Abstract
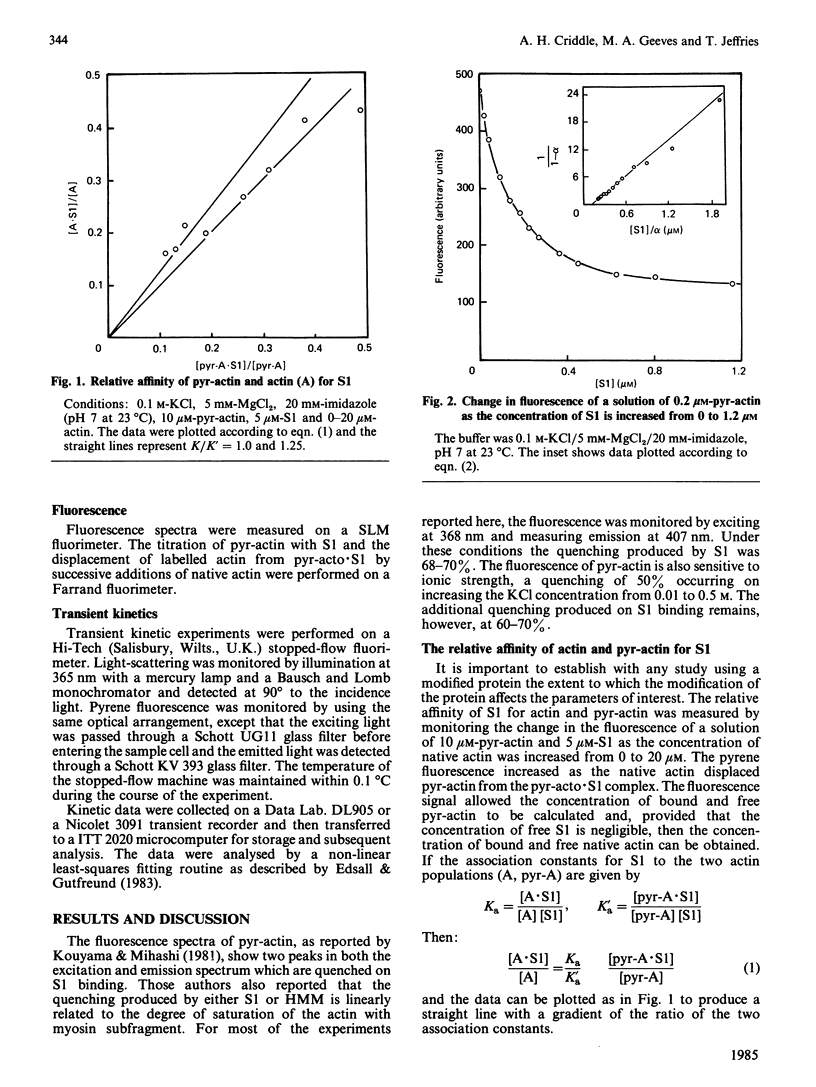
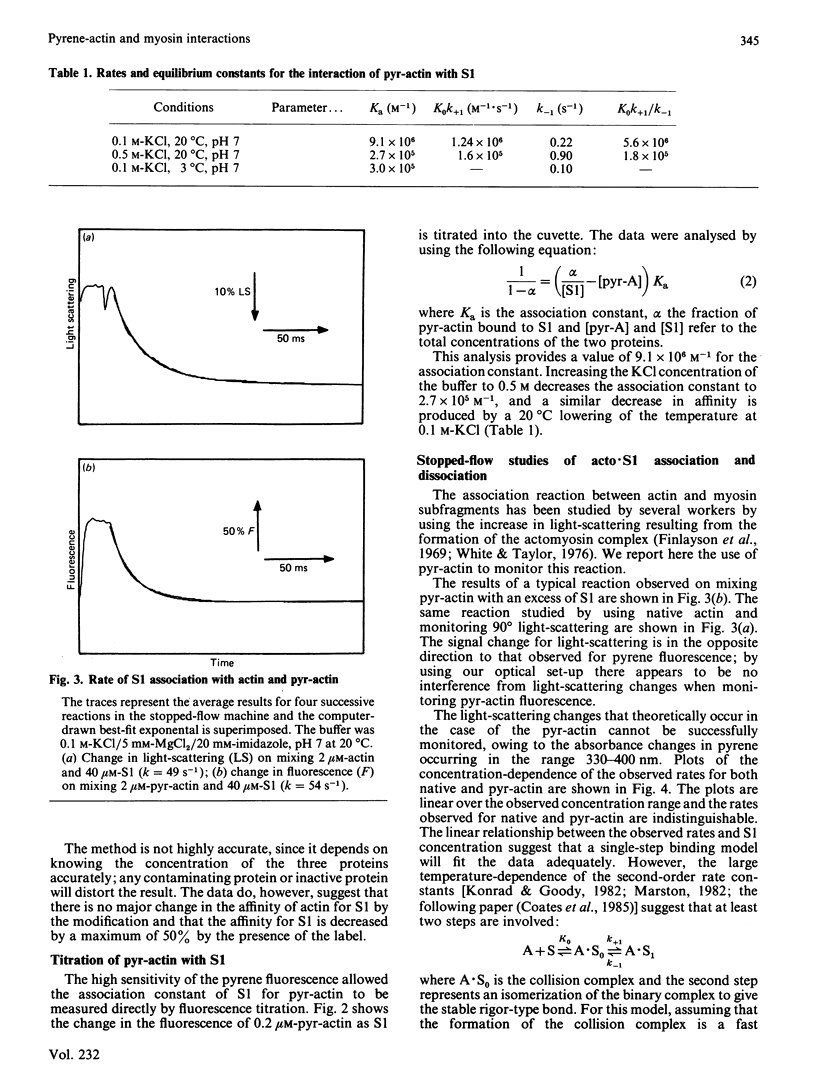
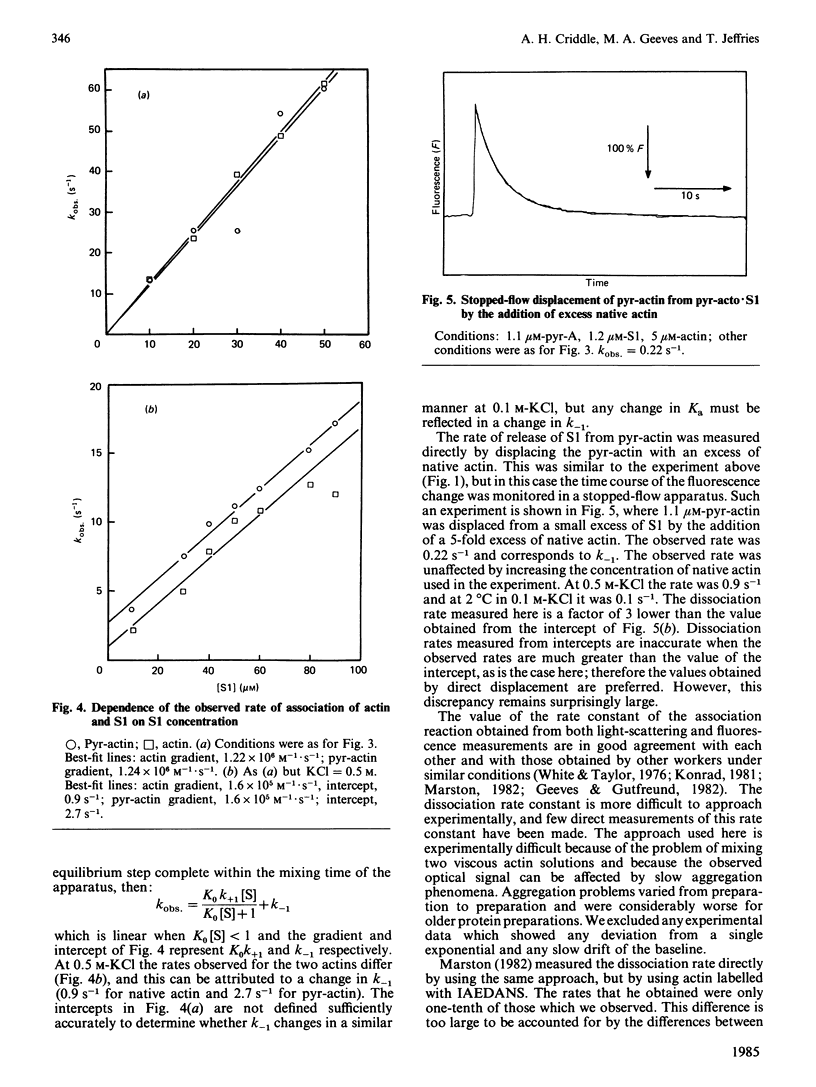
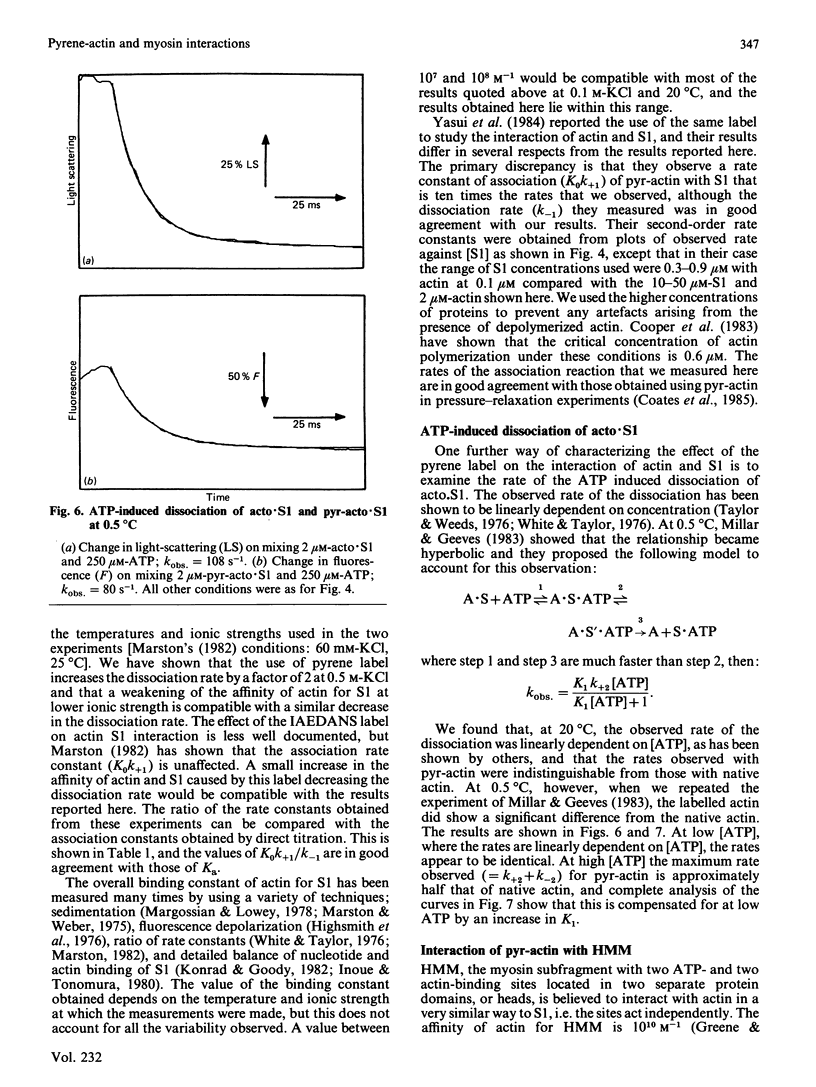
A pyrene label attached to Cys-374 of actin has been shown to be a useful probe for monitoring the interaction of actin with myosin subfragments [Kouyama & Mihashi (1981) Eur. J. Biochem. 114, 33-38]. We report that the presence of this label decreases the affinity of actin for myosin subfragment 1 by less than a factor of 2. The rate of actin binding is unaffected by the label and the dissociation rate is increased by up to a factor of 2. Both the rate of actin binding to, and the rate of actin dissociation from, heavy meromyosin show two phases when monitored by pyrene fluorescence. Thin filiments reconstituted from pyrene-labelled actin show a 5% increase in pyrene fluorescence on binding Ca2+.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
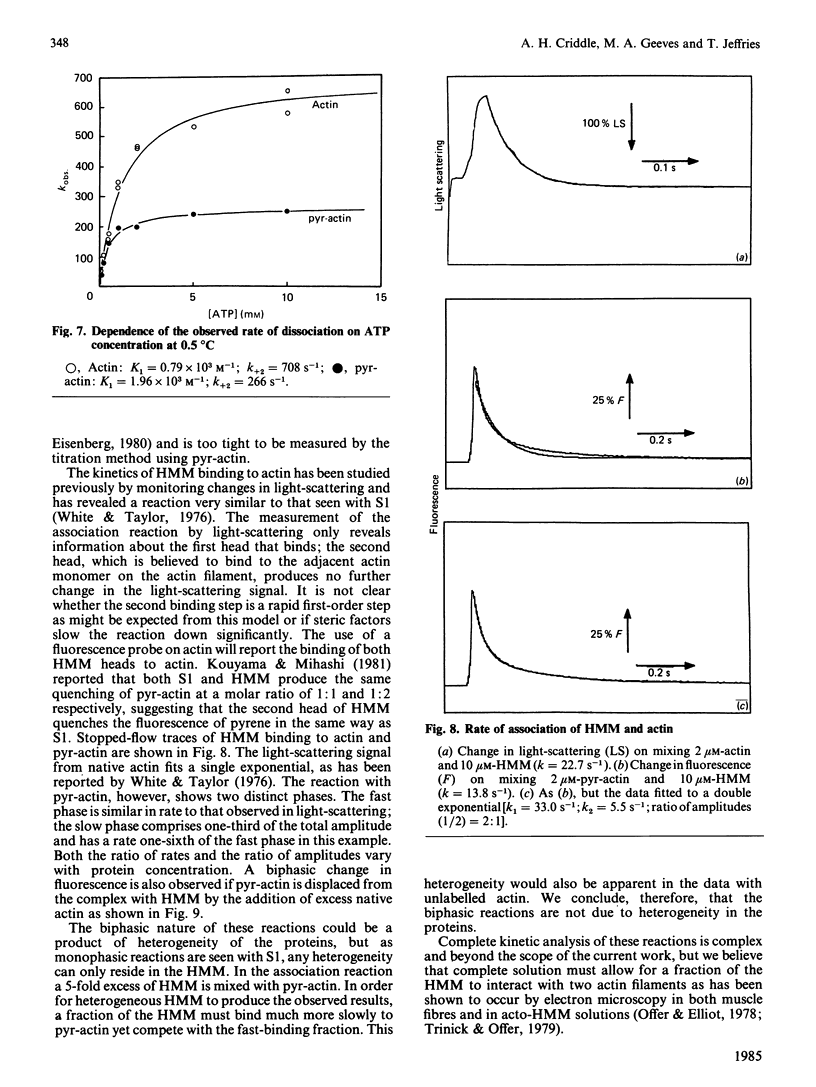
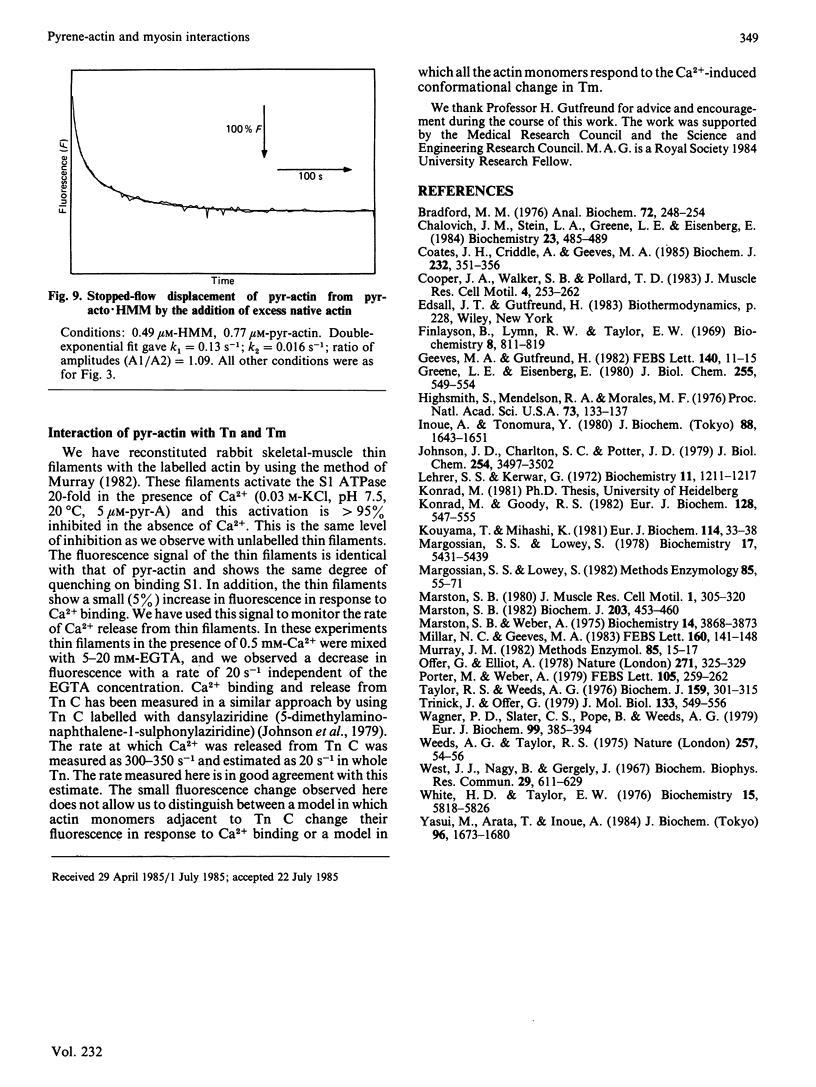
- Coates J. H., Criddle A. H., Geeves M. A. Pressure-relaxation studies of pyrene-labelled actin and myosin subfragment 1 from rabbit skeletal muscle. Evidence for two states of acto-subfragment 1. Biochem J. 1985 Dec 1;232(2):351–356. doi: 10.1042/bj2320351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Walker S. B., Pollard T. D. Pyrene actin: documentation of the validity of a sensitive assay for actin polymerization. J Muscle Res Cell Motil. 1983 Apr;4(2):253–262. doi: 10.1007/BF00712034. [DOI] [PubMed] [Google Scholar]
- Finlayson B., Lymn R. W., Taylor E. W. Studies on the kinetics of formation and dissociation of the actomyosin complex. Biochemistry. 1969 Mar;8(3):811–819. doi: 10.1021/bi00831a008. [DOI] [PubMed] [Google Scholar]
- Geeves M. A., Gutfreund H. The use of pressure perturbations to investigate the interaction of rabbit muscle myosin subfragment 1 with actin in the presence of MgADP. FEBS Lett. 1982 Apr 5;140(1):11–15. doi: 10.1016/0014-5793(82)80509-7. [DOI] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. The binding of heavy meromyosin to F-actin. J Biol Chem. 1980 Jan 25;255(2):549–554. [PubMed] [Google Scholar]
- Highsmith S., Mendelson R. A., Morales M. F. Affinity of myosin S-1 for F-actin, measured by time-resolved fluorescence anisotropy. Proc Natl Acad Sci U S A. 1976 Jan;73(1):133–137. doi: 10.1073/pnas.73.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A., Tonomura Y. Dissociation of acto-H-meromyosin and that of acto-subfragment-1 induced by adenyl-5'-yl-imidodiphosphate: evidence for a ternary complex of F-actin, myosin head, and substrate. J Biochem. 1980 Dec;88(6):1643–1651. doi: 10.1093/oxfordjournals.jbchem.a133140. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Charlton S. C., Potter J. D. A fluorescence stopped flow analysis of Ca2+ exchange with troponin C. J Biol Chem. 1979 May 10;254(9):3497–3502. [PubMed] [Google Scholar]
- Konrad M., Goody R. S. Kinetic and thermodynamic properties of the ternary complex between F-actin, myosin subfragment 1 and adenosine 5'-[beta, gamma-imido]triphosphate. Eur J Biochem. 1982 Nov 15;128(2-3):547–555. doi: 10.1111/j.1432-1033.1982.tb07000.x. [DOI] [PubMed] [Google Scholar]
- Kouyama T., Mihashi K. Fluorimetry study of N-(1-pyrenyl)iodoacetamide-labelled F-actin. Local structural change of actin protomer both on polymerization and on binding of heavy meromyosin. Eur J Biochem. 1981;114(1):33–38. [PubMed] [Google Scholar]
- Lehrer S. S., Kerwar G. Intrinsic fluorescence of actin. Biochemistry. 1972 Mar 28;11(7):1211–1217. doi: 10.1021/bi00757a015. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Interaction of myosin subfragments with F-actin. Biochemistry. 1978 Dec 12;17(25):5431–5439. doi: 10.1021/bi00618a017. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982;85(Pt B):55–71. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- Marston S. B. Evidence for an altered structure of actin-S1 complexes when Mg-adenylylimidodiphosphate binds. J Muscle Res Cell Motil. 1980 Sep;1(3):305–320. doi: 10.1007/BF00711933. [DOI] [PubMed] [Google Scholar]
- Marston S. B. The rates of formation and dissociation of actin-myosin complexes. Effects of solvent, temperature, nucleotide binding and head-head interactions. Biochem J. 1982 May 1;203(2):453–460. doi: 10.1042/bj2030453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S., Weber A. The dissociation constant of the actin-heavy meromyosin subfragment-1 complex. Biochemistry. 1975 Aug 26;14(17):3868–3873. doi: 10.1021/bi00688a021. [DOI] [PubMed] [Google Scholar]
- Millar N. C., Geeves M. A. The limiting rate of the ATP-mediated dissociation of actin from rabbit skeletal muscle myosin subfragment 1. FEBS Lett. 1983 Aug 22;160(1-2):141–148. doi: 10.1016/0014-5793(83)80954-5. [DOI] [PubMed] [Google Scholar]
- Murray J. M. Hybridization and reconstitution of the thin filament. Methods Enzymol. 1982;85(Pt B):15–17. doi: 10.1016/0076-6879(82)85006-4. [DOI] [PubMed] [Google Scholar]
- Offer G., Elliott A. Can a myosin molecule bind to two actin filaments? Nature. 1978 Jan 26;271(5643):325–329. doi: 10.1038/271325a0. [DOI] [PubMed] [Google Scholar]
- Porter M., Weber A. Non-cooperative response of actin-cystein 373 in cooperatively behaving regulated actin filaments. FEBS Lett. 1979 Sep 15;105(2):259–262. doi: 10.1016/0014-5793(79)80624-9. [DOI] [PubMed] [Google Scholar]
- Taylor R. S., Weeds A. G. The magnesium-ion-dependent adenosine triphosphatase of bovine cardiac Myosin and its subfragment-1. Biochem J. 1976 Nov;159(2):301–315. doi: 10.1042/bj1590301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinick J., Offer G. Cross-linking of actin filaments by heavy meromyosin. J Mol Biol. 1979 Oct 9;133(4):549–556. doi: 10.1016/0022-2836(79)90407-8. [DOI] [PubMed] [Google Scholar]
- Wagner P. D., Slater C. S., Pope B., Weeds A. G. Studies on the actin activation of myosin subfragment-1 isoezymes and the role of myosin light chains. Eur J Biochem. 1979 Sep;99(2):385–394. doi: 10.1111/j.1432-1033.1979.tb13267.x. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- West J. J., Nagy B., Gergely J. The effect of EDTA on spectral properties of ATP-, ADP-, and ITP-G-actin. Biochem Biophys Res Commun. 1967 Nov 30;29(4):611–616. doi: 10.1016/0006-291x(67)90530-x. [DOI] [PubMed] [Google Scholar]
- White H. D., Taylor E. W. Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry. 1976 Dec 28;15(26):5818–5826. doi: 10.1021/bi00671a020. [DOI] [PubMed] [Google Scholar]
- Yasui M., Arata T., Inoue A. Kinetic properties of binding of myosin subfragment-1 with F-actin in the absence of nucleotide. J Biochem. 1984 Dec;96(6):1673–1680. doi: 10.1093/oxfordjournals.jbchem.a134999. [DOI] [PubMed] [Google Scholar]



