Abstract
Background.
Antiretroviral therapy (ART) is being administered in developing nations at unprecedented numbers following the World Health Organization’s (WHO) development of standardized first-line drug regimens. To ensure continued efficacy of these drug regimens, WHO recommends monitoring virological responses and development of human immunodeficiency virus (HIV) drug resistance (HIVDR) in HIV-infected patients in a prospective cohort. The current study compared dried fluid spot specimens with the reference standard plasma specimens as a practical tool for viral load (VL) and HIVDR genotyping in resource-limited settings.
Methods.
Dried blood spot (DBS), dried plasma spot (DPS), and plasma specimens were collected from 173 –patients receiving ART at 2 hospital sites in Abuja, Nigeria. HIV-1 VL analysis was performed using NucliSENS EasyQ HIV-1 v1.1 RUO test kits. Genotyping of the HIV-1 pol gene was performed using a broadly sensitive in-house assay.
Results.
Direct comparison of VL levels showed that DBS specimens, and not DPS specimens, gave results comparable to those of plasma specimens (P = .0619 and .0007, respectively); however, both DBS and DPS specimens had excellent correlation with plasma specimens in predicting virological failure (VL, ≥1000 copies/mL) in patients (κ = 0.78 and 0.83, respectively). Of the 18 specimens with a plasma VL ≥1000 copies/mL, HIVDR genotyping rates were 100% in DBS and 38.9% in DPS specimens, and DBS specimens identified 61 of 65 HIVDR mutations (93.8%) identified in plasma specimens.
Conclusions.
Our results indicate that DBS specimens could be used for surveys to monitor HIVDR prevention failure in resource-limited settings.
The number of HIV (human immunodeficiency virus)–infected individuals receiving antiretroviral therapy (ART) in low- and middle-income countries has increased dramatically [1]. This massive scale-up of ART delivery in resource-limited settings has been achieved through the development of standardized first-line drug regimens promoted by the World Health Organization (WHO), which have been adopted by resource-limited countries based on need [1]. This approach has been very successful to date; however, to maintain the efficacy of the first-line regimens, WHO developed an HIV drug resistance (HIVDR) prevention and assessment strategy for countries rapidly scaling up ART [2]. One element of the strategy, the HIVDR monitoring survey, involves monitoring virological response and development of HIVDR in HIV-1–infected patients commencing ART in a prospective cohort [3].
Plasma specimens are currently the reference standard for HIV-1 viral load (VL) and HIVDR genotyping and are the only recommended specimen type for monitoring surveys that determine the VL and HIVDR genotyping of patients receiving ART [4]. This makes monitoring surveys in resource-limited settings difficult, because plasma specimens require immediate processing, cold-chain storage, and transportation. Dried fluid spot specimens have been shown to be a viable alternative to plasma specimens for polymerase chain reaction (PCR)–based early infant diagnosis of HIV-1 infection [5–7] and HIV antibody testing [8, 9], and are more manageable in resource-limited settings. Dried blood spot (DBS) specimens are particularly suited to resource-limited settings, because they require minimal training to prepare, are stable at ambient temperature for several weeks [10], are deemed nonhazardous once they are dried, and can be shipped via regular mail or courier services [11]. Thus, the use of DBS specimens could dramatically reduce the logistic complexity and cost of VL testing and HIVDR genotyping for monitoring surveys. Several studies have demonstrated that DBS specimens provided similar results compared with plasma specimens for HIVDR analysis in treatment-naive individuals [12–15]; however, they also identified limitations in the sensitivity of DBS specimens in patients with lower VLs [12, 13], suggesting that DBS specimens may not be a suitable specimen type for HIVDR analysis in patients receiving ART, who often have a low VL. Although indicated, very few studies have evaluated DBS specimens for HIVDR analysis in a cohort of treatment-experienced patients [16–21], and even fewer of these studies have examined both the VL and HIVDR from the same spot specimens collected from ART-experienced patients [16], resulting in insufficient evidence for using DBS specimens to monitor patients receiving ART with VL quantitation and HIVDR genotyping in surveys.
Our laboratory has developed a broadly sensitive HIVDR genotyping assay that amplifies the protease and reverse-transcriptase (RT) regions of the HIV-1 pol gene, can genotype multiple HIV-1 group M subtypes and circulating recombinant forms, and reduces the reagent cost by 75% when compared with commercial kits [15, 22]. We have successfully applied this assay to the surveillance of transmitted HIVDR in recently HIV-infected populations in 5 countries supported by the US President Emergency Plan for AIDS Relief (PEPFAR) [15, 23–27]. We hypothesized that with the right combination of HIV-1 VL and genotyping technologies, we could provide evidence that HIV VL measurement and genotyping could be performed with DBS specimens that meet the monitoring survey specifications. That ideal combination is the NucliSENS EasyQ HIV-1 v1.1 assay, which has the isothermal RT-PCR design to minimize the proviral contribution to VL measurement from DBS specimens, and our broadly sensitive genotyping assay.
MATERIALS AND METHODS
Participants
Between January and July 2008, 281 HIV-1-infected patients who were eligible for ART were consecutively enrolled into the monitoring survey from 2 ART sites in Abuja, Federal Capital Territory, Nigeria. They were treated with standard first-line antiretroviral drugs following the Nigeria National Treatment Guidelines [28]. Patients were monitored for clinical improvement and CD4 T-lymphocyte count during the 1-year follow-up (defined as 12–15 months after the initiation of ART) according to the routine practice of the sites. At the 12-month follow-up visit, 176 patients attended their visits at the 2 sites and blood was collected from each of them. The current study included 173 patients; 3 patients were excluded, owing to insufficient sample volumes in 2 patients and mislabeling in 1. Detailed clinical and demographic information on the participants has been provided elsewhere [29].
Specimen Collection, Preparation, and Storage
From each of the 173 patients who visited the ART sites 1 year after initiation of ART, 10 mL of whole blood was collected into an ethylenediaminetetraacetic acid vaccum blood tube. DBS specimens were prepared by spotting 100 μL of whole blood onto each of the 5 preprinted circles on a Whatman 903 filter paper (Whatman). Plasma was then separated from blood cells by centrifugation and used to make dried plasma spot (DPS) specimens (50 μL/spot), following the same instructions used for DBS specimen preparation, and the remainder was stored immediately at −70°C, to be used as the reference standard specimens. Both DBS and DPS cards were allowed to dry overnight at ambient temperature. The next day, glassine paper was folded around each DBS or DPS card, and 10–20 cards were packaged in a Bitran liquid-tight specimen bag with desiccant packs and a humidity indicator card, and then stored at ambient temperature for a mean ± standard deviation (85.31 ± 42.66 days), (median, 83.5 days) before they were shipped to the WHO Specialized Drug Resistance Laboratory at the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia, for testing. All specimens were stored at −80°C on arrival at the CDC.
Nucleic Acid Extraction and HIV-1 VL Analysis
One DBS or DPS spot was cut out per specimen and placed in 2 mL of NucliSENS lysis buffer (Biomeriuex) for 30 minutes at room temperature with gentle rotation. Plasma specimens (200 μL) were added to 2 mL of NucliSENS lysis buffer and incubated for 10 minutes at room temperature. Nucleic acid was then extracted from all specimens using the NucliSENS EasyMag (Biomeriuex) automated extraction system according to the manufacturer’s instructions. Nucleic acid was eluted in 25 μL of NucliSENS Extraction Buffer 3 and stored at −80°C until use.
HIV-1 VL was determined with the NucliSENS EasyQ automated system using NucliSENS EasyQ HIV-1 v1.1 RUO test kits (Biomeriuex), following the manufacturer’s instructions. The linear range of this assay is 50–3000000 copies/mL when 1 mL of plasma is used [30]. The VL for all specimens was normalized to a volume of 1 mL of plasma. The amount of plasma in the DBS specimen was determined by normalizing for the volume of the sample and the mean hematocrit (a generalized value of 40%) as described by Johannessen et al [31].
HIV-1 Drug-Resistance Genotyping
Genotyping of the protease and RT regions of the HIV-1 pol gene was performed using the broadly sensitive genotyping assay described in detail elsewhere [15, 22]. Briefly, a 1084–base pair segment of the 5’ region of the pol gene was generated by RT-PCR followed by nested PCR. This fragment was purified, sequenced using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems), and analyzed on the ABI Prism 3730 Genetic Analyzer (Applied Biosystems). ChromasPro software (version 1.42; Technelysium Pty Ltd) was used to edit the raw sequences and generate consensus sequences. HIVDR mutations were determined using the Stanford University Drug Resistance Database.
Statistical Analysis
All HIV-1 VL values were log10-transformed before analysis. Quantitative variables are expressed as mean values (± standard deviation) and 95% confidence intervals where relevant. The Wilcoxon signed rank test was used to assess the bias in measuring VL between plasma and DBS or DPS specimens. Bland-Altman analysis was used to determine concordance between VL measurements of plasma and DBS or DPS specimens [32]. The agreement for the detection of virological failure by DBS or DPS specimens and plasma specimens was assessed using κ statistics, and P values were determined using Fisher’s exact test. As suggested by J. L. Fleiss, κ values <0.40 were considered to indicate poor agreement; values >0.40 and <0.75, fair to good agreement; and values >0.75, excellent agreement [33]. Genotyping efficiency was determined by dividing the number of samples successfully genotyped by the total number of samples with a detectable VL or VL≥1000 copies/mL. Nucleotide identity was calculated using the BioEdit sequence alignment editor. Statistical calculations were performed using GraphPad Prism (version 5.0, GraphPad Software) or SPSS (Statistical Package for the Social Sciences) software (version 17.0; SPSS Inc).
RESULTS
HIV-1 VL Analysis
Using the NucliSENS HIV-1 v1.1 RUO kit and the NucliSENS EasyQ analyzer, we were able to measure HIV-1 VL in parallel plasma, DBS, and DPS specimens collected from all 173 patients at 12 months after the initiation of ART. The VL analysis revealed that 26 plasma, 28 DBS, and 17 DPS specimens had detectable VL. The mean log10 VLs (± standard deviation) for plasma, DBS, and DPS specimens were 3.78 ± 1.1 copies/mL (range, 2.18–6.41), 3.63 ± 0.88 copies/mL (range, 2.64–5.56), and 3.85 ± 0.82 copies/mL (range, 2.92–5.94), respectively. When we compared the mean VLs of specimens with detectable VLs in plasma and DBS specimens or plasma and DPS specimens (matched specimens), no statistically significant difference was found between plasma and DBS VL values (P = .0619); however VL values from matched plasma and DPS specimens were significantly different (P = .0007). Bland-Altman agreement analysis revealed that DBS and DPS specimens were comparable blood collection methods to plasma specimens for measuring VL, because the differences between plasma and DBS or DPS VL values for all but 1 specimen were within the 95% limits of agreement (Figure 1A and B). Notably, the mean difference between plasma and DPS specimens was >0.5 copies/mL, and all but 1 of the data points were >0, indicating that plasma VL values were consistently higher than DPS VL values by an average of 0.5 log10 copies/mL (Figure 1B). In contrast, the mean difference between plasma and DBS specimens was closer to 0, and the points were more evenly distributed above and below 0, compared with DPS specimens.
Figure 1.
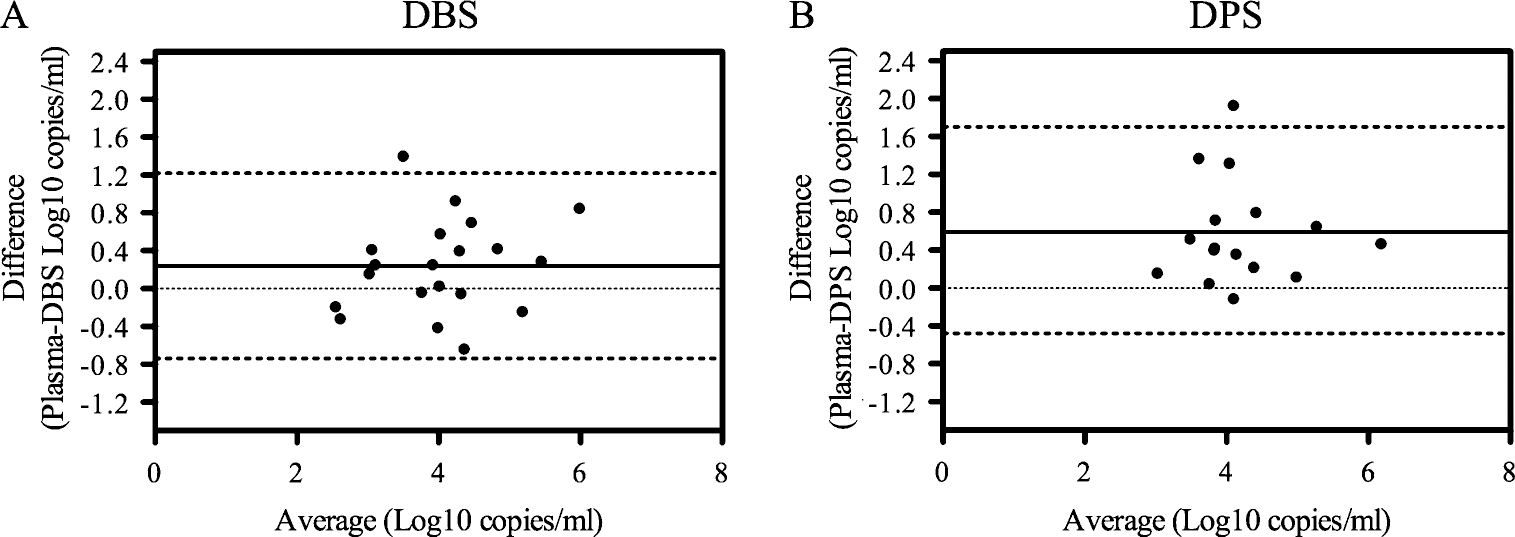
Bland-Altman analysis of viral load (VL) in dried blood spot (DBS) and dried plasma spot (DPS) specimens compared with plasma specimens. Nucleic acid was extracted from DBS, plasma, and DPS specimens using the NucliSENS EasyMag automated system, and NucliSENS HIV-1 v1.1 RUO kits were used to determine the HIV-1 viral load with the NucliSENS EasyQ analyzer. All VL values were normalized to a volume of 1 mL, and the DBS values were further normalized for a chosen mean hematocrit of 40%. Results represent DBS (n = 20) (A) and DPS (n = 16) (B) specimens with detectable VLs in both plasma and dried fluid spot specimens. Solid line represents mean difference (Log10 copies/mL) between plasma and DBS (0.23) or DPS (0.59) specimens (the mean ± standard deviation [ranges] are 0.23 ± 0.50 [0.005–0.473] Log10 copies/mL between plasma and DBS specimens, and 0.59 ± 0.55 [0.299–0.881] between plasma and DPS specimens); dotted lines represent 95% limits of agreement (mean difference ± 1.96 × standard deviation) for DBS (−0.74 and 1.22; width, 1.96) and DPS (−0.48 and 1.66; width, 2.14).
To assess the feasibility of using DBS and DPS specimens for WHO-recommended HIVDR monitoring surveys, we evaluated the ability of these dried blood collection methods to detect virological failure, defined as plasma viral RNA levels ≥1000 copies/mL as recommended by WHO [3], using plasma specimens as the reference standard. Table 1 illustrates that there is excellent overall agreement between DBS or DPS and plasma specimens for detecting virological failure in patients receiving ART, with P values <.001 for both DBS and DPS specimens and κ statistics of 0.78 (DBS) and 0.83 (DPS). DBS specimen results correctly identified virological failure in 14 of 18 patients (77.8%) and viral suppression in 152 of 155 (specificity, 98.1%; negative predictive value, 97.4%). Likewise, DPS specimen results correctly identified virological failure in 14 of 18 patients and viral suppression in 154 of 155 ( positive predictive value, 93.3%; negative predictive value, 97.5%).
Table 1.
Concordance Between DBS, DPS, and Plasma Specimens in Identifying Virological Failure
| Plasma Specimens |
||||||
|---|---|---|---|---|---|---|
| Specimen Type and VL, copies/mL | VL ≥1000 Copies/mL | VL <1000 Copies/mL | Total | κ Value, Mean ± SE (95% Confidence Interval) | P | Performance of DBS and DPS Specimens, %a |
|
| ||||||
| DBS | 0.78 ± 0.08 (0.62–0.94) | <.001 | Sensitivity, 77.8; specificity, 98.1; PPV, 82.3; NPV, 97.4 | |||
| ≥1000 | 14 | 3 | 17 | |||
| <1000 | 4 | 152 | 156 | |||
| Total | 18 | 155 | 173 | |||
| DPS | 0.83 ± 0.07 (0.69–0.98) | <.001 | Sensitivity, 77.8; specificity, 99.4; PPV, 93.3; NPV, 97.5 | |||
| ≥1000 | 14 | 1 | 15 | |||
| <1000 | 4 | 154 | 158 | |||
| Total | 18 | 155 | 173 | |||
Viral failure was defined as plasma viral RNA levels ≥1000 copies/mL.
Abbreviations: DBS, dried blood spot; DPS, dried plasma spot; NPV, negative predictive value, PPV, positive predictive value; SE, standard error; VL, viral load.
PPV and NPV were calculated using a 10.4% prevalence of virological failure
Drug Resistance Genotyping of the HIV-1 pol Gene Region
To assess whether DBS and DPS specimens were comparable to plasma specimens for HIVDR genotyping in ART-experienced patients, we determined the genotyping efficiency for DBS and DPS specimens and stratified the data by plasma VL, as well as the nucleotide sequence similarity to plasma specimens for DBS and DPS specimens that yielded genotyping results in both plasma specimens and the paired dried fluid spot specimens. The ability to successfully genotype specimens with a VL ≥1000 copies/mL was 100% in plasma and DBS specimens but only 38.9% in DPS specimens (Table 2). Despite having a substantially reduced genotyping rate, the sequences obtained from DPS specimens had a high nucleotide sequence identity with sequences obtained from plasma specimens (Table 2). DBS specimens had a comparable nucleotide sequence identity, showing 98.6% identity to plasma specimens at plasma VL <1000 copies/mL and 98.8% at VL ≥1000 copies/mL (Table 2).
Table 2.
Dried Fluid Spot Genotyping Efficiency and Pairwise Nucleotide Identity Compared to Plasma Specimens
| DBS Specimens |
DPS Specimens |
||||
|---|---|---|---|---|---|
| Plasma VL Group | Genotyping Efficiency for Plasma Specimens, % (No.) | Genotyping Efficiency, % (No.) | Nucleotide Identity to Plasma, % Mean ± SD (95% CI) | Genotyping Efficiency, % (No.) | Nucleotide Identity to Plasma, % Mean ± SD (95% CI) |
|
| |||||
| <1000 copies/mL | 87.5 (7/8) | 50.0 (4/8) | 98.6 ± 1.2 (96.7–100.5) | 12.5 (1/8) | 98.9a |
| ≥1000 copies/mL | 100 (18/18) | 100 (18/18) | 98.8 ± 0.83 (98.4–99.2) | 38.9 (7/18) | 98.2 ± 1.1 (97.2–99.2) |
Abbreviations: CI, confidence interval; DBS, dried blood spot; DPS, dried plasma spot; SD, standard deviation; VL, viral load.
This value represents the VL of a single DPS sample and is not a mean
HIV-1 Drug Resistance Mutation Profile
To further analyze HIVDR genotyping in plasma and DBS specimens, we compared drug resistance mutation profiles determined by the Stanford University HIV Drug Resistance Database, using plasma specimens as the reference standard. DPS specimens were not included in this analysis due to the poor genotyping rate (Table 2). Table 3 provides a detailed view of drug resistance mutations identified from 22 matched (genotyped in both DBS and plasma specimens) and 3 unmatched (genotyped in plasma specimens only) plasma and DBS specimens that were genotyped. In total, we identified 78 drug resistance mutations in the matched plasma specimens, 68 of which were also detected in DBS specimens. All of the discordant mutations present in plasma specimens and absent in corresponding DBS specimens were the result of base mixtures (2 A98AG, H221HY, K101KQ), indicating the presence of a subdominant virus population that was undetectable in DBS specimens. Analysis of antiretroviral susceptibility profiles determined by the HIVdb algorithm created by the Stanford Database indicates that a majority of the discordant drug resistance mutations between matched plasma and DBS specimens did not lead to changes in drug susceptibility profiles. However, we identified 3 matched specimens and 1 unmatched specimen with significant differences in drug susceptibility, 3 of which had a plasma VL <1000 copies/mL. Interestingly, the 1 discordant specimen in this group with a clinically relevant VL of ≥1000 copies/mL had detectable drug resistance in the DBS specimen due to the K65R mutation, which was not detected in the plasma specimen.
Table 3.
Mutation Profiles of Plasma and DBS Specimens
| Plasma Specimens |
DBS Specimens |
|||||||
|---|---|---|---|---|---|---|---|---|
| Specimen Type | Specimen ID | ARV Regimen | PI | NRTI | NNRTI | PI | NRTI | NNRTI |
|
| ||||||||
| Matched specimens | ||||||||
| Concordant | ||||||||
| ≥1000 copies/mL | 2-2-063 | AZT, 3TC, NVP | K101E, E138A, G190A | K101E, E138A, G190A | ||||
| 1-2-095 | 3TC, d4T, NVP | T69ST | T69ST | |||||
| 2-2-028 | AZT, 3TC, NVP | G190A | G190A | |||||
| 2-2-132 | 3TC, TDF, NVP | K65R, M184V | Y181C | K65R, M184V | Y181C | |||
| 1-2-128 | 3TC/AZT, NVP | L10I | V75IV, K219EK | Y181C | L10I | V75IV, K219EK | Y181C | |
| 2-2-010 | AZT, 3TC, NVP | K70R, M184V | A98AG, Y188L | K70KR, M184V | A98AG, Y188L | |||
| 2-2-041 | FTC/TDF, NVP | K65R, M184V | L100I, K103N | K65R, M184V | L100I, K103N | |||
| 1-2-176 | 3TC, d4T, NVP | |||||||
| 1-2-121 | 3TC, d4T, EFV | L10I | L10I | |||||
| 2-2-118 | 3TC, d4T, EFV | V118I, M184V | K103N, P225H | V118I, M184MV | K103 KN, P225HP | |||
| 1-2-030 | 3TC, d4T, NVP | L10IV | L10IV | |||||
| 1-2-016 | 3TC/AZT, NVP | T74S | K103KN | T74S | K103KN | |||
| 1-2-186 | Unknown | T74S | D67G, K70R, M184V, K219E | A98G, V108I, Y181C | T74S | D67G, K70R, M184V, K219E | A98G, V108I, Y181C | |
| 1-2-102 | 3TC, d4T, NVP | M184V | K103N, K238EK | M184V | K103N, K238E | |||
| <1000 copies/mL | 2-2-091 | AZT, 3TC, NVP | M184V | K101E, E138A, G190A, M230L | M184V | K101E, E138A, G190A, M230L | ||
| Discordant | ||||||||
| ≥1000 copies/mL | 2-2-120 a | FTC/TDF, NVP | D67DN, K70KR, M184V, K219DEKN | A98AG, V179DE, Y181I | K65R, D67N, K70KR, M184V, K219E | V179DE, Y181I | ||
| 2-2-105 | AZT, 3TC, NVP | L10I | M184MV | K103N | L10I | M184MV | K103N, H221HY | |
| 1-2-100 | 3TC, d4T, NVP | L10I | M184V | A98AG, Y181C, H221HY | L10I | M184V | Y181C, H221Y | |
| 2-2-128 | FTC/TDF, NVP | M184V | A98G, K101KQ, V108IV, Y181C, H221HY | M184V | A98G, V108I, Y181C | |||
| 2-2-116 a | FTC/TDF, NVP | L10I | M184MV | A98G, Y181C, H221Y | L10I | A98G, Y181CY, H221Y | ||
| <1000 copies/mL | 1-2-099 a | 3TC, d4T, NVP | M184V | K103N, V108IV | ||||
| 2-2-130 | FTC/TDF, NVP | A62AV, K65KR, M184V, K219KN | K103N, Y181C | L74V, M184V | K103N, Y181C | |||
| Unmatched specimens | ||||||||
| <1000 copies/mL | 2-2-053 a | AZT, 3TC, NVP | D67DN, K70KR, M184V, T215FIST | Y181C, H221HY | NDb | NDb | NDb | |
| 1-2-167 | 3TC/AZT, EFV | M46L | NDb | NDb | NDb | |||
| 2-2-060 | FTC/TDF, EFV | No | Mutations | NDb | NDb | NDb | ||
Mutation profiles from all plasma specimens that were genotyped are shown, along with the corresponding dried blood spot (DBS) specimen. Matched refers to specimens in which both the plasma and DBS specimens were genotyped; unmatched, specimens in which we were able to genotype only the plasma specimen owing to poor polymerase chain reaction (PCR) amplification of the DBS specimen. Boldface indicates mutations that were discordant between plasma and DBS specimens.
Abbreviations: 3TC, lamivudine; ARV, antiretroviral; AZT, zidovudine; d4T, stavudine; EFV, efavirenz; FTC, emtricitabine; ND, not determined; NRTI, nucleoside reverse-transcription inhibitor; NNRTI, non-NRTI; NVP, nevirapine; PI, protease inhibitor; TDF, tenofovir disoproxil fumarate.
Specimen pairs for which plasma and DBS specimens had differing drug susceptibility (susceptible, intermediate level, or high level resistance) ratings, as determined by the Stanford University HIV Drug Resistance Database HIVdb algorithm.
Not determined due to RT-PCR was negative.
DISCUSSION
In this study, we sought to determine whether dried fluid spot specimens could be used as an alternative to plasma specimens for VL and HIVDR monitoring surveys that will measure the prevalence of HIVDR prevention failure in resource-limited countries. We demonstrated that DBS and DPS specimens were comparable to plasma specimens for quantitative VL analysis, according to the 95% agreement limits of the Bland-Altman plot (Figure 1A–B); however, DPS specimens displayed a clinically relevant bias of >0.5 log10 copies/mL in this analysis (Figure 1B) and a significantly different mean VL, determined by a Wilcoxon signed rank test. These data indicate that VL values from DBS specimens were in consistent agreement with plasma specimens across multiple analyses, whereas DPS specimens showed greater variability. PCR amplification and genotyping analyses were substantially reduced in DPS specimens compared with DBS or plasma specimens (Table 2), and HIVDR genotyping results from DBS were comparable to plasma specimens at VL ≥1000 copies/mL. Overall, our results indicate that DBS specimens could be used for surveys to monitor HIVDR prevention failure in resource-limited settings; however, because of the limited sample size of our study, more studies with larger sample sizes are needed to provide sufficient evidence.
Several studies have demonstrated that DBS specimens can effectively detect HIVDR mutations [12–21, 34], yet only a few have evaluated DBS specimens from ART-experienced patients for HIVDR genotyping [15–21]. In these studies, a broad range of DBS amplification rates have been demonstrated, ranging from 58% [16, 20] to 100% [21]. Several of these studies reported substantially higher amplification efficiency from samples with a higher VL [17, 18, 20]. Consistent with these studies, we observed a 50% genotyping efficiency for DBS samples with a plasma VL <1000 copies/mL, and a 100% genotyping rate in DBS samples with a plasma VL ≥1000 copies/mL (Table 2). In addition, we observed a 98.8% nucleotide identity between specimen types (Table 2), which is in agreement with previously reported values of 99.3% [17], 98.8% [18], and 97.9% [19], providing further evidence that DBS specimens perform similarly to plasma specimens for HIVDR genotyping in patients receiving ART.
In contrast to previous studies demonstrating a high concordance in HIVDR mutation profiles between plasma and DBS specimens from ART-treated patients [17, 18, 20, 21], we identified 10 of 78 total mutations that were detected in plasma but not in DBS specimens (Table 3). The 4 discordant mutations present in plasma specimens with a VL ≥1000 copies/mL and absent in corresponding DBS specimens were the result of base mixtures, indicating an increased sensitivity for detecting subdominant quasispecies of virus in the plasma specimens of early failing patients, as compared with DBS specimens. This decreased sensitivity in genotyping observed in DBS specimens could be the result of nucleic acid degradation during the prolonged storage of the specimens at ambient temperature or could be due to the decreased sample volume (equivalent to 60 μL of plasma) of the DBS specimens compared with plasma specimens (200 μL). Conversely, it has been shown that newly acquired drug resistance mutations can be detected in plasma RNA before they can be detected in cell-associated proviral DNA [35]; therefore, the absence of these mutations in DBS specimens in this study could reflect a newly emerging drug-resistant quasispecies of virus. Because we did not differentiate between proviral DNA and RNA in DBS specimens, we cannot rule out the contribution of proviral DNA to our genotyping results. However, the detection of 3 mutations in DBS specimens that were not detected in plasma specimens (Table 3) indicates that proviral DNA may be contributing to our genotyping results from DBS specimens. Other studies have also shown that proviral DNA could contribute to HIVDR genotyping results when DBS specimens were used [10, 14, 18], but this contribution did not significantly alter the overall resistance profiles [18], which is in agreement with our current results. In our current study, the majority of discordant mutations (n = 6) between plasma and DBS specimens occurred in specimens with a plasma VL <1000 copies/mL (Table 3). Based on WHO recommendations, specimens with VLs <1000 copies/mL would not be genotyped in HIVDR monitoring surveys [3].
Unlike DBS specimens, DPS specimens performed poorly for genotyping. DPS specimens have been shown to have reduced PCR amplification rates after 1 month of storage at 20°C [10] and even faster degradation at higher temperatures and humidity [10, 36]. Our data clearly illustrate that DPS specimens stored for prolonged periods at ambient temperature (median, 83.5 days) are not suitable for HIVDR analysis.
In this study, we demonstrated that DBS specimens were comparable to plasma specimens for VL and HIVDR analyses in ART-treated patients. They require less training to collect and do not require cold-chain transport and can therefore be collected in more remote areas, increasing sampling diversity at a decreased cost. Although our data support the use of DBS specimens, more studies similar to this one with larger sample sizes are needed to prove that DBS specimens are a viable replacement for plasma specimens in HIVDR prevention monitoring surveys.
Acknowledgments.
The authors would like to express their sincere appreciation to the hospital staffers who were involved in the project and all the patients who participated in the project.
Financial support.
This study was supported in part by the US President’s Emergency Plan for AIDS Relief. E. K. R. and G. Z. are the recipients of 2009–2011 and 2010–2011 Emerging Infectious Disease Fellowships, sponsored by the American Public Health Laboratory and the US Centers for Disease Control and Prevention, respectively.
Footnotes
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Progress report. Geneva, Switzerland: World Health Organization, 2010. [Google Scholar]
- 2.Bennett DE, Bertagnolio S, Sutherland D, Gilks CF. The World Health Organization’s global strategy for prevention and assessment of HIV drug resistance. Antivir Ther 2008; 13(Suppl 2):1–13. [PubMed] [Google Scholar]
- 3.Jordan MR, Bennett DE, Bertagnolio S, Gilks CF, Sutherland D. World Health Organization surveys to monitor HIV drug resistance prevention and associated factors in sentinel antiretroviral treatment sites. Antivir Ther 2008; 13(Suppl 2):15–23. [PubMed] [Google Scholar]
- 4.World Health Organization. WHO manual for HIV drug resistance testing using dried blood spot specimens. 2010. Available at: http://www.who.int/hiv/topics/drugresistance/dbs_protocol.pdf. Accessed 4 February 2012.
- 5.Comeau AM, Pitt J, Hillyer GV, et al. Early detection of human immunodeficiency virus on dried blood spot specimens: sensitivity across serial specimens. Women and Infants Transmission Study Group. J Pediatr 1996; 129:111–18. [DOI] [PubMed] [Google Scholar]
- 6.Sherman GG, Stevens G, Jones SA, Horsfield P, Stevens WS. Dried blood spots improve access to HIV diagnosis and care for infants in low-resource settings. J Acquir Immune Defic Syndr 2005; 38:615–17. [DOI] [PubMed] [Google Scholar]
- 7.Stevens W, Erasmus L, Moloi M, Taleng T, Sarang S. Performance of a novel human immunodeficiency virus (HIV) type 1 total nucleic acid-based real-time PCR assay using whole blood and dried blood spots for diagnosis of HIV in infants. J Clin Microbiol 2008; 46:3941–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gwinn M, Pappaioanou M, George JR, et al. Prevalence of HIV infection in childbearing women in the United States. Surveillance using newborn blood samples. JAMA 1991; 265:1704–8. [PubMed] [Google Scholar]
- 9.Hoff R, Berardi VP, Weiblen BJ, Mahoney-Trout L, Mitchell ML, Grady GF. Seroprevalence of human immunodeficiency virus among childbearing women: estimation by testing samples of blood from newborns. N Engl J Med 1988; 318:525–30. [DOI] [PubMed] [Google Scholar]
- 10.Monleau M, Butel C, Delaporte E, Boillot F, Peeters M. Effect of storage conditions of dried plasma and blood spots on HIV-1 RNA quantification and PCR amplification for drug resistance genotyping. J Antimicrob Chemother 2010; 65:1562–6. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Guidance on regulations for the transport of infectious substances 2011–2012. 2011. Available at: http://whqlibdoc.who.int/hq/2010/WHO_HSE_IHR_2010.8_eng.pdf. Accessed 4 February 2012. [Google Scholar]
- 12.Bertagnolio S, Soto-Ramirez L, Pilon R, et al. HIV-1 drug resistance surveillance using dried whole blood spots. Antivir Ther 2007; 12:107–13. [PubMed] [Google Scholar]
- 13.Lira R, Valdez-Salazar H, Vazquez-Rosales G, et al. Genotypic testing for HIV-1 drug resistance using dried blood samples. Arch Virol 2010; 155:1117–25. [DOI] [PubMed] [Google Scholar]
- 14.McNulty A, Jennings C, Bennett D, et al. Evaluation of dried blood spots for human immunodeficiency virus type 1 drug resistance testing. J Clin Microbiol 2007; 45:517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang C, McNulty A, Diallo K, et al. Development and application of a broadly sensitive dried-blood-spot-based genotyping assay for global surveillance of HIV-1 drug resistance. J Clin Microbiol 2010; 48:3158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrido C, Zahonero N, Fernandes D, et al. Subtype variability, virological response and drug resistance assessed on dried blood spots collected from HIV patients on antiretroviral therapy in Angola. J Antimicrob Chemother 2008; 61:694–8. [DOI] [PubMed] [Google Scholar]
- 17.Hallack R, Doherty LE, Wethers JA, Parker MM. Evaluation of dried blood spot specimens for HIV-1 drug-resistance testing using the Trugene HIV-1 genotyping assay. J Clin Virol 2008; 41:283–7. [DOI] [PubMed] [Google Scholar]
- 18.Masciotra S, Garrido C, Youngpairoj AS, et al. High concordance between HIV-1 drug resistance genotypes generated from plasma and dried blood spots in antiretroviral-experienced patients. AIDS 2007; 21:2503–11. [DOI] [PubMed] [Google Scholar]
- 19.Steegen K, Luchters S, Demecheleer E, et al. Feasibility of detecting human immunodeficiency virus type 1 drug resistance in DNA extracted from whole blood or dried blood spots. J Clin Microbiol 2007; 45:3342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Youngpairoj AS, Masciotra S, Garrido C, Zahonero N, de Mendoza C, Garcia-Lerma JG. HIV-1 drug resistance genotyping from dried blood spots stored for 1 year at 4 degrees C. J Antimicrob Chemother 2008; 61:1217–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziemniak C, George-Agwu A, Moss WJ, Ray SC, Persaud D. A sensitive genotyping assay for detection of drug resistance mutations in reverse transcriptase of HIV-1 subtypes B and C in samples stored as dried blood spots or frozen RNA extracts. J Virol Methods 2006; 136:238–47. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Z, Wagar N, DeVos JR, et al. Optimization of a low cost and broadly sensitive genotyping assay for HIV-1 drug resistance surveillance and monitoring in resource-limited settings. PLoS One 2011; 6:e28184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bussmann H, de la Hoz Gomez F, Roels TH, et al. Prevalence of transmitted HIV drug resistance in Botswana: lessons learned from the HIVDR-threshold survey conducted among women presenting for routine antenatal care as part of the 2007 national sentinel survey. AIDS Res Hum Retroviruses 2011; 27:365–72. [DOI] [PubMed] [Google Scholar]
- 24.Kamoto K, Aberle-Grasse J. Surveillance of transmitted HIV drug resistance with the World Health Organization threshold survey method in Lilongwe, Malawi. Antivir Ther 2008; 13(Suppl 2):83–7. [PubMed] [Google Scholar]
- 25.Somi GR, Kibuka T, Diallo K, et al. Surveillance of transmitted HIV drug resistance among women attending antenatal clinics in Dar es Salaam, Tanzania. Antivir Ther 2008; 13(Suppl 2):77–82. [PubMed] [Google Scholar]
- 26.Zhang J, Kang D, Fu J, et al. Surveillance of transmitted HIV type 1 drug resistance in newly diagnosed HIV type 1-infected patients in Shandong Province, China. AIDS Res Hum Retroviruses 2010; 26:99–103. [DOI] [PubMed] [Google Scholar]
- 27.NguyeB, BuT, WagaN, et al. HIV drug resistance threshold survey using matched plasma and DBS specimens from voluntary counseling and testing sites in Ho Chi Minh City, Vietnam, 2007–2008. Clin Infect Dis 2012; doi: 10.1093/cid/cir1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National AIDS/STDs Control Program FMoH. National guideline for HIV and AIDS treatment and care in adolescents and adults. Abuja, Nigeria: Federal Ministry of Health, 2007. [Google Scholar]
- 29.Ugbena R, Aberle-Grasse J, Diallo K, et al. Virological response and HIV-1 drug resistance development profile among patients treated with first-line antiretroviral regimens in Nigeria. 2012; doi: 10.1093/cid/cir1064. [DOI] [Google Scholar]
- 30.Biomerieux. NucliSENS EasyQ HIV-1 v1.1 RUO [package insert] Durham, NC: bioMérieux, Inc., 2007. [Google Scholar]
- 31.Johannessen A, Garrido C, Zahonero N, et al. Dried blood spots perform well in viral load monitoring of patients who receive antiretroviral treatment in rural Tanzania. Clin Infect Dis 2009; 49:976–81. [DOI] [PubMed] [Google Scholar]
- 32.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1:307–10. [PubMed] [Google Scholar]
- 33.Fleiss JL, ed. Statistical methods for rates and proportions. 2nd ed. New York, NY: John Wiley, 1981. [Google Scholar]
- 34.Buckton AJ, Bissett SL, Myers RE, et al. Development and optimization of an internally controlled dried blood spot assay for surveillance of human immunodeficiency virus type-1 drug resistance. J Antimicrob Chemother 2008; 62:1191–8. [DOI] [PubMed] [Google Scholar]
- 35.Kaye S, Comber E, Tenant-Flowers M, Loveday C. The appearance of drug resistance-associated point mutations in HIV type 1 plasma RNA precedes their appearance in proviral DNA. AIDS Res Hum Retroviruses 1995; 11:1221–5. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Lerma JG, McNulty A, Jennings C, Huang D, Heneine W, Bremer JW. Rapid decline in the efficiency of HIV drug resistance genotyping from dried blood spots (DBS) and dried plasma spots (DPS) stored at 37 degrees C and high humidity. J Antimicrob Chemother 2009; 64:33–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


