Abstract
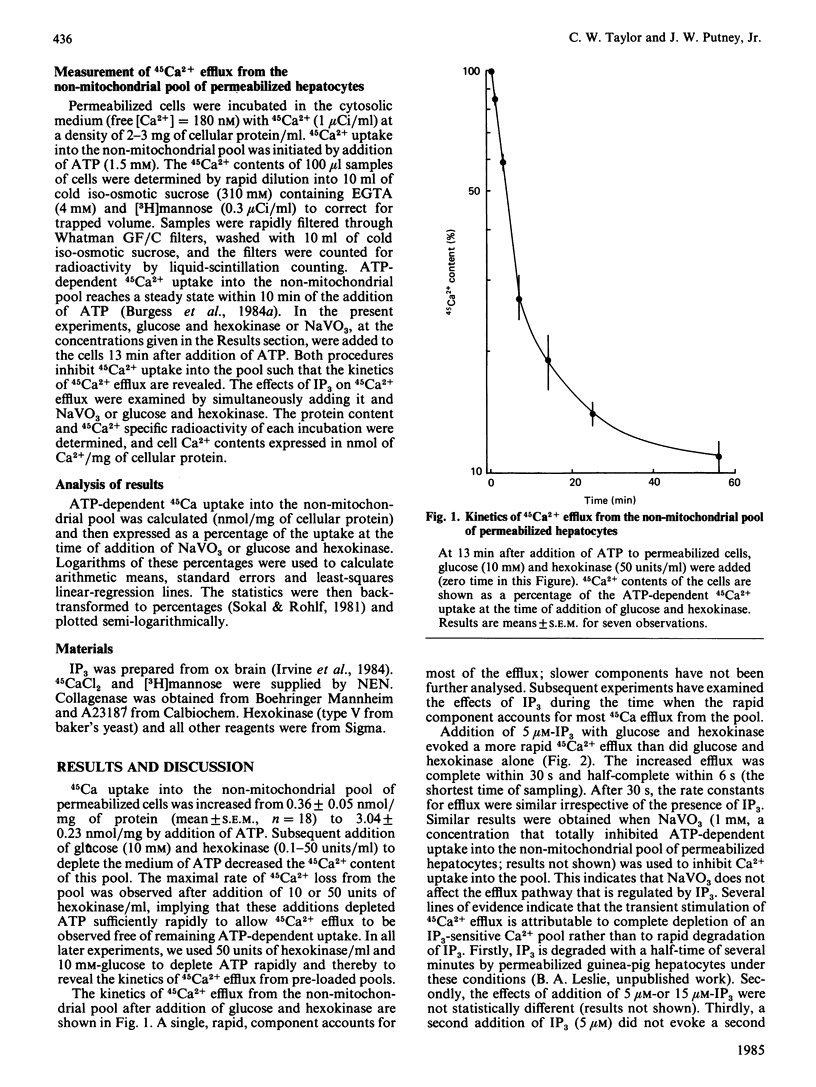
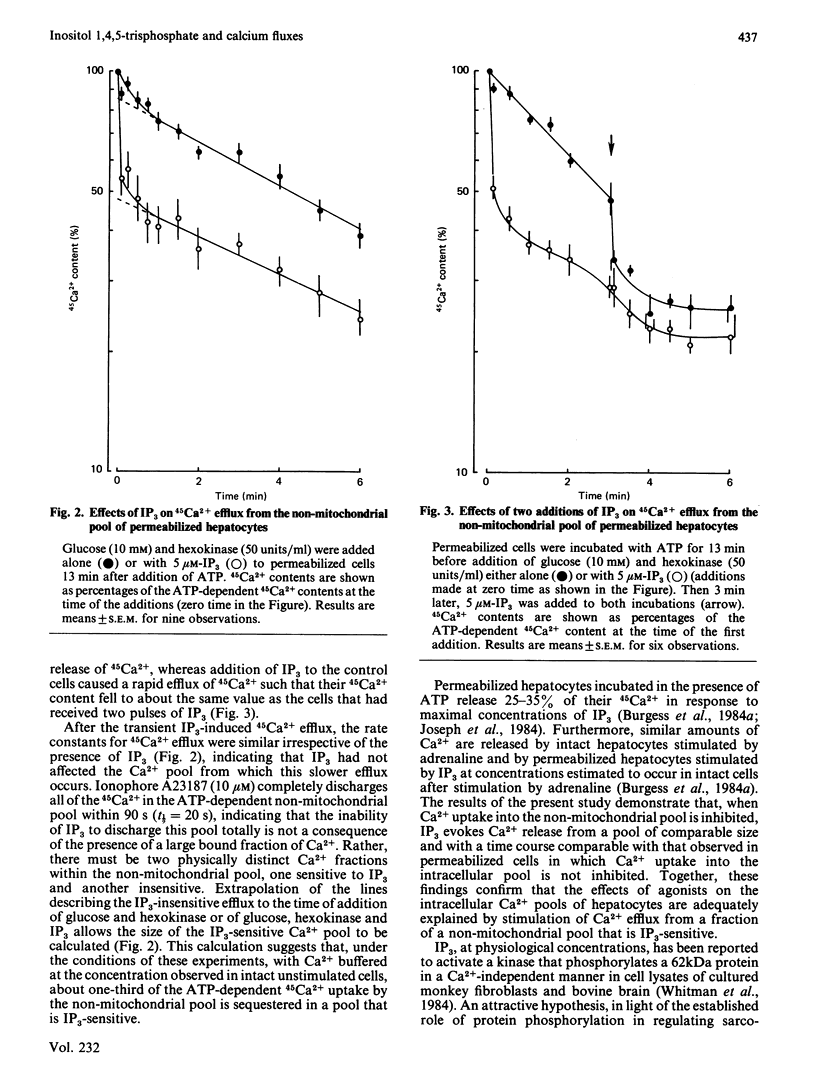
Permeabilized hepatocytes accumulated 45Ca2+ into a non-mitochondrial pool when provided with ATP. 45Ca2+ efflux from this pool was revealed by removal of ATP with glucose and hexokinase or by inhibiting uptake with NaVO3. The effect of inositol 1,4,5-trisphosphate (IP3) on 45Ca2+ efflux from the pool was investigated. IP3 (5 microM) evoked a rapid increase in the rate of 45Ca2+ efflux. Kinetic analysis of the effect of IP3 indicated the existence of two distinct Ca2+ fractions within the pool; only one, accounting for about one-third of the ATP-dependent Ca2+ content of the pool, was responsive to IP3. The effect of IP3 on 45Ca2+ efflux from the non-mitochondrial pool does not require ATP, a finding that is inconsistent with a previous suggestion that this effect may be mediated by protein phosphorylation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aub D. L., McKinney J. S., Putney J. W., Jr Nature of the receptor-regulated calcium pool in the rat parotid gland. J Physiol. 1982 Oct;331:557–565. doi: 10.1113/jphysiol.1982.sp014391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Biden T. J., Prentki M., Irvine R. F., Berridge M. J., Wollheim C. B. Inositol 1,4,5-trisphosphate mobilizes intracellular Ca2+ from permeabilized insulin-secreting cells. Biochem J. 1984 Oct 15;223(2):467–473. doi: 10.1042/bj2230467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., Claret M., Jenkinson D. H. Effects of quinine and apamin on the calcium-dependent potassium permeability of mammalian hepatocytes and red cells. J Physiol. 1981 Aug;317:67–90. doi: 10.1113/jphysiol.1981.sp013814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., Godfrey P. P., McKinney J. S., Berridge M. J., Irvine R. F., Putney J. W., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984 May 3;309(5963):63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Irvine R. F., Berridge M. J., McKinney J. S., Putney J. W., Jr Actions of inositol phosphates on Ca2+ pools in guinea-pig hepatocytes. Biochem J. 1984 Dec 15;224(3):741–746. doi: 10.1042/bj2240741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Fabiato A., Leslie B. A., Putney J. W., Jr Calcium pools in saponin-permeabilized guinea pig hepatocytes. J Biol Chem. 1983 Dec 25;258(24):15336–15345. [PubMed] [Google Scholar]
- Casteels R., Droogmans G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells or rabbit ear artery. J Physiol. 1981 Aug;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P., Irvine R. F. Inositol (1,4,5)trisphosphate-promoted Ca2+ release from microsomal fractions of rat liver. Biochem Biophys Res Commun. 1984 May 16;120(3):858–864. doi: 10.1016/s0006-291x(84)80186-2. [DOI] [PubMed] [Google Scholar]
- DeWitt L. M., Putney J. W., Jr Alpha-adrenergic stimulation of potassium efflux in guinea-pig hepatocytes may involve calcium influx and calcium release. J Physiol. 1984 Jan;346:395–407. doi: 10.1113/jphysiol.1984.sp015030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H. Mechanisms involved in alpha-adrenergic phenomena: role of calcium ions in actions of catecholamines in liver and other tissues. Am J Physiol. 1980 Jan;238(1):E3–12. doi: 10.1152/ajpendo.1980.238.1.E3. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Brown K. D., Berridge M. J. Specificity of inositol trisphosphate-induced calcium release from permeabilized Swiss-mouse 3T3 cells. Biochem J. 1984 Aug 15;222(1):269–272. doi: 10.1042/bj2220269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Le Peuch C. J., Haiech J., Demaille J. G. Concerted regulation of cardiac sarcoplasmic reticulum calcium transport by cyclic adenosine monophosphate dependent and calcium--calmodulin-dependent phosphorylations. Biochemistry. 1979 Nov 13;18(23):5150–5157. doi: 10.1021/bi00590a019. [DOI] [PubMed] [Google Scholar]
- Poggioli J., Putney J. W., Jr Net calcium fluxes in rat parotid acinar cells: evidence for a hormone-sensitive calcium pool in or near the plasma membrane. Pflugers Arch. 1982 Jan;392(3):239–243. doi: 10.1007/BF00584303. [DOI] [PubMed] [Google Scholar]
- Prentki M., Biden T. J., Janjic D., Irvine R. F., Berridge M. J., Wollheim C. B. Rapid mobilization of Ca2+ from rat insulinoma microsomes by inositol-1,4,5-trisphosphate. Nature. 1984 Jun 7;309(5968):562–564. doi: 10.1038/309562a0. [DOI] [PubMed] [Google Scholar]
- Prentki M., Wollheim C. B., Lew P. D. Ca2+ homeostasis in permeabilized human neutrophils. Characterization of Ca2+-sequestering pools and the action of inositol 1,4,5-triphosphate. J Biol Chem. 1984 Nov 25;259(22):13777–13782. [PubMed] [Google Scholar]
- Streb H., Bayerdörffer E., Haase W., Irvine R. F., Schulz I. Effect of inositol-1,4,5-trisphosphate on isolated subcellular fractions of rat pancreas. J Membr Biol. 1984;81(3):241–253. doi: 10.1007/BF01868717. [DOI] [PubMed] [Google Scholar]
- Whitman M. R., Epstein J., Cantley L. Inositol 1,4,5-trisphosphate stimulates phosphorylation of a 62,000-dalton protein in monkey fibroblast and bovine brain cell lysates. J Biol Chem. 1984 Nov 25;259(22):13652–13655. [PubMed] [Google Scholar]


