Abstract
Background
In long-term care facilities (LTCF), apathy is a prevalent issue, leading to cognitive decline, functional impairment, and increased mortality risk. Despite its significance, apathy often remains underrecognized and undermanaged in these settings. Recognizing and addressing the predictors of apathy is critical for early intervention and improved care outcomes.
Purpose
This study aims to assess the prevalence of apathy and identify its associated risk factors among newly admitted residents in the Canadian LTCF, using the InterRAI Minimum Data Set (MDS 2.0).
Methods
We conducted a cross-sectional analysis of MDS 2.0 admission assessment data between 2015 and 2019, covering 157,596 residents across six Canadian provinces and one territory. Apathy was measured using the Apathy Index of the MDS 2.0, with the biopsychosocial model guiding the analysis.
Results
The prevalence of apathy was 12.5% (19,758 individuals). The most significant predictors include cognitive impairments, specific age groups, hearing impairments, vision impairments, facility size and location.
Conclusions
The findings of this study underscore the need for tailored strategies in LTCF to address apathy, considering individual, institutional, and regional variations. Emphasis on environmental and personal factors is crucial in the management and prevention of apathy in these settings.
Keywords: Apathy, long-term care facilities, biopsychosocial model, InterRAI, MDS 2.0, Canada, healthcare system
Background & purpose
In Canada, as in many developed countries, long-term care facilities (LTCF) are vital for supporting individuals with complex health needs that exceed the scope of community-based care (Freeman et al., 2017). LTCF, also known as nursing homes or personal care homes, cater to those who require specialized care due to chronic physical, mental, or other disabilities, which is not typically available through home care or retirement services (Canadian Institute for Health Information [CIHI], 2013). The significance of LTCF in the healthcare system has been growing; in 2013, there were 2,036 LTCF across Canada, with a projection that by 2041, the number of residents requiring these facilities will increase to 320,000. However, by 2020, the number of residents in these facilities had reached 474,000, surpassing the forecasted needs (Statistics Canada, 2022).
Apathy is a complex and multidimensional construct (Marin, 1990; Marin & Wilkosz, 2005; Robert et al., 2018), that is increasingly observed among those in LTCF (Gerritsen et al., 2005; Jao et al., 2018; Roth et al., 2007; Tang et al., 2018). Cognitively, it manifests as diminished intellectual engagement such as a lack of curiosity or motivation to pursue knowledge or engage in problem-solving activities (Le Heron et al., 2018; Robert et al., 2002). Behaviorally, it is characterized by a reduction in goal-directed actions including a decrease in initiated activities, a decline in participation in previously enjoyed activities, or a lack of response to motivational cues (Marin, 1991). Emotionally, apathy leads to a blunted affective response or emotional indifference (Starkstein & Leentjens, 2008). Socially, it precipitates a withdrawal from social interactions and activities (Sockeel et al., 2006). Thus, individuals with apathy may manifest one or more symptoms depending on the domains implicated (Marin, 1990; Levy & Dubois, 2006; Robert et al., 2018). It is noteworthy that various terms are used to describe apathy in clinical settings, including abulia, negative symptoms, avolition, amotivation, anhedonia and boredom (Thant & Yager, 2019). However, these terms are distinguishable from apathy (Barch et al., 2016). For example, anhedonia is characterized by a diminished capacity to enjoy activities that are usually pleasurable, often appearing as a symptom of depression (Ang et al., 2018; Serretti, 2023). Apathy, in contrast, is marked by a broad disinterest or lack of motivation, without necessarily affecting emotional responses related to pleasure (Fahed & Steffens, 2021).
The prevalence of apathy among LTCF populations has been variably reported in literature, with estimates ranging significantly due to differences in assessment methods and population. For instance, using the Neuropsychiatric Inventory-Nursing Home Version (NPI-NH) to measure apathy, a study involving LTCF residents with Alzheimer's disease (AD) and other dementias observed that 84.1% of residents had apathy (Wood et al., 2000). An observational study which utilized the NH-version of the Apathy Evaluation Scale (AES10) to assess apathy in a sample of LTCF residents with stroke demonstrated that apathy was found in 28% of the individual within this population (van Almenkerk et al., 2015). A comprehensive review found that the frequency of apathy was 69% among 162 residents admitted to LTCF (Starkstein et al., 2006). In other systematic reviews and meta-analytic studies, it has been reported that 50% of the residents in LTCF have apathy (Leung et al., 2021; Zhao et al., 2016).
Research has consistently shown that apathy contributes to a range of adverse outcomes among LTCF residents, including accelerated cognitive decline, increased dependency in daily activities, and a higher risk of mortality (Aguera-Ortiz et al., 2015; Ayers et al., 2017; Lavretsky et al., 2015; Nijsten et al., 2017; van Reekum et al., 2005). These outcomes not only affect the individuals but also pose challenges for caregivers (formal and informal) and the healthcare system (Jao et al., 2019; Wong et al., 2020). The burden of apathy on caregivers is particularly noteworthy, as it often leads to increased caregiver stress and burnout (Wong et al., 2020). The Canadian healthcare system, with its diverse population and unique healthcare policies (Martin et al., 2018), provides a distinct context for examining apathy.
Despite the significance of apathy among LTCF residents, it is rarely diagnosed or specifically addressed (Nijsten et al., 2023). This oversight can be attributed to several factors. Firstly, there is a lack of standardized, validated tools for apathy assessment and diagnosis among the LTCF population (Agboji et al., 2024; Clarke et al., 2011; Volicer et al., 2013; Miller et al., 2021; Tay et al., 2021). Secondly, symptoms of apathy are often mistakenly attributed to other neuropsychiatric conditions like depression, leading to misdiagnosis and inappropriate management (Jao et al., 2019; Leone et al., 2013). Thirdly, prior research has primarily focused on apathy as a secondary symptom of neurodegenerative diseases including Alzheimer's and Parkinson's (Manera et al., 2020; Robert et al., 2009, 2018; Jao et al., 2019). However, recent studies suggest that apathy can exist independently (Lopez et al., 2019; Starkstein et al., 2005).
Existing literature suggests that predictors of apathy in the LTCF included a range of demographic, psychological, and social factors (Ang et al., 2018; Cummings et al., 2015; Drye et al., 2013; Robert et al., 2018; Yuen et al., 2014). Variables such as age, cognitive impairment, depression, and physical health status have been implicated, but findings are not consistent across studies. For example, in a study by Starkstein et al. (2009), it was found that older age was a risk factor for apathy, whereas, in other studies, it was reported that younger age (age below 65 years) was a predictor of apathy (Appelhof et al., 2019; Jao et al., 2018). By contrast, Holtta et al. (2012) found no differences in age among those who have apathy. With regards to sex, some studies showed that being male was a risk factor (Jao et al., 2020; Vilalta-Franch et al., 2013), while other studies found no relationship between sex and apathy (Clarke et al., 2008; Proitsi et al., 2011; Starkstein et al., 2006). These inconsistencies underscore the need for more targeted research using standardized measures.
To date, a comprehensive study examining the factors that may increase the risks of apathy in the LTCF context is sparse. The purpose of this current study was to examine the prevalence and predictors of apathy among persons living in the Canadian LTCF using the Apathy Index of the MDS 2.0, a standardized tool approved for use in LTCF across Canada.
Methods and procedures
Study design
This retrospective cross-sectional study analyzed de-identified data from residents in LTCF, using the MDS 2.0 from the Continuing Care Reporting System (CCRS) database of the Canadian Institute for Health Information (CIHI).
Setting and sample
We included residents from the Canadian LTCF across six provinces (Alberta, British Columbia, Manitoba, Newfoundland and Labrador, Ontario, and Saskatchewan) and one territory (Yukon) who participated in the admission assessments between 2015 and 2019. Exclusions were residents in comatose states or with missing relevant information.
Data collection
As aforementioned, the MDS 2.0 is a mandated assessment tool used in most Canadian LTCF. It provides comprehensive personal-level information about residents that clinicians may use to inform their decision-making when developing a care plan that reflects the individual's needs, preferences, and strengths (Hirdes et al., 2008). The assessments were completed by trained healthcare staff within 14 days of admission (admission assessment) and quarterly thereafter, involving multiple information sources, including observations and consultations with various healthcare professionals (Hirdes et al., 2008). Built into the MDS 2.0 are the clinical assessment protocols (CAPs), also referred to as outcome scales. The CAPs are used to identify residents who can improve with appropriate care or are at risk of adverse outcomes (Hirdes et al., 2008). This study incorporated several key scales including Cognitive Performance Scale (CPS), which monitors changes in cognitive status (Hirdes et al., 2008); Activity of Daily Living Self-Performance Hierarchy Scale (ADL-hierarchy), offering insight into the progression of residents’ disabilities through ADL performance analysis (Hirdes et al., 2008); the Pressure Ulcer Risk Scale (PURS), aimed at identifying the risk of developing pressure ulcers (Poss et al., 2008); Change in Health, End-Stage Disease, and Signs and Symptoms Scale (CHESS), which evaluates the resident's health instability and aids in predicting mortality (Morris et al., 2012); the Index of Social Engagement (ISE) measures residents’ sense of initiatives and social involvement, and consists of six items, including at ease interacting with others, doing planned or structured activities, doing self-initiated activities; establishes own goals; pursues involvement in the facility's life, and accepting invitations into most groups’ activities (Morris et al., 1999); and the Depression Rating Scale (DRS) measuring mood and behavior (Burrows et al., 2000).
Apathy measures
Apathy was measured using two items, termed the Apathy Index of the MDS 2.0, assessing withdrawal from activities and reduced social interactions in line with the framework for apathy assessment proposed by Volicer et al. (2013). These two items were rated on a 3-point Likert scale by the CCRS as follows: 0 = Indicator not exhibited in last 30 days; 1 = Indicator of this type exhibited up to 5 days a week; 2 = Indicator of this type exhibited daily or almost daily (6, 7 days a week). We converted these two items into a dichotomous variable. Thus, a score of 0 indicates non-apathetic and 1 is apathetic. Studies have reported a Cronbach's Alpha coefficient of 0.75–0.89 for this measure indicating a high level of internal consistency (Gerritsen et al., 2008; Stones et al., 2006; Volicer et al., 2013). Additionally, these two items are core symptoms of apathy included in the screening questions of the Neuropsychiatric Inventory (NPI) and have demonstrated a high degree of internal consistency (Cummings et al., 1994). High internal consistency indicates that the items on the apathy index are measuring the same underlying concept of apathy consistently across different assessments.
Data analysis
Data analysis was conducted using SAS version 9.4. (SAS Institute, Cary, NC, USA, 2013). Descriptive statistics described participant characteristics, and simple logistic regression identified apathy predictors. Prevalence was defined as the percentage of LTCF residents with an apathy score of 1. Statistical significance was set at an alpha level of p < .05.
Theoretical framework
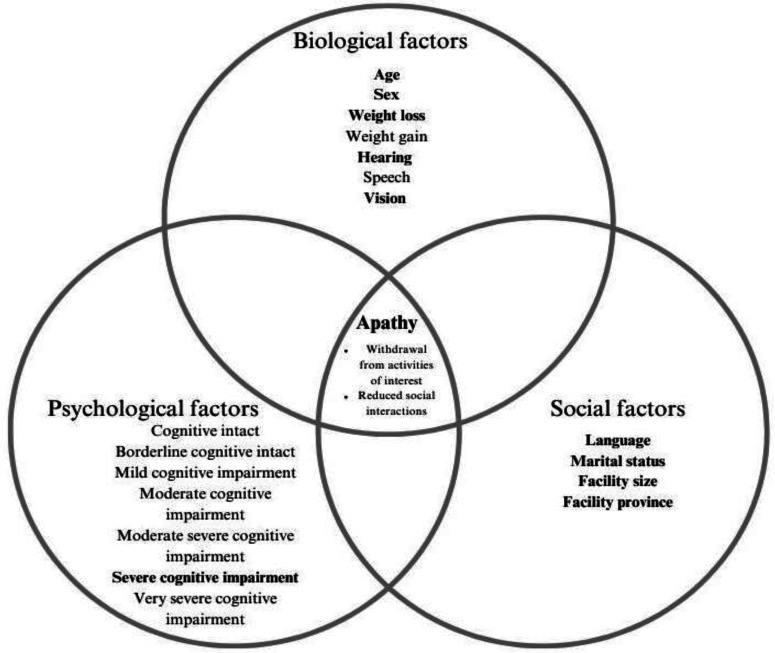
Considering the interplay of biological, psychological, and social influences on apathy, the biopsychosocial model served as the theoretical foundation for this study (Bolton & Gillett, 2019; Ghaemi, 2009). Variables were classified accordingly: biological (age, sex, weight, and sensory impairment), psychological (cognitive performance scores), and social (marital status, language, facility size, and facility province) factors.
Results
Sample characteristics
Table 1 presents the sociodemographic characteristics of the study participants. Of the 157,596 residents included in the sample, over half of the population were female (63.5%), 51.1% were aged 85 years or older, 66.6% did not have a partner or spouse (never married, widowed, divorced, or separated), 16% lived alone before admission, 84.9% were English speakers, over two-thirds lived in large LTCF (over 100 beds-Morris et al., 2012) (69.9%), and approximately one quarter entered the facilities from inpatient acute care services (27.9%).
Table 1.
Sociodemographic characteristics by status of apathy of residents newly admitted to the Canadian LTCF between 2015 and 2019 (N = 157,596).
| Variables | Total Population 100% (N = 157,596) |
Apathetic 12.5% (n = 19,758) |
Non-apathetic 87.6% (n = 137,838) |
|---|---|---|---|
| Age at admission (years) | |||
| less than 65 | 6.04 (9,519) | 14.22 (1,354) | 85.78 (8,165) |
| 65–74 | 11.95 (18,840) | 13.90 (2,619) | 86.10 (16,221) |
| 75–84 | 30.95 (48,782) | 12.73 (6,209) | 87.27 (42,573) |
| 85 and above | 51.05 (80,455) | 11.90 (9,576) | 88.10 (70,879) |
| Sex | |||
| Female | 63.46 (100, 009) | 12.05 (12,052) | 87.95 (87,957) |
| Male | 36.46 (57,452) | 13.39 (7,690) | 86.61 (49,762) |
| Other | 0.09 (135) | 11.85 (16) | 88.15 (119) |
| Marital status | |||
| Married | 29.87 (40,598) | 11.87 (4,818) | 88.13 (35,780) |
| Never married/widowed/ separated/divorced | 66.61 (90,530) | 11.70 (10,590) | 88.30 (79,940) |
| Not specified | 3.51 (4,774) | 13.57 (648) | 86.43 (4,126) |
| Lived alone before entry to LTCF | |||
| No | 84 (124,118) | 12.34 (15,322) | 87.66 (108,796) |
| Yes | 16 (23,648) | 12.50 (2,957) | 87.50 (20,691) |
| Language | |||
| English | 84.85 (133,718) | 13.06 (17,462) | 86.94 (116,256) |
| French | 2.23 (3,509) | 11.97 (420) | 88.03 (3,089) |
| Other | 12.92 (20,369) | 9.21 (1,876) | 90.79 (18,493) |
| Facility size | |||
| Large (100 + beds) | 69.78 (109,965) | 11.38 (12,513) | 88.62 (97,452) |
| Medium (30–99 beds) | 28.00 (44,134) | 14.87 (6,561) | 85.13 (37,573) |
| Small (1–29 beds) | 2.22 (3,497) | 19.56 (684) | 80.44 (2,813) |
| Entry Service Type | |||
| Ambulatory health Service | 0.63 (991) | 13.22 (131) | 86.78 (860) |
| Inpatient Acute Care Service | 27.94 (44,031) | 14.96 (6,586) | 85.04 (37,445) |
| Inpatient Rehabilitation Service | 1.55 (2,450) | 11.67 (286) | 88.33 (2,164) |
| Inpatient Continuing Care Service | 6.19 (9,758) | 13.12 (1,280) | 86.88 (8,478) |
| Residential Care Service | 15.11 (23,808) | 14.54 (3,461) | 85.46 (20,346) |
| Inpatient Psychiatric Service | 1.15 (1,818) | 20.96 (381) | 79.04 (1,437) |
| Other/Unclassified service | 0.84 (1,323) | 13.38 (177) | 86.62 (1,146) |
| Inpatient Rehabilitation Service-Specialized | 0.37 (589) | 12.05 (71) | 87.95 (518) |
| Home Care Service | 9.78 (15,415) | 11.14 (1,717) | 88.86 (13,698) |
| Residential Care Service-board and care | 15.35 (24,192) | 10.25 (2,479) | 89.75 (21,713) |
| Private Home-no home care | 21.08 (33,221) | 9.60 (3,189) | 90.40 (30,032) |
| Cognitive Performance Scale (CPS) | |||
| Intact (score = 0) | 9.39 (14,796) | 7.98 (1,181) | 92.02 (13,615) |
| Borderline Intact (score = 1) | 12.30 (19,839) | 11.59 (2,247) | 88.41 (17,142) |
| Mild impairment (score = 2) | 22.11 (34,837) | 10.80 (3,762) | 89.20 (31,075) |
| Moderate impairment (score = 3) | 36.88 (58,120) | 13.01 (7,560) | 86.99 (50,560) |
| Moderate severe impairment (score = 4) | 7.98 (12,583) | 14.11 (1,775) | 85.89 (10,808) |
| Severe impairment (score = 5) | 8.84 (13,935) | 18.43 (2,568) | 81.57 (11,367) |
| Very severe impairment (score = 6) | 2.50 (3,936) | 16.90 (665) | 83.10 (3,271) |
| Activity of daily living self performance hierarchy scale (ADL-hierarchy) | |||
| Low levels of decline (score = 0–1) | 11.80 (18,601) | 11.84 (2,202) | 88.16 (16,399) |
| Moderate levels of decline (score = 2–3) | 48.41 (76,298) | 12.12 (9,248) | 87.88 (67,050) |
| High levels of decline (score = 4–6) | 39.78 (62,697) | 13.25 (8,308) | 86.75 (54,389) |
| Pressure ulcer risk (PURS) | |||
| Low risk (score = 0–2) | 71.99 (113,447) | 12 (13,616) | 88 (99,831) |
| Moderate risk (score = 3–5) | 27.45 (43,264) | 13.82 (5,979) | 86.16 (37,285) |
| High risk (score = 6–8) | 0.56 (885) | 18.42 (163) | 81.58 (722) |
| Change in Health, End Stage Disease and Signs and Symptoms (CHESS) | |||
| Low levels of medical complexity (score = 0–1) | 84.47 (133,115) | 11.26 (14,987) | 88.74 (118,128) |
| Moderate levels of medical complexity (score = 2–3) | 14.79 (23,303) | 18.77 (4,374) | 81.23 (18,929) |
| High levels of medical complexity (score = 4–5) | 0.75 (1,178) | 33.70 (397) | 66.30 (781) |
| Index of Social engagement (ISE) | |||
| Low levels of engagement (score = 0–1) | 20.79 (32,771) | 23.33 (7,644) | 76.67(25,127) |
| Moderate levels of engagement (score = 2–3) | 40.66 (64,084) | 12.20 (7,817) | 87.80 (56,267) |
| High levels of engagement (score = 4–6) | 38.54 (60,741) | 7.07 (4,297) | 92.93 (56,444) |
| Depression Rating Scale (DRS) | |||
| Low levels of depressive symptoms (score = 0–2) | 79.64 (125,509) | 9.58 (12,020) | 90.42 (113,489) |
| Moderate levels of depressive symptoms (score = 3–5) | 15.61 (24,597) | 21.29 (5,237) | 78.71 (19,360) |
| High levels of depressive symptoms (score = 6–14) | 4.75 (7,490) | 33.39 (2,501) | 66.61 (4,989) |
| Antidepressant use | |||
| No | 54.66 (86, 136) | 11.30 (9,732) | 88.70 (76,404) |
| Yes | 45.34 (71,460) | 14.03 (10,026) | 85.97 (61,434) |
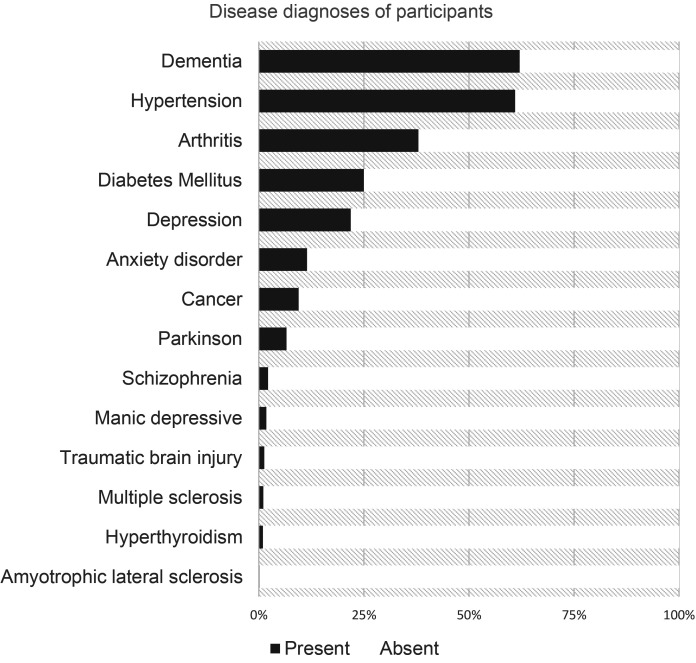
Figure 1 shows the diagnosis of the disease in the study population. Over half of the population (62.1%) was diagnosed with dementia. Hypertension was also prevalent, affecting 61% of the population. A small percentage of residents were diagnosed with amyotrophic lateral sclerosis (0.2%), multiple sclerosis (1.1%), Parkinson's disease (6.6%), manic depressive disorder (1.8%), schizophrenia (2.2%), and traumatic brain injury (1.3%). Additionally, less than a quarter of the population had anxiety disorder (11.5%) and depression (24.1%).
Figure 1.
Disease diagnoses of residents newly admitted to the Canadian LTCF between 2015 and 2019 (N = 157,596).
Prevalence of apathy
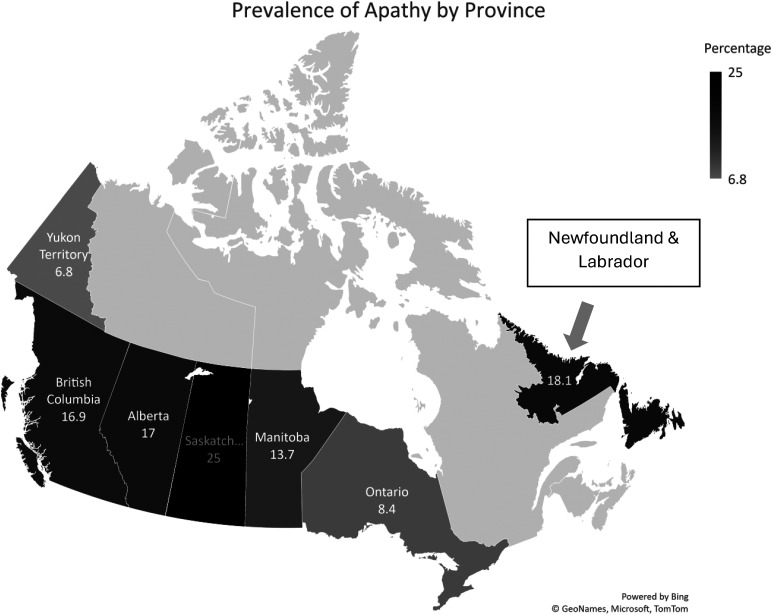
The prevalence of apathy among Canadian LTCF residents, as measured by the Apathy Index of the MDS 2.0 was 12.5% (Table 1). The prevalence of apathy was higher among residents who were under 65 years of age (14.2%) than among those aged 85 years and above (11.9%). Apathy was nearly twice as prevalent among residents of small LTCF compared to those residing in large LTCF (19.6% vs. 11.3%). Additionally, geographical region influenced the prevalence of apathy; it ranged from 6.8% in Yukon to 25.6% in Saskatchewan (Figure 2). Apathy was reported by the highest proportion of individuals (21%) entering LTCF via inpatient psychiatric services, as opposed to individuals who entered the facilities via private homes and were not receiving home care (9.6%). Apathy was more frequent among English speakers (13.1%) compared to French speakers (11.8%) and speakers of other languages (9.2%). No significant differences were found between those who lived alone before admission to LTCF and those who did not.
Figure 2.
Prevalence of apathy among the newly admitted Canadian LTCF residents by province.
Furthermore, apathy was more prevalent among residents who had a high score (4+, severe impairment) on various outcome scales than among those who had a low score (0–2, low impairment) (Table 1), including the ADLS-hierarchy (13.3% vs. 11.4%), PURS (18.4% vs 12%), CPS (16.9% vs 10%), DRS (33.4% vs. 9.6%) and CHESS scales (37.7% vs. 11.3%). In addition, the proportion of residents who had a low score on the ISE (0–1, low engagement) and exhibited apathy was higher in comparison with those who had a high score (4+, high engagement) on this item (23.3% vs. 7.1%). Apathy was more prevalent among residents who were using anti-depressants compared to those who did not (14% vs. 11.3%).
Predictors of apathy
Figure 3 shows the theoretical framework for the regression results modelling the predictors of apathy among Canadian LTCF newly admitted residents. With regards to biological variables (Table 2), the predictors of apathy were age groups- less than 65 years of age (OR 1.26 (95% CI 1.16, 1.37), between 65 and 74 years of age (OR 1.30 (95% 1.22, 1.39) and between 75 and 84 years of age (OR 1.11 (95% CI 1.06, 1.16); weight loss (OR 1.49 (95% CI 1.38, 1.2); hearing- minimal difficulty (OR 1.13 (95% CI 1.08, 1.19), hears in special situations (OR 1.19 (95% CI 1.11, 1.26), and highly impaired (OR 1.62 (95% CI 1.41, 1.85); vision- minimal impaired (OR 1.17 (95% CI 1.12, 1.23), moderately impaired (OR 1.39 (95% CI 1.29, 1.50), and severely impaired (OR 1.14 (95% CI 1.11, 1.17). Concerning psychological variables (Table 2), severe cognitive impairment (OR 2.60 (95% CI 2.42,2.28) was the strongest predictor of apathy. With respect to social variables (Table 2), the predictors were French language (OR 1.27 (95% CI 1.15,1.42); unspecified marital status (OR 1.14 (95% CI 1.04, 1.24); medium sized facility (OR 1.18 (95% CI 1.14,1.22), and facility province (Saskatchewan) (OR 1.58 (95% CI 1.49–1.68).
Figure 3.
The biopsychosocial model of apathy in LTCF.
Table 2.
The biopsychosocial predictors of apathy among Canadian LTCFs residents, 2015–2019 fiscal year
| Biological Variables | Adjusted odds ratio (95% Confidence limits) |
*p values |
|---|---|---|
| Sex (Ref = female) | ||
| Male | 1.09 (1.04–1.13) | ** < 0.0001 |
| Other (e.g., hermaphrodite) | 1.28 (0.60–2.721) | 0.5179 |
| Age group (Ref = 85 + years) | ||
| Less than 65 | 1.26 (1.16–1.37) | ** < 0.0001 |
| 65–74 | 1.30 (1.22–1.39) | ** < 0.0001 |
| 75–84 | 1.11 (1.06–1.16) | ** < 0.0001 |
| Weight gain (Ref = No) | 1.13 (0.97–1.32) | 0.1312 |
| Weight loss (Ref = No) | 1.49 (1.38–1.62) | ** < 0.0001 |
| Hearing (Ref = Adequate) | ||
| Minimal difficulty | 1.13 (1.08–1.19) | ** < 0.0001 |
| Hears in special situations | 1.19 (1.11–1.26) | ** < 0.0001 |
| Highly impaired | 1.62 (1.41–1.85) | ** < 0.0001 |
| Uses speech to communicate (Ref = No) | 0.93 (0.83–1.04) | 0.1955 |
| Vision (Ref = Adequate) | ||
| Minimal impaired | 1.17 (1.12–1.23) | ** < 0.0001 |
| Moderately impaired | 1.39 (1.29–1.50) | ** < 0.0001 |
| Highly impaired | 1.04 (0.94–1.15) | 0.5081 |
| Severely impaired | 1.37 (1.18–1.59) | ** < 0.0001 |
| Psychological variables (Ref = Intact) | ||
| Borderline Intact | 1.51 (1.40–1.63) | ** < 0.0001 |
| Mild impairment | 1.40 (1.30–1.49) | ** < 0.0001 |
| Moderate impairment | 1.72 (1.62–1.84) | ** < 0.0001 |
| Moderate severe impairment | 1.89 (1.75–2.05) | ** < 0.0001 |
| Severe impairment | 2.60 (2.42–2.80) | ** < 0.0001 |
| Very severe impairment | 2.34 (2.12–2.60) | ** < 0.0001 |
| Social Variables | ||
| Primary language (Ref = Eng) | ||
| French | 1.27 (1.15–1.42) | ** < 0.0001 |
| Other | 0.78 (0.74–0.82) | ** < 0.0001 |
| Marital status (Ref = Married) | ||
| Never married/widowed/ separated/divorced | 0.88 (0.81–0.96) | 0.1107 |
| Unspecified | 1.14 (1.04–1.24) | **0.0054 |
| Facility size (Ref = Large) | ||
| Medium | 1.18 (1.14–1.22) | ** < 0.0001 |
| Small | 1.11 (0.10–1.23) | 0.568 |
| Facility Province (Ref = British Columbia) | ||
| Manitoba | 0.80 (0.74–0.93) | ** < 0.0001 |
| Newfoundland & Labrador | 1.06 (0.98–1.16) | 0.2384 |
| Ontario | 0.46 (0.44–0.48) | ** < 0.0001 |
| Saskatchewan | 1.58 (1.49–1.68) | ** < 0.0001 |
| Yukon | 0.33 (0.21–0.53) | ** < 0.0001 |
*p value = logistic regression.
** = significant values.
Discussion
This study aimed to investigate the prevalence and predictors of apathy among residents of Canadian LTCF. Apathy was present in all age groups and various disorders. The prevalence of apathy as measured by the Apathy Index of the MDS 2.0 was 12.5% among a large sample of the Canadian LTCF residents. This corroborates with previous studies, including a Dutch study involving 199 residents that reported a prevalent rate of 12% (Aalten et al., 2005), a UK study involving 1,419 participants utilizing the Neuropsychiatric Inventory to rate apathy that reported the prevalence of clinically significant apathy to be 21.4% (Sommerlad et al., 2022), and another Dutch study comprising of 290 lTCF residents from nine LTCF which found that the prevalence of apathy was 19% (Wetzels et al., 2010). However, the prevalence rate in the current study was substantially lower than the range of 19–88% as reported in a systematic review and meta-analysis of 25 studies (Zhu et al., 2019) and a study in the Netherlands which found a 28% prevalence of apathy among LTCF residents with stroke (van Almenkerk et al., 2015). The discrepancies in the prevalence rates across studies could be attributed to various factors, including differences in the demographic characteristics of the participants, settings of the studies, and methodologies employed in assessing apathy. In the current study, the measurement tool used to assess apathy focused only on two items: “withdrawal from activities of interest (e.g., no interest in long-standing activities or being with family, friends)” and “reduced social interaction” (e.g., less talkative, more isolated) (Morris et al., 2012, p.2) suggesting that the behavioral and social domains of apathy were captured while the emotional and cognitive domains of apathy were not accounted for. Nonetheless, in the absence of a comprehensive tool to measure apathy, the Apathy Index of the MDS 2.0 provides an important starting point to increasing awareness about apathy in the Canadian LTCF.
In relation to the prevalence of apathy among different age groups, the current study revealed that apathy was more frequent among residents who were younger than 65 years compared to those in the older age group (85 years and over). This is consistent with previous studies investigating the prevalence of neuropsychiatric symptoms in young-onset dementia (Bauhuis et al., 2020; Mulders et al., 2014; 2016), but in contrast to studies involving persons with advanced dementia (Selbaek et al., 2014; van Reekum et al., 2005; Van Vliet et al., 2013; Zuidema et al., 2009). It has been suggested that apathy is one of the primary reasons for institutionalization in individuals with young-onset dementia (Bakker et al., 2013). Early identification of apathy in this age group may be valuable for testing experimental medicines aimed at this syndrome or its associated symptoms (Dujardin et al., 2014).
Furthermore, apathy and depression frequently co-occur, as observed in over half (21.3% with moderate and 33.4% with high scores on DRS) of our sample. While some symptoms overlap between apathy and depression, each condition has distinct characteristics (Levy et al., 1998; Lueken et al., 2007; Starkstein et al., 2005): apathy is marked by blunted emotional response, indifference, low social engagement, diminished initiation, and poor persistence (Ishizaki & Mimura, 2011); depression, on the other hand, includes dysphoria, suicidal ideation, self-criticism, guilt feelings, pessimism, and hopelessness (Levy et al., 1998). Furthermore, apathy and depression differ in the brain regions implicated and have different progression patterns (Fahed & Steffens, 2021). This has implication for the use of anti-depressants in this population because treatment with selective serotonin reuptake inhibitor (SSRI) antidepressants may worsen apathy (Masdrakis et al., 2023). In our study, 14% of residents who exhibited apathy were treated with antidepressants.
Regarding the biological predictors of apathy, age emerged as a predictor, demonstrating that younger residents (<65 years) are more likely to be apathetic compared to older age groups. This finding aligns with the results of previous cross-sectional studies (Appelhof et al., 2019; Jao et al., 2018) and appears to be somewhat counterintuitive given that one might expect higher frequencies of apathy in the oldest age group, suggesting that younger residents in LTCF might experience factors that predispose them to apathy, such as early onset dementia or other comorbidities that necessitate long-term care at a younger age or it might be that younger individuals in LTCF might experience greater challenges in adjusting to the institutional environment, leading to higher levels of apathy (Van Malderen et al., 2013). The relatively high prevalence of apathy in the 65–74 and 75–84 age groups emphasizes the need for targeted interventions across different age categories.
In congruence with previous research, male sex was a predictor of apathy (Jao et al., 2020; Vilalta-Franch et al., 2013). This highlights the importance of personalized and sex/gender-sensitive approaches to enhancing the well-being of LTCF residents with apathy. In addition, weight emerged as an important variable, with weight loss associated a higher likelihood of apathy compared to those without. Volicer et al. (2013) noted a similar result, however, due to the cross-sectional nature of this study, it is unclear which came first, the weight change or the apathy and to which direction the temporal sequence may occur. Nonetheless, weight loss management through avoidance of certain medications that increases the risk of anorexia such as psychoactive medications and adequate treatment of medical disorders associated with anorexia including vit B12 deficiency and gastrointestinal disorders, may play a crucial role in mitigating the development of apathy among newly admitted residents in LTCF (Volicer et al., 2013).
Sensory impairments in hearing and vision also emerged as significant predictors. Various levels of hearing impairments (minimal, moderate, and high) were associated with a higher likelihood of apathy than adequate hearing. Similarly, various levels of visual impairment were associated with apathy, with moderately impaired vision showing the highest likelihood (adjusted odds ratio: 1.39; p < 0.0001). Speech impairment, indicated by an adjusted odds ratio of 1.14 (p < 0.0001), suggests that residents with speech impairment are more likely to exhibit apathy than those without. This could be attributed to the potential isolation, communication barriers, and resultant reduced social engagement experienced by individuals with speech impairment. König et al. (2019) highlighted the efficacy of using speech characteristics, including acoustic, semantic, and prosodic features, as reliable indicators of apathy among individuals with speech impairments. Notable changes in speech, such as flatter intonation and decreased volume, alongside reduced vocabulary, and varied content, mirror the emotional and motivational deficits typical of apathy. Prosodic features such as extended pauses and speech disruptions also indicate apathy-related cognitive and emotional disturbances (König et al., 2019). In LTCF, early identification of apathy among residents with speech impairments by integrating automatic speech analysis into routine assessments can enhance care and enable timely intervention. These findings suggest that interventions aimed at improving sensory function might mitigate apathy in this population.
In terms of psychological factors, this current study showed that cognitive performance, as measured by CPS, has a significant association with apathy among newly admitted Canadian LTCF residents. This association was stronger for all levels of cognitive impairment. The early recognition of high cognitive performance scores could be instrumental in implementing early interventions and tailoring care plans for residents in LTCF. More importantly, interventions aimed at stimulating and supporting executive functions may be beneficial in mitigating the progression of apathy (Drijgers et al., 2011). Additionally, considering that apathy is associated with poor initiation, structured, and guided activities that encourage participation and engagement without relying heavily on self-initiation might be effective in promoting social interaction and mental stimulation among LTCF residents (Drijgers et al., 2011). Evidence also suggests that the presence of apathy is a potential risk factor for conversion to dementia in people with mild cognitive impairment (Lanctôt et al., 2017; Robert et al., 2006; van Dalen et al., 2018). The progression from mild to severe cognitive impairment showing a graded increase in the odds of apathy underscores the importance of early cognitive interventions and continuous monitoring to manage apathy effectively.
Social factors play an important role in predicting apathy. In this study, language, particularly French, shows a statistically significant association with apathy, with adjusted odds ratio 1.27 (p < 0.0001), indicating that residents who speak this language are more likely to exhibit apathy compared to those speaking English language. This suggests that linguistic and possibly cultural factors may play a role in the display or recognition of apathy among LTCF residents. Further, our findings suggests that those who speak other languages are at lower odds of exhibiting apathy (adjusted odds ratio: 0.78; p < 0.0001). This underscores the importance of language concordance (Hsueh et al., 2021) in the recognition of apathy among speakers of other languages in LTCF. It would be insightful to explore whether these associations are influenced by factors such as communication barriers, social integration, cultural perceptions of mental health, and emotional expression. Additionally, marital status impacts apathy, with those of unspecified marital status showing higher odds compared to residents who were married. This may point to the complexities of social support systems and their influence on residents’ health outcomes.
Facility size also emerged as a crucial predictor, with medium sized facility associated with a higher likelihood of apathy (adjusted odds ratio 1.18, p < 0.0001) compared to large sized facility, which might be due to differences in the availability of resources and activities (Mansbach et al., 2016). The relationship between the institutional environment and apathy is well documented (Chaudhury et al., 2017; Jao et al., 2015, 2019). This finding also aligns with the broader literature highlighting the protective effects of personalized care on apathy (Zuidema et al., 2010). Smaller facilities may facilitate more personalized care due to a lower staff-to-resident ratio (Zuidema et al., 2010). This personalized attention can be pivotal in recognizing and addressing early signs of apathy. In contrast, medium and larger facilities, despite having more resources, might struggle with providing individualized care to the same extent.
Furthermore, the province of the facilities emerged as a notable predictor of apathy, with residents in Manitoba, Ontario, and Yukon showing statistically significant lower likelihood of apathy while those in Saskatchewan demonstrating a higher likelihood of apathy compared to residents in British Columbia. This finding highlights the importance of considering geographical differences when developing interventions for apathy rather than just focusing on individual and institutional factors because geographical variation could be influenced by various factors, such as differences in healthcare policies, and socio-cultural contexts across provinces. This finding warrants further exploration to better understand the underlying mechanisms.
Implication for practice/policy
In this study, apathy was examined from a biopsychosocial perspective, and the results of our findings showed that various biological, psychological, and social factors are associated with apathy among residents in LTCF. To this end, health care professionals and policymakers should consider the following:
Create an awareness campaign about apathy at the institutional, provincial, and national levels.
Develop a standard assessment tool for evaluating apathy in LTCF that considers the needs and preferences of younger people.
Screen for apathy in all residents presenting with high cognitive performance scores using both systematic speech analysis and apathy screening tools to enhance early detection of apathy.
Incorporate technology-based meaningful activities into existing programs to keep residents engaged during the day while simultaneously preventing additional workloads for care staff.
Strength and limitation of the study
This study has several strengths, including the use of a large population sample, which is representative of the LTCF population. This enhances the generalizability of the findings across Canadian populations and provides a solid cross-section of apathy within the LTCF. Additionally, the results indicated that the prevalence of apathy among Canadian LTCF residents is comparable to existing studies, signifying the utility of the apathy index in the early detection of apathy among this population. This study also provides a thorough and multifaceted exploration of predictors of apathy, encompassing biological, psychological, and social factors, and offers a holistic view of the factors influencing apathy among LTCF residents. While this study was the first to investigate the prevalence of apathy among a very large sample of residents across Canadian LTCF, using data from the MDS 2.0, a validated tool approved for use in all healthcare settings across Canada, not all provinces and territories are included. This suggests that caution should be exercised when generalizing the results to all the provinces and territories in Canada. Furthermore, the use of this data, which is not primarily for clinical diagnosis or research purposes, might have made it difficult to examine more nuanced relationships between apathy and the various variables included in this study. Future studies should adopt a more rigorous methodology to examine these relationships.
Conclusion
This study examined the prevalence and predictors of apathy among residents of Canadian LTCF. The study found a complex interaction between apathy and various biological, psychological, and social factors. Apathy was found to be associated with biological indicators such as age, weight loss, and sensory deficits, highlighting the complicated relationship between apathy and physical health of LTCF residents. Psychological factors, particularly cognitive impairment, have a complex relationship with apathy. Severe cognitive impairment was associated with the highest risk for apathy than very severe impairment. This indicates that cognitive function and apathy are not linearly related. The study also found that marital status, facility size, and facility province were significantly associated with apathy. This highlights the impact of social settings and relationships on LTCF residents’ well-being. Apathy was also predicted by language, underscoring the importance of efficient communication and meaningful social interactions for preventing apathy.
Given these findings, a comprehensive and individual-focused approach to addressing and reducing LTCF apathy LTCF is essential. Interventions must address the physical, social, and psychological well-being of residents with apathy. Future research should examine the qualitative aspects of social connections and activity engagement, as well as their interactions with biological and psychological parameters, to better understand how they affect apathy in LTCF residents. This may help to develop approaches and interventions to improve the quality of life and well-being of LTCF residents. It is important to note that facility province was a strong predictor of apathy, and to the best of the author's knowledge, no research has explored this factor. Future studies should expand this factor and explore more effective interventions to mitigate apathy at the provincial level.
Author Biographies
Aderonke Agboji is a PhD candidate in the Department of Nursing, University of Northern British Columbia, Canada. She obtained a master's degree in Dementia Studies from the University of Stirling, United Kingdom. She also holds a master's degree in leadership and management from the Royal College of Surgeons of Ireland. Her research focuses on the well-being of older adults, dementia care, and the integration of technology in aging.
Shannon Freeman is an associate professor in the School of Nursing at the University of Northern British Columbia, Canada. She holds a PhD in Health Studies and Gerontology from the University of Waterloo and an MSc in Internal Medicine and Rehabilitation from Tohoku University School of Medicine in Japan. Her research focuses on the health and social care needs of older adults in rural and northern communities, with expertise in aging, hospice palliative care, and long-term care services.
Davina Banner is a professor at the University of Northern British Columbia, Canada. She holds a PhD from the University of the West of England and a BN Honors from the University of Wales. She completed post-doctoral research fellowships at McMaster University through the CIHR Strategic Training Fellowship - FUTURE Program for Cardiovascular Nurse Scientists, and at the University of Northern British Columbia. Her research interests include cardiovascular health, rural health, and the management and support of individuals living with complex cardiovascular and chronic conditions. She is also focused on developing innovative health-service delivery in rural and northern communities and advancing the science and practice of integrated knowledge translation (IKT) and patient-oriented research, particularly methods that support meaningful co-production in research.
Joshua Armstrong is a health research scientist with expertise in population health, cognitive aging, and neurological disorders. He holds a PhD in Aging, Health & Well-being from the School of Public Health and Health Systems at the University of Waterloo, and an MSc in Experimental Psychology with a focus on Applied Health and Gerontology from Lakehead University. He has a proven track record of leading research teams, designing rigorous studies, and analyzing complex data using advanced statistical methods such as SPSS, SAS, R, Matlab, and Stata. He is experienced in both university administration and national-level organizations and is passionate about translating scientific findings into actionable insights to improve health outcomes.
Melinda Martin-Khan is a health scientist and lecturer at Exeter Medical School, University of Exeter. She holds a PhD in Medicine and a Graduate Certificate in Science (Biostatistics) from the University of Queensland, along with a master's degree in health science from the University of South Australia. Her research interests include quality of care, dementia care, and the development of national assessment systems, with a focus on vulnerable populations, particularly, older adults and individuals with dementia.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Aderonke Agboji https://orcid.org/0000-0001-5262-3267
Shannon Freeman https://orcid.org/0000-0002-8129-6696
References
- Aalten P., de Vugt M. E., Jspers N., Jolles J., Verhey F. R. J. (2005). The course of neuropsychiatric symptoms in dementia. Part I: Findings from the two year longitudinal maasbed study. International Journal of Geriatric Psychiatry, 20(6), 523–530. https://doi.org/10.1002/gps.1316 [DOI] [PubMed] [Google Scholar]
- Agboji A., Freeman S., Banner D., Duchesne A., Armstrong J., Martin-Khan M. (2024). Apathy in older adults with and without dementia: An integrative review of barriers and facilitators to care. Sage Open, 14(2), 1–25. 10.1177/21582440241241882 [DOI] [Google Scholar]
- Aguera-Ortiz L., Gil-Ruiz N., Cruz-Orduna I., Ramos-Garcia I., Osorio R. S., Valenti-Soler M., Olazaran-Rodriguez J., Dobato-Ayuso J. L., Lanctot K., Martinez-Martin P. (2015). A novel rating scale for the measurement of apathy in institutionalized persons with dementia: The APADEM-NH. American Journal of Geriatric Psychiatry, 23(2), 149–159. 10.1016/j.jagp.2013.01.079 [DOI] [PubMed] [Google Scholar]
- Ang Y.-S., Lockwood P. L., Kienast A., Plant O., Drew D., Slavkova E., Tamm M., Husain M. (2018). Differential impact of behavioral, social, and emotional apathy on Parkinson's disease. Annals of Clinical and Translational Neurology, 5(10), 1286–1291. 10.1002/acn3.626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelhof B., Bakker C., Van Duinen-Van Den I. J. C. L., Zwijsen S. A., Smalbrugge M., Verhey F. R. J., De Vugt M. E., Zuidema S. U., Koopmans R. (2019). Differences in neuropsychiatric symptoms between nursing home residents with young-onset dementia and late-onset dementia. Aging Mental Health, 23(5), 581–586. 10.1080/13607863.2018.1428935 [DOI] [PubMed] [Google Scholar]
- Ayers E., Shapiro M., Holtzer R., Barzilai N., Milman S., Verghese J. (2017). Symptoms of apathy independently predict incident frailty and disability in community-dwelling older adults. Journal of Clinical Psychiatry, 78(5), e529–e536. 10.4088/JCP.15m10113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker C. M., de Vugt M. E. P., van Vliet D. M., Verhey F. R. J. M. D. P., Pijnenburg Y. A. M. D. P., Vernooij-Dassen M. J. F. J. P., Koopmans R. T. C. M. M. D. P. (2013). Predictors of time to institutionalization in young- versus late-onset dementia: Results from the needs in young onset dementia (needyd) study. Journal of the American Medical Directors Association, 14(4), 248–253. https://doi.org/10.1016/j.jamda.2012.09.011 [DOI] [PubMed] [Google Scholar]
- Barch D. M., Pagliaccio D., Luking K. (2016). Mechanisms underlying motivational deficits in psychopathology: Similarities and differences in depression and schizophrenia. Behavioral Neuroscience of Motivation, 27, 411–449. 10.1007/7854_2015_376 [DOI] [PubMed] [Google Scholar]
- Bauhuis R., Mulders A., Koopmans R. (2020). The course of neuropsychiatric symptoms in institutionalized patients with early onset dementia. Aging Mental Health, 24(3), 439–444. https://doi.org/10.1080/13607863.2018.1531379 [DOI] [PubMed] [Google Scholar]
- Bolton D., Gillett G. (2019). The biopsychosocial model of health and disease. new philosophical and scientific developments. Palgrave Open Access. https://www.palgrave.com/gp/book/9783030118983 [PubMed] [Google Scholar]
- Burrows A. B., Morris J. N., Simon S. E., Hirdes J. P., Phillips C. (2000). Development of a minimum data set-based depression rating scale for use in nursing homes. Age and Ageing, 29(2), 165–172. 10.1093/ageing/29.2.165 [DOI] [PubMed] [Google Scholar]
- Canadian Institute for Health Information. (2013). When a nursing home is home: how do canadian nursing homes measure up on quality? Retrieved January 2, 2021 from http://publications.gc.ca/collections/collection_2013/icis-cihi/H118-84-2013-eng.pdf
- Chaudhury H., Cooke H. A., Cowie H., Razaghi L. (2017). The influence of the physical environment on residents with dementia in long-term care settings: A review of the empirical literature. The Gerontologist, 58, e325–e337. 10.1093/geront/gnw259 [DOI] [PubMed] [Google Scholar]
- Clarke D. E., Ko J. Y., Kuhl E. A., van Reekum R., Salvador R., Marin R. S. (2011). Are the available apathy measures reliable and valid? A review of the psychometric evidence. Journal of Psychosomatic Research, 70(1), 73–97. 10.1016/j.jpsychores.2010.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D. E., Van Reekum R., Simard M., Streiner D. L., Conn D., Cohen T., Freedman M. (2008). Apathy in Dementia: Clinical and sociodemographic correlates. The Journal of Neuropsychiatry and Clinical Neurosciences, 20(3), 337–347. https://doi:10.1176/jnp.2008.20.3.337 [DOI] [PubMed] [Google Scholar]
- Cummings J., Friedman J. H., Garibaldi G., Jones M., Macfadden W., Marsh L., Robert P. H. (2015). Apathy in neurodegenerative diseases: Recommendations on the design of clinical trials. Journal of Geriatric Psychiatry and Neurology, 28(3), 159–173. https://doi.org/https://https://doi.org/10.1177/0891988715573534 [DOI] [PubMed] [Google Scholar]
- Cummings J. L., Mega M., Gray K., Rosenberg-Thompson S., Carusi D. A., Gornbein J. (1994). The neuropsychiatric inventory: Comprehensive assessment of psychopathology in dementia. Neurology, 44(12), 2308–2314. 10.1212/wnl.44.12.2308 [DOI] [PubMed] [Google Scholar]
- Drijgers R. L., Verhey F. R., Leentjens A. F., Kohler S. (2011). Neuropsychological correlates of apathy in mild cognitive impairment and Alzheimer's disease: The role of executive functioning. International Psychogeriatric, 23(8), 1327–1333. 10.1017/S1041610211001037 [DOI] [PubMed] [Google Scholar]
- Drye L. T., Scherer R. W., Lanctôt K. L., Rosenberg P. B., Herrmann N., Bachman D., Mintzer J. E. (2013). Designing a trial to evaluate potential treatments for apathy in dementia: The apathy in dementia methylphenidate trial (admet). The American Journal of Geriatric Psychiatry, 21(6), 549–559. https://doi.org/10.1016/j.jagp.2012.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin K., Langlois C., Plomhause L., Carette A. S., Delliaux M., Duhamel A., Defebvre L. (2014). Apathy in untreated early-stage Parkinson disease: Relationship with other non-motor symptoms. Movement Disorders: Official Journal of the Movement Disorder Society, 29(14), 1796–1801. https://doi.org/10.1002/mds.26058 [DOI] [PubMed] [Google Scholar]
- Fahed M., Steffens D. C. (2021). Apathy: Neurobiology, assessment and treatment. Clinical Psychopharmacology and Neuroscience, 19(2), 181–189. 10.9758/cpn.2021.19.2.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S., Bishop K., Spirgiene L., Koopmans E., Botelho F. C., Fyfe T., Xiong B., Patchett S., MacLeod M. (2017). Correction to: Factors affecting residents transition from long term care facilities to the community: A scoping review. BMC Health Services Research, 17(1), 694–707. 10.1186/s12913-017-2636-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen D. L., Achterberg W. P., Steverink N., Pot A. M., Frijters D. H. M., Ribbe M. W. (2008). The mds challenging behavior profile for long-term care. Aging & Mental Health, 12(1), 116–123. 10.1080/13607860701529882 [DOI] [PubMed] [Google Scholar]
- Gerritsen D. L., Jongenelis K., Steverink N., Ooms M. E., Ribbe M. W. (2005). Down and drowsy? Do apathetic nursing home residents experience low quality of life? Aging and Mental Health, 9(2), 135–141. 10.1080/13607860412331336797 [DOI] [PubMed] [Google Scholar]
- Ghaemi S. N. (2009). The rise and fall of the biopsychosocial model. British Journal of Psychiatry, 195(1), 3–4. https://doi.org/https://https://doi.org/10.1192/bjp.bp.109.063859 [DOI] [PubMed] [Google Scholar]
- Hirdes J. P., Ljunggren G., Morris J. N., Frijters D. H., Finne Soveri H., Gray L., Björkgren M., Gilgen R. (2008). Reliability of the interrai suite of assessment instruments: A 12-country study of an integrated health information system. BMC Health Services Research, 8(1), 277–288. 10.1186/1472-6963-8-277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtta E. H., Laakkonen M.-L., Laurila J. V., Strandberg T. E., Tilvis R. S., Pitkala K. H. (2012). Apathy: Prevalence, associated factors, and prognostic value among frail, older inpatients. Journal of the American Medical Directors Association, 13(6), 541–545. https://doi.org/https://dx.https://doi.org/10.1016/j.jamda.2012.04.005 [DOI] [PubMed] [Google Scholar]
- Hsueh L., Hirsh A. T., Maupomé G., Stewart J. C. (2021). Patient–provider language concordance and health outcomes: A systematic review, evidence map, and research agenda. Medical Care Research and Review, 78(1), 3–23. 10.1177/1077558719860708 [DOI] [PubMed] [Google Scholar]
- Ishizaki J., Mimura M. (2011). Dysthymia and apathy: Diagnosis and treatment. Depression Research and Treatment, 2011(1), 893–905. 10.1155/2011/893905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jao Y. L., Algase D. L., Specht J. K., Williams K. (2015). The association between characteristics of care environments and apathy in residents with dementia in long-term care facilities. Gerontologist, 55(Suppl 1), S27–S39. 10.1093/geront/gnu166 [DOI] [PubMed] [Google Scholar]
- Jao Y.-L., Liu W., Chaudhury H., Parajuli J., Holmes S., Galik E. (2020). Function-focused person-environment fit for long-term care residents with dementia: Impact on apathy. The Gerontologist, 61(3), 413–424. 10.1093/geront/gnaa111 [DOI] [PubMed] [Google Scholar]
- Jao Y. L., Mogle J., Williams K., McDermott C., Behrens L. (2018). Real-time observation of apathy in long-term care residents with dementia: Reliability of the person-environment apathy rating scale. Journal of Gerontological Nursing, 44(4), 23–28. https://doi.org/10.3928/00989134-20180131-02 [DOI] [PubMed] [Google Scholar]
- Jao Y. L., Williams K., Mogle J., Behrens L., McDermott C. (2019). Assessing apathy in long-term care residents with dementia: Who should be the rater? Dementia (Basel, Switzerland), 18(6), 2220–2229. https://doi.org/10.1177/1471301217745104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König A., Linz N., Zeghari R., Klinge X., Tröger J., Alexandersson J., Robert P. (2019). Detecting apathy in older adults with cognitive disorders using automatic speech analysis. Journal of Alzheimer's Disease: JAD, 69(4), 1183–1193. 10.3233/JAD-181033 [DOI] [PubMed] [Google Scholar]
- Lanctôt K. L., Agüera-Ortiz L., Brodaty H., Francis P. T., Geda Y. E., Ismail Z., Marshall G. A., Mortby M. E., Onyike C. U., Padala P. R., Politis A. M., Rosenberg P. B., Siegel E., Sultzer D. L., Abraham E. H. (2017). Apathy associated with neurocognitive disorders: Recent progress and future directions. Alzheimer's & Dementia: The Journal of the Alzheimer's Association, 13(1), 84–100. https://doi.org/https://https://doi.org/10.1016/j.jalz.2016.05.008 [DOI] [PubMed] [Google Scholar]
- Lavretsky H., Reinlieb M., St Cyr N., Siddarth P., Ercoli L. M., Senturk D. (2015). Citalopram, methylphenidate, or their combination in geriatric depression: A randomized, double-blind, placebo-controlled trial. American Journal of Psychiatry, 172(6), 561–569. 10.1176/appi.ajp.2014.14070889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Heron C., Apps M. A. J., Husain M. (2018). The anatomy of apathy: A neurocognitive framework for amotivated behaviour. Neuropsychologia, 118(Pt B), 54–67. 10.1016/j.neuropsychologia.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone E., Deudon A., Bauchet M., Laye M., Bordone N., Lee J. H., Piano J., Friedman L., David R., Delva F., Brocker P., Yesavage J., Robert P. H. (2013). Management of apathy in nursing homes using a teaching program for care staff: The STIMEHPAD study. International Journal of Geriatric Psychiatry, 28(4), 383–392. 10.1002/gps.3836 [DOI] [PubMed] [Google Scholar]
- Leung D. K. Y., Chan W. C., Spector A., Wong G. H. Y. (2021). Prevalence of depression, anxiety, and apathy symptoms across dementia stages: A systematic review and meta-analysis. International Journal of Geriatric Psychiatry, 36(9), 1330–1344. 10.1002/gps.5556 [DOI] [PubMed] [Google Scholar]
- Levy M. L., Cummings J. L., Fairbanks L. A., Masterman D., Miller B. L., Craig A. H., Paulsen J. S., Litvan I. (1998). Apathy is not depression. The Journal of Neuropsychiatry and Clinical Neurosciences, 10(3), 314–319. 10.1176/jnp.10.3.314 [DOI] [PubMed] [Google Scholar]
- Levy R., Dubois B. (2006). Apathy and the Functional anatomy of the prefrontal cortex-basal ganglia circuits. Cerebral Cortex, 16(7), 916–928. 10.1093/cercor/bhj043 [DOI] [PubMed] [Google Scholar]
- Lopez F. V., Eglit G. M., Schiehser D. M., Pirogovsky-Turk E., Litvan I., Lessig S., Filoteo J. V. (2019). Factor analysis of the apathy scale in Parkinson's disease. Movement Disorders Clinical Practice, 6(5), 379–386. 10.1002/mdc3.12767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueken U., Seidl U., Völker L., Schweiger E., Kruse A., Schröder J. (2007). Development of a short version of the apathy evaluation scale specifically adapted for demented nursing home residents. The American Journal of Geriatric Psychiatry, 15(5), 376–385. https://doi.org/https://https://doi.org/10.1097/jgp.0b013e3180437db3 [DOI] [PubMed] [Google Scholar]
- Manera V., Abrahams S., Agüera-Ortiz L., Bremond F., David R., Fairchild K., … Robert P. (2020). Recommendations for the nonpharmacological treatment of apathy in brain disorders. The American Journal of Geriatric Psychiatry, 28(4), 410–420. https://doi.org/https://https://doi.org/10.1016/j.jagp.2019.07.014 [DOI] [PubMed] [Google Scholar]
- Mansbach W. E., Mace R. A., Clark K. M., Firth I. M. (2016). Meaningful activity for long-term care residents with dementia: A comparison of activities and raters. The Gerontologist, 57(3), 461–468. 10.1093/geront/gnv694 [DOI] [PubMed] [Google Scholar]
- Marin R. S. (1990). Differential diagnosis and classification of apathy. The American Journal of Psychiatry, 147(1), 22–30. 10.1176/ajp.147.1.22 [DOI] [PubMed] [Google Scholar]
- Marin R. S. (1991). Apathy: A neuropsychiatric syndrome. The Journal of Neuropsychiatry and Clinical Neurosciences, 3(3), 243–254. 10.1176/jnp.3.3.243 [DOI] [PubMed] [Google Scholar]
- Marin R., Wilkosz P. (2005). Disorders of diminished motivation. The Journal of Head Trauma Rehabilitation, 20(4), 377–388. https://doi.org/https://doi.10.1097/00001199-200507000-00009 [DOI] [PubMed] [Google Scholar]
- Martin D., Miller A. P., Quesnel-Vallée A., Caron N. R., Vissandjée B., Marchildon G. P. (2018). Canada's universal health-care system: Achieving its potential. Lancet (London, England), 391(10131), 1718–1735. 10.1016/S0140-6736(18)30181-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masdrakis V. G., Markianos M., Baldwin D. S. (2023). Apathy associated with antidepressant drugs: A systematic review. Acta Neuropsychiatrica, 35(4), 189–204. https://doi.org/https://https://doi.org/10.1017/neu.2023.6 [DOI] [PubMed] [Google Scholar]
- Miller D. S. Robert P. Ereshefsky L. Adler L. Bateman D. Cummings J. Dekosky S. T. Fischer C. E. Husain M. Ismail Z. Jaeger J. Lerner A. J. Li A. Lyketsos C. G. Manera V. Mintzer J. Moebius H. J. Mortby M. Meulien D.… Lanctot K. L. (2021). Diagnostic criteria for apathy in neurocognitive disorders. Alzheimers Dementia, 17(12), 1892–1904. 10.1002/alz.12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. N., Fries B. E., Morris S. A. (1999). Scaling adls within the mds. The Journals of Gerontology: Series A, 54(11), M546–M553. https://doi.org/https://https://doi.org/10.1093/gerona/54.11.m546 [DOI] [PubMed] [Google Scholar]
- Morris J. N., Hawes C., Mor V., Phillips C., Fries B. E., Nonemaker S., Murphy K. (2012). Resident Assessment Instrument (RAI) RAI-MDS 2.0 User’s Manual: Canadian Version . [DOI] [PubMed]
- Mulders A. J., Fick I. W., Bor H., Verhey F. R., Zuidema S. U., Koopmans R. T. (2016). Prevalence and correlates of neuropsychiatric symptoms in nursing home patients with young-onset dementia: The BEYOnD study. Journal of the American Medical Directors Association, 17(6), 495–500. https://doi.org/10.1016/j.jamda.2016.01.002 [DOI] [PubMed] [Google Scholar]
- Mulders A. J., Zuidema S. U., Verhey F. R., Koopmans R. T. (2014). Characteristics of institutionalized young onset dementia patients–the BEYOnD study. International Psychogeriatrics, 26(12), 1973–1981. https://doi.org/https://https://doi.org/10.1017/S1041610214001859 [DOI] [PubMed] [Google Scholar]
- Nijsten J. M. H., Leontjevas R., Pat-El R., Smalbrugge M., Koopmans R., Gerritsen D. L. (2017). Apathy: Risk factor for mortality in nursing home patients. Journal of America Geriatric Society, 65(10), 2182–2189. 10.1111/jgs.15007 [DOI] [PubMed] [Google Scholar]
- Nijsten J. M. H., Smalbrugge M., Plouvier A. O. A., Koopmans R. T. C. M., Leontjevas R., Gerritsen D. L. (2023). Identifying and managing apathy in people with dementia living in nursing homes: A qualitative study. BMC geriatrics, 23(1), 727–738. 10.1186/s12877-023-04422-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss J. W., Jutan N. M., Hirdes J. P., Fries B. E., Morris J. N., Teare G. F., Reidel K. (2008). A review of evidence on the reliability and validity of minimum data set. Healthcare Management Forum, 21(1), 33–39. 10.1016/S0840-4704(10)60127-5 [DOI] [PubMed] [Google Scholar]
- Proitsi P., Hamilton G., Tsolaki M., Lupton M., Daniilidou M., Hollingworth P., Archer N., Foy C., Stylios F., McGuinness B., Todd S., Lawlor B., Gill M., Brayne C., Rubinsztein D. C., Owen M., Williams J., Craig D., Passmore P., Lovestone S., Powell J. F. (2011). A multiple indicators multiple causes (mimic) model of behavioural and psychological symptoms in dementia (bpsd). Neurobiology of Aging, 32(3), 434–442. 10.1016/j.neurobiolaging.2009.03.005 [DOI] [PubMed] [Google Scholar]
- Robert P. H., Berr C., Volteau M., Bertogliati C., Benoit M., Sarazin M., Legrain S., Dubois B.; PréAL study. (2006). Apathy in patients with mild cognitive impairment and the risk of developing dementia of Alzheimer's disease: A one-year follow-up study. Clinical Neurology and Neurosurgery, 108(8), 733–736. https://doi.org/https://https://doi.org/10.1016/j.clineuro.2006.02.003 [DOI] [PubMed] [Google Scholar]
- Robert P. H., Clairet S., Benoit M., Koutaich J., Bertogliati C., Tible O., Caci H., Borg M., Brocker P., Bedoucha P. (2002). The Apathy Inventory: Assessment of apathy and awareness in Alzheimer's disease, Parkinson's disease and mild cognitive impairment. International Journal of Geriatric Psychiatry, 17(12), 1099–1105. 10.1002/gps.755 [DOI] [PubMed] [Google Scholar]
- Robert P., Lanctôt K. L., Agüera-Ortiz L., Aalten P., Bremond F., Defrancesco M., Hanon C., David R., Dubois B., Dujardin K., Husain M., König A., Levy R., Mantua V., Meulien D., Miller D., Moebius H. J., Rasmussen J., Robert G., Manera V. (2018). Is it time to revise the diagnostic criteria for apathy in brain disorders? The 2018 international consensus group. European Psychiatry, 54, 71–76. https://doi.org/https://https://doi.org/10.1016/j.eurpsy.2018.07.008 [DOI] [PubMed] [Google Scholar]
- Robert P., Onyike C. U., Leentjens A. F., Dujardin K., Aalten P., Starkstein S., Verhey F. R., Yessavage J., Clement J. P., Drapier D., Bayle F., Benoit M., Boyer P., Lorca P. M., Thibaut F., Gauthier S., Grossberg G., Vellas B., Byrne J. (2009). Proposed diagnostic criteria for apathy in Alzheimer's disease and other neuropsychiatric disorders. European Psychiatry: The Journal of the Association of European Psychiatrists, 24(2), 98–104. 10.1016/j.eurpsy.2008.09.001 [DOI] [PubMed] [Google Scholar]
- Roth R. M., Flashman L. A., McAllister T. W. (2007). Apathy and its treatment. Current Treatment Options in Neurology, 9(5), 363–370. 10.1007/s11940-007-0022-5 [DOI] [PubMed] [Google Scholar]
- SAS Institute, Cary, NC, USA (2013). [Computer software].
- Selbaek G., Engedal K., Benth J. S., Bergh S. (2014). The course of neuropsychiatric symptoms in nursing-home patients with dementia over a 53-month follow-up period. International Psychogeriatrics, 26(1), 81–91. https://doi.org/https://https://doi.org/10.1017/S1041610213001609 [DOI] [PubMed] [Google Scholar]
- Serretti A. (2023). Anhedonia and depressive disorders. Clinical Psychopharmacology and Neuroscience, 21(3), 401–409. 10.9758/cpn.23.1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockeel P., Dujardin K., Devos D., Denève C., Destée A., Defebvre L. (2006). The lille apathy rating scale (LARS), a new instrument for detecting and quantifying apathy: Validation in Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry, 77(5), 579–584. 10.1136/jnnp.2005.075929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerlad A., Park H. K., Marston L., Livingston G. (2022). Apathy in UK care home residents with dementia: Longitudinal course and determinants. Journal of Alzheimer's Disease: JAD, 87(2), 731–740. 10.3233/JAD-215623 [DOI] [PubMed] [Google Scholar]
- Starkstein S. E., Ingram L., Garau M. L., Mizrahi R. (2005). On the overlap between apathy and depression in dementia. Neurosurgery, and Psychiatry, 76(8), 1070–1074. 10.1136/jnnp.2004.052795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein S. E., Jorge R., Mizrahi R. (2006). The prevalence, clinical correlates and treatment of apathy in Alzheimer’s disease. European Journal of Psychiatry, 20(2), 96–106. https://doi.org/https://https://doi.org/10.4321/S0213-61632006000200005 [Google Scholar]
- Starkstein S. E., Leentjens A. F. G. (2008). The nosological position of apathy in clinical practice. Journal of Neurology, Neurosurgery & Psychiatry, 79(10), 1088–1092. 10.1136/jnnp.2007.136895 [DOI] [PubMed] [Google Scholar]
- Starkstein S. E., Merello M., Jorge R., Brockman S., Bruce D., Power B. (2009). The syndromal validity and nosological position of apathy in Parkinson's disease. Movement Disorders: Official Journal of the Movement Disorder Society, 24(8), 1211–1216. 10.1002/mds.22577 [DOI] [PubMed] [Google Scholar]
- Statistics Canada (2022). Nursing and residential care facilities in 2020, . Government of Canada, Canada. Available from https://www150.statcan.gc.ca/n1/en/daily-quotidien/221102/dq221102c-eng.pdf?st=80LUWtLd. Accessed November 25th, 2023. [Google Scholar]
- Stones M. J. J., Clyburn L. D., Gibson M. C., Woodbury M. G. (2006). Predicting diagnosed depression and anti-depressant treatment in institutionalized older adults by symptom profiles: A closer Look at anhedonia and dysphoria. Canadian Journal on Aging / La Revue Canadienne du Vieillissement, 25(2), 153–159. https://doi.org/https://www.muse.jhu.edu/article/203422 [DOI] [PubMed] [Google Scholar]
- Tang Q., Zhou Y., Yang S., Thomas W. K. S., Smith G. D., Yang Z., Yuan L. (2018). Effect of music intervention on apathy in nursing home residents with dementia. Geriatric Nurse, 39(4), 471–476. 10.1016/j.gerinurse.2018.02.003 [DOI] [PubMed] [Google Scholar]
- Tay J., Morris R. G., Markus H. S. (2021). Apathy after stroke: Diagnosis, mechanisms, consequences, and treatment. International Journal of Stroke, 16(5), 510–518. 10.1177/1747493021990906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thant T., Yager J. (2019). Updating apathy: Using research domain criteria to inform clinical assessment and diagnosis of disorders of motivation. The Journal of Nervous and Mental Disease, 207(9), 707–714. 10.1097/NMD.0000000000000860 [DOI] [PubMed] [Google Scholar]
- van Almenkerk S., Smalbrugge M., Depla M. F., Eefsting J. A., Hertogh C. M. (2015). Apathy among institutionalized stroke patients: Prevalence and clinical correlates. America Journal of Geriatric Psychiatry, 23(2), 180–188. https://doi.org/https://https://doi.org/10.1016/j.jagp.2014.03.011 [DOI] [PubMed] [Google Scholar]
- van Dalen J. W., van Wanrooij L. L., Moll van Charante E. P., Brayne C., van Gool W. A., Richard E. (2018). Association of apathy with risk of incident dementia: A systematic review and meta-analysis. JAMA Psychiatry, 75(10), 1012–1021. https://doi.org/https://https://doi.org/10.1001/jamapsychiatry.2018.1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Malderen L., Mets T., Gorus E. (2013). Interventions to enhance the quality of life of older people in residential long-term care: A systematic review. Ageing Research Reviews, 12(1), 141–150. 10.1016/j.arr.2012.03.007 [DOI] [PubMed] [Google Scholar]
- van Reekum R., Stuss D. T., Ostrander L. (2005). Apathy: Why care? The Journal of Neuropsychiatry and Clinical Neurosciences, 17(1), 7–19. https://doi.org/https://https://doi.org/10.1176/appi.neuropsych.17.1.7 [DOI] [PubMed] [Google Scholar]
- Van Vliet D., De Vugt M. E., Aalten P., Bakker C., Pijnenburg Y. A., Vernooij-Dassen M. J., Koopmans R. T., Verhey F. R. (2013). Prevalence of neuropsychiatric symptoms in young-onset compared to late-onset Alzheimer’s disease-part 1: Findings of the two-year longitudinal NeedYD-study. Dementia and Geriatric Cognitive Disorders, 34(5-6), 319–327. 10.1159/000342824 [DOI] [PubMed] [Google Scholar]
- Vilalta-Franch J., Calvó-Perxas L., Garre-Olmo J., Turró-Garriga O., López-Pousa S. (2013). Apathy syndrome in Alzheimer's disease epidemiology: Prevalence, incidence, persistence, and risk and mortality factors. Journal of Alzheimer's Disease: JAD, 33(2), 535–543. 10.3233/JAD-2012-120913 [DOI] [PubMed] [Google Scholar]
- Volicer L., Frijters D. H., van der Steen J. T. (2013). Apathy and weight loss in nursing home residents: Longitudinal study. Journal of the American Medical Directors Association, 14(6), 417–420. 10.1016/j.jamda.2012.12.004 [DOI] [PubMed] [Google Scholar]
- Wetzels R. B., Zuidema S. U., de Jonghe J. F. M., Verhey F. R. J., Koopmans R. T. C. M. (2010). Course of neuropsychiatric symptoms in residents with dementia in nursing homes over 2-year period. American Journal of Geriatric Psychiatry, 18(12), 1054–1065. 10.1097/JGP.0b013e3181f60fa1 [DOI] [PubMed] [Google Scholar]
- Wong S., Irish M., Husain M., Hodges J. R., Piguet O., Kumfor F. (2020). Apathy and its impact on carer burden and psychological wellbeing in primary progressive aphasia. Journal of the Neurological Sciences, 416, 117007. https://doi.org/10.1016/j.jns.2020.117007https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Wood S., Cummings J. L., Hsu M.-A., Barclay T., Wheatley M. V., Yarema K. T., Schnelle J. F. (2000). The use of the neuropsychiatric inventory in nursing home residents: Characterization and measurement. The American Journal of Geriatric Psychiatry, 8(1), 75–83. https://doi.org/https://https://doi.org/10.1097/00019442-200002000-00010 [DOI] [PubMed] [Google Scholar]
- Yuen G. S., Gunning-Dixon F. M., Hoptman M. J., Abdelmalak B., Mcgovern A. R., Seirup J. K. (2014). The salience network in the apathy of late-life depression. International Journal of Geriatric Psychiatry, 29(11), 1116–1124. 10.1002/gps.4171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q. F., Tan L., Wang H. F., Jiang T., Tan M. S., Tan L., Xu W., Li J. Q., Wang J., Lai T. J. (2016). The prevalence of neuropsychiatric symptoms in Alzheimer's disease: Systematic review and meta-analysis. Journal of Affective Disorder, 190, 264–271. 10.1016/j.jad.2015.09.069 [DOI] [PubMed] [Google Scholar]
- Zhu C. W., Grossman H. T., Sano M. (2019). Why do they just sit? Apathy as a core symptom of Alzheimer disease. The American Journal of Geriatric Psychiatry, 27(4), 395–405. https://doi.org/https://https://doi.org/10.1016/j.jagp.2018.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuidema S. U., de Jonghe J. F., Verhey F. R., Koopmans R. T. (2009). Predictors of neuropsychiatric symptoms in nursing home patients: Influence of gender and dementia severity. International Journal of Geriatric Psychiatry, 24(10), 1079–1086. https://doi.org/https://https://doi.org/10.1002/gps.2225 [DOI] [PubMed] [Google Scholar]
- Zuidema S. U., De Jonghe J. F., Verhey F. R., Koopmans R. T. (2010). Environmental correlates of neuropsychiatric symptoms in nursing home patients with dementia. International Journal of Geriatric Psychiatry, 25(1), 14–22. 10.1002/gps.2292 [DOI] [PubMed] [Google Scholar]