Abstract
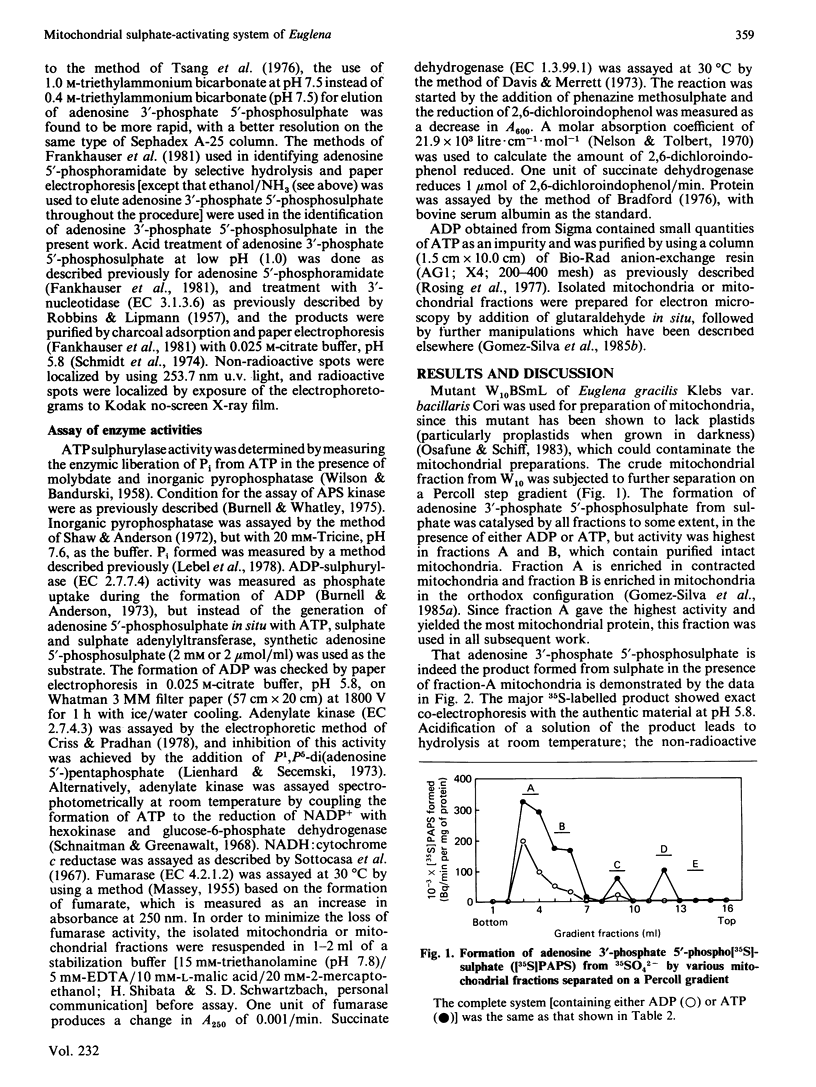
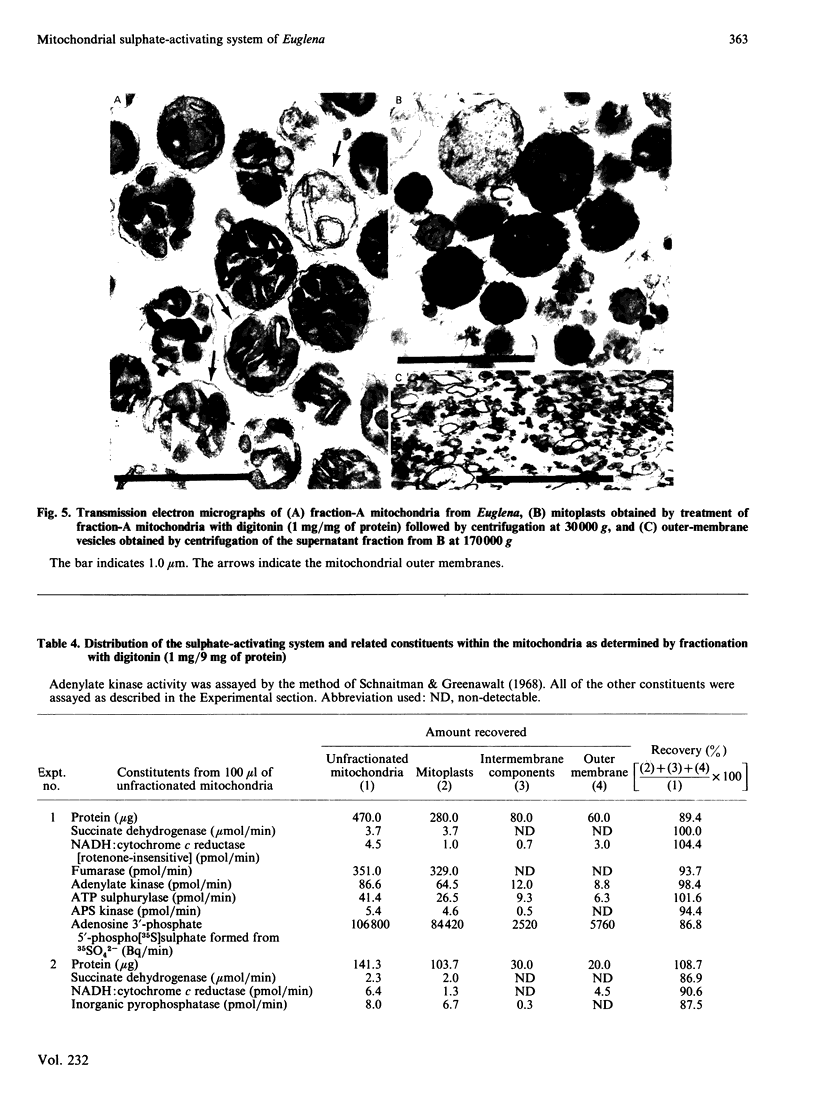
Intact mitochondria, obtained from Euglena gracilis Klebs var. bacillaris Cori mutant W10BSmL, which lacks plastids, and purified on Percoll density gradients, form adenosine 3'-phosphate 5'-phosphosulphate from sulphate. The optimal conditions include addition of 17 mM-Tricine/KOH, pH 7.6, 18 mM-MgCl2, 250 mM-sucrose, 5.66 mM-sodium ADP (or 0.94 mM-sodium ATP), 1 mM-K2SO4, carrier-free 35SO4(2-) (32.1 microCi) and 1.0 mg of mitochondrial protein in a total volume of 2.65 ml and incubation at 30 degrees C. Experiments with the inhibitor of adenylate kinase P1, P5-di(adenosine 5'-)pentaphosphate indicate that ATP is the preferred substrate for sulphate activation; ADP is utilized by conversion into ATP via adenylate kinase. ATP sulphurylase, adenylylsulphate kinase (APS kinase) and inorganic pyrophosphatase constitute the sulphate-activating system; ADP sulphurylase is undetectable. Fractionation of Euglena mitochondria with digitonin and centrifugation allowed the separation of outer-membrane vesicles and mitoplasts as judged by electron microscopy and selected enzymic markers. The detergent-labile association of the sulphate-activating system with the mitoplasts (similar to that of adenylate kinase), the fact that most of the adenosine 3'-phosphate 5'-phosphosulphate formed by intact mitochondria is found in the surrounding medium, and the ease with which nucleotide substrates reach the activating system in intact organelles, suggest that the enzymes of sulphate activation are located on the outer surface of the mitochondrial inner membrane.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM A., BACHHAWATBK Effect of light and streptomycin on the incorporation of sulfate into sulfolipids in Euglena gracilis. Biochim Biophys Acta. 1963 Feb 19;70:104–106. doi: 10.1016/0006-3002(63)90729-7. [DOI] [PubMed] [Google Scholar]
- Arron G. P., Henry L., Palmer J. M., Hall D. O. Superoxide dismutases in mitochondria from Helianthls tuberosus and Neurospora crassa. Biochem Soc Trans. 1976;4(4):618–620. doi: 10.1042/bst0040618. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brunold C., Schiff J. A. Studies of sulfate utilization of algae: 15. Enzymes of assimilatory sulfate reduction in euglena and their cellular localization. Plant Physiol. 1976 Mar;57(3):430–436. doi: 10.1104/pp.57.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell J. N., Anderson J. W. Adenosine diphosphate sulphurylase activity in leaf tissue. Biochem J. 1973 Jul;133(3):417–428. doi: 10.1042/bj1330417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell J. N., Whatley F. R. A new, rapid, and sensitive assay for adenosine 5'-phosphosulphate (APS) kinase. Anal Biochem. 1975 Sep;68(1):281–288. doi: 10.1016/0003-2697(75)90706-x. [DOI] [PubMed] [Google Scholar]
- Criss W. E., Pradhan T. K. Purification and characterization of adenylate kinase from rat liver. Methods Enzymol. 1978;51:459–467. doi: 10.1016/s0076-6879(78)51063-x. [DOI] [PubMed] [Google Scholar]
- Davies W. H., Mercer E. I., Goodwin T. W. Some observations on the biosynthesis of the plant sulpholipid by Euglena gracilis. Biochem J. 1966 Feb;98(2):369–373. doi: 10.1042/bj0980369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B., Merrett M. J. Malate dehydrogenase isoenzymes in division synchronized cultures of euglena. Plant Physiol. 1973 Jun;51(6):1127–1132. doi: 10.1104/pp.51.6.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. Isolation and properties of the outer membrane of plant mitochondria. Arch Biochem Biophys. 1975 Nov;171(1):117–123. doi: 10.1016/0003-9861(75)90014-4. [DOI] [PubMed] [Google Scholar]
- Fankhauser H., Schiff J. A., Garber L. J. Purification and properties of adenylyl sulphate:ammonia adenylyltransferase from Chlorella catalysing the formation of adenosine 5' -phosphoramidate from adenosine 5' -phosphosulphate and ammonia. Biochem J. 1981 Jun 1;195(3):545–560. doi: 10.1042/bj1950545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBel D., Poirier G. G., Beaudoin A. R. A convenient method for the ATPase assay. Anal Biochem. 1978 Mar;85(1):86–89. doi: 10.1016/0003-2697(78)90277-4. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Secemski I. I. P 1 ,P 5 -Di(adenosine-5')pentaphosphate, a potent multisubstrate inhibitor of adenylate kinase. J Biol Chem. 1973 Feb 10;248(3):1121–1123. [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E. Glycolate dehydrogenase in green algae. Arch Biochem Biophys. 1970 Nov;141(1):102–110. doi: 10.1016/0003-9861(70)90112-8. [DOI] [PubMed] [Google Scholar]
- Nissen P., Benson A. A. Choline Sulfate in Higher Plants. Science. 1961 Dec 1;134(3492):1759–1759. doi: 10.1126/science.134.3492.1759. [DOI] [PubMed] [Google Scholar]
- Osafune T., Schiff J. A. W10BSmL, a mutant of Euglena gracilis var. bacillaris lacking plastids. Exp Cell Res. 1983 Oct 15;148(2):530–535. doi: 10.1016/0014-4827(83)90176-3. [DOI] [PubMed] [Google Scholar]
- Palmieri F., Klingenberg M. Direct methods for measuring metabolite transport and distribution in mitochondria. Methods Enzymol. 1979;56:279–301. doi: 10.1016/0076-6879(79)56029-7. [DOI] [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Isolation and identification of active sulfate. J Biol Chem. 1957 Dec;229(2):837–851. [PubMed] [Google Scholar]
- Rosing J., Kayalar C., Boyer P. D. Evidence for energy-dependent change in phosphate binding for mitochondrial oxidative phosphorylation based on measurements of medium and intermediate phosphate-water exchanges. J Biol Chem. 1977 Apr 25;252(8):2478–2485. [PubMed] [Google Scholar]
- Schiff J. A. A green safelight for the study of chloroplast development and other photomorphogenetic. Methods Enzymol. 1972;24:321–322. doi: 10.1016/0076-6879(72)24079-4. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Abrams W. R., Schiff J. A. Reduction of adenosine 5'-phosphosulfate to cysteine in extracts from Chlorella and mutants blocked for sulfate reduction. Eur J Biochem. 1974 Sep 16;47(3):423–434. doi: 10.1111/j.1432-1033.1974.tb03709.x. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Erwin V. G., Greenawalt J. W. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1967 Mar;32(3):719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. H., Anderson J. W. Purification, properties and substrate specificity of adenosine triphosphate sulphurylase from spinach leaf tissue. Biochem J. 1972 Mar;127(1):237–247. doi: 10.1042/bj1270237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang M. L., Lemieux J., Schiff J. A., Bojarski T. B. Preparation of adenosine 5'-phosphosulfate (APS) from adenosine 3'-phosphate 5'-phosphosulfate (PAPS) prepared by an improved procedure. Anal Biochem. 1976 Aug;74(2):623–626. doi: 10.1016/0003-2697(76)90249-9. [DOI] [PubMed] [Google Scholar]
- Vignais P. V. Molecular and physiological aspects of adenine nucleotide transport in mitochondria. Biochim Biophys Acta. 1976 Apr 30;456(1):1–38. doi: 10.1016/0304-4173(76)90007-0. [DOI] [PubMed] [Google Scholar]
- WILSON L. G., BANDURSKI R. S. An enzymatic reaction involving adenosine triphosphate and selenate. Arch Biochem Biophys. 1956 Jun;62(2):503–506. doi: 10.1016/0003-9861(56)90151-5. [DOI] [PubMed] [Google Scholar]
- WILSON L. G., BANDURSKI R. S. Enzymatic reactions involving sulfate, sulfite, selenate, and molybdate. J Biol Chem. 1958 Oct;233(4):975–981. [PubMed] [Google Scholar]
- Zeldin M. H., Schiff J. A. RNA metabolism during light-induced chloroplast development in euglena. Plant Physiol. 1967 Jul;42(7):922–932. doi: 10.1104/pp.42.7.922. [DOI] [PMC free article] [PubMed] [Google Scholar]




