Abstract
COVID-19 vaccination recommendations include healthcare workers (HCWs). We measured COVID-19 vaccine effectiveness (CVE) of the autumn 2023 dose against laboratory-confirmed SARS-CoV-2 infection in a prospective cohort study of 1,305 HCWs from 13 European hospitals. Overall CVE was 22% (95% CI: −17 to 48), 49% (95% CI: −8 to 76) before and −11% (95% CI: −84 to 34) after the start of BA.2.86/JN.1 predominant circulation. Autumn 2023 COVID-19 vaccination led to a moderate-to-low reduction in SARS-CoV-2 infection incidence in HCWs. Monitoring of CVE is crucial for COVID-19 prevention.
Keywords: Vaccine effectiveness, COVID-19, SARS-CoV-2, Healthcare workers, Europe
COVID-19 vaccination recommendations prioritise healthcare workers (HCWs), considering their exposure to severe acute respiratory coronavirus 2 (SARS-CoV-2) and their key role in the functioning of healthcare systems. In the European Union/European Economic Area (EU/EEA), HCWs were considered a priority for COVID-19 revaccination during the autumn 2023 campaign [1], and the World Health Organization (WHO) recommended revaccination of HCWs 12 months after their last dose [2]. Because the Omicron sub-lineage XBB.1.5 predominated in spring 2023, the COVID-19 vaccines were adapted to target this emerging strain, and the first XBB.1.5 vaccine was authorised for use in the EU/EEA in August 2023. Omicron BA.2.86/JN.1 emerged in the EU/EEA at the end of 2023, according to data available on the European Respiratory Virus Surveillance Summary (ERVISS) [3]. Evidence for COVID-19 vaccine recommendation in the HCW population remains scarce. Within the Vaccine Effectiveness, Burden and Impact (VEBIS) project, we aimed to measure the COVID-19 vaccine effectiveness (CVE) in HCWs, in the winter season 2023/24.
VEBIS healthcare worker cohort
In this prospective cohort study [4], we recruited HCWs from 13 hospitals in seven countries (Estonia, Ireland, Italy, Latvia, Portugal, Romania, and Spain). At a weekly follow-up, HCWs provided nasopharyngeal or saliva samples to detect incident SARS-CoV-2 infections and completed a questionnaire to update vaccination and exposure information. We excluded HCWs who did not provide informed consent, missed important information for analysis (e.g. vaccination status and laboratory results) or presented discordant serology and virology results.
Definition of exposures, outcomes, covariates
We defined current vaccination as HCWs who received a dose of any COVID-19 vaccine brand during the autumn 2023 campaign and unvaccinated as HCWs who did not receive a vaccine dose during this campaign, regardless of the number of doses and timing of previous vaccination(s). We stratified previous vaccination in (i) more than 365 days or unvaccinated before the autumn 2023 vaccination campaign and (ii) 90–365 days before the autumn 2023 campaign. We grouped the time since current vaccination in 7–59, 60–119 and ≥ 120 days.
The main outcome of the study was time to the first incident SARS-CoV-2 infection, detected by RT-PCR, regardless of symptoms. Secondary outcomes included symptomatic and asymptomatic COVID-19 in HCWs, depending on whether or not symptoms were reported from 14 days before to 7 days after the first positive test.
Recent previous SARS-CoV-2 infection was defined as self-reported SARS-CoV-2 infection after 1 November 2022 (the month with the start of predominant circulation of Omicron XBB sub-lineage in the participating countries). Non-recent previous infection was defined as self-reported previous SARS-CoV-2 infection before 1 November 2022. We excluded from all analyses a period of 60 days after a positive RT-PCR sample [5].
Vaccine effectiveness analysis
First, we measured the CVE of the autumn 2023 vaccine dose, comparing the current vaccinated with unvaccinated HCWs. In secondary analyses, we measured the CVE by time since previous vaccination, by time since current vaccination, by recent previous infection overall, and by symptomatic status of the SARS-CoV-2 infection, stratified before and after the start of predominant circulation of the Omicron BA.2.86/JN.1 virus sub-lineage. Using Cox regression, we calculated effectiveness as:
| CVE = (1 − hazard ratio of current vaccination) × 100. |
We adjusted the CVE for hospital, age, sex, at least one underlying condition, and recent SARS-CoV-2 infection.
Descriptive and vaccine effectiveness results
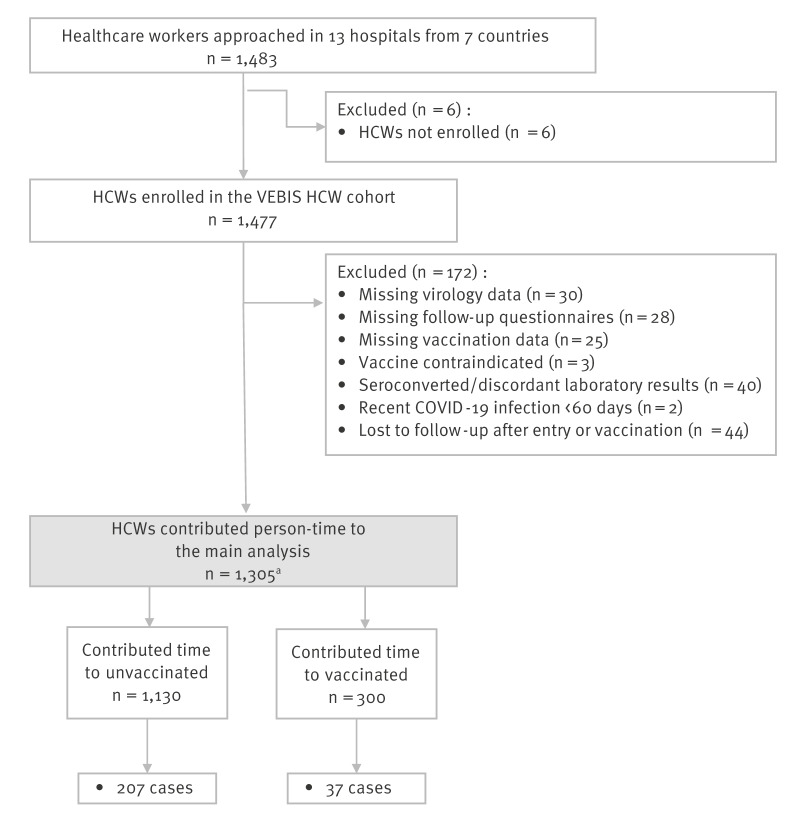
Between October 2023 and May 2024, out of 1,483 HCWs approached, 1,477 were enrolled, ranging from 160 in Italy to 304 in Romania. After applying the inclusion and exclusion criteria, 1,305 HCWs remained in the CVE analysis (Figure 1). Vaccinated HCWs were more likely to be older or to work as medical doctors, and less likely to be female or current smokers (Table 1).
Figure 1.
Inclusion and exclusion criteria, VEBIS healthcare worker multicentre cohort study on COVID-19 vaccine effectiveness, seven European countries, season 2023/24 (n = 1,483)
HCW: healthcare worker.
a HCWs could contribute to both vaccinated and unvaccinated groups.
Table 1. Main characteristics of participants at enrolment by vaccination status, VEBIS healthcare worker multicentre cohort study on COVID-19 vaccine effectiveness, seven European countries, season 2023/24 (n = 1,305).
| Characteristic | Vaccinated in season 2023/24 (n = 300) | Not vaccinated in season 2023/24 (n = 1,005) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Sex | ||||
| Female | 235 | 78.3 | 849 | 84.5 |
| Male | 65 | 21.7 | 156 | 15.5 |
| Age group (years) | ||||
| 18–34 | 40 | 13.3 | 224 | 22.3 |
| 35–39 | 33 | 11.0 | 111 | 11.1 |
| 40–44 | 47 | 15.7 | 143 | 14.2 |
| 45–49 | 52 | 17.3 | 177 | 17.6 |
| 50–54 | 42 | 14.0 | 154 | 15.3 |
| ≥ 55 | 86 | 28.7 | 196 | 19.5 |
| Role | ||||
| Medical doctor | 53 | 17.7 | 161 | 16.0 |
| Nurse | 74 | 24.7 | 491 | 48.9 |
| Administration/reception | 48 | 16.0 | 134 | 13.3 |
| Ancillary | 8 | 2.7 | 40 | 4.0 |
| Allied | 10 | 3.3 | 23 | 2.3 |
| Laboratory | 13 | 4.3 | 47 | 4.7 |
| Other | 38 | 12.7 | 98 | 9.8 |
| Missing | 56 | 18.7 | 11 | 1.1 |
| Smoking | ||||
| Never smoked | 170 | 56.7 | 509 | 50.6 |
| Ex-smoker | 70 | 23.3 | 255 | 25.4 |
| Current smoker | 32 | 10.7 | 234 | 23.3 |
| Missing | 28 | 9.3 | 7 | 0.7 |
| Underlying conditions | ||||
| At least one | 78 | 26.0 | 278 | 27.7 |
| No underlying condition | 212 | 70.7 | 698 | 69.4 |
| Missing | 10 | 3.3 | 29 | 2.9 |
| Recent previous COVID-19 episode | ||||
| Yes | 72 | 24.0 | 324 | 32.2 |
| No | 184 | 61.3 | 653 | 65.0 |
| Missing | 44 | 14.7 | 28 | 2.8 |
| Time since last previous COVID-19 episode | ||||
| Median time in days (range) | 521 (60–1371) | 493 (60–1,481) | ||
| Brand autumn vaccination dose | ||||
| Not XBB1.5-adapted | 55 | 18.3 | Not applicable | |
| XBB1.5-adapted | 245 | 81.6 | ||
| Time since last vaccination dose | ||||
| Median time since previous dose in days (range) | 895 (224–1,122) | 741 (132–1,107) | ||
| Number of vaccine doses ever received before the autumn 2023 vaccination campaign | ||||
| Unvaccinated | 0 | 0.0 | 58 | 5.8 |
| 1 dose | 2 | 0.7 | 44 | 4.4 |
| 2 doses | 7 | 2.3 | 216 | 21.5 |
| 3 doses | 38 | 12.7 | 502 | 49.9 |
| 4 doses | 134 | 44.7 | 183 | 18.2 |
| 5 doses | 119 | 39.7 | 2 | 0.2 |
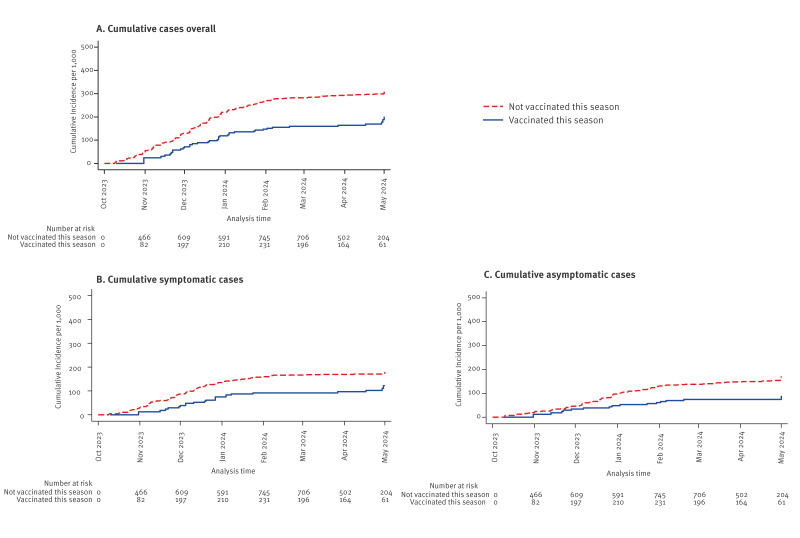
We detected 244 SARS-CoV-2 infections (Figure 1): 37 among vaccinated (1.03 per 1,000 person-days of observation) and 207 among unvaccinated HCW (1.7 per 1,000 person-days). Of these infections, 128 (52%) were symptomatic. The cumulative incidence was lower among the vaccinated throughout the entire follow-up regardless of the outcome used (Figure 2).
Figure 2.
Kaplan-Meier plots of time (days) from enrolment to SARS-CoV-2 infection in VEBIS healthcare worker multicentre cohort study on COVID-19 vaccine effectiveness, by vaccination status, seven European countries, season 2023/24 (n = 1,305)
The adjusted CVE against SARS-CoV-2 infection was 22% (95% confidence interval (CI): −17 to 58) overall, with a CVE point estimate of 26% against asymptomatic and 17% against symptomatic infection, with overlapping confidence intervals. The CVE point estimates were 33% in HCWs with no recent prior infection and 23% in HCWs with a previous vaccination > 365 days. The CVE was 49% (95% CI: −8 to 76) before the start of BA2.86/JN1 circulation and below 0 during the BA2.86/JN1 circulation, with higher CVE point estimates 7–59 days after vaccination. The CVE point estimates were below 0 in HCWs with recent prior infection and vaccinated 90–365 days before the autumn dose, as well as ≥ 120 days after vaccination (Table 2).
Table 2. Adjusted vaccine effectiveness in the primary and secondary analyses, VEBIS healthcare worker multicentre cohort study on COVID-19 vaccine effectiveness, seven European countries, season 2023/24 (n = 1,305).
| Analysis | Vaccinated | Unvaccinated | Adjusteda CVE | ||||
|---|---|---|---|---|---|---|---|
| Number HCWs | Events | Person-days | Number HCWs | Events | Person-days | ||
| Overall effect | |||||||
| SARS-CoV-2 infection | 300 | 37 | 35,657 | 1,130 | 207 | 121,394 | 22 (−17 to 48) |
| Asymptomatic infection | 300 | 15 | 35,657 | 1,130 | 101 | 121,394 | 26 (−43 to 61) |
| Symptomatic COVID-19 | 300 | 22 | 35,657 | 1,130 | 106 | 121,394 | 17 (−40 to 51) |
| By recent prior infection | |||||||
| No recent prior infection | 184 | 25 | 21,135 | 738 | 150 | 70,024 | 33 (−9 to 58) |
| Recent prior infection | 92 | 8 | 9,848 | 486 | 53 | 48,601 | −9 (−139 to 50) |
| Time since previous vaccination | |||||||
| Vaccinated 90–365 days before the current season vaccination | 300 | 37 | 35,657 | 165 | 10 | 5,186 | −31 (−245 to 50) |
| Vaccinated > 365 days before the current season vaccination | 300 | 37 | 35,657 | 1,082 | 197 | 116,208 | 23 (−16 to 49) |
| Before/after BA.2.86 predominant circulation and time since vaccination in season | |||||||
| Before | 190 | 9 | 4,999 | 871 | 108 | 36,503 | 49 (−8 to 76) |
| 7–59 days vs > 365 days before | 190 | 9 | 4,854 | 801 | 100 | 32,278 | 47 (−11 to 75) |
| ≥ 60 days vs > 365 days before | 21 | 0 | 145 | 801 | 100 | 32,278 | Not calculated |
| After | 296 | 28 | 30,658 | 963 | 99 | 84,891 | −11 (−84 to 34) |
| 7–59 days vs > 365 days before | 231 | 9 | 5,849 | 958 | 97 | 83,930 | 16 (−88 to 63) |
| ≥ 60 days vs > 365 days before | 283 | 19 | 24,809 | 958 | 97 | 83,930 | −25 (−128 to 31) |
| Time since vaccination in season vs > 365 days before | |||||||
| 7–59 days | 267 | 18 | 10,703 | 1,082 | 197 | 116,208 | 24 (−29 to 55) |
| 60–119 days | 251 | 9 | 6,658 | 1,082 | 197 | 116,208 | 39 (−43 to 74) |
| 120–149 days | 243 | 9 | 16,080 | 1,082 | 197 | 116,208 | −2 (−114 to 52) |
CVE: COVID-19 vaccine effectiveness; HCW: healthcare worker; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
a Adjusted by age sex, site, at least one underlying condition, recent previous SARS-CoV-2 infection (Participants with at least one covariate missing were not included in the adjusted model).
Discussion
We estimated the effectiveness of an autumn 2023 COVID-19 vaccine dose in HCWs from 13 European hospitals of the VEBIS HCW prospective cohort study. The results suggest a moderate-to-low CVE among HCWs against SARS-CoV-2 infection overall. However, the CVE point estimates suggest a higher protection of COVID-19 vaccines against XBB.1.5 sub-lineages, in circulation before the start of predominant circulation of Omicron BA.2.86/JN.1. The CVE point estimates were higher in HCWs with non-recent previous SARS-CoV-2 infection, suggesting higher benefit of vaccination in these HCWs. The CVE point estimates were also higher for recent vaccination (< 60 days), even during predominant circulation of BA2.86/JN1 when CVE became lower than 0 after 60 days. The null CVE and wide confidence intervals in those with a recent prior vaccination suggest that, in the described scenario, vaccination more frequently than annually may not provide additional protection against SARS-CoV-2 infection overall.
Our results were similar to those of a study conducted in the United States in HCWs with similar vaccination coverage before (CVE = 42%) and during JN1 circulation (CVE = 19%) [6]. They were also similar to the overall estimates by time since vaccination during a 6-month season 2023/24 and to the estimates in HCWs with no recent previous infection in a study from the United Kingdom with higher vaccination coverage [7].
Disentangling the effect of time since last vaccination from the effect of virus evolution was of particular importance for CVE studies during the 2023/24 season [8,9]. As the protection remains at moderate level for about 4 months after vaccination and SARS-CoV-2 variants and sub-lineages continuously emerge, efforts need to be made to better predict the immune evasion [10] and take into account the antigenic distance in CVE estimation [11]. Meanwhile it remains necessary, in addition to vaccination, to recommend frequent testing for HCWs in contact with suspected cases in hospital and in the community, and to regularly reinforce the use of protective equipment when in contact with vulnerable patients, especially when new virus strains emerge.
One of the main strengths of our study was the frequent testing regardless of symptoms, which captured asymptomatic and milder infections; this is important in studies on emerging variants/sub-lineages and in the HCW population. Another strength was the thorough collection of vaccination and previous SARS-CoV-2 infection status.
The main limitation of the study was its low precision of CVE estimates, due to low uptake of the vaccine at the participating hospitals and to the limited number of events, resulting in small sample size particularly when further adjusting by other confounders such as the number of previous vaccine doses or professional role. Adding these covariates in the regression model increased the overall CVE point estimate by 8%, but with a poorer fit of the data than the reported model and with concerns around corelation with existing covariates in the model. Secondly, vaccinated participants seemed to be more likely to accept further vaccination: 45% had four doses compared with 18% in those unvaccinated during the 2023 vaccination campaign, potentially overestimating the CVE results (which was not the case when adjusting by number of previous vaccination doses, as described above). Finally, the studied season was characterised by an initial circulation of Omicron XBB.1.5, later replaced by sub-lineage BA.2.86 and its offspring JN.1. As eight hospitals lacked sequencing information to more accurately define the periods with predominant circulation of the Omicron sub-lineages, we used ERVISS data reported at country level as a proxy; further investigation is needed to check the consistency of our approach.
Conclusion
Our results indicate that an autumn 2023 COVID-19 vaccine dose presented a moderate-to-low reduction of 22% in the risk of SARS-CoV-2 infection in HCWs overall. Nevertheless, the vaccine protected almost one in two HCWs in the period before the predominant circulation of BA.2.86/JN.1 sub-lineage and during less than 60 days after vaccination. Timely deployment of vaccines is crucial for the COVID-19 vaccination programme. With increased sample size, our VEBIS HCW cohort study can provide more precise information to inform key vaccination policies and public health interventions for HCWs in the following seasons.
Ethical statement
The planning, conduct and reporting of the current study was in line with the Declaration of Helsinki, as revised in 2013 (https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects). Informed consent was obtained from each participant at the enrolment in the study. The study was approved by the Ethical review committees from each hospital: Tartu Ülikooli inimuuringute eetika komitee, Estonia: 382/M-7; Clinical research ethics committee, Galway University Hospital, Ireland: C.A. 2693; SJH/TUH Joint Research Ethics Committee, Ireland: 0513; Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, Dipartimento di Sicurezza e Bioetica, Rome, Italy : 00372/23; Ethics Committee for Clinical Research at Pauls Stradins Clinical University Hospital, Latvia: 241023 - 3L; Ethics Committee for Clinical Research at Pauls Stradins Clinical University Hospital, extended to Children Clinical University Hospital, Latvia: 241023 - 10L; Comissão de Ética para a Saúde, INSA Doutor Ricardo Jorge, Portugal: INSA-IM60_05; Comisia de Etica a Spitalului Clinic de Boli Infectioase si Tropicale “Dr. Victor Babes”, Romania: 14420; Comisia Locala de Etica a Spitalului Universitar de Urgenta Militar Central “Carol Davila”, Romania: 646; Comité de Ética de la Investigación de la Comunidad Autónoma de Aragón (CEICA), HU Miguel Servet, Zaragoza, Spain: PI22/483; Comité De Ética De La Investigación Con Medicamentos, Sant Joan de Déu, Fundacio de Recerca, Barcelona, Spain: 17/2023.
Funding statement
This study was funded by European Centre for Disease Prevention and Control through “Vaccine Effectiveness, Burden and Impact Studies” (VEBIS) Lot 2 “Assessment of COVID-19 and influenza vaccine effectiveness among healthcare workers” framework contract ECDC/2021/017.
Use of artificial intelligence tools
None declared.
Data availability
Data will be made available on request.
Acknowledgements
We thank all participants, hospital staff and study investigators not listed above for their efforts to conduct this study. We are also grateful to the following study team members:
Estonia: Kadri Kõivumägi, Natalia Nikitina, Anna Aleksandrova, Anastassia Kuželko.
Ireland: Irene Flynn Dowling; David Byrne; Noeleen Maher; Nicola Murphy.
Portugal: Ana Catarina Dias, Filipe Pimenta, Rui Pedro Lopes, Pedro Nunes, Filipa Chaves, Catarina Barradas.
Romania: Camelia Grancea, Oana Popescu, Catalina Pascu, Sorin Dinu, Alina Elena Ivanciuc, Iulia Bistriceanu, Mihaela Oprea, Maria Elena Mihai.
Spain: Lourdes Roc Alfaro, Sonia Usón Lucea, Beatriz Órpez Villen, Víctor Cantín-Lahoz, Emilia Ferrer López, María Pilar Martínez López, Miriam Infante Garza, Pilar Díaz Díaz, (HUMS, Zaragoza); Jesus Marquez, Ana Codina, Cristina Jou (Biobank- Hospital Sant Joan de Deu), Marta Cubells and Felipe Pérez-Soler (Clinical Research Unit-Institut de Recerca Sant Joan de Deu).
We also thank Esther Kissling and Baltazar Nunes for valuable comments on early draft of the manuscript, as well as to Valerie Nancey and Djenaba Bamba for their support in the study.
Conflict of interest: APU reported payment under EMA DARWIN EU project outside of the submitted work. MLM, AM, LC reported additional support received from ISIDORe (EATRIS) Network for carrying out the local SARS-CoV-2 sequencing. CPP, SAF reported speaker fees from and participation in Advisory board of Pfizer and MSD. SAF reports also participation in Advisory board of Gilead. CMA reported speaker fees from MSD, Pfizer and Sanofi. JS reported support for attending ESID conference 2022 from Takeda Pharmaceutical. RH and SP reported grant attributed to their institution on “PSCD Longitudinal evaluation of SARS CoV-2 immune status post-natural infection and post-vaccination”. All other authors declare no conflicts of interest related to this work.
Authors’ contributions: CS coordinated the VEBIS HCW VE network, wrote the study protocol, contributed to the data analysis and interpretation of the results and wrote the first draft of the manuscript. APU undertook the statistical analysis on which the research article is based, helped interpret results, and contributed to first draft of the manuscript. KB and SB were involved in the study design, interpretation of results, and the review of all versions of the manuscript. AN was involved in the original methodological design of the study (generic protocol), coordinated the VEBIS HCW VE network, helped interpret results, and contributed to all versions of the manuscript. AU, CB, CF, RM, VZ, DZ, VG, CPP, RH, MC, MLM, LL, JM, LF, KGD, IA, DG, AM, SAF, ML, PS, LCC, JS, CK, RS, DK, EAB, CVH, AGK, SMP, CMA, AM and the VEBIS HCW VE study group, were responsible for the coordination of the study at the national/regional/hospital level, contributed to developing the study site-specific protocols, were in charge of the collection, management and validation of the clinical and laboratory data. They interpreted the results, reviewed and contributed to all versions of the manuscript. All authors approved the final version of the manuscript.
References
- 1.European Centre for Disease Prevention and Control (ECDC). ECDC-EMA statement on updating COVID-19 vaccines composition for new SARS-CoV-2 virus variants. Amsterdam: EMA; Stockholm: ECDC; 2023. Available from: https://www.ecdc.europa.eu/en/news-events/ecdc-ema-statement-updating-covid-19-vaccines-composition-new-sars-cov-2-virus-variants
- 2.World Health Organization (WHO). WHO roadmap on uses of COVID-19 vaccines in the context of Omicron and high population immunity. Geneva: WHO; 2023. Available from: https://iris.who.int/bitstream/handle/10665/373987/WHO-2019-nCoV-Vaccines-SAGE-Prioritization-2023.2-eng.pdf?sequence=1
- 3.European Centre for Disease Prevention and Control (ECDC). The European Respiratory Virus Surveillance Summary (ERVISS). Stockholm: ECDC. [Accessed: 11 Jun 2024]. Available from: https://www.ecdc.europa.eu/en/publications-data/european-respiratory-virus-surveillance-summary-erviss
- 4.European Centre for Disease Prevention and Control (ECDC). Generic protocol for ECDC studies of COVID-19 vaccine effectiveness against confirmed SARS-CoV-2 using healthcare worker cohorts, version 3.0 . Stockholm: ECDC; 2024. Available from: https://www.ecdc.europa.eu/en/publications-data/generic-protocol-ecdc-studies-covid-19-vaccine-effectiveness
- 5.European Centre for Disease Prevention and Control (ECDC). Reinfection with SARS-CoV-2: implementation of a surveillance case definition within the EU/EEA. Stockholm: ECDC; 2021. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/Reinfection-with-SARSCoV2-implementation-of-a-surveillance-case-definition.pdf
- 6. Shrestha NK, Burke PC, Nowacki AS, Gordon SM. Effectiveness of the 2023-2024 Formulation of the coronavirus disease 2019 messenger RNA vaccine. Clin Infect Dis. 2024;79(2):405-1. [DOI] [PubMed] [Google Scholar]
- 7. Kirwan PD, Foulkes S, Munro K, Sparkes D, Singh J, Henry A, et al. Protection of vaccine boosters and prior infection against mild/asymptomatic and moderate COVID-19 infection in the UK SIREN healthcare worker cohort: October 2023 to March 2024. J Infect. 2024;89(5):106293. 10.1016/j.jinf.2024.106293 [DOI] [PubMed] [Google Scholar]
- 8. Link-Gelles R, Ciesla AA, Mak J, Miller JD, Silk BJ, Lambrou AS, et al. Early estimates of updated 2023-2024 (Monovalent XBB.1.5) COVID-19 vaccine effectiveness against symptomatic SARS-CoV-2 infection attributable to co-circulating Omicron variants among immunocompetent adults - increasing community access to testing program, United States, September 2023-January 2024. MMWR Morb Mortal Wkly Rep. 2024;73(4):77-83. 10.15585/mmwr.mm7304a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laniece Delaunay C, Melo A, Maurel M, Mazagatos C, Goerlitz L, O’Donnell J, et al. Effectiveness of COVID-19 vaccines administered in the 2023 autumnal campaigns in Europe: Results from the VEBIS primary care test-negative design study, September 2023-January 2024. Vaccine. 2024;42(19):3931-7. 10.1016/j.vaccine.2024.05.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thadani NN, Gurev S, Notin P, Youssef N, Rollins NJ, Ritter D, et al. Learning from prepandemic data to forecast viral escape. Nature. 2023;622(7984):818-25. 10.1038/s41586-023-06617-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao L, Lou J, Chan SY, Zheng H, Liu C, Zhao S, et al. Rapid evaluation of COVID-19 vaccine effectiveness against symptomatic infection with SARS-CoV-2 variants by analysis of genetic distance. Nat Med. 2022;28(8):1715-22. 10.1038/s41591-022-01877-1 [DOI] [PMC free article] [PubMed] [Google Scholar]