Abstract
Background
Following the COVID-19 pandemic, millions of people continue to experience ongoing physical and mental health sequelae after recovery from acute infection. There is currently no specific treatment for the diverse symptoms associated with post-COVID-19 condition. Physical and mental health rehabilitation may help improve quality of life in such patients. This study reports the cost-effectiveness of a programme of physical and mental health rehabilitation compared to best practice usual care in people with post-COVID-19 condition who were previously hospitalised.
Methods
We conducted an economic evaluation within a randomised controlled trial from the perspective of the UK national health service (NHS) and personnel social services perspective (PSS). Resource used and health-related quality of life were collected using bespoke questionnaire and the EQ-5D-5 L questionnaire at three, six, and 12 months. Incremental costs and quality adjusted life years accrued over the follow-up period were estimated and reported as the incremental cost-effectiveness ratio. Estimate uncertainty was managed by multiple imputation and bootstrapping cost-effectiveness estimates; and displayed graphically on the cost-effectiveness plane.
Results
Over a 12-month time horizon, incremental costs and QALYs were £305 (95% CI: -123 to 732) and 0.026 (95% CI: -0.005 to 0.052) respectively. The ICER was £11,941 per QALY indicating cost-effective care. Sensitivity analyses supported the base case findings. The probability of the intervention being cost-effective at a £30,000 per QALY willingness-to-pay threshold was 84%.
Conclusion
The within-trial economic evaluation suggested that people with post-COVID-19 condition after hospitalisation should be offered a programme of physical and mental health rehabilitation as it likely reflects a cost-effective use of NHS resources. Hospitalisation for COVID-19 has become less commonplace: further evaluation in non-hospitalised patients may be worthwhile.
Trial registration
ISRCTN registry ISRCTN11466448 23rd November 2020.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12913-024-11679-5.
Keywords: Randomised controlled trials, RCT, COVID-19, Long COVID, Post-COVID-19 condition, Cost-effectiveness analysis, Cost-utility analysis, Physical rehabilitation, Mental health rehabilitation, Rehabilitation
Introduction
Post-COVID-19 condition is a term used to describe persistent or new symptoms lasting for weeks or months after COVID-19 infection [1]. Common symptoms include fatigue, shortness of breath, muscle soreness and neurocognitive dysfunction [1]. Post-COVID-19 condition affects about 65 million people globally [2] and about 1.9 million people in the UK reported COVID-19 symptoms lasting more than 12 weeks after an acute infection, and 762,000 people reported enduring symptoms beyond two years [3]. There are no official data on the proportion of the 1.9 million people with prolonged COVID-19 symptoms who were previously hospitalised; however, 1.5 million of these reported a severe impact on their day to day activities. Data from the UK Health Security Agency reported over 1.1 million hospitalisations for COVID-19 in the UK since March 2020 [4], and another prospective UK study found that 92.8% of adults hospitalised with COVID-19 reported experiencing at least one symptom persisting beyond six months from discharge [5]. A 2023 global study suggested that in hospitalised and non-hospitalised patients with COVID-19, the pooled prevalence of survivors experiencing at least one symptom after an average of 126 days of infection was 53% and 35% respectively [6].
There is currently no specific treatment for the diverse symptoms associated with post-COVID-19 condition and existing therapeutic strategies are limited in effectiveness and generalisability [7]. Exercise-based rehabilitation interventions might help improve outcomes in people with long-term conditions but there is little evidence of clinical or cost effectiveness in people with post-COVID-19 condition due to a lack of high quality research [8]. Cost-effectiveness analysis is the evaluation of alternative health interventions based on costs and outcomes. Budget constraints underscore the need for resource allocation decisions in health care, and countries with publicly funded health systems often rely on normative cost-effectiveness criteria to inform such decisions [9].
The 2024 REGAIN randomised controlled trial (n = 585), in adults with the post-COVID-19 condition, following a hospital admission, showed that an eight week, supervised, home-based, online group rehabilitation programme led to statistically significant improvements in health-related quality of life (measured using the patient reported outcomes measurement information system (PROMIS) preference (PROPr) score) compared to best practice usual care (a single online session of advice and support with a trained practitioner). An increase in PROMIS-PROPr score of 0.03 [95% confidence interval (CI): 0.01 to 0.05] was observed at 3 months and 12 months (PROMIS-PROPr score of 0.03; 95%CI:0.01 to 0.06) [10]. At six months the difference was not statistically significant, 0.02 (95% CI -0.003 to 0.05). The cost-effectiveness of the REGAIN intervention in managing post-COVID-19 condition is unknown and such evidence can help further inform resource allocation decisions in people with post-COVID-19 condition. This is the first study to report the cost-effectiveness of a rehabilitation programme in people with post-COVID-19 condition following hospital admission.
Methods
Trial background
The trial protocol, main results, and intervention development are published elsewhere [10–12]. The REGAIN study was a multicentre, parallel group, superiority randomised controlled trial (RCT) of rehabilitation approaches in people with post-COVID-19 condition. Individuals were eligible to participate if they were adults who had been discharged from hospital three or more months previously after COVID-19 admission and had ongoing physical and mental health sequelae. Participants were randomised to receive either best practice usual care, consisting of a 30 min, online one-to-one consultation with a trained practitioner, or the REGAIN (Rehabilitation Exercise and psycholoGical support After COVID-19 InfectioN) intervention which comprised of a one-hour one-to one consultation with a trained practitioner followed by an eight week, online, home based, supervised, group rehabilitation programme [13]. Participants randomised to receive the REGAIN intervention were offered eight weekly live online group exercise session and six live online group psychological support sessions. Participants were recruited between January 2021 and July 2022. The REGAIN study was approved by the East of England, Cambridge South Research Ethics Committee (reference 20/EE/0235). Informed consent was obtained from all participants in the study.
Recruitment and demographics
We randomised 298 people to receive the REGAIN intervention and 287 people to receive usual care. Participants’ mean age was 56 years and 52% were women. The mean time from hospital discharge to randomisation was 10.6 months.
Data collection and valuation
Resource use
Data on health and social care services used were recorded using questionnaires at three, six, and 12 months. The questionnaires captured details of the following resource use categories: medication, out-patient and emergency attendances, in-patient admission, personal and social services, primary and community care contacts, nursing home care, private care costs, personal expenses, and number of days off work due to illness. Societal costs included private medical costs, personal expenses and productivity loses due to post-COVID-19 condition. The resource use questionnaire developed for the study can be found in the supplementary appendix. We did not triangulate medical records captured using participant-completed questionnaires with hospital records due to the costs, and considerable delays, associated with requesting Hospital Episode Statistics data from NHS England.
Intervention costs
Best practice usual care consisted of a 30-minute one-to-one consultation with a REGAIN practitioner (clinical exercise physiologist or physiotherapist). The REGAIN intervention consisted of an hour-long online one-to-one consultation with a REGAIN practitioner, a weekly one-hour group exercise session over eight weeks and a one-hour group behavioural session over 6 weeks [13]. Each online, group session had up to eight participants and was led by a REGAIN practitioner. Costs for best practice usual care and the intervention were determined using the unit cost of a Band 6 clinical exercise physiologist and health psychologist from the Department of Health and Social Care Reference Costs [14]. Training costs were not included as practitioners who delivered both the REGAIN and best practice usual care intervention received similar training.
Given the rehabilitation intervention was the offer of a series of fixed appointments, we assumed full session attendance and compliance with the intervention as our base case analysis. We explored a scenario as part of the sensitivity analysis where data on the actual number of sessions each participant attended were used. The overall cost to the NHS of sessions remained the same as the base case, but in the scenario, the cost per patient varied by the number of sessions attended.
Valuation of resource use
Resources used were valued in accordance with methods recommended by NICE Guide to Methods of Technology Appraisal [15]. Unit costs were derived for each resource use item from national databases. The key databases used to derive unit costs for resource use items included: Department of Health and Social Care Reference Costs [14], Personnel and Social Services Research Unit (PSSRU) unit cost compendium [16], 2022 NHS Prescription Cost Analysis database for England [17] and 2022 volumes of the British National Formulary [12] Data from the Office for National Statistics (ONS) was used to estimate loss of earnings due to time off work [18]. All costs are expressed in Pound Sterling at 2022/2023 prices. If required, costs were adjusted to current prices using the NHS Cost Inflation Index [7].
Health outcomes
The main health measure used for economic evaluation was the quality-adjusted life year (QALY) as recommended by NICE [15]. Health-related quality of life was evaluated at baseline, three months, six months, and twelve months after randomization using the EQ-5D-5 L instrument [19]. EQ-5D-5 L responses were transformed into utilities by mapping EQ-5D-5 L responses to the EQ-5D-3 L valuation set using the recommended mapping function at the time of analysis planning [20–22]. Responses were summarised at each time point by trial group. Quality-adjusted life years (QALYs) were generated for each patient using the area under the curve assuming linear interpolation across each temporal measurement point and summarised by trial group.
Statistical analysis
Effects were estimated and summarised for those with complete data in each randomised group. Statistical significance was assessed through an independent samples t-test using a two-sided significance of 5%.
The base case cost-effectiveness analysis involved bootstrapping a multiple imputed bivariate model. Development of the imputation model followed best practice guidelines [23].
Missing data
Missing data are common in RCTs. Participants are likely to be lost to follow-up for various reasons. Participants with missing data may systematically differ from those with fully observed data. Hence, analytic techniques for handling missing data should be underpinned by the missing data mechanism. Missing costs and health utility data were imputed under the Missing at Random (MAR) assumption, at each time point using fully conditional multiple imputation by chain equations implemented using the MICE package in STATA 18 [24]. The suitability of using a MAR assumption was assessed by examining the missing data patterns and comparing subjects with and without missing data at each time point. Predictors for missingness were identified using a stepwise logistic regression model at each time point, adjusting for baseline co-variates (see supplementary appendix for further details). The imputation model was adjusted for the following covariates: age, gender, level of hospital care—either ward or intensive care unit, and presence of mental health symptoms (measured using self-reported impact of event scale-6 (IES-6) post-traumatic stress disorder score ≥ 11/24, or hospital anxiety and depression scale (HADS) anxiety subscore ≥ 11/21, or HADS depression subscore ≥ 11/21; compared with IES-6 post-traumatic stress disorder score < 11/24, or HADS anxiety subscore < 11/21, or HADS depression subscore < 11/21) to align with the randomisation strata.
Missing costs and utility were imputed at each time point using observed values. The imputation model was evaluated for algorithmic convergence, and graphical comparisons were made between the distributions of observed, imputed, and completed data. The imputation was carried out separately for each trial arm [25] and ran 50 times, following methodological guidance that the number of imputations be determined by the fraction of missing information rather than the proportion of missing data [26].
Bivariate regression using seemingly unrelated regression model (sureg), within the Stata MI framework, was used to estimate the costs and QALYs over the time horizon, controlling for baseline covariates (age, gender, level of care and presence of mental health). The analytic model used to estimate QALYs was adjusted for baseline utility. There were no significant interactions between the interventions and any of the baseline co-variates. Joint distributions of estimates from the original data set were derived through bootstrapping of the MI model [24], and incremental costs and QALYs were calculated. This method provides adjusted estimates using Rubin’s rule [27] to reflect the variability within and across imputations.
Cost-utility analysis
The primary analysis followed a National Health Service (NHS) and Personnel Social Service (PSS) perspective as recommended by the National Institute of Health and Care Excellence (NICE) [15]. Cost-utility analysis followed the health economics analysis plan (see supplementary appendix) and was presented as an incremental cost-effectiveness ratio (ICER), calculated as the ratio of the incremental costs and QALYs with best practice usual care as the reference treatment.
Bootstrapped estimates of the incremental costs and QALYs were graphically represented on the ICER plane. The net monetary benefit (NMB) was calculated across willingness-to-pay thresholds ranging from £0 to £100,000 per QALY. A positive incremental NMB shows that REGAIN is a cost-effective alternative to best practice usual care at the specified threshold. A cost-effectiveness acceptability curve (CEAC) showed the probability that REGAIN is cost-effective compared to best practice usual care across similar willingness-to-pay thresholds.
The expected value of perfect information (EVPI) and expected value of sampled information (EVSI) was estimated at similar willingness to pay thresholds and represented graphically. The expected value of perfect information reflects the monetary value of removing uncertainty from the cost-effectiveness estimates at a specified willingness-to-pay threshold. The expected value of sampled information reflects the monetary value of reducing uncertainty with a given study design and sample size. Further details on the estimation of EVPI and EVSI can be found in the supplementary appendix.
Sensitivity and secondary analysis
Sensitivity analyses were undertaken to re-estimate the cost-effectiveness results under the following scenarios: (i) using complete data (ii) Broadening the evaluative space from an NHS perspective to a societal perspective that additionally includes private costs and productivity loses due to illness (iii) using data on the actual number of sessions participants attended. We did not undertake pre-specified sensitivity analysis of development and training costs (to simulate wider rollout and inclusion of development costs) as practitioners delivering both interventions received similar training.
Results
Completeness of data
Data were complete for 436 of 585 (75%) participants at the three month time point, 438 of 585 (75%) participants at the six month time point, and 430 of 585 (74%) participants at the 12 month time point. Across the entire follow-up period, data were complete for 371 of 585 participants (63%). Completion rates were slightly higher (3–5%) in the usual care group compared to the REGAIN intervention group. At the aggregate level, data were considered missing if any resource use category were missing in any timepoint. Similarly, an EQ-5D-5 L response needed to be completed in all five domains at each timepoint to be non-missing. A detailed breakdown of completeness of data can be found in Supplementary Table i.
Resource use and economic costs
NHS and PSS costs were similar at each time point as shown in Table 1. The small non-significant differences in costs between the groups was largely driven by visits to out-patient services and hospital admissions as shown in Table 2. Total NHS and PSS costs were lower in the REGAIN intervention group compared to usual care but higher when intervention costs were added. However, between group differences in costs were statistically similar at each timepoint and over the follow-up period (0–12 m). A detailed breakdown of costs per resource use category and follow-up time point can be found in Supplementary Table ii.
Table 1.
Unadjusted total NHS and PSS costs at each timepoint and across the follow-up period for those with complete data in 2022/2023 prices
| REGAIN | Usual Care | Between group difference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Follow-up period | n | Mean (£) | sd | n | Mean (£) | sd | Mean difference (£) | 95% CI | p−value |
| 0–3 months | 216 | 378.36 | 720.01 | 220 | 384.60 | 737.17 | -6.24 | -143.02 to 130.54 | 0.93 |
| 4–6 months | 215 | 395.44 | 731.61 | 223 | 445.56 | 849.90 | -50.11 | -198.46 to 98.24 | 0.51 |
| 7–12 months | 212 | 818.20 | 1533.50 | 218 | 769.70 | 1284.09 | 48.50 | -219.21 to 316.22 | 0.72 |
| Total | 182 | 1484.68 | 2344.57 | 189 | 1639.16 | 2201.94 | -154.48 | -617.71 to 308.75 | 0.51 |
| Intervention costs | 298 | 399.92 | 0 | 287 | 72.71 | 0 | 327.21 | . | |
| Totala | 182 | 1884.60 | 2344.57 | 189 | 1711.87 | 2201.94 | 172.73 | -290.50 to 635.96 | 0.47 |
aIncluding base case intervention costs
Table 2.
Mean NHS and PSS costs per resource use category in GBP (£) between 0 and 12 months for cases with complete data in 2022/2023 prices
| REGAIN | Usual care | Between group difference | ||||
|---|---|---|---|---|---|---|
| Category | n | Mean (SD) | n | Mean (SD) | Mean difference | 95% CI |
| Medication | 185 | 15.57 (36.16) | 191 | 12.62 (24.05) | 2.95 | -3.28 to 9.18 |
| Outpatient services | 185 | 880.05 (1346.77) | 190 | 937.92 | -57.86 | -319.72 to 203.99 |
| Community care | 185 | 153.25 (399.97) | 189 | 108.62 (199.08) | 44.62 | -11.99 to 101.24 |
| Accident and Emergency | 185 | 87.82 (214.12) | 189 | 95.83 (212.79) | -8.01 | -51.28 to 35.26 |
| In-patient admission | 187 | 258.99 (871.36) | 190 | 456.60 (1351.98) | -197.61 | -426.86 to 31.64 |
| PSS | 184 | 86.64 (814.27) | 189 | 20.93 (269.57) | 65.71 | -58.06 to 189.48 |
| Nursing home care | 186 | 0.00 (0.00) | 190 | 10.61 (141.52) | -10.61 | -30.73 to 9.52 |
Health outcomes
Unadjusted EQ-5D-5 L utility estimates at each timepoint and QALYs for the follow-up period, for cases with complete data are summarised in Table 3. While health outcomes in both groups remained poor, there were no statistically significant differences in utility estimates between the groups at each time point. Over the follow-up period, participants who received the REGAIN intervention had a non-statistically significant increase in QALYs of 0.039 (95% CI: -0.009 to 0.087).
Table 3.
Unadjusted EQ-5D-5 L utility estimates at each time point and QALY estimates across the follow-up period for those with complete data
| REGAIN | Usual care | Between group difference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Follow-up period | n | Mean | sd | n | Mean | sd | Mean difference (£) | 95% CI | p−value |
| Baseline | 298 | 0.531 | 0.290 | 287 | 0.528 | 0.268 | 0.003 | -0.042 to 0.048 | 0.90 |
| 3 months | 237 | 0.584 | 0.292 | 245 | 0.561 | 0.275 | 0.024 | --0.027 to 0.074 | 0.36 |
| 6 months | 225 | 0.590 | 0.286 | 234 | 0.574 | 0.276 | 0.015 | -0.036 to 0.67 | 0.56 |
| 12 months | 217 | 0.598 | 0.284 | 225 | 0.557 | 0.286 | 0.042 | -0.012 to 0.095 | 0.13 |
| Follow-upa | 201 | 0.603 | 0.245 | 209 | 0.564 | 0.246 | 0.039 | -0.009 to 0.087 | 0.134 |
aUtility values calculated as area under the curve
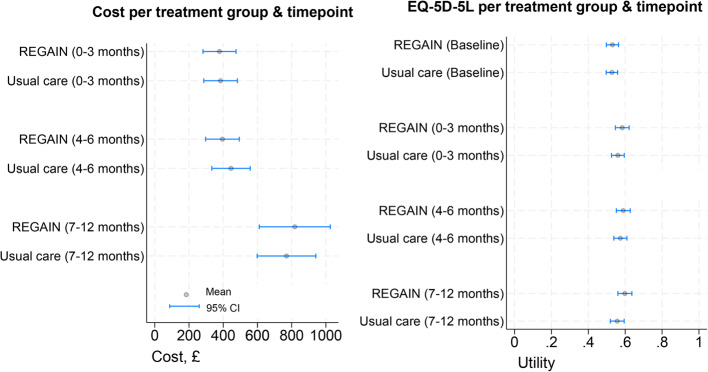
Both costs and EQ-5D-5 L utility estimates were statistically similar at each time point and over the time horizon as graphically shown in Fig. 1.
Fig. 1.
NHS and PSS costs and EQ-5D-5 L utility estimates at each timepoint per trial group
Cost-effectiveness analysis
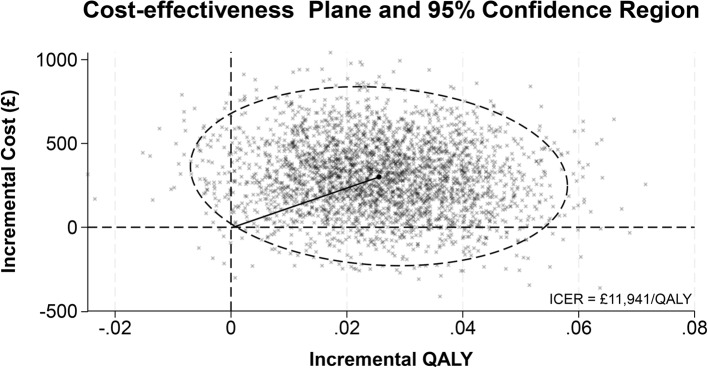
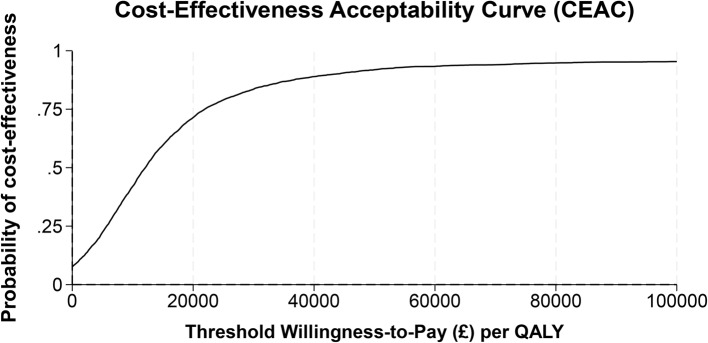
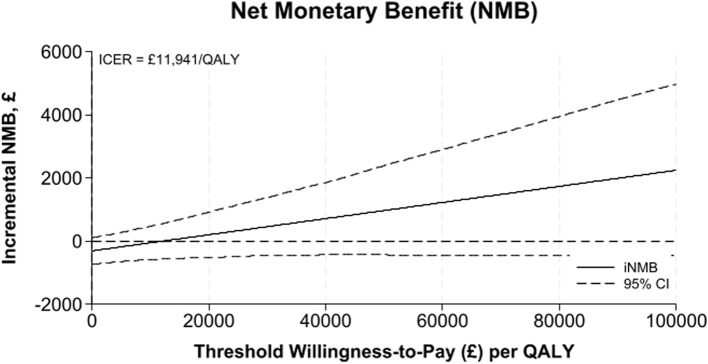
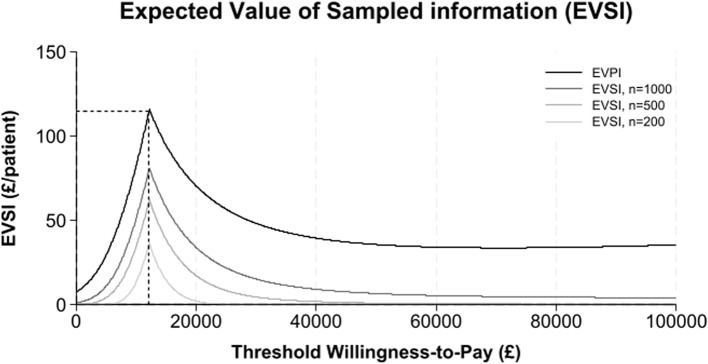
Cost-effectiveness results are presented in Table 4 with REGAIN intervention group as the reference treatment and usual care as the comparator. The analytic time horizons encompass the period from randomisation to 12-months post-randomisation. Estimates of the costs and QALYs are shown with 95% credible intervals. Credible intervals show the probability that the true cost-effectiveness estimate lie within a defined interval given the observed data. The probability of the REGAIN intervention being cost-effective and net monetary benefit is shown at willingness to pay thresholds of £15,000, £20,000, and £30,000. The distribution of the ICER with a 95% credible region is shown graphically (Fig. 2). The cost-effectiveness acceptability curve, net monetary benefit and expected value of perfect and sampled information are shown graphically (Figs. 3, 4 and 5), at a range of willingness to pay thresholds (£0 – £100,000).
Table 4.
Cost-effectiveness results of REGAIN intervention compared to usual care at 12 months (£, 2022)
| REGAIN cost (£) (95% CI) |
Usual care cost (£) (95% CI) |
Incremental costs (£) (95% CI) |
REGAIN QALY (95% CI) |
Usual care QALY (95% CI) |
Incremental QALYs (95% CI) |
ICER (£/QALY) |
Pce1 | Pce2 | Pce3 | NMB1 (95% CI) |
NMB2 (95% CI) |
NMB3 (95% CI) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base case abc |
1987 (1666 to 2307) |
1681 (1406 to 1955) |
305 (-123 to 732) |
0.582 (0.555 to 0.608) |
0.556 (0.530 to 0.583) |
0.026 (-0.005 to 0.052) |
11,941 | 0.596 | 0.714 | 0.836 |
78 (-538 to 683) |
206 (-506 to 920) |
461 (-458 to 1338) |
| Complete case ad |
1884 (1561 to 2209) |
1711 (1394 to 2030) |
173 (-282 to 627) |
0.600 (0.579 to 0.618) |
0.567 (0.548 to 0.586) |
0.032 (0.004 to 0.059) |
5410 | 0.823 | 0.888 | 0.941 |
306 (-627 to 953) |
466 (-285 to 1217) |
785 (-200 to 1770) |
| Societal perspective abd |
3995 (3204 to 4785) |
3839 (3062 to 4616) |
156 (-949 to 1260) |
0.581 (0.562 to 0.601) |
0.557 (0.538 to 0.575) |
0.025 (-0.002 to 0.052) |
6242 | 0.636 | 0.699 | 0.788 |
219 (-1261 to 1446) |
343 (-950 to 1636) |
593 (-860 to 2045) |
| Compliance data abd |
1995 (1695 to 2295) |
1676 (1380 to 1972) |
319 (-103 to 741) |
0.581 (0.562 to 0.601) |
0.557 (0.538 to 0.575) |
0.025 (-0.002 to 0.052) |
12,708 | 0.573 | 0.691 | 0.815 |
58 (-741 to 674) |
183 (-537 to 903) |
434 (-516 to 1383) |
a Adjusted for age, gender, level of hospital care and presence of mental health symptoms
b Imputed costs and QALYs
c Estimates derived from non-parametric bootstrapping using 3000 replications
d Estimates derived using parametric methods
NMB1 NMB2 NMB3 Mean NMB with 95% CI at cost effectiveness thresholds £15000, £20000, £30000
Pce1 Pce2 Pce3 Probability of cost-effectiveness at thresholds £15000, £20,000, and £30,000
Fig. 2.
Cost-effectiveness plane with 95% credible region
Fig. 3.
Cost-effectiveness acceptability curve
Fig. 4.
Net monetary benefit
Fig. 5.
Expected value of perfect and sampled information
Base case analysis
The results for the base case analysis are presented in Table 4. Patients in the REGAIN group had increased costs and gained additional QALYs of £305 (95% CI: -123 to 732) and 0.026 (95% CI: -0.005 to 0.052) respectively. The ICER for the base case analysis of £11,941 suggests that the REGAIN intervention is a cost-effective alternative to usual care. The probability of REGAIN being cost effective at the £30,000/QALY threshold is 0.836. At a threshold of £30,000/QALY, the EVPI is £48 per patient. In the UK, an estimated 1.5 million experience post-COVID-19 condition which adversely affects day-to-day activities [3] and there have been over 1.1 million hospitalisations with COVID-19 since March 2020 [4]. However, we have no information on the number of people with post-COVID-19 condition who were previously hospitalised.
Assuming a post-COVID-19 syndrome prevalence of 52.6% among patients hospitalised with COVID-19 in the UK, the monetary value of removing all uncertainty from the cost-effectiveness estimate would be about £28 million per annum and could rise to £72 million per annum if the estimated 1.5 million people who experience post-COVID-19 condition which adversely affected day-to-day activities is considered. However, the future incidence of COVID-19 is uncertain and the estimated 1.5 million people with post-COVID 19 conditions in the UK reflects an upper bound as it likely includes non-hospitalised individuals with post-COVID-19 condition. Further we do not know if changes in COVID-19 strains and treatments for those admitted to hospital will affect incidence of prolonged post-COVID-19 symptoms. Given, the opportunity loss from uncertainty further research to reduce uncertainty is likely to be justified. Figure 5 graphically displays the expected value of perfect and sampled information at different willingness-to-pay thresholds.
Sensitivity analysis
Incremental costs and QALYs were compared under different analytic scenarios (i.e., complete case, societal perspective and using compliance data) and presented in Table 4. These sensitivity analyses support the base case findings. In the complete case analysis, the REGAIN intervention had a non-significant incremental cost of £173 (95% CI: -282 to 627) and QALYs of 0.032 (95% CI: 0.004 to 0.059). An ICER of £5410 per QALY indicates that REGAIN is cost-effective. The probability of REGAIN being cost-effective at a £30,000 per QALY threshold is 0.941. For the societal perspective, REGAIN had a non-significant incremental cost of £156 (95% CI: -949 to 1260) and incremental QALYs of 0.025 (95% CI: -0.002 to 0.052). While acknowledging that the combination of health benefits with broader societal costs is problematic, the ICER reduced to £6242 per QALY reflecting higher private care costs, personal expenses and productivity loses in the usual care group. However, reflecting greater uncertainty, the probability of the REGAIN intervention being cost-effective at £30,000 per QALY is 0.788. The cost-effectiveness results were re-evaluated using alternative distribution (gamma distribution) for costs in the analytic model and using an alternative mapping algorithm [20] for the EQ-5D-5 L response rather than the currently recommended algorithm [21]. Both findings were similar to the base case.
Accounting for the actual number of exercise and psychological support sessions attended, findings were similar to the base case findings. The REGAIN group had a non-significant incremental cost of £319 (95% CI: -103 to 741) and QALYs of 0.025 (95% CI: -0.002 to 0.052). The ICER slightly increased to £12,708 per QALY and the probability of REGAIN being cost-effective at £30,000 per QALY remained at 0.815.
The protocol allowed for decision analytic making to estimate longer term cost-effectiveness if costs and outcomes do not converge over the analytic time horizon. As seen in Fig. 1, incremental costs and QALYs were small and non-significant over the analytic time horizon hence longer extrapolation of cost-effectiveness is unlikely to change the base case findings or provide new information.
Discussion
REGAIN was a pragmatic RCT, nationally recruiting 585 adults discharged following hospital admission for COVID-19. The REGAIN intervention was clinically effective when compared to usual care with improvements in health-related quality of life driven predominantly by improvements in fatigue, depression, and pain interference sub-scales [10]. The findings from the within trial economic analysis are consistent with the clinical findings with modest and non-significant overall changes in cost and quality-of-life. The uncertainty in the incremental costs and QALYs was reflected in the cost-effectiveness plane with the 95% credible region ranging from REGAIN being dominated to REGAIN being dominant over usual care. The distribution of the incremental costs and QALYs suggest that the REGAIN intervention is likely to be cost-effective at a willingness-to-pay threshold of £30,000/QALY with p = 0.836. However, given the large monetary value of reducing the uncertainty of the cost-effectiveness findings, further research may be merited.
Previous studies, albeit very small and often non-randomised, have shown that rehabilitation-based interventions may help to reduce disability [28] and improve outcomes in patients with long COVID [29–31]. Such interventions can improve health-related quality of life [32] and physical functioning [33] in patients with long COVID who were previously hospitalised with COVID-19. Likewise, systematic reviews of rehabilitation interventions (including observational and quasi-experimental studies) in patients with long COVID showed improved outcomes [34, 35]. However, no previous study has considered the cost-effectiveness of rehabilitation strategies for patients with long COVID. This is the first study to examine the cost-effectiveness of physical and mental health rehabilitation in the management of people with post-COVID-19 condition after hospitalisation.
The delivery of the REGAIN intervention digitally, through practitioner-led online sessions can facilitate adoption in other settings and ease burden on health systems given labour shortages [36, 37]. However, barriers to the access and use of digital technology may limit digital application in older individuals and settings with poor digital infrastructure. While the World Health Organisation recommends various rehabilitation strategies to manage the diverse symptoms of post-COVID-19 syndrome [38], there is no definitive rehabilitation-based standard of care for such patients. In studies comparing rehabilitation-based interventions in patients with post-COVID syndrome, standard of care varied considerably between studies reflecting variation in clinical practice across health systems [34]. These differences in standard-of-care approaches could impact the cost-effectiveness of the REGAIN intervention. Furthermore, labour costs of health care practitioners tasked with delivering the interventions is also expected to vary which could also impact the cost-effectiveness. Willingness to pay for additional unit of QALYs varies across health systems [39] which could impact the decision making in different settings given the uncertainties in the cost-effectiveness results.
The study is strengthened by the large number of participants recruited. The pragmatic nature of the study intervention which was delivered by community and NHS staff underscores its external validity. Only 11% of the trial participants were non-white which may limit its generalisability to minority ethnic groups. Treatment allocation was not masked to trial participants and practitioners delivering the intervention which may lead to bias. All participants in the study were previously hospitalised with COVID-19 which might limit its generalisability to non-hospitalised patients with post-COVID-19 condition. We did not adjust for compliance to treatment and baseline costs which might affect the cost-effectiveness results. Furthermore, the use of questionnaires to collect information on resources used might be susceptible to recall bias.
The substantial global burden of post-COVID-19 [2, 40] and the large economic consequence of post-COVID-19 condition [41],underscores the need for decision-makers globally to encourage the use of physical and mental rehabilitation to improve quality of life.
Conclusion
Physical and mental health rehabilitation improves quality of life in patients with long-term post-COVID-19 condition and is likely to be a cost-effective use of NHS resources. Given the substantial burden of this condition and the demonstrated clinical effectiveness providing improvements in post-COVID-19 symptoms such as fatigue, depression and pain, decision makers should offer rehabilitation to people with ongoing physical and mental sequalae following hospitalisation with COVID-19 infection.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- REGAIN
Rehabilitation Exercise and psycholoGical support After COVID-19 InfectioN
- CRF
Case report forms
- PROMIS-PROPr
Patient reported outcomes measurement information system preference score
- RCT
Randomised controlled trial
- NHS
National health service
- PSS
Personnel social services
- UK
United Kingdom
- PSSRU
Personnel and Social Services Research Unit
- ONS
Office for national statistics
- QALY
Quality adjusted life year
- NICE
National institute of health and care excellence
- ICER
Incremental cost-effectiveness ratio
- NMB
Net monetary benefit
- CEAC
Cost-effectiveness acceptability curve
- EVPI
Expected value of perfect information
- MAR
Missing at random
- MICE
Multiple imputation using chained equations
- Sureg
Seemingly unrelated regression
Authors’ contributions
HN drafted the paper, interpreted the data, and conducted the analysis. JM participated in the conceptualisation and design of the study, conducted the analysis, interpreted the data, critically revised the paper. MU conceived and designed the study, provided clinical input for cost estimates, critically revised the paper. JB participated in the conceptualisation and design of the study, critically revised the paper. RL participated in the conceptualisation and design of the study, critically revised the paper. CJ interpreted the data and critically revised the paper. MR interpreted the data and critically revised the paper. GM conceived and designed the study, provided clinical input for cost estimates, critically revised the paper.
Funding
This trial was funded by the UK National Institute for Health and Care Research Health Technology Assessment Programme. All researchers can confirm their independence from funders, and all authors, had full access to all the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Availability of data and materials
The datasets used and/or analysed for this study are stored in the University of Warwick databases and available from the corresponding author on reasonable request. Requests can be sent to wctudataaccess@warwick.ac.uk.
Declarations
Ethics approval and consent to participate
The REGAIN study was approved by the East of England, Cambridge South Research Ethics Committee (reference 20/EE/0235) and the Health Research Authority/Health and Care Research Wales on 6 November 2020. Informed consent was obtained from all participants in the study.
Consent for publication
Not applicable in this section.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Raveendran A, Jayadevan R, Sashidharan S. Long COVID: an overview. Diabetes Metabolic Syndrome: Clin Res Reviews. 2021;15(3):869–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis HE, et al. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Office for National Statistics. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK: 30 March 2023. 2023 [cited 2024 14/02]; https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/30march2023.
- 4.UK Health Security Agency. COVID-19 Hospital Activity. 2024. [cited 2024 05/09]. Available from: https://ukhsa-dashboard.data.gov.uk/topics/covid-19.
- 5.Evans RA, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK Multicentre, prospective cohort study. Lancet Respiratory Med. 2021;9(11):1275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Mahoney LL, et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. eClinicalMedicine. 2023;55:101762. [DOI] [PMC free article] [PubMed]
- 7.Cha C, Baek G. Symptoms and management of long COVID: a scoping review. J Clin Nurs. 2024;33(1):11–28. [DOI] [PubMed] [Google Scholar]
- 8.Huizinga F, et al. Home-based physical activity to alleviate fatigue in cancer survivors: a systematic review and meta-analysis. Med Sci Sports Exerc. 2021;53(12):2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Organization WH. 2015 global survey on health technology assessment by national authorities: main findings, in 2015 global survey on health technology assessment by national authorities: main findings. 2015. [Google Scholar]
- 10.McGregor G, et al. Clinical effectiveness of an online supervised group physical and mental health rehabilitation programme for adults with post-covid-19 condition (REGAIN study): multicentre randomised controlled trial. BMJ. 2024;384:e076506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGregor G, et al. Rehabilitation Exercise and psycholoGical support after covid-19 InfectioN’(REGAIN): a structured summary of a study protocol for a randomised controlled trial. Trials. 2021;22(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.British National Formulary. British National Formulary. 2022;81. [cited 2022 01/12]. Available from: https://bnf.nice.org.uk/.
- 13.Ennis S, et al. Development of an online intervention for the Rehabilitation Exercise and psycholoGical support after covid-19 InfectioN (REGAIN) trial. NIHR Open Res. 2023;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NHS Improvement, NHS Improvement. Highlights, analysis and introduction to the data, in 2021/22 National Cost Collection Data Publication, National Health Service England. 2022.
- 15.National Institute for Health and Care Excellence. Guide to the Methods of Technology Appraisal 2013. 2013. [PubMed] [Google Scholar]
- 16.Jones KC, Weatherly H, Birch S, Castelli A, Chalkley M, Dargan A, Forder JE, Gao J, Hinde S, Markham S, Ogunleye D. Unit Costs of Health and Social Care 2022 Manual. Technical report. Personal Social Services Research Unit (University of Kent) & Centre for Health Economics (University of York), Kent. 2023. 10.22024/UniKent/01.02.100519.
- 17.NHS Business Services Authority. Prescription Cost Analysis-England. 2022. [cited 2023 02/02]. Available from: https://www.nhsbsa.nhs.uk/statistical-collections/prescription-cost-analysis-england/prescription-cost-analysis-england-202122.
- 18.Office for National Statistics. Employee earnings in the UK: 2021. London: Office for National Statistics; 2021. [Google Scholar]
- 19.Herdman M, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Hout B, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–15. [DOI] [PubMed] [Google Scholar]
- 21.Hernández Alava M, Pudney S, Wailoo A. Estimating the relationship between EQ-5D-5L and EQ-5D-3L: results from a UK Population Study. Pharmacoeconomics. 2023;41(2):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institute for Health and Care Excellence. Position statement on use of the EQ-5D-5L value set for England (updated October. 2019). 2019; https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l.
- 23.Gomes M, et al. Multiple imputation methods for handling missing data in cost-effectiveness analyses that use data from hierarchical studies: an application to cluster randomized trials. Med Decis Making. 2013;33(8):1051–63. [DOI] [PubMed] [Google Scholar]
- 24.StataCorp. College Station, TX: StataCorp LP; 2023. Stata Stat Software: Release. 2023;19:19. [Google Scholar]
- 25.Faria R, et al. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics. 2014;32(12):1157–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madley-Dowd P, et al. The proportion of missing data should not be used to guide decisions on multiple imputation. J Clin Epidemiol. 2019;110:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Little RJ, Rubin DB. Statistical analysis with missing data, vol. 793. College Station: John Wiley & Sons; 2019.
- 28.Grishechkina IA, Lobanov AA, Andronov SV, Rachin AP, Fesyun AD, Ivanova EP, Masiero S, Maccarone MC. Long-term outcomes of different rehabilitation programs in patients with long COVID syndrome: a cohort prospective study. Eur J Transl Myol. 2023;33(2):11063. 10.4081/ejtm.2023.11063. [DOI] [PMC free article] [PubMed]
- 29.Nopp S, et al. Outpatient pulmonary rehabilitation in patients with long COVID improves exercise capacity, functional status, dyspnea, fatigue, and quality of life. Respiration. 2022;101(6):593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Compagno S, et al. Physical and psychological reconditioning in long COVID syndrome: results of an out-of-hospital exercise and psychological-based rehabilitation program. IJC Heart Vasculature. 2022;41:101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jimeno-Almazán A, et al. Rehabilitation for post-COVID-19 condition through a supervised exercise intervention: a randomized controlled trial. Scand J Med Sci Sports. 2022;32(12):1791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Longobardi I, et al. Effects of a 16-week home-based exercise training programme on health-related quality of life, functional capacity, and persistent symptoms in survivors of severe/critical COVID-19: a randomised controlled trial. Br J Sports Med. 2023;57(20):1295–303. [DOI] [PubMed] [Google Scholar]
- 33.Berentschot JC, Heijenbrok-Kal MH, Bek LM, Huijts SM, van Bommel J, van Genderen ME, Aerts JGJV, Ribbers GM, Hellemons ME, van den Berg-Emons RJG; CO-FLOW Collaboration Group. Physical recovery across care pathways up to 12 months after hospitalization for COVID-19: A multicenter prospective cohort study (CO-FLOW). Lancet Reg Health Eur. 2022;22:100485. 10.1016/j.lanepe.2022.100485. [DOI] [PMC free article] [PubMed]
- 34.Sánchez-García JC, Reinoso-Cobo A, Piqueras-Sola B, Cortés-Martín J, Menor-Rodríguez MJ, Alabau-Dasi R, Rodríguez-Blanque R. Long COVID and Physical Therapy: a Systematic Review. Diseases. 2023;11(4):163. 10.3390/diseases11040163. [DOI] [PMC free article] [PubMed]
- 35.Melendez-Oliva E, et al. Efficacy of pulmonary rehabilitation in post-COVID-19: a systematic review and meta-analysis. Biomedicines. 2023;11(8):2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erku D, Khatri R, Endalamaw A, Wolka E, Nigatu F, Zewdie A, Assefa Y. Digital Health Interventions to Improve Access to and Quality of Primary Health Care Services: a scoping review. Int J Environ Res Public Health. 2023;20(19):6854. 10.3390/ijerph20196854. [DOI] [PMC free article] [PubMed]
- 37.Khan N, et al. Post-COVID-19: can digital solutions lead to a more equitable global healthcare workforce? BJPsych Int. 2023;20(1):18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clinical management of COVID-19: living guideline, 13 January 2023. Geneva: World Health Organization; 2023. (WHO/2019-nCoV/clinical/2023.1). Licence: CC BY-NC-SA 3.0 IGO.
- 39.Pichon-Riviere A, et al. Determining the efficiency path to universal health coverage: cost-effectiveness thresholds for 174 countries based on growth in life expectancy and health expenditures. Lancet Global Health. 2023;11(6):e833–42. [DOI] [PubMed] [Google Scholar]
- 40.Collaborators G. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. 2022;328(16):1604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cutler DM. The economic cost of long COVID: an update. Publish Online July; 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed for this study are stored in the University of Warwick databases and available from the corresponding author on reasonable request. Requests can be sent to wctudataaccess@warwick.ac.uk.