Abstract
An understanding of the biosynthesis and metabolism of dimethylamine (DMA) is important because it is a precursor of dimethylnitrosamine (nitroso-DMA). DMA is the major short-chain aliphatic amine in human and rat urine. DMA is formed from trimethylamine (TMA), which, in turn, is a breakdown product of dietary choline. Enzymes within gut bacteria catalyse both of these reactions; it is not known whether mammalian cells can form DMA. To determine the relative importance of dietary choline, bacteria and other mechanisms for the formation of DMA, we measured DMA excretion in the urine of rats fed on a diet devoid of choline, and in urine of rats with no bacterial colonization of the intestines. We also describe an improved gas-chromatographic method for the measurement of methylamines in biological fluids. In control rats there were significant amounts of DMA within several biological fluids [urine, 54.2 +/- 3.0 mumol/kg body wt. per 24 h (556.2 +/- 37.5 nmol/ml); blood, 18.8 +/- 1.9 nmol/ml; gastric juice, 33.5 +/- 10.5 nmol/ml; means +/- S.E.M.]. Animals eating a diet containing no choline excreted as much MMA and DMA as did choline-supplemented rats (25-35 mumol/kg per 24 h), and they excreted slightly less TMA (2 versus 2.5 mumol/kg per 24 h). Rats with no gut bacteria excreted the same amount of DMA in their urine as did the control animals (45-55 mumol/kg per 24 h). They excreted much less MMA (16.3 +/- 1.5 versus 40.3 +/- 2.6 mumol/kg per 24 h; mean +/- S.E.M.; P less than 0.01), TMA (0.7 +/- 0.2 versus 2.5 +/- 0.5 mumol/kg per 24 h; mean +/- S.E.M.; P less than 0.01) and piperidine (2.0 +/- 0.3 versus 6.3 +/- 0.6 mumol/kg per 24 h; mean +/- S.E.M.; P less than 0.01) in their urine. From our studies we conclude that DMA is present in significant amounts within gastric fluid, an environment that is ideal for nitrosamine formation (under acidic conditions, nitroso-DMA is chemically formed by the reaction of nitrite with DMA). Results also indicate that dietary choline was not the sole precursor for DMA formation and that gut bacteria are not essential for the formation of DMA. Hence in mammals there must be endogenous pathways that are capable of forming DMA; however, these endogenous mechanisms remain unidentified.
Full text
PDF

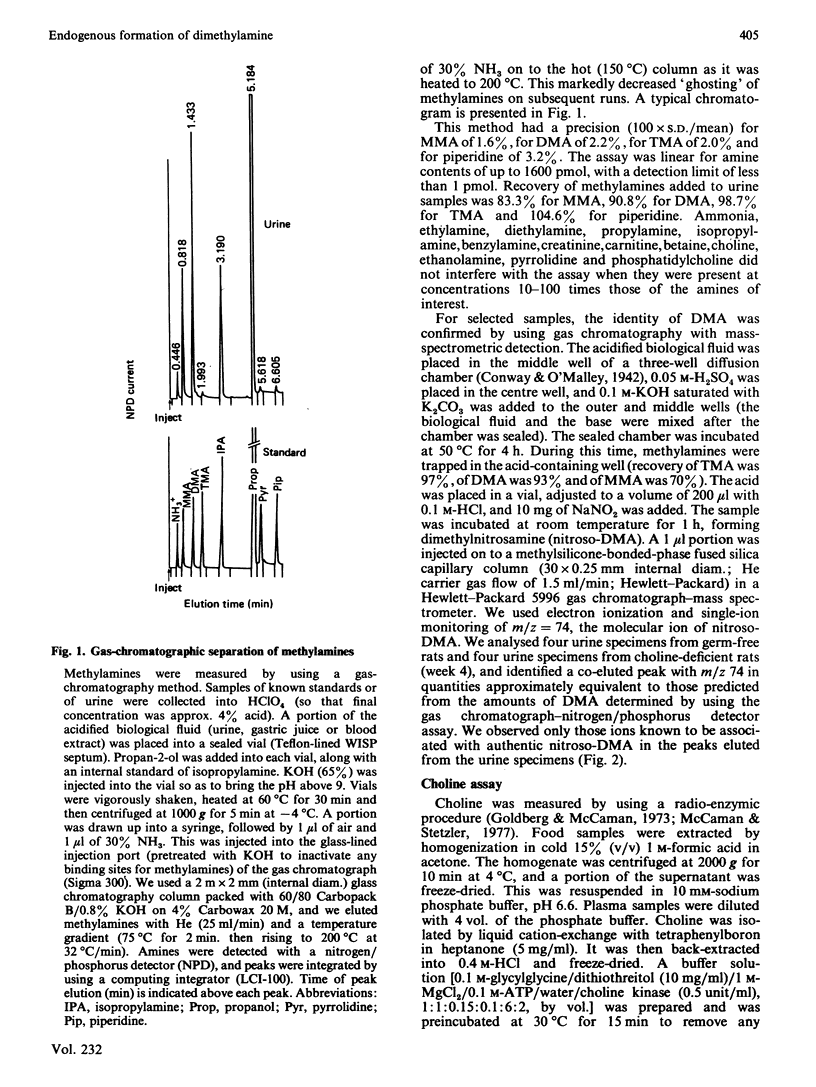
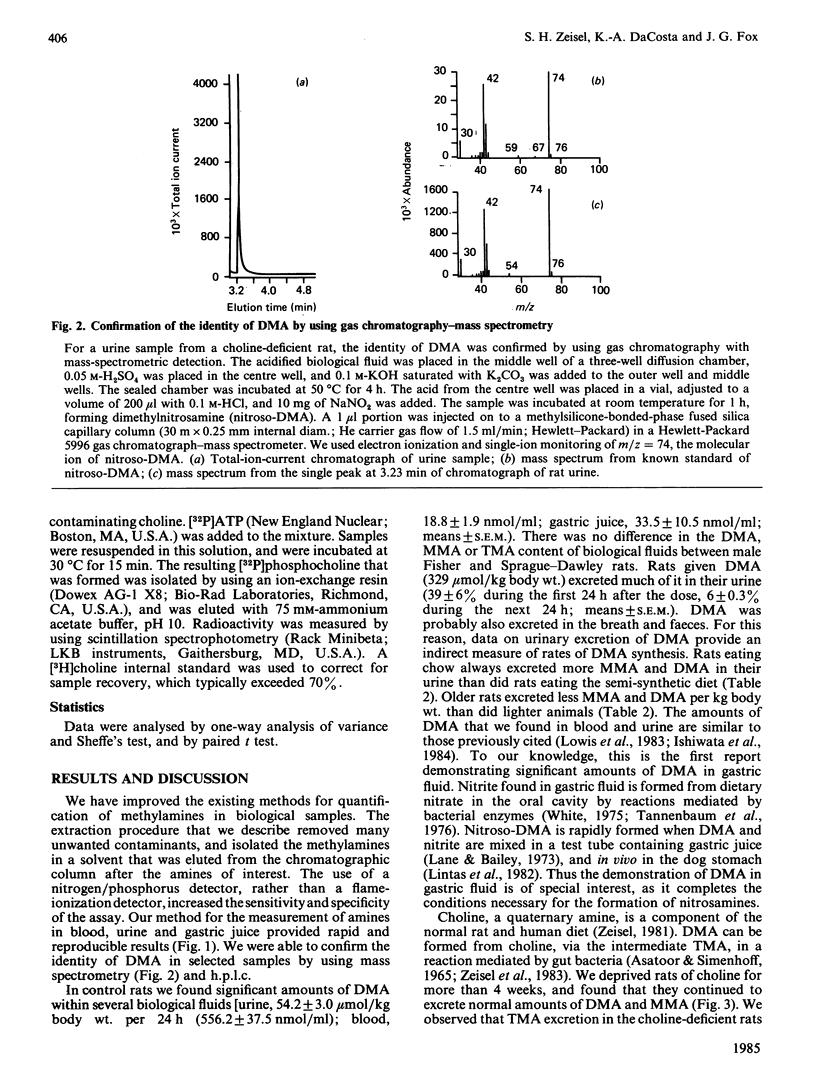
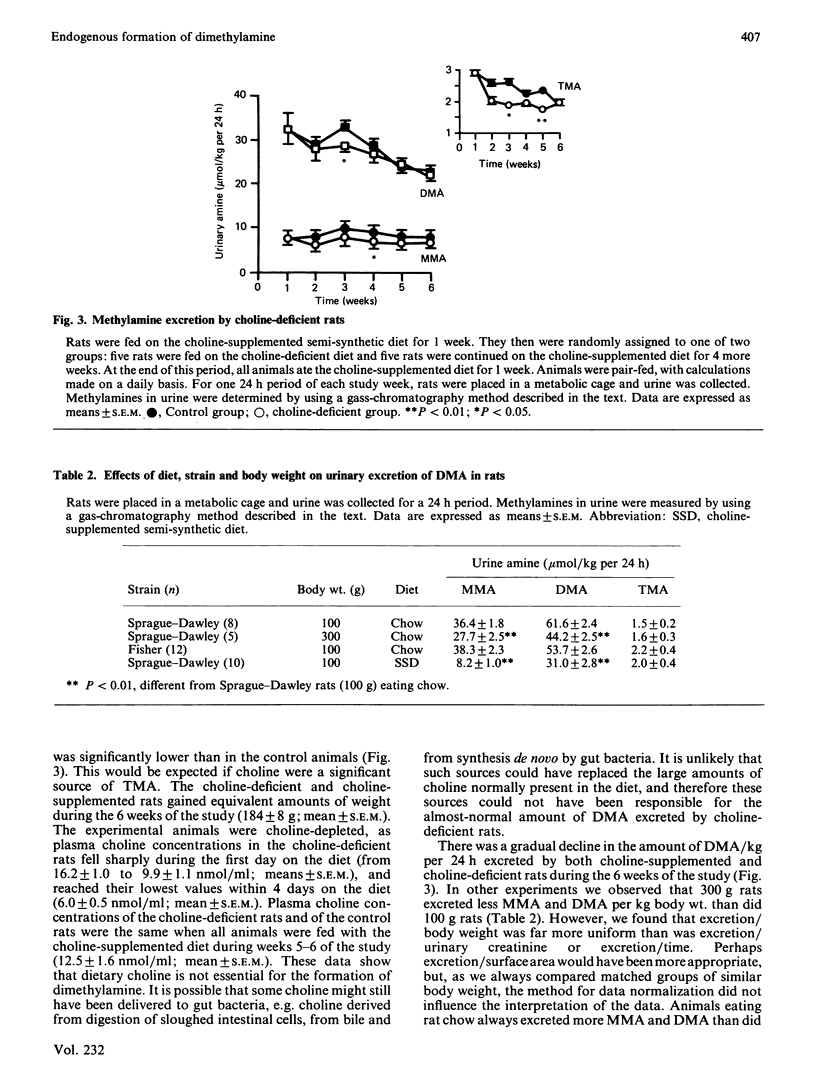

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asatoor A. M., Simenhoff M. L. The origin of urinary dimethylamine. Biochim Biophys Acta. 1965 Dec 16;111(2):384–392. doi: 10.1016/0304-4165(65)90048-6. [DOI] [PubMed] [Google Scholar]
- Bartsch H., Montesano R. Relevance of nitrosamines to human cancer. Carcinogenesis. 1984 Nov;5(11):1381–1393. doi: 10.1093/carcin/5.11.1381. [DOI] [PubMed] [Google Scholar]
- Blau K. Chromatographic methods for the study of amines from biological material. Biochem J. 1961 Jul;80(1):193–200. doi: 10.1042/bj0800193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byington M. H., Howe J. M., Clark H. E. Effect of different levels and proportions of methionine, cystine, choline, and inorganic sulfur on growth and body composition of young rats. J Nutr. 1972 Feb;102(2):219–227. doi: 10.1093/jn/102.2.219. [DOI] [PubMed] [Google Scholar]
- Conway E. J., O'malley E. Microdiffusion methods. Ammonia and urea using buffered absorbents (revised methods for ranges greater than 10mug. N). Biochem J. 1942 Sep;36(7-9):655–661. doi: 10.1042/bj0360655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. M., McCaman R. E. The determination of picomole amounts of acetylcholine in mammalian brain. J Neurochem. 1973 Jan;20(1):1–8. doi: 10.1111/j.1471-4159.1973.tb12097.x. [DOI] [PubMed] [Google Scholar]
- Ishiwata H., Iwata R., Tanimura A. Intestinal distribution, absorption and secretion of dimethylamine and its biliary and urinary excretion in rats. Food Chem Toxicol. 1984 Aug;22(8):649–653. doi: 10.1016/0278-6915(84)90274-6. [DOI] [PubMed] [Google Scholar]
- Lane R. P., Bailey M. E. The effect of pH on dimethylnitrosamine formation in human gastric juice. Food Cosmet Toxicol. 1973 Oct;11(5):851–854. doi: 10.1016/0015-6264(73)90144-2. [DOI] [PubMed] [Google Scholar]
- Lintas C. L., Clark A., Fox J., Tannenbaum S. R., Newberne P. M. In vivo stability of nitrite and nitrosamine formation in the dog stomach: effect of nitrite and amine concentration and of ascorbic acid. Carcinogenesis. 1982;3(2):161–165. doi: 10.1093/carcin/3.2.161. [DOI] [PubMed] [Google Scholar]
- Lowis S., Eastwood M. A., Brydon W. G. The measurement of methylamines in biological material using a gas chromatographic head space gas technique. J Chromatogr. 1983 Nov 11;278(1):139–143. doi: 10.1016/s0378-4347(00)84764-7. [DOI] [PubMed] [Google Scholar]
- Lundstrom R. C., Racicot L. D. Gas chromatographic determination of dimethylamine and trimethylamine in seafoods. J Assoc Off Anal Chem. 1983 Sep;66(5):1158–1163. [PubMed] [Google Scholar]
- McCaman R. E., Stetzler J. Radiochemical assay for ACh: modifications for sub-picomole measurements. J Neurochem. 1977 Mar;28(3):669–671. doi: 10.1111/j.1471-4159.1977.tb10442.x. [DOI] [PubMed] [Google Scholar]
- Singer G. M., Lijinsky W. Naturally occurring nitrosatable compounds. I. Secondary amines in foodstuffs. J Agric Food Chem. 1976 May-Jun;24(3):550–553. doi: 10.1021/jf60205a044. [DOI] [PubMed] [Google Scholar]
- Tannenbaum S. R., Weisman M., Fett D. The effect of nitrate intake on nitrite formation in human saliva. Food Cosmet Toxicol. 1976 Dec;14(6):549–552. doi: 10.1016/s0015-6264(76)80006-5. [DOI] [PubMed] [Google Scholar]
- White J. W., Jr Relative significance of dietary sources of nitrate and nitrite. J Agric Food Chem. 1975 Sep-Oct;23(5):886–891. doi: 10.1021/jf60201a034. [DOI] [PubMed] [Google Scholar]
- Zeisel S. H. Dietary choline: biochemistry, physiology, and pharmacology. Annu Rev Nutr. 1981;1:95–121. doi: 10.1146/annurev.nu.01.070181.000523. [DOI] [PubMed] [Google Scholar]
- Zeisel S. H., Wishnok J. S., Blusztajn J. K. Formation of methylamines from ingested choline and lecithin. J Pharmacol Exp Ther. 1983 May;225(2):320–324. [PubMed] [Google Scholar]



