Abstract
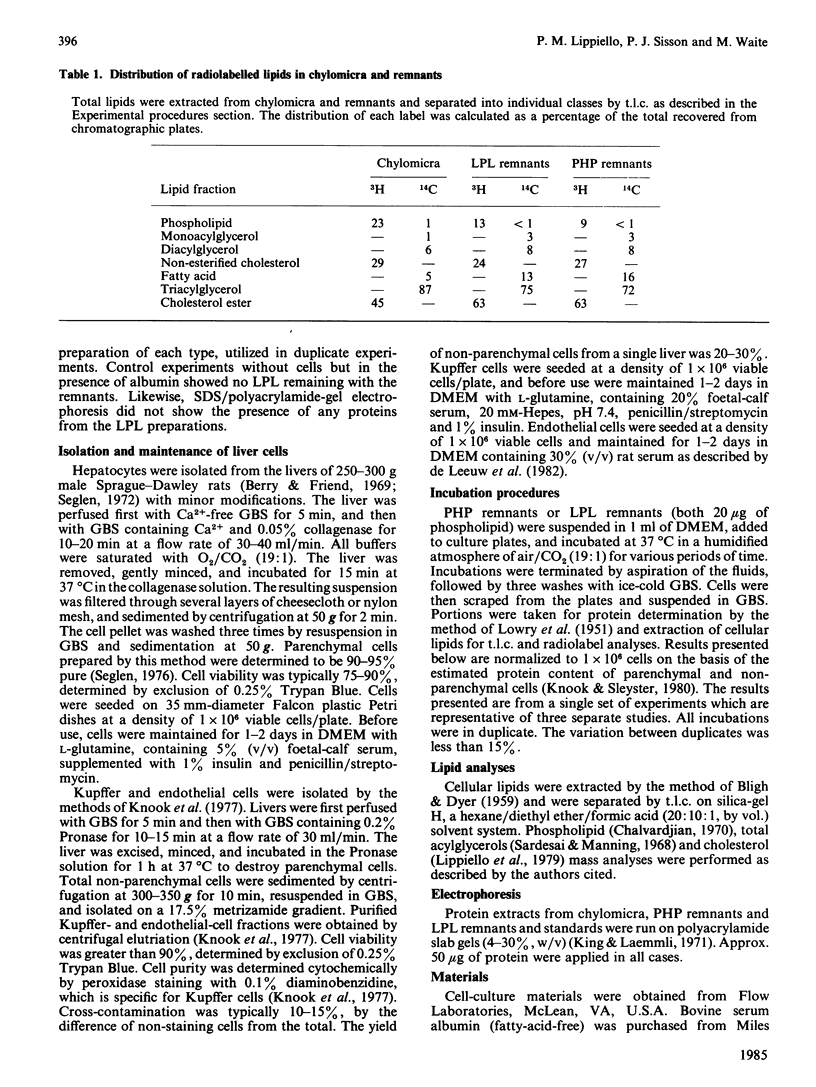
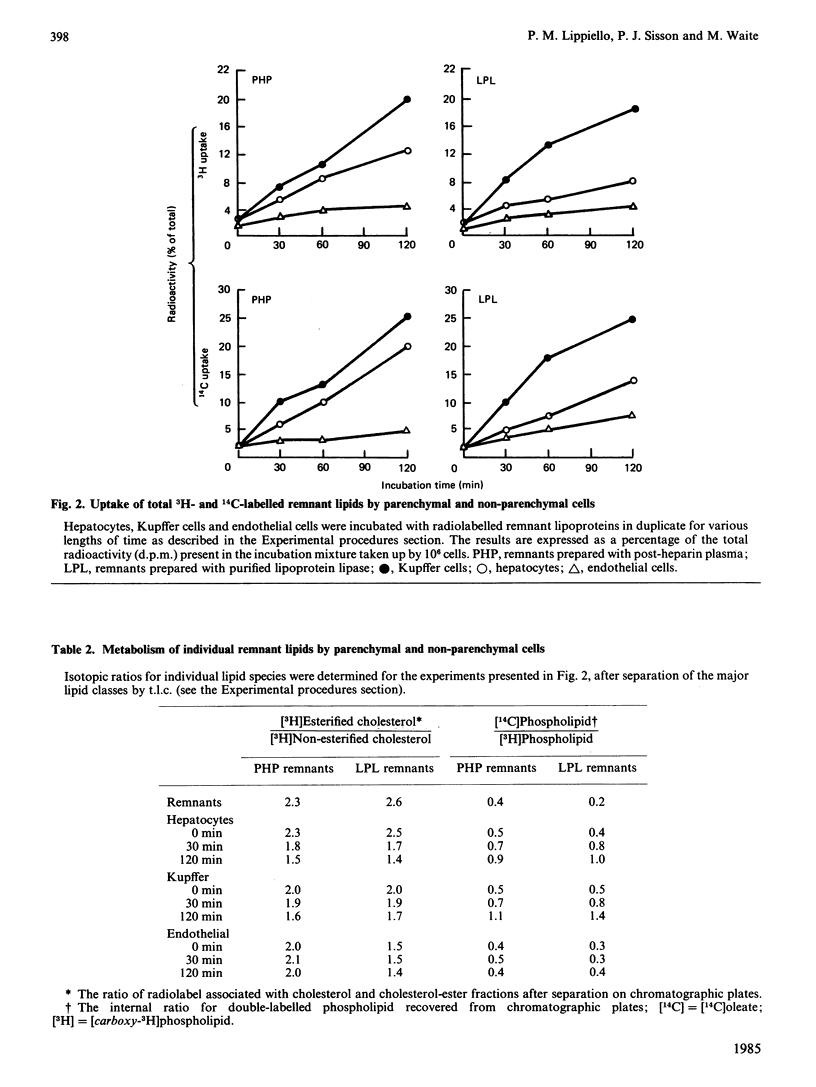
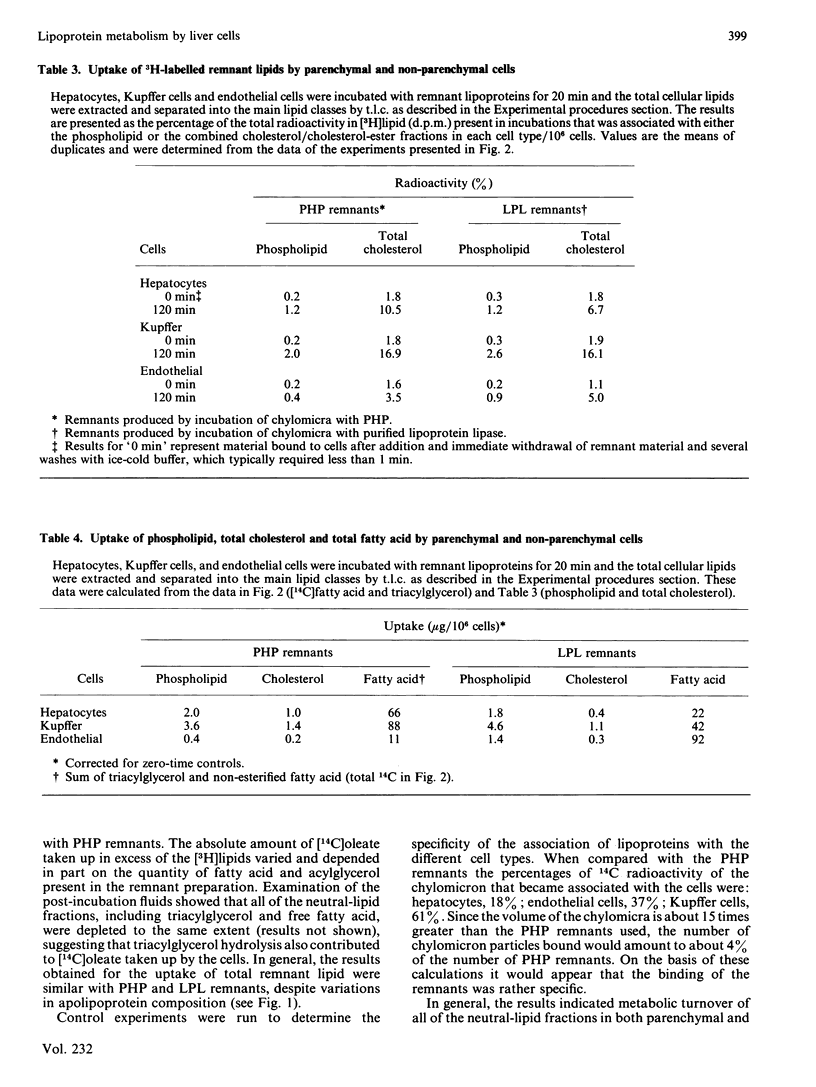
The uptake and metabolism of chylomicron-remnant lipids by individual liver cell types was examined by incubating remnants with monolayer cultures of hepatocytes, Kupffer cells, and endothelial cells from rat liver. Remnants were prepared in vitro from radiolabelled mesenteric-lymph chylomicra, utilizing either purified lipoprotein lipase from bovine milk, or plasma isolated from heparinized rats. The resulting particles contained [3H]phosphatidylcholine and cholesterol, and [14C]oleate in the acylglycerol, phospholipid, fatty-acid and cholesterol-ester fractions. The capacities of the three cell types for uptake of both [3H]lipids and [14C]lipids were determined to be, on a per-cell basis, in the order: Kupffer greater than hepatocytes greater than endothelial. The relative proportions of [3H]phospholipid and total [3H]cholesterol taken up by hepatocytes and non-parenchymal cells remained constant with time. The uptake of [14C]oleoyl lipids by all three cell types was slightly greater than that of the total [3H]cholesterol and [3H]phospholipid components. There was evidence of cholesterol-ester hydrolysis and turnover of [14C]oleate in the phospholipid fraction in hepatocytes and Kupffer cells, but not endothelial cells, over the first 2 h. With both remnant preparations, these observations indicate that significant differences exist between the three major liver cell types with respect to the uptake and metabolism of remnant lipid components.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J. M., Nervi F. O., Dietschy J. M. Rate constants for the uptake of cholesterol from various intestinal and serum lipoprotein fractions by the liver of the rat in vivo. Biochim Biophys Acta. 1977 Feb 23;486(2):298–307. doi: 10.1016/0005-2760(77)90025-x. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalvardjian A., Rudnicki E. Determination of lipid phosphorus in the nanomolar range. Anal Biochem. 1970 Jul;36(1):225–226. doi: 10.1016/0003-2697(70)90352-0. [DOI] [PubMed] [Google Scholar]
- Cooper A. D., Yu P. Y. Rates of removal and degradation of chylomicron remnants by isolated perfused rat liver. J Lipid Res. 1978 Jul;19(5):635–643. [PubMed] [Google Scholar]
- Eisenberg S., Rachmilewitz D. Interaction of rat plasma very low density lipoprotein with lipoprotein lipase-rich (postheparin) plasma. J Lipid Res. 1975 Sep;16(5):341–351. [PubMed] [Google Scholar]
- El-Maghrabi M. R., Waite M., Rudel L. L., King V. L. Metabolism of lipoprotein acylglycerols by liver parenchymal cells. Biochim Biophys Acta. 1979 Jan 29;572(1):52–63. doi: 10.1016/0005-2760(79)90199-1. [DOI] [PubMed] [Google Scholar]
- El-Maghrabi M. R., Waite M., Rudel L. L., Sisson P. Hydrolysis of monoacylglycerol in lipoprotein remnants catalyzed by the liver plasma membrane monoacylglycerol acyltransferase. J Biol Chem. 1978 Feb 10;253(3):974–981. [PubMed] [Google Scholar]
- Faergeman O., Havel R. J. Metabolism of cholesteryl esters of rat very low density lipoproteins. J Clin Invest. 1975 Jun;55(6):1210–1218. doi: 10.1172/JCI108039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R. V., Pottenger L. A., Reingold A. L., Getz G. S., Wissler R. W. Degradation of I 125 -labelled serum low density lipoprotein in normal and estrogen-treated male rats. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1471–1477. doi: 10.1016/s0006-291x(71)80251-6. [DOI] [PubMed] [Google Scholar]
- Imaizumi K., Fainaru M., Havel R. J. Composition of proteins of mesenteric lymph chylomicrons in the rat and alterations produced upon exposure of chylomicrons to blood serum and serum proteins. J Lipid Res. 1978 Aug;19(6):712–722. [PubMed] [Google Scholar]
- Jansen H., Kalkman C., Zonneveld A. J., Hülsmann W. C. Secretion of triacylglycerol hydrolase activity by isolated parenchymal rat liver cells. FEBS Lett. 1979 Feb 15;98(2):299–302. doi: 10.1016/0014-5793(79)80204-5. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- Knook D. L., Blansjaar N., Sleyster E. C. Isolation and characterization of Kupffer and endothelial cells from the rat liver. Exp Cell Res. 1977 Oct 15;109(2):317–329. doi: 10.1016/0014-4827(77)90011-8. [DOI] [PubMed] [Google Scholar]
- Knook D. L., Sleyster E. C. Isolated parenchymal, Kupffer and endothelial rat liver cells characterized by their lysosomal enzyme content. Biochem Biophys Res Commun. 1980 Sep 16;96(1):250–257. doi: 10.1016/0006-291x(80)91207-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lippiello P. M., Dijkstra J., van Galen M., Scherphof G., Waite B. M. The uptake and metabolism of chylomicron-remnant lipids by nonparenchymal cells in perfused liver and by Kupffer cells in culture. J Biol Chem. 1981 Jul 25;256(14):7454–7460. [PubMed] [Google Scholar]
- Lippiello P. M., Holloway C. T., Garfield S. A., Holloway P. W. The effects of estradiol on stearyl-CoA desaturase activity and microsomal membrane properties in rooster liver. J Biol Chem. 1979 Mar 25;254(6):2004–2009. [PubMed] [Google Scholar]
- Nagelkerke J. F., Barto K. P., van Berkel T. J. In vivo and in vitro uptake and degradation of acetylated low density lipoprotein by rat liver endothelial, Kupffer, and parenchymal cells. J Biol Chem. 1983 Oct 25;258(20):12221–12227. [PubMed] [Google Scholar]
- Nicoll A., Miller N. E., Lewis B. High-density lipoprotein metabolism. Adv Lipid Res. 1980;17:53–106. doi: 10.1016/b978-0-12-024917-6.50008-2. [DOI] [PubMed] [Google Scholar]
- Nilsson A., Ehnholm C., Florén C. H. Uptake and degradation of rat chylomicron remnants, produced in vivo and in vitro, in rat hepatocyte monolayers. Biochim Biophys Acta. 1981 Feb 23;663(2):408–420. doi: 10.1016/0005-2760(81)90170-3. [DOI] [PubMed] [Google Scholar]
- Redgrave T. G. Formation of cholesteryl ester-rich particulate lipid during metabolism of chylomicrons. J Clin Invest. 1970 Mar;49(3):465–471. doi: 10.1172/JCI106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of rat liver cells. I. Effect of Ca 2+ on enzymatic dispersion of isolated, perfused liver. Exp Cell Res. 1972 Oct;74(2):450–454. doi: 10.1016/0014-4827(72)90400-4. [DOI] [PubMed] [Google Scholar]
- Sherrill B. C., Dietschy J. M. Characterization of the sinusoidal transport process responsible for uptake of chylomicrons by the liver. J Biol Chem. 1978 Mar 25;253(6):1859–1867. [PubMed] [Google Scholar]
- Smith L. C., Pownall H. J., Gotto A. M., Jr The plasma lipoproteins: structure and metabolism. Annu Rev Biochem. 1978;47:751–757. doi: 10.1146/annurev.bi.47.070178.003535. [DOI] [PubMed] [Google Scholar]
- Stoffel W. Chemical synthesis of choline-labeled lecithins and sphingomyelins. Methods Enzymol. 1975;35:533–541. doi: 10.1016/0076-6879(75)35181-1. [DOI] [PubMed] [Google Scholar]
- Van Berkel T. J., Kruijt J. K., Koster J. F. Identity and activities of lysosomal enzymes in parenchymal and non-parenchymal cells from rat liver. Eur J Biochem. 1975 Oct 1;58(1):145–152. doi: 10.1111/j.1432-1033.1975.tb02358.x. [DOI] [PubMed] [Google Scholar]
- Van Berkel T. J., Van Tol A. Role of parenchymal and non-parenchymal rat liver cells in the uptake of cholesterolester-labeled serum lipoproteins. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1097–1101. doi: 10.1016/0006-291x(79)92120-x. [DOI] [PubMed] [Google Scholar]
- Windler E., Chao Y., Havel R. J. Determinants of hepatic uptake of triglyceride-rich lipoproteins and their remnants in the rat. J Biol Chem. 1980 Jun 10;255(11):5475–5480. [PubMed] [Google Scholar]
- de Leeuw A. M., Barelds R. J., de Zanger R., Knook D. L. Primary cultures of endothelial cells of the rat liver: a model for ultrastructural and functional studies. Cell Tissue Res. 1982;223(1):201–215. doi: 10.1007/BF00221510. [DOI] [PubMed] [Google Scholar]
- van Berkel T. J., van Tol A. In vivo uptake of human and rat low density and high density lipoprotein by parenchymal and nonparenchymal cells from rat liver. Biochim Biophys Acta. 1978 Aug 25;530(2):299–304. doi: 10.1016/0005-2760(78)90015-2. [DOI] [PubMed] [Google Scholar]
- van Tol A., van Berkel T. J. Uptake and degradation of rat and human very low density (remnant) apolipoprotein by parenchymal and non-parenchymal rat liver cells. Biochim Biophys Acta. 1980 Jul 14;619(1):156–166. doi: 10.1016/0005-2760(80)90251-9. [DOI] [PubMed] [Google Scholar]