday, all new participants receive the basic explanations of the program. These webinars are repeated every 3 weeks. Monday-Webinar 1: The WATCH project and the Body Intervention, Monday-Webinar 2: PACING, Monday-Webinar 3: Introduction to the tracker data. On Thursday, the webinars will be held on specific topics. Table 3 provides an overview of the content of the WATCH webinar for Thursday. In webinars patients receive further information on how to use pacing strategies correctly and how to recognize early warning signs of worsening symptoms. Objective data such as resting heart rate are also used. In these webinars, anonymous, group-aggregated data from the Pulsatio® app are used to explain the theoretical background of pacing, PEM, and heart rate monitoring. Participants are given opportunities to ask the project personnel questions anonymously.
Table 3.
Overview of the content of the WATCH-webinar (every Thursday at 16:30).
| Week | Theme | Date (Thursday) |
|---|---|---|
| 1 | The components of PACING | July 04 |
| 2 | Respiratory regulation with and without position change | July 11 |
| 3 | PACING/PEM component 1 and diaphragm self-mobilization | July 25 |
| 4 | Body activity - standing up / sitting down (sit-to-stand) | August 01 |
| 5 | What do the tracker data mean? | August 08 |
| 6 | PACING component 2 and cyclical breathing | August 15 |
| 7 | Energy, metabolism, PEM and preparation for Body | August 22 |
| 8 | PACING/PEM component 3 and 4-7-8 breathing | August 29 |
| 9 | Nutrition | September 05 |
| 10 | PACING/PEM component 4 and pranayama breathing | September 12 |
| 11 | What happens after WATCH? | September 19 |
| 12 | What do I take away from WATCH? | September 26 |
To ensure knowledge communication for all participants, the webinars are recorded and made available as an on-demand-service on the project homepage.
Methods: data collection, management, and analysis
Outcome measures
As the primary outcome, we chose the difference in quality of life, as defined by the SF36-PCS subscore between week 1 and week 12. A difference of 2.5 points is considered as clinically relevant (see above). The SF36 is delivered to the patients at each of the three visits in the post-COVID-19 outpatient clinic in paper questionnaire format and twice between visits via the Pulsatio® app.
Further variables collected during the project include heart rate, sleep quality, and steps walked, all of which are collected continuously for 24 weeks with the Pulsatio® app and the wearable devices provided. Additionally, questionnaires for fatigue (Fatigue Assessment Scale 37 ), depression (Patient Health Questionnaire 38 ), sleep quality, 39 working ability, 40 cognitive failures, 41 and PEM are delivered via the Pulsatio® App. The detailed timeline is provided in Table 4.
Table 4.
Overview of questionnaires used in the WATCH module and the timepoints of assessment.
| Variables and parameters | Measurement time | Instrument | Items (number) |
|---|---|---|---|
| Primary endpoint | |||
| Physical health | T0p, W4a, W8a, T1p, T2p | SF-36 Physcial component Subscore (PCS) | 36 |
| Secondary endpoints / Ecological momentary assessment (EMA) - diagnostic equipment in the bus | |||
| Mental health | T0p, W4a, W8a, T1p, T2p | SF-36 Mental component Subscore (MCS) | 36 |
| Tablet-based concentration, attention and memory test | T0, T1, T2 | Oxford screening Plus (OCS-plus) | |
| Test to assess mild cognitive dysfunction | T0, T1, T2 | Montreal Cognitive Assessment (MoCA) | |
| Cardiorespiratory physical performance | T0, T1, T2 | 1-Minute Sit-to-Stand-Test inklusive Herzfrequenz, Sauerstoffsättigung | |
| Muscular physical performance | T0, T1, T2 | Measurement of hand grip strength | |
| Ventilation disorders | T0, T1, T2 | Lung monitoring with electrical impedance tomography | |
| Ecological momentary assessment (EMA) | |||
| Screening Depression | T0p, W6a, T1p, W18a, T2p | Patient Health Questionnaire (PHQ-9) | 9 |
| Current coping efforts to cope with the illness (emotional, cognitive) | T0p, W6a, T1p, T2p | Essener Fragebogen zur Krankheitsverarbeitung (EFK) | 45 |
| Status and severity of mental disorders | T0p, W6a, W12p, W24p | ICD-10-Symptom-Rating-Scale (ISR) | 29 |
| Physical and mental fatigue | T0p, W2a, W4a, W6a, …T1a, …T2a | Fatigue assessment scale (FAS) | 10 |
| Daily cognitive problems | T0p, W3a, W6a, W9a, …T1a, …T2a | Cognitive failure questionaire (CFQ) | 25 |
| Sleep quality | T0p, W2a, W4a, W6a, …T1a, …T2a | Pittsburgh Sleep Quality Index (PSQI) | 19 |
| Immunological recovery and stress | T0p, W2a, W4a, W6a, …T1a, …T2a | Immunological inventory acute recovery, stress (II-EBZ) | 18 |
| Post exertional malaise | T0a-T2a (wöchentlich) | Post exertional mailaise (PEM) | 10 |
| Current and future subjective work ability | T0p, T1p, T2p | Work Ability Index (WAI) | 10 |
| Interview: Usage behavior, adherence, acceptance | W3i, W9i, W18i | Survey by the DScK (interviews): 10–12 per BRAIN, BODY, SOUL | 30–45 min |
| Wearable-Data | |||
| Physical exertion: number of steps, metabolic equivalent, distance traveled per day | Continuously | Pulsatio-App | |
| Physical demands: resting heart rate night and day, sleep regularity and duration, peak heart rate day | Continuously | Pulsatio-App | |
Variables, outcomes, measurement times, Instruments and Items in WATCH. Measurement time: T0: bus visit 1a; T1: bus visit 2; T2: bus visit 3; week (W); p: paper; a: Pulsatio app; i: Interview telephone or video
Changes in the parameters of physical and cognitive assessment (sit to stand test, handgrip, MoCA, OCS-plus, subjective and objective exertion) between the three visits in the PoCo bus will be evaluated as secondary outcomes. Acceptance and user experience of the WATCH concept will be assessed through structured interviews performed in a subgroup of participants during the course of the project as well as interviews and a survey with general practitioners recruiting participants for the project and with project personnel.
Wearables and Pulsatio® App
A core aspect of evaluating the aims of WATCH is therapy guidance through continuous heart rate monitoring. Therefore, every participant in WATCH receives a wearable (Garmin VivoSmart4®) for continuous monitoring of heart rate, daily steps walked, and sleep quality. If a participant already uses a smartwatch or similar device compatible with the Pulsatio® app, that device can instead be used within the project. The data measured by the device are recorded with the Pulsatio® app, which is the study-version of the former Corona-Datenspende-App. All participants are instructed in using the device and the Pulsatio® app at the beginning of the project, and receive a unique QR code to identify themselves in the app and ensure linkage with the project database.
Beyond data collection, the Pulsatio® app is used for delivering questionnaires to assess the feasibility and effectiveness of WATCH throughout the project duration. Participants can directly answer the questionnaires in the app. A detailed list of questionnaires and time points is provided in Table 4. Furthermore, participants can monitor their own heart rate, sleep quality, and walked steps in the Pulsatio® app, and use the information when performing the multimodal intervention.
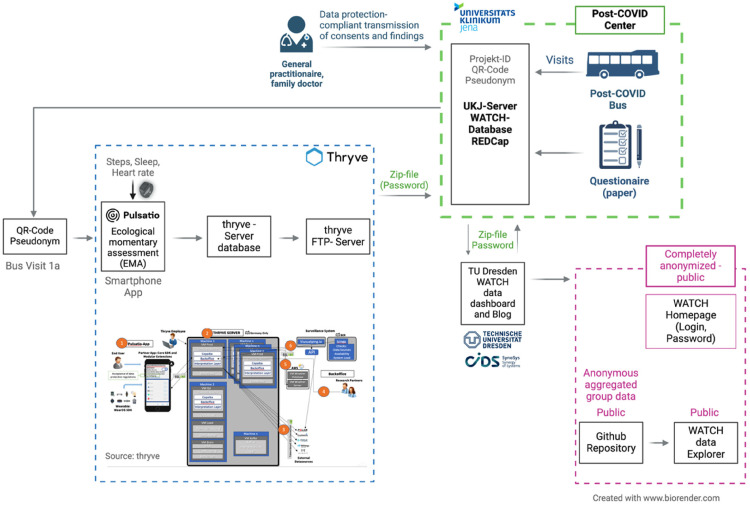
The detailed data flow is illustrated in Figure 4. The vital parameter data are transferred to a Germany-based server to ensure adherence to data protection agreements and are analyzed in two ways. First, the QR code allows individual, pseudonymized data to be combined with information collected in the PoCo bus and demographic data. Second, data will be anonymized and aggregated for use in webinars for instructing patients.
Figure 4.
Detailed data flow within WATCH and the Pulsatio® app.
Statistical analysis
As described above, the primary outcome measure is the absolute change in SF36-PCS score between week 1 and week 12. Given the multimodal nature of the WATCH intervention and the typical symptoms of cognitive dysfunction (BRAIN), physical stress insufficiency (BODY), and depressive moods (SOUL) reported by patients with post-COVID-19 condition, we have intensively examined whether additional endpoints in (neuro-)psychological health might be upgraded to primary endpoints.15,42,43 We decided on testing hierarchically ordered hypotheses as a framework for the confirmatory test with strict control of the familywise error. 44
Thus, the SF36-PCS is initially considered the primary endpoint. If a significant difference (α = 0.05) is observed between groups, the confirmatory analysis will be repeated for the Mental Component Score of the SF-36 (SF36-MCS). According to the principle of hierarchical testing, no adjustment of the α-level is required. On the basis of the recommended procedure for handling potentially confounding variables, the two groups will be compared through descriptive statistics considering the variables age, sex, pre-existing conditions, GAF scale (acute illness and post-COVID-19 severity), and COVID-19 vaccination status. The primary endpoints will be analyzed using a linear mixed regression model, with treatment arm and the SF36-PCS/ SF36-MCS from week one as fixed effects, and the district stratum as a random effect, adjusting for further covariates in case of a misbalance between groups (see below).
Only patients with no missing values for the required variables will be considered. The analysis will be performed according to the intention-to-treat concept. Dropouts and the corresponding reasons for early withdrawal from the study, if voluntary information is provided, will be reported. Accordingly, participants who do not fully implement the intervention will also be analyzed, provided that the data necessary for the analysis are available.
In addition to the main analysis, we will perform a per-protocol analysis, taking into account the number of attended intervention appointments.
The protocol does not include interim analysis; therefore, we do not have any preliminary data until the end of the study.
Additional analyses
The secondary endpoints will also be analyzed with a linear mixed regression model. All additional analyses will be exploratory; effect estimates will be reported with confidence intervals, but no significance testing will be performed.
The regression analyses will be repeated by considering variables that are imbalanced between groups. A difference of more than two percentage points in the categorical variables, a difference in the median age of more than 2 years, and a difference in the mean GAF scale of more than two points will be considered to indicate imbalance.
Furthermore, subgroup analyses will be conducted with stratification by sex as well as certain comorbidities. To evaluate the sustainability of the intervention, the primary and secondary endpoints will be analyzed again 12 weeks post-intervention in the early intervention group (compared to the initial values).
Ethics
The protocol has been approved by the local ethics committee of Jena University Hospital (No 2022-2634-BO), and all patients will provide written informed consent before participating in the project.
Funding
The WATCH consortium is funded by the Innovationsfonds des Gemeinsamen Bundesausschusses in Germany (01NVF22114). The funder did not have any influence on the project design, and will have no influence on data interpretation and publication.
Discussion
The WATCH study is aimed at enhancing post-COVID-19 care by using a mobile ambulance and a multimodal telemedicine intervention. This innovative study involves the first integration of three distinct data streams: three-point measurements of patient data from a mobile post-COVID-19 clinic, weekly disease parameters via questionnaires, and, most importantly, daily data from wearable devices. An additional aim is improving disease management through patient education provided via manuals and webinars.
This study uses a multimodal intervention designed to address the most important and relevant symptoms in patients with post-COVID-19 condition, as reported by affected individuals. Data from our outpatient clinic have revealed that cognitive dysfunction, impaired physical fitness including fatigue, and depressive disorders are the most relevant problems in these patients.4,8,42 A recent Delphi statement has emphasized the importance of interdisciplinary and individual concepts in therapy for post-COVID-19 condition. 43 WATCH provides excellent opportunities for patients to individualize their therapeutic schedules. Patients can combine different modules throughout the week according to their daily routines and current feelings/exercise capacity, thus creating their own schedules. The basic rules for this schedule are simple and are explained to the patients at the beginning of the intervention. As part of the intervention, the NeuroNation Med® app used for the BRAIN module automatically adjusts to the individual skills of the patients. In the BODY module, patients can tailor their daily tasks according to their symptoms of PEM and exercise capacity. By using these mechanisms, we aim to prevent triggering of exercise-induced PEM episodes. SOUL improves disease management and the psychological burden of disease.
Current concepts for PEM have described heart rate as a potentially easily measured parameter for avoiding PEM. 44 However, these recommendations have been based on traditional concepts in which the heart rate is measured at single points. The use of wearable devices for continuous measurement provides an opportunity to gather information on heart rate even in special situations, such as during exercise, and therefore has the potential to enhance knowledge regarding the value of heart rate as a diagnostic tool, and consequently enable better activity management concepts in the future. Despite these theoretical benefits and the current broad availability of these devices in the population, data regarding device usability in therapeutic concepts remain lacking. 44
We have chosen quality of life as the primary endpoint in WATCH, as assessed via the SF36, an established scoring system. In previous studies, we have observed significant differences in people reporting any symptoms after SARS-CoV-2 infection and those with relevant symptoms affecting their daily lives 5 ; the latter group requires medical help. These two groups are effectively distinguished by the SF36-PCS; therefore because we aimed to include the second group, we have chosen the SF-36 as an inclusion criterion. Additionally, the cutoff of 45 points was selected on the basis of this post-COVID-19 cohort and data from our outpatient clinic.5,10 Another aspect of WATCH is educating patients in handling PEM in their daily lives; this education is delivered through regular webinars, and disease processing is enhanced through a video-based psychological intervention.
In planning this study, we considered various approaches to measure clinical symptoms. The use of multiple measurement approaches in clinical trials is a well-established concept aimed at providing a comprehensive understanding of intervention or treatment effectiveness. The use of multiple methods to assess key outcomes is intended to elucidate treatment outcomes, thus facilitating the development of more effective interventions. 45 Conducting self-assessments or measurements at different time points and using diverse instruments to evaluate the same construct can mitigate biases associated with self-report measures and offer a more comprehensive depiction of disease changes during an intervention. The evaluation of multiple measurement approaches can provide additional insights into responders and diverse analysis options for interventions, including response timing. 46 Notably, ecological momentary assessments offer the potential to gather more detailed information about intervention effects than can be achieved through relying on pre- and post-results, or even weekly symptom point measurements. In order to tailor the intervention to the needs of the patients, they were already involved in the planning of the study.
Conclusion
In summary, WATCH integrates a mobile post-COVID-19 outpatient clinic with multimodal holistic telemedicine interventions to provide high-quality therapy, particularly for patients with post-COVID-19 condition in rural regions. Highly innovative elements include the incorporation of continuous daily monitoring of physical performance through wearable devices, considering PEM together with self-education components.
Footnotes
Contributorship: AS had the original idea for the project. All authors jointly developed the project and will conduct it jointly. All authors wrote the MS and approved the final version.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics: The protocol has been approved by the local ethics committee of Jena University Hospital (No 2022-2634-BO), and all patients will provide written informed consent before participating in the project.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The WATCH consortium is funded by the Innovationsfonds des Gemeinsamen Bundesausschusses in Germany (01NVF22114). The funder did not have any influence on the project design, and will have no influence on data interpretation and publication.
Guarantor: Corresponding author
ORCID iDs: Philipp A Reuken https://orcid.org/0000-0002-7696-475X
Bettina Zippel-Schultz https://orcid.org/0000-0003-4791-7222
References
- 1.Lundberg-Morris L, Leach S, Xu Y, et al. COVID-19 vaccine effectiveness against post-COVID-19 condition among 589 722 individuals in Sweden: population based cohort study. Br Med J 2023; 383: e076990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021; 27: 601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis 2022; 22: e102–e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis HE, McCorkell L, Vogel JMet al. et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 2023; 21: 133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giszas B, Trommer S, Schüßler N, et al. Post-COVID-19 condition is not only a question of persistent symptoms: structured screening including health-related quality of life reveals two separate clusters of post-COVID. Infection 2023; 51: 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diexer S, Klee B, Gottschick C, et al. Association between virus variants, vaccination, previous infections, and post-COVID-19 risk. Int J Infect Dis 2023; 136: 14–21. [DOI] [PubMed] [Google Scholar]
- 7.Reuken PA, Besteher B, Finke K, et al. Longterm course of neuropsychological symptoms and ME/CFS after SARS-CoV-2-infection: a prospective registry study. Eur Arch Psychiatry Clin Neurosci [Internet] 2023. Aug 18; 1–8. DOI: 10.1007/s00406-023-01661-3 . [DOI] [PubMed] [Google Scholar]
- 8.Jason LA, Islam MF, Conroy K, et al. COVID-19 symptoms over time: comparing long-haulers to ME/CFS. Fatigue Biomed Health Behav 2021; 9: 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kedor C, Freitag H, Meyer-Arndt L, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun 2022; 13: 5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stallmach A, Kesselmeier M, Bauer M, et al. Comparison of fatigue, cognitive dysfunction and psychological disorders in post-COVID patients and patients after sepsis: is there a specific constellation? Infection 2022; 50: 661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemhöfer C, Sturm C, Loudovicki-Krug D, et al. Quality of life and ability to work of patients with Post-COVID syndrome in relation to the number of existing symptoms and the duration since infection up to 12 months: a cross-sectional study. Qual Life Res 2023 Jul; 32(7): 1991–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu L, Valencia IJ, Garvert DWet al. et al. Deconstructing post-exertional malaise in myalgic encephalomyelitis/ chronic fatigue syndrome: a patient-centered, cross-sectional survey. PloS One 2018; 13: e0197811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill CF, Powers BW, Jain SH, et al. Mobile health clinics in the era of reform. Am J Manag Care 2014; 20: 261–264. [PubMed] [Google Scholar]
- 14.Malone NC, Williams MM, Smith Fawzi MC, et al. Mobile health clinics in the United States. Int J Equity Health 2020; 19: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stallmach A, Katzer K, Besteher B, et al. Mobile primary healthcare for post-COVID patients in rural areas: a proof-of-concept study. Infection 2023; 51: 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohannon RW, Crouch R. 1-Minute sit-to-stand test: systematic review of procedures, performance, and clinimetric properties. J Cardiopulm Rehabil Prev 2019; 39: 2–8. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton GF, McDonald C, Chenier TC. Measurement of grip strength: validity and reliability of the sphygmomanometer and jamar grip dynamometer. J Orthop Sports Phys Ther 1992; 16: 215–219. [DOI] [PubMed] [Google Scholar]
- 18.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment: MOCA: a BRIEF SCREENING TOOL FOR MCI. J Am Geriatr Soc 2005; 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 19.Demeyere N, Haupt M, Webb SS, et al. Introducing the tablet-based Oxford cognitive screen-plus (OCS-plus) as an assessment tool for subtle cognitive impairments. Sci Rep 2021; 11: 8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao S, Martin EM, Reuken PA, et al. Long COVID is associated with severe cognitive slowing: a multicentre cross-sectional study. eClinicalMedicine 2024; 68: 102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katzer K, Gremme Y, Moshmosh Alsabbagh M, et al. Electrical impedance tomography (EIT) in a patient suffering from post-COVID syndrome with dyspnea: a case report. Diagnostics 2022; 12: 2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strobach T, Huestegge L. Evaluating the effectiveness of commercial brain game training with working-memory tasks. J Cogn Enhanc 2017; 1: 539–558. [Google Scholar]
- 23.Ophey A, Giehl K, Rehberg S, et al. Effects of working memory training in patients with Parkinson’s disease without cognitive impairment: a randomized controlled trial. Parkinsonism Relat Disord 2020; 72: 13–22. [DOI] [PubMed] [Google Scholar]
- 24.Bartlett DM, Govus A, Rankin T, et al. The effects of multidisciplinary rehabilitation on neuroimaging, biological, cognitive and motor outcomes in individuals with premanifest Huntington’s disease. J Neurol Sci 2020; 416: 117022. [DOI] [PubMed] [Google Scholar]
- 25.Salman D, Vishnubala D, Le Feuvre P, et al. Returning to physical activity after COVID-19. Br Med J 2021; 372: m4721. [DOI] [PubMed] [Google Scholar]
- 26.Puta C, Haunhorst S, Bloch W. Post-akutes COVID-19 (“long-COVID”): andauernde symptome, mögliche ursachen und symptomgeleitetes post-akut COVID-19 management zur wiedererlangung der körperlichen leistungsfähigkeit (scoping review). Sports Orthop Traumatol 2021; 37: 214–225. [Google Scholar]
- 27.Jason LA, Melrose H, Lerman A, et al. Managing chronic fatigue syndrome: overview and case study. AAOHN J Off J Am Assoc Occup Health Nurses 1999; 47: 17–21. [PubMed] [Google Scholar]
- 28.Jason L, Benton M, Torres-Harding Set al. et al. The impact of energy modulation on physical functioning and fatigue severity among patients with ME/CFS. Patient Educ Couns 2009; 77: 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghali A, Lacombe V, Ravaiau C, et al. The relevance of pacing strategies in managing symptoms of post-COVID-19 syndrome. J Transl Med 2023; 21: 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abonie US, Hoekstra F, Seves BL, et al. Associations between activity pacing, fatigue, and physical activity in adults with multiple sclerosis: a cross sectional study. J Funct Morphol Kinesiol 2020; 5: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garber CE, Blissmer B, Deschenes MR, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43: 1334–1359. [DOI] [PubMed] [Google Scholar]
- 32.Sang L, Zhao Z, Lin Z, et al. A narrative review of electrical impedance tomography in lung diseases with flow limitation and hyperinflation: methodologies and applications. Ann Transl Med 2020; 8: 1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021; 38: 101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krisenintervention und Suizidverhütung Gernot Sonneck, Nestor Kapusta, Gerald Tomandl, Martin Voracek (Herausgeber) Buch | Softcover 352 Seiten 2016 | 3. aktual. Aufl. UTB (Verlag) 978-3-8252-4641-9 (ISBN). In.
- 35.Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977; 196: 129–136. [DOI] [PubMed] [Google Scholar]
- 36.Kabat-Zinn J. Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. New York: Bantam Dell, 2013. ISBN 978-0345539724. [Google Scholar]
- 37.Vries J, Michielsen H, Heck GLet al. et al. Measuring fatigue in sarcoidosis: the fatigue assessment scale (FAS). Br J Health Psychol 2004; 9: 279–291. [DOI] [PubMed] [Google Scholar]
- 38.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16: 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193–213. [DOI] [PubMed] [Google Scholar]
- 40.Tuomi K, Ilmarinen J, Jahkola A, et al. Work ability index. 2nd ed. Helsinki: Finnish Institute of Occupational Health, 1998. [Google Scholar]
- 41.Broadbent DE, Cooper PF, FitzGerald Pet al. et al. The cognitive failures questionnaire (CFQ) and its correlates. Br J Clin Psychol 1982; 21: 1–16. [DOI] [PubMed] [Google Scholar]
- 42.Han Q, Zheng B, Daines Let al. et al. Long-Term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens 2022; 11: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rochmawati E, Iskandar AC, Kamilah F. Persistent symptoms among post-COVID -19 survivors: a systematic review and meta-analysis. J Clin Nurs 2024; 33: 29–39. [DOI] [PubMed] [Google Scholar]
- 44.Marcus R, Eric P, Gabriel KR. On closed testing procedures with special reference to ordered analysis of variance. Biometrika 1976; 63: 655–660. [Google Scholar]
- 45.Kazdin AE. Single-case research designs: Methods for clinical and applied settings. Oxford, UK: Oxford University Press, 2017. [Google Scholar]
- 46.Vickerstaff V, Ambler G, Omar RZ. A comparison of methods for analysing multiple outcome measures in randomised controlled trials using a simulation study. Biom J 2021; 63: 599–615. [DOI] [PMC free article] [PubMed] [Google Scholar]