Abstract
Background
Physical activities performed under free-living conditions that are unsupervised in the home or community have the potential to modulate non-motor symptoms in people with Parkinson's disease.
Objective
This systematic review investigates the relationships between physical activities performed in free-living conditions and non-motor symptoms in people with Parkinson's disease: cognition, anxiety, apathy, depression, sleep disturbances, fatigue, and pain.
Data sources
A database search was performed on Scopus, Web of Science, Ovid (PsycINFO), CINAHL, PubMed, and ProQuest (Health and Medicine).
Review methods
Observational studies published from 2000 to 2024 that examined the relationships between physical activity and non-motor symptoms were included. The methodological quality of reports was evaluated using critical appraisal checklists appropriate to the study design. Where appropriate, a meta-analysis was conducted to combine data from the included articles.
Results
A total of 14 articles met the criteria and used various tools to evaluate non-motor symptoms and physical activity. Meta-analyses showed that people with Parkinson's who are more physically active have better global cognition [β ranged from 0.12 to 0.28; p = 0.00–0.02] and less affective disorders [β -0.20, p = 0.00]. Increased physical activity levels were also associated with better sleep quality (n = 1) and less chronic pain (n = 1). The overall methodological quality of the included articles was considered high.
Conclusion
Engagement in increased levels of physical activities performed under free-living conditions is associated with better cognition and less anxiety, apathy, and depression in people with Parkinson's disease.
Keywords: Parkinson's, physical activity, non-motor symptoms
Introduction
The global burden of neurological conditions is significant and increasing, 1 and as the population ages, the prevalence of Parkinson's disease is particularly fast growing. 2 Parkinson's is often characterised by motor impairments, such as bradykinesia and postural instability, rigidity, or tremor. 3 In more recent times, research has highlighted the recognition of non-motor symptoms which present in more than 90% of people with Parkinson's.4,5 Compared to healthy older adults, the prevalence and severity of these symptoms, such as cognitive impairment, fatigue, affective disorders (e.g. depression, anxiety, apathy), pain, and sleep problems, are greater among people with Parkinson's.6,7 Non-motor symptoms typically increase with disease progression8,9 and negatively affect health-related quality of life,10,11 daily routines, planning/socialising, and independence. 12
While there is growing evidence from intervention studies supporting the benefits of exercise13–16 and physical activities, such as dance, 17 aquatic therapy, 18 tai chi, 19 and Nordic walking, 20 for improving physical domains of health, such as muscle strength, balance, and gait speed, few have focused on non-motor symptoms as the primary outcome. Additionally, such interventions have been critiqued for being highly supervised and not reflecting a sustainable frequency or intensity of activity under free-living conditions. 21 Although supervision ensures exercise safety and provides encouragement and support for participants, upon cessation of the intervention, many participants return toward baseline activity levels without this supervision.21–24 In contrast, participation in unsupervised physical activities that are performed under free-living conditions, such as gardening, housework, and community exercise classes, which are part of an individual's day-to-day life,25–27 tends to be more sustainable throughout the lifespan.28–30 The potential for participation in physical activities under free-living conditions to benefit non-motor symptoms is important, particularly when pharmaceutical interventions offer limited effectiveness or unwanted side effects.31–36 It is also important for people with Parkinson's, who might otherwise be sedentary due to factors, such as access or resource constraints, and low self-efficacy for exercise37–41; yet there is a dearth of reviews to determine this relationship.
Therefore, the purpose of this systematic review was to analyse the research literature to understand whether there is a relationship between physical activities performed under free-living conditions and non-motor symptoms in people with Parkinson's: cognitive impairment, fatigue, sleep problems, affective disorders, and pain.
Methods
Search and identification of articles
The review has been performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.42,43 The protocol has been published elsewhere. 44
The primary reviewer (AS) conducted a systematic search (first run in October 2021, subsequently updated in June 2023 and June 2024) on the following six databases: Scopus, Web of Science, Ovid (PsycINFO), CINAHL, PubMed, and ProQuest (Health and Medicine). A keyword search strategy 44 was designed and developed in consultation with an experienced subject librarian (TF). The search strategy, filters, and limits were tailored appropriately to the database. The full search strategy for all databases can be found in Supplementary Tables S1 and S2. Titles identified in the database search were exported to the Rayyan software. After removing the duplicates, AS screened the titles and abstracts of all citations for possible inclusion. Two reviewers (AS and PJ) then independently screened the full texts to identify relevant articles. A manual search of reference lists of included articles was carried out to locate any relevant publications that may have been missed in the electronic search. A third reviewer resolved any disagreement between the reviewers if a consensus could not be reached. Articles were considered based on the inclusion and exclusion criteria below.
Inclusion criteria
Adults aged 18 years and above diagnosed with idiopathic Parkinson's disease or atypical parkinsonism: atypical parkinsonism was limited to multiple system atrophy, progressive supranuclear palsy, corticobasal degeneration, and dementia with Lewy bodies;
Cross-sectional and longitudinal studies published after the year 2000 that investigated the relationship between at least one non-motor symptom domain (i.e. cognition, affective disorders, sleep, pain, and fatigue) and at least one physical activity characteristic (e.g. metabolic equivalent minutes per week, daily steps, frequency, intensity, duration, and type);
Studies used quantitative measurement tools to assess physical activities performed under free-living conditions and each non-motor symptom domain of interest.
Exclusion criteria
Studies that included participants with a diagnosis of secondary parkinsonism (e.g. vascular and drug-induced parkinsonism);
Studies that included participants with various diagnoses (e.g. Alzheimer's and idiopathic Parkinson's disease), but did not present separate statistical analysis results for the diagnoses of interest;
Studies on animals;
Conference publications, abstracts, editorials, commentaries, case reports, reviews, randomised controlled trials, clinical trials, theses, or qualitative study designs;
Studies published in languages other than English;
Studies that used a single measurement tool for multiple non-motor symptom domains, with no individual domain scores in statistical analyses.
Data extraction and analysis
Relevant data were extracted from the included articles by the primary reviewer (AS) using predetermined data extraction tables (Supplementary Tables S3 and S4) and verified by a second reviewer (SA). The following data were extracted: study characteristics, such as authors, year published, study design, sample size, recruitment country and setting; participant characteristics, such as diagnosis, sex, disease stage, mean (SD) age of participations, disease duration, and Levodopa dosage equivalent [LEDD]; measurement tools used to evaluate non-motor symptoms of interest and physical activity measures; and relevant statistical findings. The measures of interest were correlation and regression coefficients measuring the relationship between physical activity and the five major non-motor symptoms of interest: cognition, affective disorders, sleep, pain, and fatigue. The sleep problems of interest were sleep quality, total sleep time, and excessive daytime sleepiness. The affective disorders of interest include depression, anxiety, and apathy. The pain types of interest included musculoskeletal, dystonic, radicular, central, or akathisia. Results from univariate and multivariable analyses were included if they were reported. Physical activity categories included total weekly physical activity, daily light-intensity physical activity time, daily moderate-to-vigorous physical activity time, daily steps, daily energy expenditure, and monthly physical activity days.
Methodological quality assessment
The methodological quality of each article was assessed independently by two reviewers (AS and SA) using either the JBI critical appraisal checklist for analytical cross-sectional studies, the JBI critical appraisal checklist for case–control studies (Supplementary Figure S1), 45 or the Critical Appraisal Skills Programme checklist for cohort studies, as appropriate (Supplementary Figure S2).45–47 Any reviewer disagreements were resolved by a discussion with a third reviewer (PJ). A percentage score of methodological quality was estimated for each article. The JBI scores were classified as high quality if values were ≥70%, medium quality if between 50% and 70%, and low quality if values were <50%. 48 However, no a-priori decision was made to exclude articles based on the methodological quality scores.
Data synthesis
Where appropriate, a meta-analysis of continuous variables was conducted to combine data from the included articles and presented visually as forest plots. Standardised regression coefficients (β) were used as the effect size estimate as different measurement methods, and metrics were used across the included articles. 49 Correlation coefficients and unstandardised regression coefficients were transformed into standardised regression coefficients using the formulas and approximations presented by Nieminen et al. 49 (Supplementary Figure S3). The meta-analysis did not include articles reporting odds ratios (OR) or relative risks (RR).
The corresponding authors of the included articles were contacted to obtain additional data where required (e.g. β, 95% CI(β), SD(X), and SD(Y)). When β coefficients were not reported, it was estimated using SD(X) and SD(Y)), as recommended by Nieminen et al. 49 Where SD(X) and SD(Y) were not attainable from authors, these were imputed from another article within this review or from another review with meta-analysis, as described by Higgins et al. 50
To ensure that all pooled β coefficients are interpreted with the same directionality (i.e. higher outcome scores indicate better symptoms), β coefficients and corresponding 95% CIs were reversed for outcomes where a higher score indicated worse symptoms. 50
The methodological diversity (measurement tools) and statistical diversity (statistical analyses, covariates) of the included articles were evaluated collaboratively by two reviewers (AS and PJ) to determine the homogeneity for quantitative synthesis. Initially, effect size estimates from both unadjusted and adjusted models (‘combined model’) were pooled. Where data from at least two articles were available, separate statistical pooling was undertaken for adjusted models with similar predictor variables and unadjusted models. In situations where a study reported multiple effect size estimates for the same non-motor symptom domain and physical activity association, the strongest estimate from the most comprehensive non-motor symptom measure (i.e. assessed multiple symptoms within one domain) and objective physical activity measure (e.g. estimate taken from accelerometry data over subjective recall of physical activity), which had the greatest adjustment for covariates, was selected unless this selection method reduced the homogeneity between articles, in which case, homogeneity was prioritised.
A random-effects model was used to control for any unobserved heterogeneity between articles. Further, I2 statistics were used to determine the heterogeneity of the estimates and interpreted using the following criteria: <30%, not important; ≥30%, moderate; ≥60%, substantial; and ≥90%, considerable heterogeneity. 51 The strength of the pooled effect size (β) was interpreted as small if values ranged between 0.10 and 0.19, medium if between 0.20 and 0.29, and large if ≥0.30. 49 To assess the level of certainty in the pooled effect size estimates, 95% confidence intervals (CIs) and p-values were reported. Meta-analyses were performed using Stata/SE 16.0 software for Mac.
Results
Study selection
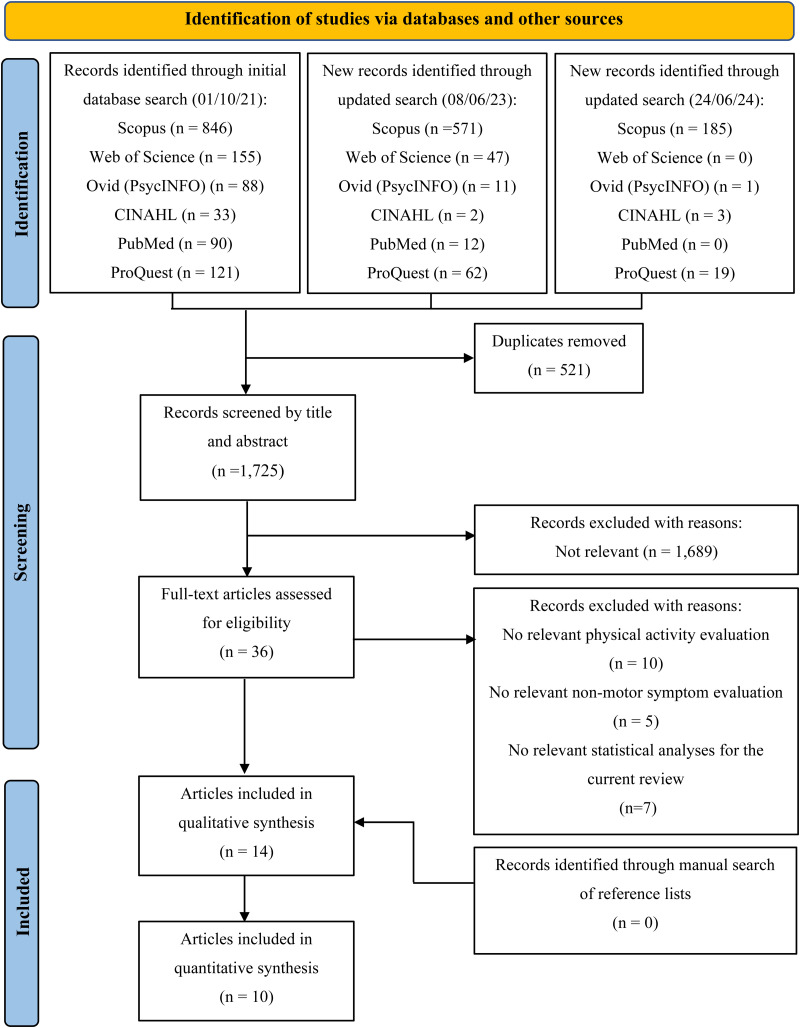
A total of 2246 references were identified through an electronic database search. After removing duplicates, 1752 titles were screened for relevant articles (Figure 1). The full texts of 36 articles were screened independently by two reviewers (AS and PJ) with a substantial inter-rater agreement (Cohen's k 0.70). After conflicts were resolved by discussion, 12 of the articles met the criteria for inclusion after the full-text screening. An additional two articles were identified from the updated searches and included in the review. The remaining 22 articles were excluded due to no relevant physical activity evaluation (n = 10),52–61 no relevant non-motor symptom evaluation (n = 4),39,62–64 or no relevant statistical analyses for the current review (n = 6).39,65–71 No additional articles were identified in the manual search of reference lists.
Figure 1.
PRISMA flow diagram of the study selection process.
Characteristics of included articles
A summary of descriptive data extracted from the 14 articles can be found in Table 1. Participants were recruited from various settings: community, local support groups, hospital-based neurology units, movement disorder clinics, Parkinson's registries, and research databases. A total of 13 investigations had participants diagnosed with idiopathic Parkinson's. One was restricted to mild Parkinson's signs. 72 No eligible articles included individuals with atypical parkinsonism.
Table 1.
Descriptive characteristics of included articles.
| Author, year, and study design | Recruitment country | PD sample size and gender (n, % Male) | Diagnosis | Recruitment setting | Age, years (Mean, [SD])a | Disease duration, years (Mean, [SD])a | Disease stage, original H&Y Scale (n, [%])a | MDS-UPDRS motor examination score (Mean, [SD])a | LEDD, mg/day (Mean, [SD])a |
|---|---|---|---|---|---|---|---|---|---|
| Alwardat et al. (2019) 73 /Cross-sectional | ITA | 128 (60) | IPD | Hospital-based neurology unit | 61.98 (7.95) | 4.99 (4.05) | NR | 25.21 (10.49)o | 493.14 (309.39) |
| Amara et al. (2019) 47 /Longitudinal case–control | USA, EUR | 380 (65.5) | IPD | NR | 63.55 (9.8) | 2.6 (0.56) | 0 (n = 2 [0.71]) 1 (n = 71 [25.09]) 2 (n = 197 [69.61]) 3–5 (n = 13 [4.59]) |
27.16 (11.3) | 389.06 (302.5) |
| Cerff et al. (2017) 74 /Cross-sectional | DEU | PD-NC: 17 (59) PD-MCI: 22 (77) PDD: 9 (100) |
IPD | Community | PD-NC: 71 (44/80)f PD-MCI: 68 (57/78)f PDD: 72 (67/75)f |
PD-NC: 6 (1/13)f PD-MCI: 6 (1/20)f PDD: 6 (5/18)f |
PD-NC: 1 (n = 6 [35]) 2 (n = 10 [59]) 3 (n = 1 [6]) PD-MCI: 1 (n = 3 [14]) 2 (n = 13 [59]) 3 (n = 4 [18]) 4 (n = 2 [9]) PDD: 2 (n = 3 [33]) 3 (n = 3 [33]) 4 (n = 2 [22]) 5 (n = 1 [11]) |
PD-NC: 20 (11/58)fo PD-MCI: 24 (10/62)fo PDD: 36 (14/ 56)fo |
PD-NC: 620 (160/2420)f PD-MCI: 763 (210/2378)f PDD: 496 (100/1139)f |
| Donahue et al. (2022) 75 /Cross-sectional | USA | 96 (57.3%) | IPD | NR | 66.76 (8.60) | 4.72 (4.81) | NR | 23.99 (10.33) | 568.51 (383.41) |
| Dontje et al. (2013) 76 /Cross-sectional | NLD | 467 (66) | IPD | Hospital-based neurology unit | 65.7 (7.4) | 5.15 (4.38) | 1 (n = 8 [2])m 1.5 (n = 14 [3])m 2 (n = 350 [75])m 2.5 (n = 69 [15])m 3 (n = 26 [6])m |
32.9 (10.5) | 480.0 (389.7) |
| Duvdevani et al. (2024) 77 /Cross-sectional | ISR | 88 (65.9) | IPD | Movement Disorder Institute, Neurology Department, Health Care Campus | 66.84 (8.8) | NR | 1 (n = 4 [4.6]) 2 (n = 52 [59.8]) 3 (n = 18 [20.7]) 4 (n = 13 [14.9]) 5 (n = 0 [0]) |
31 (1–94)f | 763.86 (573.01) |
| Ellingson et al. (2017)78,79/Cross-sectional | USA | 52 (56) | IPD | Community | 67.8 (7.9) | 10.0 (6.7) | NR | 57.4 (19.4) | NR |
| Leavy et al. (2021) 80 /Cross-sectional | SWE | 89 (54) | IPD | Community and research database | 71.0 (6.0) | 6.0 (4.3) | NR | NR | 580 (291) |
| Loprinzi et al. (2018) 81 /Cross-sectional | USA | 23 (57) | IPD | Community | 68.7 (NR) | NR | 2.2 (NR)b | NR | NR |
| Ng et al. (2021) 82 /Longitudinal cohort | Singapore | 121 (61.2) | IPD | Movement disorder clinics | 64.5 (8.2) | NR | NR | 22.33 (8.71) | 186.96 (143.42) |
| Nguy et al. (2020) 83 /Cross-sectional | AUS | 52 (69) | IPD | Community, research clinic and database | 67.8 (7.8) | 7.8 (6.5) | NR | 33.7 (12.8) | NR |
| Oguh et al. (2014) 84 /Longitudinal cohort | NLD, N. Amer, IL | 4866 (63) | IPD | Research database | 67.0 (9.8) | 5.5 (2.0–10.0)c | ≥3 (n = 1699 [37.4]) m | NR | NR |
| Santos et al. (2018) 72 /Longitudinal cohort | USA | 130 (49) | MPS | Community | 78.45 (7.06) | NR | NR | NR | NR |
| Shih et al. (2019) 85 /Cross-sectional | USA | 174 (68) | IPD | Community | 65.79 (9.48) | 5.59 (0.54) | 1.96 (0.39)b | 29.94 (11.13) | NR |
Abbreviations: n, number; SD, standard deviation; NR, not reported; PD, Parkinson's disease; H&Y, Hoehn and Yahr Scale; MDS-UPDRS, International Parkinson and Movement Disorder Society — Unified Parkinson's Disease Rating Scale; LEDD, Levodopa equivalent daily dosage; IPD, Idiopathic Parkinson's disease; MPS, Mild Parkinsonian Signs; PD-NC, Parkinson's disease — non-cognitively impaired; PD-MCI, Parkinson's disease — mild cognitive impairment; PDD, Parkinson's disease dementia; ITA, Italy; USA, United States of America; EUR, Europe; DNK, Denmark; DEU, Germany; NLD, Netherlands; ISR, Israel; SWE, Sweden; AUS = Australia; N. Amer, North America; IL, Israel.
Except where indicated; bMean (SD); cMean (range); dn,(%); eMedian (IQR), fMedian (min/max); mModified Hoehn and Yahr Scale with stages 1–5; oOriginal UPDRS motor examination.
The sample sizes in the included studies ranged from 23 to 4866 people. All studies included females and males with a mean age that ranged from 62 to 78 years. Disease stage ranged from 0 (no clinical signs present) to 5 (wheelchair-bound or bedridden unless aided) on the original Hoehn and Yahr (H&Y) scale. The mean MDS-UPDRS part III motor score (0–132) reported by eight articles ranged from 22 to 57. The mean Levodopa equivalent daily dose reported by five articles ranged from 187 to 764 mg/day. Six studies were conducted in Europe,47,73,74,76,80,84 seven in North America,47,72,75,78,81,84,85 three in Asia,77,82,84 and one in Oceania. 83
Physical activity was assessed objectively using accelerometry in seven articles74–76,78,80,81,83 and assessed using participant self-reported questionnaires in nine articles.47,72,73,77,78,82–85 The physical activity measures of interest were total weekly physical activity, moderate-to-vigorous intensity physical activity, and light-intensity physical activity. 86 The following physical activity data were also extracted as potentially relevant: total daily steps, daily energy expenditure, and the number of days per month considered physically active.
Non-motor symptoms were typically evaluated using participant self-reported questionnaires, except for cognition, which was often evaluated using researcher-administered measures in eight articles.47,72,74–76,81,82,85 Relevant correlation estimates were presented in eight articles.74–78,80,84,85 Regression estimates were also presented in eight articles. Four of these articles72,81–83 reported standardised β-values, while the remaining four47,73,75,84 reported unstandardised b-values. The standard error (SE) of correlation (rs) and regression estimates (β, b) were not reported in any of the articles. SE (β) for these articles were obtained from the respective association's 95% CI. The computed standardised regression coefficient (β) with standard error SE (β) and 95% CI for each article can be found in Supplementary Table S5. Where multivariable regression models were used, estimates were usually adjusted for age, gender, Levodopa equivalent daily dose, MDS-UPDRS part III motor score, and disease duration.
The results from individual articles (Supplementary Table S5) found that increases in physical activity levels that are performed under free-living conditions were associated with better cognitive function (eight out of 11 articles),47,72,75–77,81,84,85 less affective disorders (five out of eight articles),47,73,77,82,84 better sleep quality (one out of two articles), 47 and less pain (one out of three articles). 84 Another article 83 found that pain was significantly exacerbated when physical activity increased. None of the articles found a significant relationship between physical activity and fatigue or excessive daytime sleepiness.
Combined results
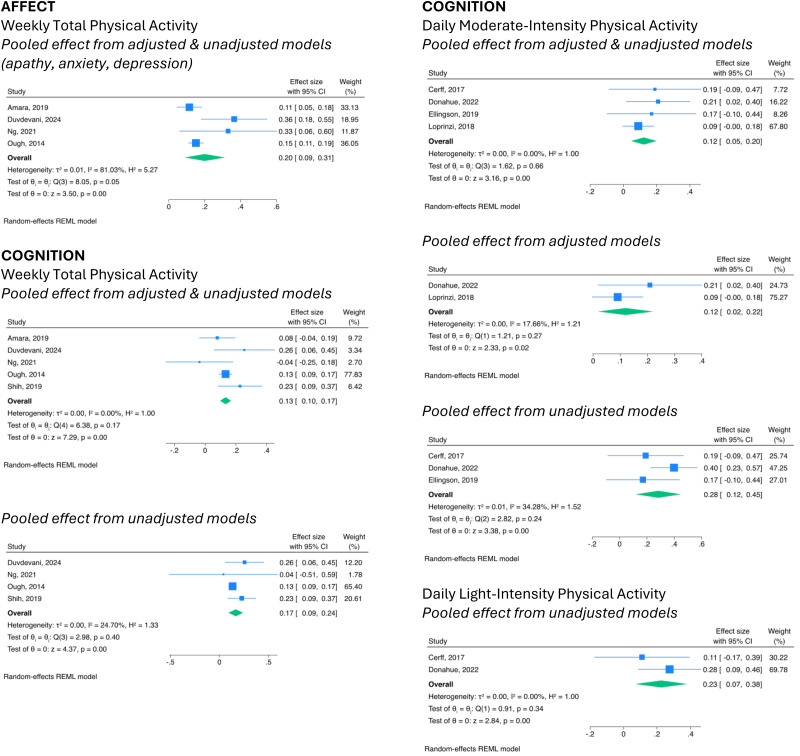
The meta-analysis of five articles,47,77,82,84,85 which evaluated cognitive function, indicated significant positive effects with total weekly physical activity (combined model = β, 0.13; 95% CI, 0.10–0.17; I2, 0%; unadjusted model = β, 0.17; 95% CI, 0.09–0.24; I2, 24.70%). A significant positive relationship was also found with daily moderate-to-vigorous intensity physical activity74,75,78,81 (combined model = β, 0.12; 95% CI, 0.05–0.20; I2, 0%; adjusted model = β, 0.12; 95% CI, 0.02–0.22; I2, 17.66%.; unadjusted model = β, 0.28; 95% CI, 0.12–0.45; I2, 34.28%) and light-intensity physical activity74,75 (unadjusted model = β, 0.23; 95% CI, 0.07–0.38; I2, 0%) (Figure 2).
Figure 2.
Forest plots with pooled estimates from articles estimating the relationship between physical activity categories and non-motor symptoms in Parkinson's disease.
The meta-analysis for four articles,47,77,82,84 which evaluated anxiety, depression or apathy, also indicated significant positive effects with the total weekly physical activity (combined model = β, 0.20; 95% CI, 0.09–0.31; I2, 81.03%) (Figure 2).
No significant associations were found from the meta-analyses of articles that evaluated sleep quality (adjusted model, p = 0.15),47,82 excessive daytime sleepiness (adjusted model, p = 0.69),47,82 fatigue (adjusted model, p = 0.58),47,82 or pain (combine model, p = 0.78; unadjusted model, p = 0.63)78,83,84 with total weekly physical activity levels. No significant pooled effect was found between pain and moderate-to-vigorous intensity physical activity (unadjusted model, p = 0.49).78,82 See Supplementary Figure S4.
Methodological quality assessment
All articles were classified as having high methodological quality (72.7–100%). 48 Tables 2, 3, and 4 present the results of the methodological quality assessment for the included articles.
Table 2.
Methodological quality assessment using the JBI critical appraisal checklist for cross-sectional studies.
| Report citation | Inclusion criteria defined | Participants and setting described | Describes the use of valid and reliable exposure (physical activity) measure | PD diagnostic criteria used | Confounding factors identified | Strategies used to deal with confounding factors | Describes the use of valid and reliable outcome (non-motor symptom) measures | Appropriate statistical analyses used | Total score |
|---|---|---|---|---|---|---|---|---|---|
| Alwardat et al. (2019) 73 | Y | Y | N | Y | Y | Y | Y | Y | 87.5%* |
| Cerff et al. (2017) 74 | Y | Y | Y | Y | Y | Y | Y | Y | 100%* |
| Donahue et al. (2022) 75 | Y | Y | Y | Y | Y | Y | Y | Y | 100%* |
| Dontje et al. (2013) 76 | Y | Y | Y | Y | Y | Y | Y | Y | 100%* |
| Ellingson et al. (2017) 78 | Y | Y | Y | Y | Y | U | Y | Y | 87.5%* |
| Leavy et al. (2021) 80 | Y | Y | Y | U | Y | Y | Y | Y | 87.5%* |
| Loprinzi et al. (2018) 81 | N | Y | Y | Y | Y | Y | Y | Y | 100%* |
| Nguy et al. (2020) 83 | Y | Y | Y | Y | Y | Y | Y | Y | 100%* |
| Shih et al. (2019) 85 | Y | Y | N | Y | Y | Y | Y | Y | 87.5%* |
Abbreviations: Y = yes; N = no; U = unclear. Identification of confounding factors was rated ‘Y’ if typical confounders, such as age, gender, Levodopa dosage equivalent, disease duration, and motor symptom severity, were identified and measured. The use of valid and reliable measures was rated ‘Y’ if psychometric properties have been previously evaluated and shown acceptable levels in people with Parkinson's disease. Study quality is defined as follows: a total score greater than 70% as high quality, a score between 50% and 70% as medium quality, and a score less than 50% as low quality. *High quality articles, >70%.
Table 3.
Methodological quality assessment using the JBI critical appraisal checklist for case–control studies.
| Report Citation | Addressed a clearly focused issue | Acceptable recruitment | Exposure (physical activity) accurately measured | Outcomes (non-motor symptoms) accurately measured | Important confounding factors identified | Strategies used to deal with confounding factors | Was the follow-up of subjects complete enough | Was the follow-up of subjects long enough | Do you believe the results | Can the results be applied to the local population | Results fit with other available evidence | Total score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ng et al. (2021) 82 | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | 90% |
| Oguh et al. (2014) 84 | Y | Y | N | Y | Y | Y | U | Y | Y | Y | Y | 81.2% |
| Santos et al. (2018) 72 | Y | N | N | Y | Y | Y | N | Y | Y | Y | Y | 72.7% |
Abbreviations: Y = yes; N = no; U = unclear. Identification of confounding factors was rated ‘Y’ if typical confounders, such as age, gender, Levodopa dosage equivalent, disease duration, and motor symptom severity, were identified and measured. The use of valid and reliable measures was rated ‘Y’ if psychometric properties have been previously evaluated and shown acceptable levels in people with Parkinson's disease. Study quality is defined as follows: a total score greater than 70% as high quality, a score between 50% and 70% as medium quality, and a score less than 50% as low quality. *High quality articles, >70%.
Table 4.
Critical appraisal skills programme checklist for cohort studies.
| Report citation | Groups comparable | Cases and controls matched | Same criteria used for identification of cases and controls | Exposure (physical activity) is measured in a standard, valid and reliable way | Exposure measured in the same way for cases and controls | Confounding factors identified | Strategies used to deal with confounding factors | Outcomes (non-motor symptoms) assessed in a standard, valid and reliable way for cases and controls | Exposure period of interest was long enough to be meaningful | Appropriate statistical analyses used | Total score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amara et al. (2019) 47 | Y | U | Y | N | Y | Y | Y | Y | Y | Y | 80%* |
Abbreviations: Y = yes; N = no; U = unclear. Identification of confounding factors was rated ‘Y’ if typical confounders, such as age, gender, Levodopa dosage equivalent, disease duration, and motor symptom severity, were identified and measured. The use of valid and reliable measures was rated ‘Y’ if psychometric properties have been previously evaluated and shown acceptable levels in people with Parkinson's disease. Study quality is defined as follows: a total score greater than 70% as high quality, a score between 50% and 70% as medium quality, and a score less than 50% as low quality. *High quality articles, >70%.
Articles excluded from meta-analysis
Four articles were not included in the meta-analysis. Alwardat et al. 73 did not contain sufficient information to compute an effect size for statistical pooling. This article reported improvement in anxiety and depression with increases in the weekly physical activity. The methodological heterogeneity of the remaining four articles by Dontje et al., 76 Santos et al., 72 Leavy et al., 80 and Bonde-Jensen et al. 71 precluded their inclusion in the meta-analysis.
Discussion
This review showed emerging evidence that people with Parkinson's who are more physically active in their daily lives have better global cognition and less anxiety, apathy, and depression. The prevalence of cognitive impairment and affective disorders is relatively high in people with Parkinson's and contributes to a poor quality of life.87–90 While the benefits of physical activity on these symptoms may be independent of each other, 91 other reports suggest the cognitive benefits of physical activity may be mediated through improvements in depression, anxiety, and apathy.92–94 A recent report by Engels et al. 93 explored interactions among cognition, affective disorders, sleep, fatigue, and pain in people with Parkinson's. Significant positive associations were found among all symptoms, with the strongest association between cognition and affective disorders. Cognitive benefits may also be mediated through less affective symptoms facilitating greater physical activity engagement. 94 Similarly, improved cognition, such as executive function, may facilitate greater physical activity engagement and therefore the benefits of physical activity on affective symptoms. 95
Physical activity refers to any bodily movement produced by skeletal muscles that results in energy expenditure, such as walking, work activities, self-care, household activities and sports. 96 Exercise is a form of physical activity that is planned, structured, repetitive, and performed with the intention of improving or maintaining physical fitness. 96 Physical activities in free-living conditions refer to leisure activities (e.g. walking, swimming, gardening, golf, dance), non-leisure activities (e.g. transportation, occupational, housework), and planned exercise (e.g. gym, community exercise class) that people do in their everyday life. 27 The benefits of exercise and physical activity for symptoms experienced by people with Parkinson's may be mediated via a wide range of factors.97–99 Among these factors are the increase in endogenous dopamine, serotonin, noradrenaline, and acetylcholine100–102 which are depleted in people with Parkinson's who present with non-motor symptoms. 103 The benefits may also be related to exercise increasing the absorption and transportation of Levodopa across the blood–brain barrier, which enhances endogenous dopamine production.104,105
Many people with Parkinson's reduce the amount and intensity of their physical activities, particularly moderate-intensity activity as the disease progresses.69,70,106 In contrast, the prevalence and severity of non-motor symptoms increase. 107 Given the progressive nature of Parkinson's and the known benefits of being physically active, it is important that people with Parkinson's can participate in physical activity recommendations that are sustainable. 22 Recommendations to increase current free-living leisure, non-leisure, and planned exercise activities is one solution to encourage greater physical activity engagement. 21
This systematic review suggested that increases in physical activity are associated with changes in some non-motor symptoms; however, the results should be interpreted with caution due to the nature of the observational data, which prevents the assessment of causal relationships. It is also possible that increases in non-motor symptoms are associated with changes in physical activity behaviours. Non-motor symptoms, such as cognition difficulties, 74 depression,37–39 apathy, 108 fatigue, 38 excessive daytime sleepiness, 38 and pain, 40 are argued to correlate with low physical activity levels. This review did not explore the effect of non-motor symptoms on physical activity behaviour. People with Parkinson's who experience motor impairments, fear of falling, low self-efficacy for exercise, negative perceptions of exercise, access, and resource constraints may further limit their participation in physical activity and contribute to a sedentary lifestyle.37–41 There is a need for further research to assess the causal relationship between non-motor symptoms and physical activity in people with Parkinson's with more appropriate designs such as a systematic review of randomised controlled trials.
There were several limitations of this review. No eligible articles included individuals with atypical parkinsonism, so the findings may not be generalisable to this population. However, it is likely that the eligible studies included participants with atypical parkinsonism, but were not identified because they can be indistinguishable from idiopathic Parkinson's due to symptom overlap, particularly in the early stages of the disease.109,110 Many articles used retrospective questionnaires, which are inherently subject to recall bias, with participants being more likely to over or underestimate their activity and symptoms.111–113 Most investigations assessed few and varied physical activity characteristics (e.g. total weekly physical activity minutes, daily light-, moderate-, and vigorous-intensity activity time, daily steps, and total daily energy expenditure) and used different measurement tools to evaluate non-motor symptoms. This heterogeneity limited the number of articles that could be sensibly pooled in the meta-analyses. While we are confident that our search strategy was comprehensive, having a second reviewer screen titles and abstracts would have ensured no relevant articles were missed. Including non-English language publications and qualitative study designs may have provided this review with a greater breadth and depth of information. In addition, including randomised controlled trials would have provided the review with more detail about exercise dosage (e.g. type, intensity, duration, and frequency).
There is emerging evidence of an association between increased levels of physical activity performed under free-living conditions and better cognitive function and less affective disorder symptoms, especially anxiety, depression, and apathy in people with Parkinson's. There is a need for further research to identify feasible and sustainable physical activity recommendations as such behaviour might slow the rate of functional decline and symptom presentation.13,14
Clinical messages.
Supporting people with Parkinson's to maintain or increase their usual daily activities affords them a sustainable approach to managing non-motor symptoms
Participation in physical activities in free-living conditions is related to better cognition and less anxiety, apathy, and depression in people living with Parkinson's disease
Supplemental Material
Supplemental material, sj-docx-1-cre-10.1177_02692155241272967 for Relationships between physical activities performed under free-living conditions and non-motor symptoms in people with Parkinson's: A systematic review and meta-analysis by Amanda Still, Leigh Hale, Sarfaraz Alam, Meg E. Morris and Prasath Jayakaran in Clinical Rehabilitation
Acknowledgements
The authors thank the University of Otago Librarian, Thelma Fisher (TF), for her contribution to the search strategy development.
Footnotes
Data Availability: Data are available on request to the corresponding author.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Amanda Still https://orcid.org/0000-0002-5179-1602
Supplemental Material: Supplemental material for this article is available online.
References
- 1.GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18: 459–480. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin VL, Abajobir AA, Abate KH, et al. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 2017; 16: 877–897. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 2015; 30: 1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhuri KR, Odin P, Antonini Aet al. et al. Parkinson’s disease: the non-motor issues. Parkinsonism Relat Disord 2011; 17: 717–723. doi: 10.1016/j.parkreldis.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Grażyńska A, Urbaś W, Antoniuk S, et al. Comparative analysis of non-motor symptoms in patients with Parkinson’s disease and atypical parkinsonisms. Clin Neurol Neurosurg 2020; 197: 106088. doi: 10.1016/j.clineuro.2020.106088. [DOI] [PubMed] [Google Scholar]
- 6.Kim H-S, Cheon S-M, Seo J-W, et al. Nonmotor symptoms more closely related to Parkinson’s disease: comparison with normal elderly. J Neurol Sci 2013; 324: 70–73. doi: 10.1016/j.jns.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan S Sarma G, Sarma S. et al. et al. Do nonmotor symptoms in Parkinson’s disease differ from normal aging? Mov Disord 2011; 26: 2110–2113. [DOI] [PubMed] [Google Scholar]
- 8.Breen KC, Drutyte G. Non-motor symptoms of Parkinson’s disease: the patient’s perspective. J Neural Transm 2013; 120: 531–535. doi: 10.1007/s00702-012-0928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shulman LM, Taback RL, Bean Jet al. et al. Comorbidity of the nonmotor symptoms of Parkinson’s disease. Mov Disord 2001; 16: 507–510. doi: 10.1002/mds.1099. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord 2011; 26: 399–406. doi: 10.1002/mds.23462. [DOI] [PubMed] [Google Scholar]
- 11.Soh S-E, Morris ME, McGinley JL. Determinants of health-related quality of life in Parkinson’s disease: a systematic review. Parkinsonism Relat Disord 2011; 17: 1–9. doi: 10.1016/j.parkreldis.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Hermanowicz N, Jones SA, Hauser RA. Impact of non-motor symptoms in Parkinson’s disease: a PMDAlliance survey. Neuropsychiatr Dis Treat 2019; 15: 2205–2212. doi: 10.2147/NDT.S213917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ernst M, Folkerts A-K, Gollan R, et al. Physical exercise for people with Parkinson’s disease: a systematic review and network meta-analysis. Cochrane Database Syst Rev 2023; 1: CD013856. doi: 10.1002/14651858.CD013856.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tonkin P, Miller T, Hartmann Tet al. et al. The effects of exercise on non-motor experiences of daily living experienced in Parkinson’s disease: a systematic review and network meta-analysis. Clin Park Relat Disord 2023; 9: 100203. doi: 10.1016/j.prdoa.2023.100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gollan R, Ernst M, Lieker E, et al. Effects of resistance training on motor-and non-motor symptoms in patients with Parkinson’s disease: a systematic review and meta-analysis. J Parkinson’s Dis 2022; 12: 1783–1806. doi: 10.3233/JPD-223252. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin VA, Richards SH, Taylor RS, et al. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov Disord 2008; 23: 631–640. doi: 10.1002/mds.21922. [DOI] [PubMed] [Google Scholar]
- 17.Emmanouilidis S, Hackney ME, Slade SC, et al. Dance is an accessible physical activity for people with Parkinson’s disease. Parkinson’s Disease 2021; 2021: 7516504. doi: 10.1155/2021/7516504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carroll LM, Morris ME, O’Connor WTet al. et al. Is aquatic therapy optimally prescribed for Parkinson’s disease? A systematic review and meta-analysis. J Parkinson’s Dis 2020; 10: 59–76. doi: 10.3233/JPD-191784. [DOI] [PubMed] [Google Scholar]
- 19.Song R, Grabowska W, Park M, et al. The impact of Tai Chi and Qigong mind–body exercises on motor and non-motor function and quality of life in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord 2017; 41: 3–13. doi: 10.1016/j.parkreldis.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Santis KK, Kaplan I. The motor and the non-motor outcomes of Nordic walking in Parkinson’s disease: a systematic review. J Bodyw Mov Ther 2020; 24: 4–10. doi: 10.1016/j.jbmt.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Hunter H, Lovegrove C, Haas B, et al. Experiences of people with Parkinson’s disease and their views on physical activity interventions: a qualitative systematic review. JBI Evidence Synthesis 2019; 17: 548–613. doi: 10.11124/jbisrir-2017-003901. [DOI] [PubMed] [Google Scholar]
- 22.Ene H, McRae C, Schenkman M. Attitudes toward exercise following participation in an exercise intervention study. J Neurol Phys Ther 2011; 35: 34–40. 10.1097/NPT.0b013e31820cb917. [DOI] [PubMed] [Google Scholar]
- 23.Comella CL, Stebbins GT, Brown-Toms Net al. et al. Physical therapy and Parkinson’s disease: a controlled clinical trial. Neurology 1994; 44: 376–378. doi: 10.1212/WNL.44.3_Part_1.376. [DOI] [PubMed] [Google Scholar]
- 24.Lai B, Kim Y, Wilroy J, et al. Sustainability of exercise intervention outcomes among people with disabilities: a secondary review. Disabil Rehabil 2019; 41: 1584–1595. doi: 10.1080/09638288.2018.1432704. [DOI] [PubMed] [Google Scholar]
- 25.Del Din S, Godfrey A, Mazzà C, et al. Free-living monitoring of Parkinson’s disease: lessons from the field. Mov Disord 2016; 31: 1293–1313. doi: 10.1002/mds.26718. [DOI] [PubMed] [Google Scholar]
- 26.Berlin JE, Storti KL, Brach JS. Using activity monitors to measure physical activity in free-living conditions. Phys Ther 2006; 86: 1137–1145. doi: 10.1002/mds.26170. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. Global recommendations on physical activity for health [internet]. Geneva: World Health Organization, 2010. Available from: https://www.ncbi.nlm.nih.gov/books/NBK305057/. [PubMed] [Google Scholar]
- 28.Li F, Harmer P, Liu Y, et al. A randomized controlled trial of patient-reported outcomes with Tai Chi exercise in Parkinson’s disease. Mov Disord 2014; 29: 539–545. doi: 10.1002/mds.25787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiPietro L. Physical activity in aging: changes in patterns and their relationship to health and function. J Gerontol A Biol Sci Med Sci 2001; 56: 13–22. doi: 10.1093/gerona/56.suppl_2.13. [DOI] [PubMed] [Google Scholar]
- 30.Scanlon-Mogel J, Roberto K. Older adults’ beliefs about physical activity and exercise: life course influences and transitions. Qual Ageing Older Adults 2004; 5: 33–44. doi: 10.1108/14717794200400017. [DOI] [Google Scholar]
- 31.Zesiewicz T, Sullivan K, Arnulf I, et al. Practice parameter: treatment of nonmotor symptoms of Parkinson disease: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2010; 74: 924–931. doi: 10.1212/WNL.0B013E3181D55F24. [DOI] [PubMed] [Google Scholar]
- 32.Litvan I. Atypical parkinsonian disorders clinical and research aspects [internet]. Totowa, NJ: Humana Press, 2005. Available from: https://doi.org/10.1385/159259834X. [Google Scholar]
- 33.Giagkou N, Stamelou M. Therapeutic management of the overlapping syndromes of atypical parkinsonism. CNS Drugs 2018; 32: 827–837. doi: 10.1007/s40263-018-0551-3. [DOI] [PubMed] [Google Scholar]
- 34.Chaudhuri KR, Schapira AHV. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol 2009; 8: 464–474. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- 35.Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA 2014; 311: 1670–1683. doi: 10.1007/s00415-009-5255-7. [DOI] [PubMed] [Google Scholar]
- 36.Zgaljardic D, Foldi N, Borod J. Cognitive and behavioral dysfunction in Parkinson’s disease: neurochemical and clinicopathological contributions. J Neural Transm 2004; 111: 1287–1301. doi: 10.1007/s00702-004-0178-z. [DOI] [PubMed] [Google Scholar]
- 37.Feliciano Jéssica S, Rodrigues Samara Maria A, de Carvalho Lana Ret al. et al. Predictors of physical activity levels in individuals with Parkinson’s disease: a cross-sectional study. Neurol Sci 2021; 42: 1499–1505. doi: 10.1007/s10072-020-04701-1. [DOI] [PubMed] [Google Scholar]
- 38.Afshari M, Yang A, Bega D. Motivators and barriers to exercise in Parkinson’s disease. J Parkinson’s Dis 2017; 7: 703–711. doi: 10.3233/JPD-171173. [DOI] [PubMed] [Google Scholar]
- 39.Ellis T, Cavanaugh JT, Earhart GM, et al. Factors associated with exercise behavior in people with Parkinson disease. Phys Ther 2011; 91: 1838–1848. doi: 10.2522/ptj.20100390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ellis T, Boudreau JK, DeAngelis TR, et al. Barriers to exercise in people with Parkinson disease. Phys Ther 2013; 93: 628–636. doi: 10.2522/ptj.20120279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schootemeijer S, van der Kolk NM, Ellis T, et al. Barriers and motivators to engage in exercise for persons with Parkinson’s disease. J Parkinson’s Dis 2020; 10: 1293–1299. doi: 10.3233/JPD-202247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shamseer L, Moher D, Clarke M, et al. Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) 2015: elaboration and explanation. Br Med J 2015; 349: 1–9. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 43.Moher D, Liberati A, Tetzlaff Jet al. et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8: 336–341. doi: 10.1136/bmj.b2535. [DOI] [PubMed] [Google Scholar]
- 44.Still A, Hale L, Jayakaran P. The inter-relationship between various non-motor symptoms and with habitual physical activity in parkinsonism: a scoping review protocol. Phys Ther Rev 2022; 27: 444–452. doi: 10.1080/10833196.2022.2133885. [DOI] [Google Scholar]
- 45.Moola S, Munn Z, Tufanaru C, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z. (eds) JBI manual for evidence synthesis. JBI, 2020. Available from: https://doi.org/10.46658/JBIMES-24-01. [Google Scholar]
- 46.Critical Appraisal Skills Programme. CASP Cohort Study Checklist 2018. Available from: https://casp-uk.net/checklists/casp-cohort-studies-checklist-fillable.pdf.
- 47.Amara AW, Chahine L, Seedorff N, et al. Self-reported physical activity levels and clinical progression in early Parkinson’s disease. Parkinsonism Relat Disord 2019; 61: 118–125. doi: 10.1016/j.parkreldis.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Pimsen A, Kao C-Y, Hsu S-Tet al. et al. The effect of advance care planning intervention on hospitalization among nursing home residents: a systematic review and meta-analysis. J Am Med Dir Assoc 2022: 1448–1460. doi: 10.1016/j.jamda.2022.07.017. [DOI] [PubMed] [Google Scholar]
- 49.Nieminen P. Application of standardized regression coefficient in meta-analysis. BioMedInformatics [internet]. 2022; 2: 434–458. doi: 10.3390/biomedinformatics2030028 [DOI] [Google Scholar]
- 50.Higgins JPT, Li T Deeks JJ (eds). Chapter 6: Choosing effect measures and computing estimates of effect [internet]. In: Higgins JPT, Thomas J, Chandler J, et al. (eds) Cochrane handbook for systematic reviews of interventions. 2nd Ed. Chichester, UK: John Wiley & Sons, 2019: 143–176. 10.1002/9781119536604.ch6. [DOI] [Google Scholar]
- 51.Deeks JJ, Higgins JPT, Altman DG. (editors). Chapter 10: Analysing data and undertaking meta-analyses [internet]. In: Higgins JPT, Thomas J, Chandler J, et al. (editors) Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester (UK): John Wiley & Sons, 2019: 241–284. 10.1002/9781119536604.ch10. [DOI] [Google Scholar]
- 52.Bernhard FP, Sartor J, Bettecken K, et al. Wearables for gait and balance assessment in the neurological ward — study design and first results of a prospective cross-sectional feasibility study with 384 inpatients. BMC Neurol 2018; 18: 1–8. doi: 10.1186/s12883-018-1111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bohnen JLB, Müller MLTM, Haugen Jet al. et al. Mentally stimulating activities associate with better cognitive performance in Parkinson disease. J Neural Transm 2017; 124: 1205–1212. doi: 10.1007/s00702-017-1761-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bryant S, Rintala DH, Hou J-Get al. et al. Relationship of falls and fear of falling to activity limitations and physical inactivity in Parkinson’s disease. J Aging Phys Act 2015; 23: 187–193. doi: 10.1123/japa.2013-0244. [DOI] [PubMed] [Google Scholar]
- 55.Hultqvist J, Sahlstrom T, Timpka J, et al. Everyday occupations and other factors in relation to mental well-being among persons with advanced Parkinson’s disease. Occup Ther Health Care 2020; 34: 1–18. doi: 10.1080/07380577.2019.1692269. [DOI] [PubMed] [Google Scholar]
- 56.Kataoka H, Saeki K, Yamagami Y, et al. Quantitative associations between objective sleep measures and early-morning mobility in Parkinson’s disease: cross-sectional analysis of the PHASE study. Sleep 2020; 43: zsz203. doi: 10.1093/sleep/zsz203. [DOI] [PubMed] [Google Scholar]
- 57.Lana RC, de Araujo LN, Cardoso Fet al. et al. Main determinants of physical activity levels in individuals with Parkinson’s disease. Arq Neuropsiquiatr 2016; 74: 112–116. doi: 10.1590/0004-282X20160009. [DOI] [PubMed] [Google Scholar]
- 58.Morley D, Dummett S, Kelly L, et al. Predictors of activity and participation across neurodegenerative conditions: a comparison of people with motor neurone disease, multiple sclerosis and Parkinson’s disease. BMC Neurol 2018; 18: 1–6. doi: 10.1186/s12883-018-1024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nilsson MH, Jonasson SB, Zijlstra GAR. Predictive factors of fall-related activity avoidance in people with Parkinson disease — A longitudinal study with a 3-year follow-up. J Neurol Phys Ther 2020; 44: 188–194. doi: 10.1097/npt.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 60.Rantakokko M, Iwarsson S, Slaug Bet al. et al. Life–space mobility in Parkinson’s disease: associations with motor and non-motor symptoms. J Gerontol A Biol Sci Med Sci 2019; 74: 507–512. doi: 10.1093/gerona/gly074. [DOI] [PubMed] [Google Scholar]
- 61.Fellows RP, Schmitter-Edgecombe M. Multimethod assessment of everyday functioning and memory abilities in Parkinson’s disease. Neuropsychol 2019; 33: 169–177. doi: 10.1037/neu0000505. [DOI] [PubMed] [Google Scholar]
- 62.Duncan RP, Van Dillen LR, Garbutt JM, et al. Low back pain-related disability in Parkinson disease: impact on functional mobility, physical activity, and quality of life. Phys Ther 2019; 99: 1346–1353. doi: 10.1093/ptj/pzz094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rafferty MR, Schmidt PN, Luo ST, et al. Regular exercise, quality of life, and mobility in Parkinson’s disease: a longitudinal analysis of National Parkinson Foundation quality improvement initiative data. J Parkinson’s Dis 2017; 7: 193–202. doi: 10.3233/JPD-160912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raggi A, Leonardi M, Ajovalasit D, et al. Disability and profiles of functioning of patients with Parkinson’s disease described with ICF classification. Int J Rehabil Res 2011; 34: 141–150. doi: 10.1097/MRR.0b013e328344ae09. [DOI] [PubMed] [Google Scholar]
- 65.Kumar N, Gupta R, Kumar H, et al. Impact of home confinement during COVID-19 pandemic on sleep parameters in Parkinson’s disease. Sleep Med 2021; 77: 15–22. doi: 10.1016/j.sleep.2020.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lana RD, de Paula AR, Silva AFS, et al. Validity of mHealth devices for counting steps in individuals with Parkinson’s disease. J Bodyw Mov Ther 2021; 28: 496–501. doi: 10.1016/j.jbmt.2021.06.018. [DOI] [PubMed] [Google Scholar]
- 67.Pradhan S, Kelly VE. Quantifying physical activity in early Parkinson disease using a commercial activity monitor. Parkinsonism Relat Disord 2019; 66: 171–175. doi: 10.1016/j.parkreldis.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prusynski RA, Kelly VE, Fogelberg DJet al. et al. The association between sleep deficits and sedentary behavior in people with mild Parkinson disease. Disabil Rehabil 2021. doi: 10.1080/09638288.2021.1940320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Nimwegen M, Speelman AD, Hofman-van Rossum EJ, et al. Physical inactivity in Parkinson’s disease. J Neurol 2011; 258: 2214–2221. doi: 10.1007/s00415-011-6097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cavanaugh JT, Ellis TD, Earhart GM, et al. Toward understanding ambulatory activity decline in Parkinson disease. Phys Ther 2015; 95: 1142–1150. doi: 10.2522/ptj.20140498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bonde-Jensen F, Dalgas U, Langeskov-Christensen M. Are physical activity levels, cardiorespiratory fitness and peak power associated with Parkinson’s disease severity? J Neurol Sci 2024; 460: 122996. doi: 10.1016/j.jns.2024.122996. [DOI] [PubMed] [Google Scholar]
- 72.Santos D, Mahoney JR, Allali Get al. et al. Physical activity in older adults with mild parkinsonian signs: a cohort study. J Gerontol A Biol Sci Med Sci 2018; 73: 1682–1687. doi: 10.1093/gerona/glx133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alwardat M, Schirinzi T, Di Lazzaro G, et al. Association between physical activity and dementia’s risk factors in patients with Parkinson’s disease. J Neural Transm 2019; 126: 319–325. doi: 10.1007/s00702-019-01979-0. [DOI] [PubMed] [Google Scholar]
- 74.Cerff B, Maetzler W, Sulzer P, et al. Home-based physical behavior in late stage Parkinson disease dementia: differences between cognitive subtypes. Neurodegener Dis 2017; 17: 135–144. doi: 10.1159/000460251. [DOI] [PubMed] [Google Scholar]
- 75.Donahue EK, Venkadesh S, Bui V, et al. Physical activity intensity is associated with cognition and functional connectivity in Parkinson’s disease. Parkinsonism Relat Disord 2022; 104: 7–14. doi: 10.1016/j.parkreldis.2022.09.005. [DOI] [PubMed] [Google Scholar]
- 76.Dontje ML, de Greef MHG, Speelman AD, et al. Quantifying daily physical activity and determinants in sedentary patients with Parkinson’s disease. Parkinsonism Relat Disord 2013; 19: 878–882. doi: 10.1016/j.parkreldis.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 77.Duvdevani M, Yogev-Seligmann G, Schlesinger I, et al. Association of health behaviors with function and health-related quality of life among patients with Parkinson’s disease. Isr J Health Policy Res 2024; 13: 1–10. doi: 10.1186/s13584-023-00588-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ellingson LD, Zaman A, Stegemöller EL. Sedentary behavior and quality of life in individuals with Parkinson’s disease. Neurorehabil Neural Repair 2019; 33: 595–601. doi: 10.1177/1545968319856893. [DOI] [PubMed] [Google Scholar]
- 79.Fava GA, Kellner R, Munari Fet al. et al. The Hamilton Depression Rating Scale in normals and depressives. Acta Psychiatr Scand 1982; 66: 26–32. doi: 10.1111/j.1600-0447.1982.tb00911.x. [DOI] [PubMed] [Google Scholar]
- 80.Leavy B, Hagstromer M, Conradsson DMet al. et al. Physical activity and perceived health in people with Parkinson disease during the first wave of COVID-19 pandemic: a cross-sectional study from Sweden. J Neurol Phys Ther 2021; 45: 266–272. doi: 10.1097/NPT.0000000000000372. [DOI] [PubMed] [Google Scholar]
- 81.Loprinzi PD, Danzl MM, Ulanowski Eet al. et al. A pilot study evaluating the association between physical activity and cognition among individuals with Parkinson’s disease. Disabil Health J 2018; 11: 165–168. doi: 10.1016/j.dhjo.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 82.Ng SY-E, Chia NS-Y, Abbas MM, et al. Physical activity improves anxiety and apathy in early Parkinson’s disease: a longitudinal follow-up study. Front Neurol 2021; 11: 625897. doi: 10.3389/fneur.2020.625897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nguy V, Barry BK, Moloney N, et al. The associations between physical activity, sleep, and mood with pain in people with Parkinson’s disease: an observational cross-sectional study. J Parkinson’s Dis 2020; 10: 1161–1170. doi: 10.3233/jpd-201938. [DOI] [PubMed] [Google Scholar]
- 84.Oguh O, Eisenstein A, Kwasny Met al. et al. Back to the basics: regular exercise matters in Parkinson’s disease: results from the National Parkinson Foundation QII Registry Study. Parkinsonism Relat Disord 2014; 20: 1221–1225. doi: 10.1016/j.parkreldis.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 85.Shih CH, Moore K, Browner N, et al. Physical activity mediates the association between striatal dopamine transporter availability and cognition in Parkinson’s disease. Parkinsonism Relat Disord 2019; 62: 68–72. doi: 10.1016/j.parkreldis.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 86.Ramsey KA, Rojer AGM, D’Andrea L, et al. The association of objectively measured physical activity and sedentary behavior with skeletal muscle strength and muscle power in older adults: a systematic review and meta-analysis. Ageing Res Rev 2021; 67: 101266. doi: 10.1016/j.arr.2021.101266. [DOI] [PubMed] [Google Scholar]
- 87.Martinez-Martin P, Schapira AHV, Stocchi F, et al. Prevalence of nonmotor symptoms in Parkinson’s disease in an international setting; study using nonmotor symptoms questionnaire in 545 patients. Mov Disord 2007; 22: 1623–1629. doi: 10.1002/mds.21586. [DOI] [PubMed] [Google Scholar]
- 88.Barone P, Antonini A, Colosimo C, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord 2009; 24: 1641–1649. doi: 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- 89.Colosimo C, Morgante L, Antonini A, et al. Non-motor symptoms in atypical and secondary parkinsonism: the PRIAMO study. J Neurol 2010; 257: 5–14. doi: 10.1007/s00415-009-5255-7. [DOI] [PubMed] [Google Scholar]
- 90.Rodriguez-Blazquez C, Schrag A, Rizos A, et al. Prevalence of non-motor symptoms and non-motor fluctuations in Parkinson’s disease using the MDS–NMS. Mov Disord Clin Pract 2021; 8: 231–239. doi: 10.1002/mdc3.13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hopkins ME, Davis FC, VanTieghem MR, et al. Differential effects of acute and regular physical exercise on cognition and affect. Neurosci 2012; 215: 59–68. doi: 10.1016/j.neuroscience.2012.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Poletti M, De Rosa A, Bonuccelli U. Affective symptoms and cognitive functions in Parkinson’s disease. J Neurol Sci 2012; 317: 97–102. doi: 10.1016/j.jns.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 93.Engels G, Douw L, Kerst Y, et al. Non-motor symptoms in Parkinson’s disease: an explorative network study. Parkinsonism Relat Disord 2019; 66: 237–240. doi: 10.1016/j.parkreldis.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 94.Liu J, Qiang F, Dang Jet al. et al. Depressive symptoms as mediator on the link between physical activity and cognitive function: longitudinal evidence from older adults in China. Clin Gerontol 2023; 46: 808–818. doi: 10.1080/07317115.2022.2077158. [DOI] [PubMed] [Google Scholar]
- 95.Bode M, Sulzer P, Schulte C, et al. Multidomain cognitive training increases physical activity in people with Parkinson’s disease with mild cognitive impairment. Parkinsonism Relat Disord 2023; 113: 105330. doi: 10.1016/j.parkreldis.2023.105330. [DOI] [PubMed] [Google Scholar]
- 96.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 1985; 100: 126–131. Available from: https://www.jstor.org/stable/20056429. [PMC free article] [PubMed] [Google Scholar]
- 97.Emig M, George T, Zhang JKet al. et al. The role of exercise in Parkinson’s disease. J Geriatr Psychiatry Neurol 2021; 34: 321–330. doi: 10.1177/08919887211018273. [DOI] [PubMed] [Google Scholar]
- 98.Ellis T, Rochester L. Mobilizing Parkinson’s disease: the future of exercise. J Parkinson’s Dis 2018; 8: S95–S100. doi: 10.3233/JPD-181489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Speelman AD, Van De Warrenburg BP, Van Nimwegen M, et al. How might physical activity benefit patients with Parkinson disease? Nat Rev Neurol 2011; 7: 528–534. doi: 10.1038/nrneurol.2011.107. [DOI] [PubMed] [Google Scholar]
- 100.Meeusen R, De Meirleir K. Exercise and brain neurotransmission. Sports Med 1995; 20: 160–188. doi: 10.2165/00007256-199520030-00004. [DOI] [PubMed] [Google Scholar]
- 101.Lin TW, Kuo YM. Exercise benefits brain function: the monoamine connection. Brain Sci 2013; 3: 39–53. doi: 10.3390/brainsci3010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zong B, Yu F, Zhang X, et al. Understanding how physical exercise improves Alzheimer’s disease: cholinergic and monoaminergic systems. Front Aging Neurosci 2022; 14: 869507. doi: 10.3389/fnagi.2022.869507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barone P. Neurotransmission in Parkinson’s disease: beyond dopamine. Eur J Neurol 2010; 17: 364–376. doi: 10.1111/j.1468-1331.2009.02900.x. [DOI] [PubMed] [Google Scholar]
- 104.Muhlack S, Welnic J, Woitalla Det al. et al. Exercise improves efficacy of levodopa in patients with Parkinson’s disease. Mov Disord 2007; 22: 427–430. doi: 10.1002/mds.21346. [DOI] [PubMed] [Google Scholar]
- 105.Reuter I, Harder S, Engelhardt Met al. et al. The effect of exercise on pharmacokinetics and pharmacodynamics of levodopa. Mov Disord 2000; 15: 862–868. doi:. [DOI] [PubMed] [Google Scholar]
- 106.Cavanaugh JT, Ellis TD, Earhart GM, et al. Capturing ambulatory activity decline in Parkinson’s disease. J Neurol Phys Ther 2012; 36: 51–57. doi: 10.1097/NPT.0b013e318254ba7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Borroni B, Turla M, Bertasi V, et al. Cognitive and behavioral assessment in the early stages of neurodegenerative extrapyramidal syndromes. Arch Gerontol Geriatr 2008; 47: 53–61. doi: 10.1016/j.archger.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 108.Gorzkowska A, Cholewa J, Małecki A, et al. What determines spontaneous physical activity in patients with Parkinson’s disease? J Clin Med 2020; 9: 1296. doi: 10.3390/jcm9051296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Litvan I, Bhatia KP, Burn DJ, et al. SIC task force appraisal of clinical diagnostic criteria for parkinsonian disorders. Mov Disord 2003; 18: 467–486. doi: 10.1002/mds.10459. [DOI] [PubMed] [Google Scholar]
- 110.Marek K, Chowdhury S, Siderowf A, et al. The Parkinson’s progression markers initiative (PPMI)—establishing a PD biomarker cohort. Ann Clin Transl Neurol 2018; 5: 1460–1477. doi: 10.1002/acn3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mantri S, Wood S, Duda JEet al. et al. Comparing self-reported and objective monitoring of physical activity in Parkinson disease. Parkinsonism Relat Disord 2019; 67: 56–59. doi: 10.1016/j.parkreldis.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 112.Knell G, Gabriel KP, Businelle MS, et al. Ecological momentary assessment of physical activity: validation study. JMIR 2017; 19: e7602. doi: 10.2196/jmir.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Colombo D, Suso-Ribera C, Fernandez-Álvarez J, et al. Exploring affect recall bias and the impact of mild depressive symptoms: an ecological momentary study. In: Pervasive Computing Paradigms for Mental Health: 9th International Conference, MindCare 2019, Buenos Aires, Argentina April 23-24, 2019, Proceedings 9 (pp.208–215).Cham, Switzerland: Springer. https://doi.org/10.1007/978-3-030-25872-6_17. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cre-10.1177_02692155241272967 for Relationships between physical activities performed under free-living conditions and non-motor symptoms in people with Parkinson's: A systematic review and meta-analysis by Amanda Still, Leigh Hale, Sarfaraz Alam, Meg E. Morris and Prasath Jayakaran in Clinical Rehabilitation