Abstract
Background
Secondary autoimmune disease (SAID) in the context of alemtuzumab treatment is one of the main safety concerns that may arise following administration in people with multiple sclerosis (pwMS). Contributing factors underlying this adverse event are not well understood. The purpose of this systematic review was to appraise the literature investigating the role of alemtuzumab in the development of SAID in pwMS following treatment and identify potential biomarkers/ risk factors that may be predictive of onset of this manifestation.
Methods
Relevant publications were retrieved from PubMed, Embase, and Web of Science using a three-pronged search strategy containing the following keywords: “multiple sclerosis”; “alemtuzumab”; and “autoimmunity”. Studies that fulfilled the specified eligibility criteria and investigated SAID development after alemtuzumab in pwMS were included in the final analysis.
Results
19 papers were included in the final review. Approximately, 47.92% of pwMS treated with alemtuzumab experienced SAID. A variety of biomarkers and risk factors were noted in the development of SAID, with a focus on immunological changes, including: increased homeostatic proliferation and T cell cycling, along with consistently elevated baseline serum IL-21 levels and thyroid autoantibodies. There was no significant association between known human leukocyte antigen (HLA) risk alleles, lymphocyte profile or dynamics and SAID development.
Conclusions
While the mechanism underlying SAID following alemtuzumab is not fully understood, potential biomarkers and risk factors that may assist in elucidating mechanisms underlying this phenomenon have been documented in several independent studies. Following immunodepletion from alemtuzumab, an IL-21 driven increase in homeostatic proliferation and T cell cycling may disrupt tolerance mechanisms leading to an increase in the propensity toward alemtuzumab-induced autoimmunity. Further research is necessary to clarify the physiological changes after alemtuzumab therapy that trigger SAID in pwMS.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12974-024-03263-9.
Keywords: Multiple Sclerosis, Alemtuzumab, Secondary autoimmune disease
Introduction
Multiple sclerosis (MS) is a chronic autoimmune disorder that affects the central nervous system (CNS) and is one of the leading causes of disability in young adults [1]. In MS, self-reactive B and T lymphocytes cross an impaired blood–brain barrier (BBB) to enter the CNS and attack the myelin sheath, leading to axonal loss and neuronal degeneration [2, 3]. The neuroinflammation caused by a self-reactive immune system leads to the appearance of demyelinating plaques, leading to a variety of motor, sensory, and cognitive symptoms [4]. MS can lead to progressive nerve damage and increased disability when left untreated. Whilst available treatments for MS can reduce relapse frequency and slow disease progression, MS remains incurable. Disease modifying therapies (DMT), such as alemtuzumab (Lemtrada®) offer strong therapeutic promise for treating MS by targeting specific immune cells.
The European Medicines Agency and the United States Food and Drug Administration have approved the use of the anti-CD52 monoclonal antibody, alemtuzumab, in highly active forms of MS [5]. Alemtuzumab is administered as a pulsed immune reconstitution therapy over 5 days during the first year, followed by a 3-day cycle, 12 months after the first infusion. The rapid and profound lymphodepletion caused by alemtuzumab is followed by repletion of immune cells skewed towards a more anti-inflammatory phenotype, and expansion of regulatory T cells with enhanced suppressive function [6]. Alemtuzumab decreased the risk of relapse by 49–74% and significantly reduced the rate of sustained disability progression across multiple trials including CAMMS223 (hazard ratio: 0.29) and CARE-MS II (hazard ratio: 0.58) compared to interferon beta‐1a [7]. Unlike conventional therapies that require regular administration, alemtuzumab’s unique mechanism of action provides pwMS durable therapeutic protection without the need for ongoing treatment [8]. The convenience of alemtuzumab’s dose regime increases treatment compliance and simplifies overall disease management [9]. Additionally, the two-course administration regime offers a cost-effective alternative when compared to other DMTs that require ongoing administration for many years.
While alemtuzumab is currently one of the most effective therapies to halt disease progression in MS, the high incidence of SAID following treatment limits its use in practice [9]. This phenomenon has also been described in other conditions following alemtuzumab treatment including vasculitis [10], and haematological conditions such as chronic lymphocytic leukaemia [11]. However, this uniquely occurs at a much higher frequency in pwMS [12]. SAID has been observed in around 40–50% of pwMS treated with alemtuzumab and most commonly include, but are not limited to, autoimmune thyroid disease (AITD, 16.7–41%), immune thrombocytopenic purpura (2%) and autoimmune kidney disease (0.2%) [13–16]. Despite B-cell driven autoimmune conditions being most frequently described in the literature, the development of T-cell-mediated autoimmune diseases such as vitiligo and alopecia have also been reported following alemtuzumab treatment in pwMS [17–19]. Onset of these conditions is delayed, peaking between 2–3 years post first infusion and is monitored alongside other adverse effects for up to five years through frequent monthly urine and blood tests [8, 20]. Although ongoing monitoring over such a significant time period is intensive, the implementation of accessible online support programs such as Bloodwatch® in Australia assists in retaining compliance [20].
While there are various studies published on this topic, it is still not fully understood how alemtuzumab has proved to be effective in mitigating one immune-mediated disease, while increasing the risk of developing a secondary one [21]. Further research is needed to clarify the main drivers contributing to susceptibility to autoimmunity secondary to alemtuzumab in MS. This may also shed light on the mechanisms of autoimmunity more broadly. Therefore, the aim of this systematic review was to compile and appraise literature investigating SAID development following alemtuzumab for the treatment of MS. This may assist in elucidating the mechanisms underlying this manifestation, to inform safety risks, as well as highlight potential therapeutic candidates to counteract this adverse outcome and improve the overall risk:benefit ratio of alemtuzumab [22].
Methods
Literature search
This systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) and Cochrane review guidelines [23, 24]. The protocol was prospectively compared with other published and unpublished systematic reviews to assess duplication using the PROSPERO database (University of York, UK) and was registered to the server (ID: CRD42023445973). The literature search was initially conducted on the 24th of May 2023 and a subsequent and final search was conducted on 26th July 2024. The online databases PubMed, Embase, and Web of Science were systematically searched for articles containing ‘multiple sclerosis’ AND ‘alemtuzumab’ AND ‘secondary autoimmune disease’ keywords (full search code can be found in Additional file 1). The Boolean operator “AND” was used to combine the terms and “OR” was used to broaden the search to include all relevant terms including Medical Subject Headings (MeSH). These terms were confined to abstract and title using [abstract/title] filters. An English language filter was applied. No date filter was used to ensure inclusion of all relevant studies since the first reported use of alemtuzumab for MS treatment in 1991. To ensure that all relevant publications were included a reference list search of all included publications was also conducted.
Eligibility criteria
Studies were included if they fulfilled the following inclusion criteria: (1) peer-reviewed, full-text studies published in English; (2) studies of people diagnosed with MS who received alemtuzumab as a first- or second-line therapy, who did or did not develop secondary autoimmunity; (3) and original research articles that investigated predictive biomarkers and/or risk factors of alemtuzumab-associated secondary autoimmunity in pwMS. Studies were excluded if they investigated diseases other than MS or if they did not examine potential biomarkers of alemtuzumab-induced SAID in MS.
Study selection
Endnote v20 (Endnote®, Clarivate, Philadelphia, USA) was used to sort and store all articles retrieved from the databases. Duplicates were removed using Endnote’s automated removal tool and any other duplicates that were not detected through the automated process were manually removed. Abstract and full-text screening were conducted independently by SJS and RM and all differences were compared and adjudicated through discussion. All included publications were validated by the remaining authors.
Data extraction
The following data was manually extracted from each of the publications: (1) author and year of publication; (2) study type; (3) location; (4) recruitment; (5) study enrolment; (6) sample size; (7) age; (8) sex (proportion female); (9) diagnosis; (10) treatment; (11) follow-up period; (12) SAID incidence; (13) time from first dose of alemtuzumab to SAID onset; (14) EDSS scores; (15) markers/predictors of SAID; (16) analysis method; (17) and findings.
Quality analysis
The Joanna Briggs Institute Critical Appraisal checklists were used to assess the quality and bias of the included studies [25–27]. The quality analysis was conducted independently by SJS and RM and discrepancies in the quality analysis were resolved by discussion. Any further discrepancies that were not resolved by discussion were independently assessed by a third reviewer to reach a consensus (NEF). The results from this analysis were reviewed and validated by all remaining authors. No studies were excluded based on the results from this analysis.
Results
A total of 1,157 records were retrieved from PubMed (n = 274), Embase (n = 606), and Web of Science (n = 277). After de-duplication and initial title and abstract screening, 80 full-text articles were assessed for eligibility based on specific inclusion and exclusion criteria. A total of 19 articles were included in the final review [6, 8, 12, 20, 21, 28–41]. This full process is outlined in Fig. 1.
Fig. 1.
PRISMA 2020 flow diagram of systematic search investigating the mechanisms underlying SAID development following Alemtuzumab in pwMS
Participant and study characteristics
Study and participant information, as well as summarised findings for each of the studies are reported in additional file 2. Out of the 19 included studies: 6 were case–control studies [20, 21, 28, 32, 34, 38], 11 were cohort studies [6, 8, 12, 31, 33, 35–37, 39–41]. The remaining two studies included an open-label study [30] and a randomised control trial (RCT) [29]. Almost all included studies were conducted longitudinally [6, 8, 12, 28–31, 33–41], except one which was a cross-sectional study [20] and two studies that incorporated a mixed longitudinal and cross-sectional design [21, 32]. Seven of the studies were add-on mechanistic sub-studies for larger clinical trials [6, 8, 21, 32, 35, 36, 40].
Across the included studies, there were a total of 2,236 participants. Due to variability in subgrouping and data format aggregate participant characteristics (e.g. age and sex) means were not calculated. Raw data is presented in Additional file 2. Almost all included participants were diagnosed with relapsing–remitting MS (RRMS) [6, 8, 12, 20, 21, 28, 29, 32–41], except for two studies that recruited people with progressive MS [30, 31]. All participants were treated with a minimum dose of alemtuzumab of 96 mg except one study, where one participant received a total of 60 mg [31]. Further cycles were administered, as needed, to those who experienced a further relapse. The follow-up duration following alemtuzumab across the studies ranged from 2 to 12 years [6, 8, 12, 20, 28, 29, 31, 33–37, 39–41]. Approximately 952.13 (47.92%) pwMS who were treated with alemtuzumab went on to develop SAID. Only 11 studies reported the time of onset of SAID from the first infusion [6, 12, 28–30, 33–37, 41]. Time of onset varied between and across the studies, ranging from 6 to 123 months.
Immunological, genetic, endocrine, and neurological markers were measured across the studies, and outcomes have been simplified in Table 1. A variety of different techniques were used across the studies to measure these markers. The most frequently used was flow cytometry which was incorporated in 11 studies either in isolation or in combination with other techniques [6, 8, 21, 29–32, 38–41]. Five studies specifically used the specialised flow cytometry technique fluorescence-activated cell sorting (FACS) [6, 8, 32, 38–40]. PCR was also used in four studies [21, 30, 32, 33]. In the four studies that measured baseline thyroid autoantibodies, two utilised electrochemiluminescence [34, 35], one utilised ELISA [36], while the remaining study utilised an in-house luciferase assay in combination with flow cytometry [41]. Two studies exclusively analysed clinical data including routine blood tests and MRI [12, 20].
Table 1.
Summarised results of potential markers for SAID following Alemtuzumab treatment in PwMS
| Markers | Finding (refs.) |
|---|---|
| Immunological markers | |
| Rate of lymphocyte reconstitution | NS [21, 31, 32] |
| Lymphocyte reconstitution/immune repertoire changes |
NS |
| T cell homeostatic proliferation | ↑[8, 32] |
| T cell cycling | ↑ [21] |
| Fas-mediated apoptosis of T-cells | ↑ [21] |
| Total B and T cell levels | ↑ [39] |
| Ratio of CD4 + CD45RO:CD45R | ↓ [30] |
| Innate immune cell levels | NS [39] |
| Serum IL-21 | ↑ [21, 28] |
| IgG4 | ↑ [37] |
| Genetic markers | |
| FCGR3A and FCGR2A allele frequencies | NS [33] |
| Overexpression of thyroid autoimmunity HLA-II risk alleles | NS [30] |
| rs13151961 A/A, rs6822844 G/G, rs6840978 C/C risk alleles | NS [21] |
| Endocrine markers | |
| Vitamin D levels | NS [20] |
| Thyroid autoantibodies (anti-TG and/or anti-TPO and/or anti-TRAb) at baseline | ↑ [34–36, 41] |
| Neurological markers | |
| Brainstem involvement | S [12] |
| Lifestyle markers | |
| Smoking | S [36] |
| NS [34] | |
FCGR Fc gamma receptor, IL Interleukin, HLA Human Leukocyte Antigens, Ref Reference, Tg Thyroglobulin, TPO Thyroid Peroxidase
↑ indicates significantly elevated levels of markers in SAID
↓ indicates significantly reduced levels of markers in SAID
NS represents no significant change between SAID and no secondary autoimmune disease (NSAID)
S represents a significant change in SAID compared to NSAID
Immunological markers for SAID following alemtuzumab treatment for MS
Eleven studies investigated immune involvement in the development of SAID following alemtuzumab for MS [6, 8, 21, 28, 29, 31, 32, 37–40]. The rate of lymphocyte repopulation was investigated in three studies [21, 31, 32]. These studies found no association between the rate of lymphocyte repopulation after alemtuzumab therapy and the development of SAID. Eight studies found no link between the immune repertoire profile changes during the immune reconstitution stage after alemtuzumab therapy and the development of SAID [6, 8, 21, 31, 32, 37, 38, 40]. One study found elevated levels of total T and B lymphocytes, CD4 memory and naive B lymphocytes in pwMS with SAID after alemtuzumab [39]. However, this study found no involvement of innate immune cells and regulatory T cells in those developing SAID. Another study identified a lower CD45RO:CD45RA ratio in participants that developed Grave’s disease (GD) (p < 0.05)[30]. CD8 counts were also significantly higher in pwMS that went on to develop alemtuzumab-associated GD. Two studies reported an association between the occurrence of SAID and the reconstitution of lymphocytes by homeostatic proliferation processes rather than thymopoiesis [8, 32]. One study demonstrated this mechanism through determining clonal repertoire using two distinct methods: spectratyping and sequencing [32]. These techniques validated that pwMS that develop SAID had greater clonal restriction compared to NSAID (p < 0.05). The other study identified elevated levels of baseline hyperexpanded T cell clones in SAID (CD8 + : 10–55%, CD4: 0–5%) compared to NSAID (CD8 + : 0%-25%, CD4 + : not detected, p < 0.05). SAID development was associated with a greater expansion of persisting CD4 + and CD8 + T- cell clones (p < 0.05). Additionally, one study reported an increase in apoptotic cell death in SAID (unstimulated: 14.4%, Fas-mediated: 32.1%, TSHr: 25.5%) compared to NSAID (unstimulated: 4.7%, Fas-mediated, 18.32% TSHr: 9.5%, p < 0.01 for all comparisons). This study also identified an association between IL-21 and increased proliferation of CD8 + (proliferation index, IL-21-: 11.59 vs. IL-21 + : 19.39, p = 0.012) [21]. Another study did not explicitly investigate immune profile changes, they reported an inverse relationship between the administration of Palifermin therapy and thymopoiesis including a reduction in CD4 + T cells in palifermin-treated (2.229 × 107) compared to untreated (7.733 × 107) on alemtuzumab (p = 0.007) [29]. However, there was no significant difference in the incidence of autoimmunity in alemtuzumab-treated pwMS between the palifermin and placebo arms [29].
Two studies investigated baseline levels of the cytokine IL-21 in those with SAID compared to those without [21, 28]. One of the studies found elevated baseline levels of serum IL-21 in those who went on to develop alemtuzumab-induced SAID (430 pg/mL) compared to those who did not (206 pg/mL) and heathy controls (213 pg/mL)[21]. Another study corroborated these findings, using the redundant eBioscience Ready-Set-Go ELISA capture antibody 3A3-N2, detection antibody 2B2-G20 kit whereby there was a two-fold difference in baseline IL-21 levels in those who developed SAID (542.4 ± 91.3 pg/mL) compared to pwMS who did not (222.5 ± 32.8 pg/mL) (p < 0.001) [28]. IgG4 levels were measured in one study. This study reported significantly higher levels of IgG4 in those with SAID, irrespective of the timing of alemtuzumab administration [37]. Diagnostic potential of IgG4 in determining pwMS that will go onto develop SAID following alemtuzumab was assessed using receiver operator characteristics (ROC) analysis where the area under the curve was calculated as 0.7909 (95% CI 0.6199–0.9618) [37].
Genetic, endocrine, and neurological markers for SAID following alemtuzumab treatment for MS
Three studies investigated genetic predispositions to SAID secondary to alemtuzumab treatment in MS [21, 30, 33]. One study found that FCGR allelic variants, known to contribute to the efficacy of depleting antibodies, were not associated with the development of SAID [33]. Another study found no association between the overexpression of HLA-II and the appearance of autoimmune thyroid disease [30]. The remaining study found that the single nucleotide polymorphisms (SNPs): rs13151961 A/A (Gasdermin B gene, chromosome 17q12, p = 0.0076), rs6822844 G/G (IL-2/IL-21 gene, Chromosome 4q27, p = 0.0098), rs6840978 C/C (protein Tyrosine Phosphatase, Non-Receptor Type 2, chromosome 18p11.21, p = 0.0067) correlated with elevated levels of IL-21 in alemtuzumab-treated pwMS. While there were trends observed between rs13151961 A/A and SAID development in pwMS these did not reach significance [21].
Five studies investigated potential endocrine markers to differentiate pwMS that did or did not develop SAID following alemtuzumab treatment [20, 34–36]. One study investigated the association between baseline vitamin D levels and SAID development post alemtuzumab treatment but found no significance [20]. Four studies investigated baseline levels of anti-thyroid antibodies between SAID and NSAID subjects [34–36]. All these studies reported a link between the increased baseline levels of anti-thyroid antibodies and the increased risk of secondary autoimmunity [20, 34–36]. One of these studies identified that TRAbs (7.3 IU/L offer diagnostic potential for predicting onset of GD following alemtuzumab (AUC: 0.893, p = 0.0001) [34]. The diagnostic potential of TRAbs was also corroborated by another study that found that pre-treatment TRAbs detected using in-house flow cytometry and luciferase assays (ihTRAb) were present in 31.2% of pwMS that went on to develop AITD following alemtuzumab treatment compared to 0% in NAITD (p = 0.017). ihTRAb preceded AITD development by a median of 1.2 years (28 days–7.3 years) in 32.1% of alemtuzumab-treated pwMS [41]. The remaining study showed an association between onset of thyroid autoimmunity and combined anti-TGO and anti-TG (Hazard ratio: 12.15, 95% CI 95% CI 4.73–31.2)[35].
One study investigated neurological involvement in the development of SAID following alemtuzumab treatment for MS. This study found an association between brainstem involvement in pwMS at disease onset and a higher risk of developing symptomatic GD [12]. Brainstem involvement at onset of MS was associated with a relative risk (RR) for developing symptomatic GD of 11.1 (95% CI 1.4–86.2, p = 0.01), and 3 × more likely to develop TRAb (RR = 3.3, 95% CI 1.1–9.9, p = 0.05) following alemtuzumab treatment. Brainstem involvement was present in 83.3% of pwMS that had symptomatic GD following alemtuzumab treatment [12].
The presence of autoimmune conditions other than MS at baseline and the risk of developing alemtuzumab-induced AITD was also studied, with no association being found [36]. This study did report a link between current and previous smoking status and risk of AITD development. This finding was not corroborated by an earlier study [34]. This study also found no association between family history of thyroid disease and onset of AITDs.
Quality analysis
The quality and bias analysis is presented in Additional file 3. The JBI checklist for case–control [26], RCT [26], cohort [27], and quasi-experimental [25] studies were selected on the basis of individual study design. Quality and potential bias varied across the studies [6, 8, 12, 20, 21, 28–41]. In almost all cases except two (88.9%), standard and validated methods were used to investigate the mechanisms of SAID following alemtuzumab treatment in MS. Statistical quality (Case–control 60%; RCT 50%; Cohort 36.4%) was inconsistent. Majority of the studies reported confounding variables (Case–control 100%; Cohort 90.9%) and provided methods to mitigate them (Case–control 83%; Cohort 90%). Providing defined criteria for cases and controls was also only fulfilled in 60% of the studies. In case–control studies, exposures were only measured in three studies. Two of these studies provided information on the methods used to measure the exposure and whether it was consistent between cases and controls [20, 28]. In the RCT study, information on the placebo constituents and randomisation methods used were provided [29]. While outcome assessors were blinded, information of blinding of participants and those delivering the treatment were not indicated [29]. The open label study addressed 70% of criteria [30]. Items pertaining to the reporting of follow up and whether appropriate statistics were used were not fulfilled. In the cohort studies the least addressed items pertained to providing information on follow up including whether follow up was complete (36.4%) and if there were strategies utilised to address incomplete follow up (0%) [6, 8, 12, 31, 33, 35–37, 39–41]. Nine (81.8%) cohort studies had provided a sufficient follow up time for outcomes to occur [6, 8, 12, 31, 33, 35–37, 39, 41].
Discussion
Although comprehensive reviews on the mechanism of action of alemtuzumab in MS as well as the incidence of adverse events have been previously published [42], there is limited knowledge regarding the processes by which alemtuzumab leads to secondary autoimmunity in MS. This systematic review investigated the role of alemtuzumab in the development of secondary autoimmunity in pwMS. This review aims to highlight possible biomarkers and risk factors and elucidate underlying mechanisms that may lead to the development of this adverse event.
This review primarily included pwMS diagnosed with RRMS due to the known efficacy of alemtuzumab in this subtype [6, 8, 12, 20, 21, 28, 29, 32–41]. Additionally, two early studies recruited participants diagnosed with progressive MS [30, 31]. However, there was no evidence of benefit for alemtuzumab in reducing disability progression in progressive MS [43]. It has been suggested that the limited efficacy of alemtuzumab in progressive MS could be due to this subtype arising from prior lesion accumulation and neurodegeneration rather than inflammation which is the main target for alemtuzumab therapy. Consequently, later studies only included people with RRMS [6, 8, 12, 20, 21, 28, 29, 32–41].
Potential biomarkers of SAID following alemtuzumab treatment were investigated using a variety of different methods across all the studies including ELISA, PCR, and flow cytometry. Flow cytometry was the most consistent technique used [6, 8, 21, 29–32, 38–41]. Flow cytometry is regarded as the gold standard technique for immune cell quantification due to its sensitivity and ability to provide in-depth analysis of different heterogeneous cellular populations [44]. While other techniques lack single-cell discrimination, flow cytometry has cell-specific resolution. Additionally, flow cytometry can be high throughput and is widely accessible both in a benchside and clinical diagnostic setting therefore emphasising its utility in investigating alemtuzumab-induced immune changes in pwMS [44, 45].
Across the studies, approximately 47.92% of people with MS went on to develop SAID following alemtuzumab treatment. It is important to note the variations in criteria used to define SAID across the studies that may influence this proportion [6, 8, 12, 20, 21, 28–41]. However, this value closely aligns with previously published data that reports that an estimated 40% of alemtuzumab-treated MS patients will develop SAID. Onset time of SAID following the first infusion of alemtuzumab ranged widely from 6 months to 10 years [6, 8, 12, 20, 21, 28–41]. This variability is typical as the prior literature reports that alemtuzumab-induced autoimmunity occurs from 6 months up to 12 years, peaking in years 2 and 3 and declining afterwards [46]. Overall, this systematic review corroborates previously reported findings demonstrating a delay in onset of SAID following alemtuzumab treatment with the earliest cases being seen at least six months after initial treatment [29, 30, 33]. There is currently limited understanding on the cause of this delay, highlighting the need for further research to better understand the factors contributing to this pattern in the onset of alemtuzumab-induced SAID [8].
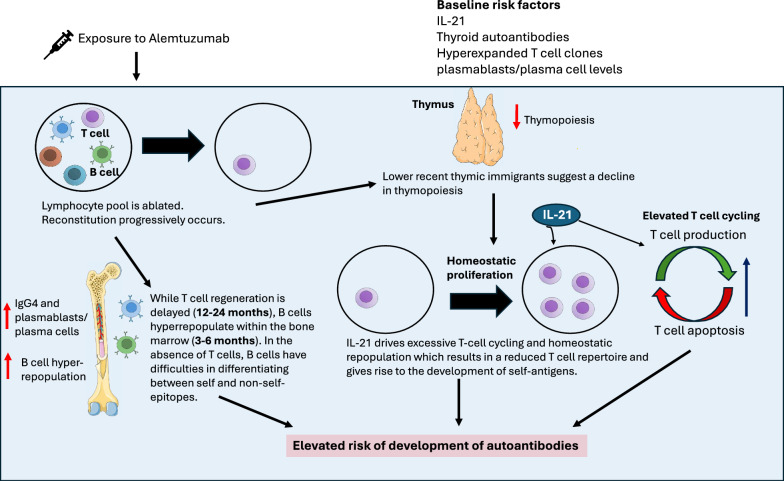
Contrary to previous speculation on the cause of SAID following alemtuzumab treatment in pwMS, there was no consistent evidence of an association between lymphocyte repopulation dynamics or profile changes and SAID [6, 8, 21, 31, 32, 37, 38, 40]. An interplay between increased homeostatic proliferation and IL-21 dependent T cell cycling has been highlighted as a potential driving force of SAID in pwMS following alemtuzumab across the studies [8, 21, 28, 32]. This postulated pathway is illustrated in Fig. 2. A single risk factor may not be sufficient to result in manifestation of the secondary autoimmune condition, but rather, an accumulation of these factors lead to its onset [21]. Following lymphocyte depletion by alemtuzumab, IL-21-driven suppression of thymic output may instead result in repopulation of T cells via homeostatic proliferation; a process that leads to expansion of lymphocytes that escaped alemtuzumab depletion [8, 32]. This causes a restricted TCR repertoire and allows for the expansion of autoreactive T lymphocytes, increasing the risk of SAID following alemtuzumab treatment in pwMS.
Fig. 2.
Postulated mechanisms underlying SAID development following Alemtuzumab treatment in pwMS. Following exposure to alemtuzumab, the lymphocyte pool is ablated, and reconstitution progressively occurs. There is evidence of both T and B cell involvement in SAID development. T cell repopulation occurs predominantly via homeostatic repopulation due to a decline in thymic function, as suggested by lower recent thymic immigrants. Increased T cell cycling is also evident. Both these processes are postulated to be IL-21 driven. An accumulation of these risk factors results in a reduced T cell repertoire and gives rise to the development of self-antigens. While T cell regeneration takes approximately 12–24 months, B cells hyperrepopulate within the bone marrow. In the absence of T cells, B cells have difficulties in differentiating between self and non-self-epitopes therefore also elevating the risk of the development of self-reactive antigens. This figure was designed in Microsoft PowerPoint 16 using some open sourced images from Servier Medical Art, https://smart.servier.com
Changes in baseline cytokine levels have also been correlated with an increased susceptibility to the onset of autoimmune events following alemtuzumab treatment [21, 28]. It has been postulated that higher levels of IL-21 increase T cell apoptosis and cell cycling in response to alemtuzumab leading to the development of alemtuzumab induced autoimmunity. Previously published literature also has indicated that IL-21 promotes homeostatic proliferation. It is important to note that IL-21’s use as a prognostic tool for the development of SAID is limited by the reduced predictive utility of commercially available ELISA kits. Moreover, IL-21 is a ubiquitous autoimmune marker for various autoimmune conditions including rheumatoid arthritis, inflammatory bowel disease and autoimmune diabetes, potentially limiting its specificity for alemtuzumab-induced SAID [28]. Additionally, a genetic study indicated a correlation with SNPs mapped to Gasdermin B (rs13151961 A/A), IL-2/IL-21 (rs6822844 G/G), protein Tyrosine Phosphatase, Non-Receptor Type 2 (rs6840978 C/C) genes and elevated IL-21 [21]. However, these risk alleles were not significantly associated with SAID. As many of the investigations into these risk-alleles and SAID susceptibility contain a relatively small sample size this limits the ability to make clear inferences [21, 30, 33]. Therefore, further large-scale investigations are required to fully understand the pathological impact of risk alleles in alemtuzumab-induced SAID.
There is currently no validated treatment to mitigate SAID following alemtuzumab for pwMS. Only one study in this review investigated potential therapeutic avenues for SAID. This study trialled the effect of keratinocyte growth factor, palifermin, that has shown improvements in thymopoiesis in animal studies. In human clinical trials, this treatment further impaired thymic recovery after alemtuzumab therapy, and there were no differences observed in incidence of SAID in palifermin-treated and placebo groups [29]. Vitamin D deficiency has been implicated as a risk factor in several autoimmune conditions, including autoimmune thyroid disease and MS [47–50]. As a result, concurrent vitamin D supplementation at optimal concentrations in combination with alemtuzumab has been postulated as a potential therapeutic avenue to reduce SAID development in MS [51]. A retrospective study in alemtuzumab-treated pwMS, found no association between vitamin D levels and risk of SAID [20]. However, as these results are purely observational, this does not fully infer the efficacy of vitamin D supplementation in alemtuzumab-induced SAID and further longitudinal based interventional studies are required for validation. While elevated baseline levels of IgG4 and total B cells, especially, plasmablasts, and plasma cells were reported risk factors [37, 39], there was limited emphasis overall on the role of B cells in SAID. As the incidence of antibody-mediated secondary autoimmune conditions is most prominent following alemtuzumab further understanding of B cell involvement in this pathomechanism is pivotal. An immune reconstitution analysis found that a marked hyperrepopulation of CD19+ B cells (180% increase) follows initial depletion (< 85%) post-treatment resulting in the rapid development of binding and neutralising antibodies against alemtuzumab [52]. However, this study was not included in the final analysis as the observations were made across all alemtuzumab-treated pwMS and were not specific for SAID. B cell repopulation also occurs significantly earlier (3–6 months) compared to T cell repopulation (12–24 months). T cells are involved in the B cell-mediated self-antigen differentiation process, therefore in their absence, B cells are unable to appropriately differentiate between self- and non-self epitopes further increasing the risk of developing self-reactive autoantibodies [15]. The chimeric anti-CD20 monoclonal antibody, rituximab, has been evaluated as a potential therapeutic candidate to mitigate B cell repopulation to synchronize the repopulation of B and T cell subsets reducing SAID risk. A pilot study using a low-dose of rituximab (50–150 mg/m2) has shown promising results in reducing alemtuzumab-induced SAID risk in pwMS, providing valuable insight for further exploration of anti-CD20 therapies that may potentially halt the development of SAID after alemtuzumab administration [15]. This study was also not included in this review due to the low number of enrolled participants. The active Phase II, placebo-controlled clinical trial, RAMBLE (Reducing the frequency of Autoimmune adverse events in the treatment of Multiple sclerosis with alemtuzumab using B-celL dEpletion) (ACTRN12621001502820) is designed to further assess the effectiveness and safety of B cell targeted therapy such as rituximab in mitigating autoimmunity following alemtuzumab therapy.
While none of the studies directly compared SAID risk following alemtuzumab with other immune reconstitution therapies such as autologous haematopoietic stem cell transplantation (AHSCT), SAID following AHSCT has been reported in published literature [53]. There is possible mechanistic overlap between AHSCT and alemtuzumab-related immune changes following treatments including a surge of regulatory T cells, and lymphocyte repopulation of naive T and B cells [8]. Homeostatic proliferation has also been described as a potential underlying mechanism in the development of SAID following both AHSCT and alemtuzumab. One study included in this review indirectly compared previously published data on SAID following AHSCT [54] with their alemtuzumab dataset [8]. In this analysis, the occurrence of SAID was found to be approximately 10 times higher in alemtuzumab-treated pwMS compared to AHSCT [8]. While direct comparisons into possible mechanisms underlying this difference is limited, incomplete CD4 + TCR receptor exchange 12 months post-alemtuzumab treatment has been shown while there is almost a complete repertoire renewal following AHSCT [8]. Independent studies have also reported a reduction in thymopoiesis in pwMS following alemtuzumab treatment, resulting in a reduced and more self-reactive TCR repertoire, meanwhile, extended thymopoiesis and greater clonal T cell diversity has been described following AHSCT [8, 32, 53]. Further research is warranted to better understand the factors underlying these differences.
Research in this area presents challenges due to the delay in the onset of alemtuzumab-induced SAID. This is generally mitigated through the incorporation of prolonged longitudinal studies of at least 5 years and possibly longer [6, 8, 12, 28–31, 33–41]. This also emphasises the need for early risk indicators for secondary autoimmunity development following alemtuzumab treatment of MS in contrast to markers that are observed post-administration to better equip clinicians to prospectively determine the safety profile prior to administration. Potential early-stage markers were described including baseline thyroid autoantibodies, hyperexpanded T cell clones, IL-21, IgG4, and Plasmablast/Plasma cells (PB/PC) levels [21, 28, 34–36, 39, 41]. Further diagnostic validation is required.
Reporting of possible confounding and/or risk factors for alemtuzumab-acquired SAID development in pwMS including previous use of DMT and smoking were limited across the studies. Ten studies stated if the participants were previously treated with another DMT but, in all but one [39], pwMS were not stratified to enable comparisons with those who did not have previous exposure to DMT [6, 20, 29, 33, 35–39]. Therefore, the compounding risk of developing alemtuzumab-associated SAID following previous DMT therapy could not be determined. There is currently limited understanding on the impact of previous DMT use and alemtuzumab-induced SAID risk. One study that did not meet eligibility criteria for inclusion did find that prior DMT use resulted in a significantly greater risk of SAID development [55]. Although the mechanism behind these differences has not been investigated it was suggested that the accumulated immune changes from prior immune therapies without sufficient refractory period resulted in further susceptibility to SAID. Additionally, there currently appears to be limited corroboration of the association between age and SAID risk [12, 34, 36]. Future research that investigates the effects of prior MS therapies, demographic factors, and lifestyle factors before alemtuzumab therapy might help to clarify whether they pose any additional risks of SAID development. This will allow for more informed clinical decision-making and observations in research.
Levels of quality and bias varied across the studies [6, 8, 12, 20, 21, 28–41]. No studies addressed all criteria. Key considerations for future studies to improve quality and reduce potential sources of bias in this field involve use of power-based sample sizes, appropriate selection of statistical tests based on distribution as well as adjusting for multiple comparisons to reduce the risk of type two errors. In RCTs, incorporation of multiple levels of blinding in addition to the outcome assessor including the participant and those who are delivering the treatment will ensure internal validity [29]. Moreover, providing clear information on loss of participants during follow up and possible methods to address incomplete follow up in cohort studies is required for a comprehensive and accurate representation of the cohort [6, 8, 12, 31, 33, 35–37, 39, 41].
Conclusions
This systematic review aimed to examine possible biomarkers and risk factors predictive of alemtuzumab-acquired SAID in pwMS based on appraisal of published literature. Multi-system changes observed across the studies emphasises the need for multidisciplinary collaboration between a variety of different clinical specialties for comprehensive management of alemtuzumab-induced SAID in pwMS [6, 8, 12, 20, 21, 28–40]. Immunological involvement in the development of alemtuzumab-associated SAID was most profound [6, 8, 21, 28, 29, 31, 32, 37–40] where IL-21-mediated homeostatic proliferation and T cell cycling was considered possible driving forces for alemtuzumab-associated SAID in pwMS [8, 21, 28, 32]. Additionally, reduced thymic function has been shown to reduce TCR clonality leading to the expansion of self-reactive T cells in alemtuzumab-induced SAID [8, 32]. Additionally, baseline thyroid autoantibodies and IL-21 levels are suggested to be possible early risk indicators for SAID development [8, 32, 34–36, 41]. These findings may assist in potentially identifying pwMS at higher risk of developing alemtuzumab-induced SAID and advising healthcare professionals on the optimal and safest MS treatment. Studies comparing findings after AHSCT and alemtuzumab, and after alemtuzumab in other disease states may be informative. Furthermore, understanding factors contributing to SAID development after alemtuzumab may allow treatment avenues to be explored to mitigate the development of this secondary condition and provide valuable insights into the causation of autoimmunity more broadly [29].
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- TG
Anti-thyroglobulin
- TPO
Anti-thyroid peroxidase
- AUC
Area under the curve
- AITD
Autoimmune thyroid disease
- AHSCT
Autologous haematopoietic stem cell transplantation
- CNS
Central nervous system
- CI
Confidence interval
- DMT
Disease-modifying therapies
- ELISA
Enzyme-linked immunosorbent assay
- FACS
Fluorescence-activated cell sorting
- FCGR
Fc gamma receptor
- GD
Grave's disease
- HLA
Human leukocyte antigen
- IL
Interleukin
- NSAID
No secondary autoimmune disease
- MeSH
Medical subject headings
- MRI
Magnetic resonance imaging
- MS
Multiple sclerosis
- pwMS
People with multiple sclerosis
- PB/PC
Plasmablast/plasma cells
- PCR
Polymerase chain reaction
- PRISMA
Preferred reporting items for systematic reviews and meta-analysis
- RCT
Randomised control trial
- ROC
Receiver operator characteristics
- RAMBLE
Reducing the frequency of autoimmune adverse events in the treatment of Multiple sclerosis with alemtuzumab using B-celL dEpletion
- Ref
Reference
- RRMS
Relapsing–remitting multiple sclerosis
- RR
Relative risk
- SAID
Secondary autoimmune disease
- TCR
T cell receptor
- TRAbs
Thyroid antibodies
- USA
United States of America
Author contributions
This study was conceptualised by SJS, SAB, NF, and SMG. Systematic review methodology was designed by SJS and RM and both authors independently conducted database searches and quality assessment. SJS and RM also extracted publication data with significant input from SAB on data presentation. SJS wrote the first draft of the manuscript with support from RM. NEF provided substantial feedback on early versions of the manuscript. All authors critically reviewed and approved the final version of the submission.
Funding
SJS is the recipient of a Postgraduate Scholarship from MS Australia (22-3-052). This research was also supported by The Disability and Rehabilitation Program, Griffith University.
Availability of data and materials
All data supporting the conclusions of this article are included within the article and its additional files.
Declarations
Ethics approval and consent to participate
This systematic review utilised existing published data and did not directly involve the recruitment of participants therefore no consent was required. The authors also advise that there are no other ethical conflicts relating to this research project to disclose.
Consent for publication
Not applicable.
Competing interests
The authors wish to declare that SJS and SAB are investigators on the RAMBLE trial (ACTRN12621001502820). The RAMBLE study has been funded by MS Australia (Project Grant 21-2-060). SAB has received support through Griffith University in relation to service on advisory boards, conference organising committees and speakers honoraria from Novartis, Roche and Alexion, and has been a principal investigator on clinical trials sponsored by ATARA, Biogen-Idec, Sanofi and UCB. RM, NEF, and SMG declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jakimovski D, et al. Multiple sclerosis. Lancet. 2024;403(10422):183–202. [DOI] [PubMed] [Google Scholar]
- 2.Mey GM, Mahajan KR, DeSilva TM. Neurodegeneration in multiple sclerosis. WIREs Mech Dis. 2023;15(1):e1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaskow BJ, Baecher-Allan C. Effector T cells in multiple sclerosis. Cold Spring Harb Perspect Med. 2018. 10.1101/cshperspect.a029025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christogianni A, et al. Temperature sensitivity in multiple sclerosis: an overview of its impact on sensory and cognitive symptoms. Temperature (Austin). 2018;5(3):208–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riera R, Porfírio GJ, Torloni MR. Alemtuzumab for multiple sclerosis. Cochrane Database Syst Rev. 2016. 10.1002/14651858.CD011203.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilmore W, et al. Repopulation of T, B, and NK cells following alemtuzumab treatment in relapsing-remitting multiple sclerosis. J Neuroinflamm. 2020;17(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JW, Coles AJ. Alemtuzumab: evidence for its potential in relapsing-remitting multiple sclerosis. Drug Des Devel Ther. 2013;7:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruck T, et al. Alemtuzumab-induced immune phenotype and repertoire changes: implications for secondary autoimmunity. Brain. 2022;145(5):1711–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alroughani R, et al. The use of alemtuzumab in patients with relapsing-remitting multiple sclerosis: the Gulf perspective. Ther Adv Neurol Disord. 2020;13:1756286420954119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gopaluni S, et al. Alemtuzumab for refractory primary systemic vasculitis-a randomised controlled dose ranging clinical trial of efficacy and safety (ALEVIATE). Arthritis Res Ther. 2022;24(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodgson K, et al. Chronic lymphocytic leukemia and autoimmunity: a systematic review. Haematologica. 2011;96(5):752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yap SM, et al. Alemtuzumab-related thyroid disease in people with multiple sclerosis is associated with age and brainstem phenotype at disease onset. Multiple Scler J Exp Transl Clin. 2020; 10.1177/2055217320933928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuker A, et al. Immune thrombocytopenia in alemtuzumab-treated MS patients: incidence, detection, and management. Mult Scler J. 2020;26(1):48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leibowitz SM, et al. A case of relapsing anti-GBM disease secondary to alemtuzumab therapy. CEN Case Rep. 2024;13(3):209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meltzer E, et al. Mitigating alemtuzumab-associated autoimmunity in MS: a “whack-a-mole” B-cell depletion strategy. Neurol Neuroimmunol Neuroinflamm. 2020;7(6):e868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rotondi M, et al. Autoimmune thyroid diseases in patients treated with alemtuzumab for multiple sclerosis: an example of selective anti-TSH-receptor immune response. Front Endocrinol (Lausanne). 2017;8:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan JK, Traboulsee AL, Sayao AL. Case of alemtuzumab-related alopecia areata management in MS. Neurol Neuroimmunol Neuroinflamm. 2019;6(1):e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borriello G, Ianniello A, Toosy AT. Alopecia universalis occurring after alemtuzumab treatment for multiple sclerosis. A two-year follow-up of two patients. Int J Environ Res Public Health. 2021;18(14):7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruck T, et al. Vitiligo after alemtuzumab treatment: secondary autoimmunity is not all about B cells. Neurology. 2018;91(24):e2233–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnett S, et al. Low vitamin D levels do not predict risk of autoimmune disease following alemtuzumab treatment for multiple sclerosis. Multiple Scler Relat Disord. 2022. 10.1016/j.msard.2022.103511. [DOI] [PubMed] [Google Scholar]
- 21.Jones JL, et al. IL-21 drives secondary autoimmunity in patients with multiple sclerosis, following therapeutic lymphocyte depletion with alemtuzumab (Campath-1H). J Clin Investig. 2009;119(7):2052–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costa-Frossard França L, et al. Real-world retrospective analysis of alemtuzumab outcomes in relapsing-remitting multiple sclerosis: the LEMCAM study. CNS Drugs. 2024;38(3):231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cochrane handbook for systematic reviews of interventions version 6.4. In T.J. Higgins JPT, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Chichester, United Kingdom: Cochrane; 2023. Available from www.training.cochrane.org/handbook.
- 25.Barker TH, et al. The revised JBI critical appraisal tool for the assessment of risk of bias for quasi-experimental studies. JBI Evid Synth. 2024;22(3):378–88. [DOI] [PubMed] [Google Scholar]
- 26.Barker TH, et al. The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid Synth. 2023;21(3):494–506. [DOI] [PubMed] [Google Scholar]
- 27.Moola SMZ, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, Lisy K, Mu P-F. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris MZE, editor. JBI manual for evidence synthesis. Adelaide, South Australia: JBI; 2020. Available from: https://reviewersmanual.joannabriggs.org/.
- 28.Azzopardi L, et al. Predicting autoimmunity after alemtuzumab treatment of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2014;85(7):795–8. [DOI] [PubMed] [Google Scholar]
- 29.Coles AJ, et al. Keratinocyte growth factor impairs human thymic recovery from lymphopenia. JCI Insight. 2019. 10.1172/jci.insight.125377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coles AJ, et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet. 1999;354(9191):1691–5. [DOI] [PubMed] [Google Scholar]
- 31.Hill-Cawthorne GA, et al. Long term lymphocyte reconstitution after alemtuzumab treatment of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2012;83(3):298–304. [DOI] [PubMed] [Google Scholar]
- 32.Jones JL, et al. Human autoimmunity after lymphocyte depletion is caused by homeostatic T-cell proliferation. Proc Natl Acad Sci U S A. 2013;110(50):20200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller CW, et al. Impact of FcγR variants on the response to alemtuzumab in multiple sclerosis. Ann Clin Transl Neurol. 2019;6(12):2586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manso J, et al. Alemtuzumab-induced autoimmune thyroid events in patients with relapsing-remitting multiple sclerosis: a real-life and monocentric experience at a tertiary-level centre. Clin Endocrinol. 2022;97(3):331–8. [DOI] [PubMed] [Google Scholar]
- 35.Ruck T, et al. Pretreatment anti-thyroid autoantibodies indicate increased risk for thyroid autoimmunity secondary to alemtuzumab: a prospective cohort study. EBioMedicine. 2019;46:381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandgren S, et al. The role of autoimmune antibodies to predict secondary autoimmunity in patients with relapsing-remitting multiple sclerosis treated with alemtuzumab: a nationwide prospective survey. Front Neurol. 2023. 10.3389/fneur.2023.1137665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vakrakou AG, et al. IgG4-related autoimmune manifestations in Alemtuzumab-treated multiple sclerosis patients. J Neuroimmunol. 2021. 10.1016/j.jneuroim.2021.577759. [DOI] [PubMed] [Google Scholar]
- 38.von Essen MR, et al. Immune reconstitution following alemtuzumab therapy is characterized by exhausted T cells, increased regulatory control of proinflammatory T cells and reduced B cell control. Front Immunol. 2023; 10.3389/fimmu.2023.1249201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walo-Delgado PE, et al. Role of B cell profile for predicting secondary autoimmunity in patients treated with alemtuzumab. Front Immunol. 2021. 10.3389/fimmu.2021.760546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiendl H, et al. Lymphocyte pharmacodynamics are not associated with autoimmunity or efficacy after alemtuzumab. Neurol Neuroimmunol Neuroinflamm. 2020;7(1):e635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller I, et al. Longitudinal characterization of autoantibodies to the thyrotropin receptor (TRAb) during alemtuzumab therapy: evidence that TRAb may precede thyroid dysfunction by many years. Thyroid. 2018;28(12):1682–93. [DOI] [PubMed] [Google Scholar]
- 42.Sabahi Z, et al. A systematic review of the safety and efficacy of monoclonal antibodies for progressive multiple sclerosis. Int Immunopharmacol. 2023; 10.1016/j.intimp.2023.110266. [DOI] [PubMed] [Google Scholar]
- 43.Ruck T, et al. Alemtuzumab in multiple sclerosis: mechanism of action and beyond. Int J Mol Sci. 2015;16(7):16414–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson JP, et al. Flow cytometry: the next revolution. Cells. 2023;12(14):1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Black CB, et al. Cell-based screening using high-throughput flow cytometry. Assay Drug Dev Technol. 2011;9(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coles AJ, et al. Safety and efficacy with alemtuzumab over 13 years in relapsing-remitting multiple sclerosis: final results from the open-label TOPAZ study. Ther Adv Neurol Disord. 2023; 10.1177/17562864231194823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sintzel MB, Rametta M, Reder AT. Vitamin D and multiple sclerosis: a comprehensive review. Neurol Ther. 2018;7(1):59–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Antico A, et al. Hypovitaminosis D as predisposing factor for atrophic type A gastritis: a case-control study and review of the literature on the interaction of Vitamin D with the immune system. Clin Rev Allergy Immunol. 2012;42(3):355–64. [DOI] [PubMed] [Google Scholar]
- 49.Orgaz-Molina J, et al. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: a case-control study. J Am Acad Dermatol. 2012;67(5):931–8. [DOI] [PubMed] [Google Scholar]
- 50.Zhao R, et al. Immunomodulatory function of vitamin D and its role in autoimmune thyroid disease. Front Immunol. 2021. 10.3389/fimmu.2021.574967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goischke HK. Prevention or reduction of alemtuzumab-induced secondary autoimmune diseases through high-dose daily vitamin D supplementation? Mult Scler Relat Disord. 2022. 10.1016/j.msard.2022.103781. [DOI] [PubMed] [Google Scholar]
- 52.Baker D, et al. Interpreting lymphocyte reconstitution data from the pivotal phase 3 trials of alemtuzumab. JAMA Neurol. 2017;74(8):961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Massey J, et al. Haematopoietic stem cell transplantation results in extensive remodelling of the clonal T cell repertoire in multiple sclerosis. Front Immunol. 2022. 10.3389/fimmu.2022.798300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muraro PA, et al. T cell repertoire following autologous stem cell transplantation for multiple sclerosis. J Clin Invest. 2014;124(3):1168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pfeuffer S, et al. Impact of previous disease-modifying treatment on effectiveness and safety outcomes, among patients with multiple sclerosis treated with alemtuzumab. J Neurol Neurosurg Psychiatry. 2021;92(9):1007–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the conclusions of this article are included within the article and its additional files.