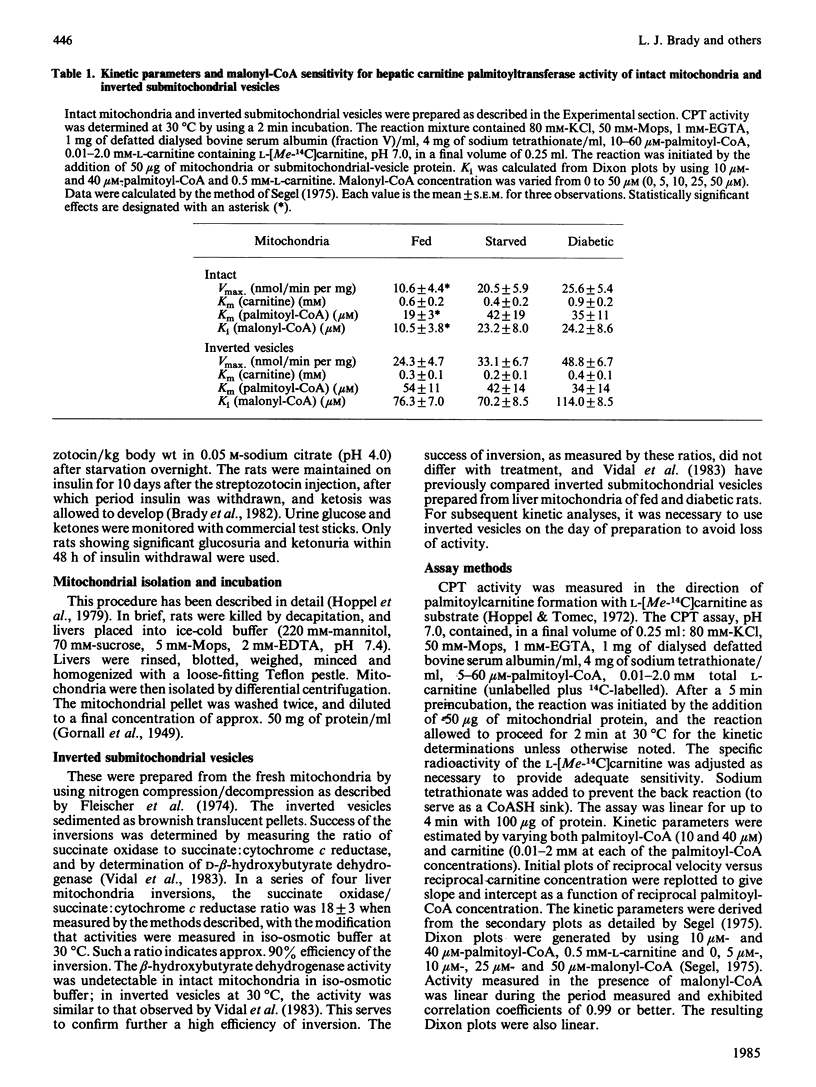
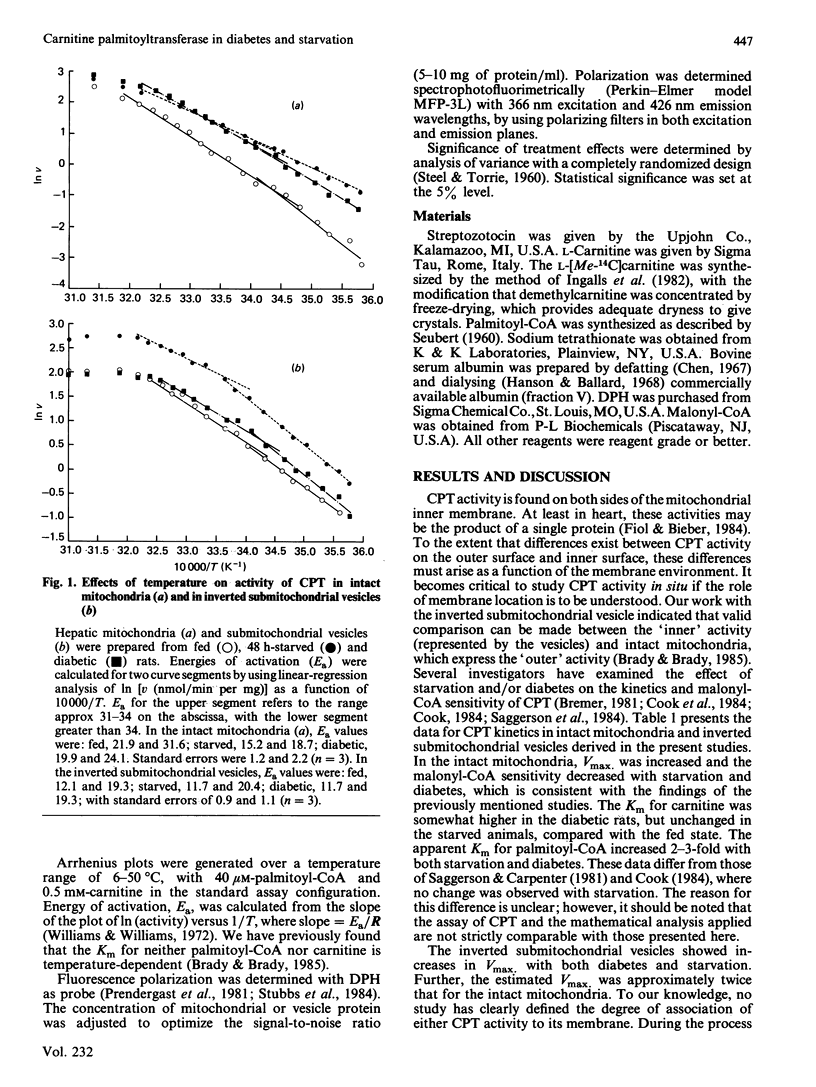
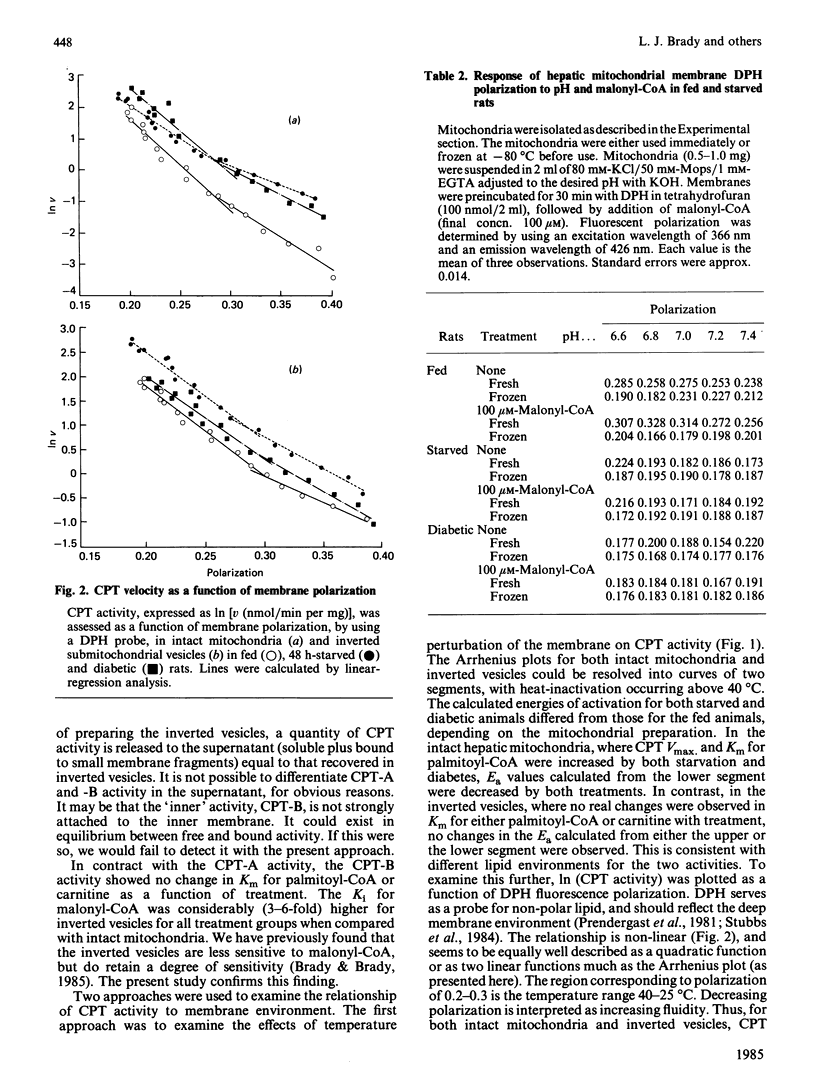
Abstract
Intact mitochondria and inverted submitochondrial vesicles were prepared from the liver of fed, starved (48 h) and streptozotocin-diabetic rats in order to characterize carnitine palmitoyltransferase kinetics and malonyl-CoA sensitivity in situ. In intact mitochondria, both starved and diabetic rats exhibited increased Vmax., increased Km for palmitoyl-CoA, and decreased sensitivity to malonyl-CoA inhibition. Inverted submitochondrial vesicles also showed increased Vmax. with starvation and diabetes, with no change in Km for either palmitoyl-CoA or carnitine. Inverted vesicles were uniformly less sensitive to malonyl-CoA regardless of treatment, and diabetes resulted in a further decrease in sensitivity. In part, differences in the response of carnitine palmitoyltransferase to starvation and diabetes may reside in differences in the membrane environment, as observed with Arrhenius plots, and the relation of enzyme activity and membrane fluidity. In all cases, whether rats were fed, starved or diabetic, and whether intact or inverted vesicles were examined, increasing membrane fluidity was associated with increasing activity. Malonyl-CoA was found to produce a decrease in intact mitochondrial membrane fluidity in the fed state, particularly at pH 7.0 or less. No effect was observed in intact mitochondria from starved or diabetic rats, or in inverted vesicles from any of the treatment groups. Through its effect on membrane fluidity, malonyl-CoA could regulate carnitine palmitoyltransferase activity on both surfaces of the inner membrane through an interaction with only the outer surface.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergstrom J. D., Reitz R. C. Studies on carnitine palmitoyl transferase: the similar nature of CPTi (inner form) and CPTo (outer form). Arch Biochem Biophys. 1980 Oct 1;204(1):71–79. doi: 10.1016/0003-9861(80)90008-9. [DOI] [PubMed] [Google Scholar]
- Brady P. S., Schumann W. C., Ohgaku S., Scofield R. F., Landau B. R. Evidence for an underestimation of the shunt pathway of mevalonate metabolism in slices of livers and kidneys from fasted rats and rats in diabetic ketosis. J Lipid Res. 1982 Dec;23(9):1317–1320. [PubMed] [Google Scholar]
- Bremer J. The effect of fasting on the activity of liver carnitine palmitoyltransferase and its inhibition by malonyl-CoA. Biochim Biophys Acta. 1981 Sep 24;665(3):628–631. doi: 10.1016/0005-2760(81)90282-4. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Clarke P. R., Bieber L. L. Isolation and purification of mitochondrial carnitine octanoyltransferase activities from beef heart. J Biol Chem. 1981 Oct 10;256(19):9861–9868. [PubMed] [Google Scholar]
- Cook G. A. Differences in the sensitivity of carnitine palmitoyltransferase to inhibition by malonyl-CoA are due to differences in Ki values. J Biol Chem. 1984 Oct 10;259(19):12030–12033. [PubMed] [Google Scholar]
- Cook G. A., Stephens T. W., Harris R. A. Altered sensitivity of carnitine palmitoyltransferase to inhibition by malonyl-CoA in ketotic diabetic rats. Biochem J. 1984 Apr 1;219(1):337–339. doi: 10.1042/bj2190337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer S., Meissner G., Smigel M., Wood R. Preparation of submitochondrial vesicles using nitrogen decompression. Methods Enzymol. 1974;31:292–299. doi: 10.1016/0076-6879(74)31030-0. [DOI] [PubMed] [Google Scholar]
- Hanson R. W., Ballard F. J. Citrate, pyruvate, and lactate contaminants of commercial serum albumin. J Lipid Res. 1968 Sep;9(5):667–668. [PubMed] [Google Scholar]
- Harano Y., Kowal J., Yamazaki R., Lavine L., Miller M. Carnitine palmitoyltransferase activities (1 and 2) and the rate of palmitate oxidation in liver mitochondria from diabetic rats. Arch Biochem Biophys. 1972 Dec;153(2):426–437. doi: 10.1016/0003-9861(72)90360-8. [DOI] [PubMed] [Google Scholar]
- Hoppel C. L. Carnitine and carnitine palmitoyltransferase in fatty acid oxidation and ketosis. Fed Proc. 1982 Oct;41(12):2853–2857. [PubMed] [Google Scholar]
- Hoppel C. L., Tomec R. J. Carnitine palmityltransferase. Location of two enzymatic activities in rat liver mitochondria. J Biol Chem. 1972 Feb 10;247(3):832–841. [PubMed] [Google Scholar]
- Hoppel C., DiMarco J. P., Tandler B. Riboflavin and rat hepatic cell structure and function. Mitochondrial oxidative metabolism in deficiency states. J Biol Chem. 1979 May 25;254(10):4164–4170. [PubMed] [Google Scholar]
- Kiorpes T. C., Hoerr D., Ho W., Weaner L. E., Inman M. G., Tutwiler G. F. Identification of 2-tetradecylglycidyl coenzyme A as the active form of methyl 2-tetradecylglycidate (methyl palmoxirate) and its characterization as an irreversible, active site-directed inhibitor of carnitine palmitoyltransferase A in isolated rat liver mitochondria. J Biol Chem. 1984 Aug 10;259(15):9750–9755. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- Prendergast F. G., Haugland R. P., Callahan P. J. 1-[4-(Trimethylamino)phenyl]-6-phenylhexa-1,3,5-triene: synthesis, fluorescence properties, and use as a fluorescence probe of lipid bilayers. Biochemistry. 1981 Dec 22;20(26):7333–7338. doi: 10.1021/bi00529a002. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Bird M. I., Carpenter C. A., Winter K. A., Wright J. J. Cycloheximide blocks changes in rat liver carnitine palmitoyltransferase 1 activity in starvation. Biochem J. 1984 Nov 15;224(1):201–206. doi: 10.1042/bj2240201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Effects of fasting, adrenalectomy and streptozotocin-diabetes on sensitivity of hepatic carnitine acyltransferase to malonyl CoA. FEBS Lett. 1981 Jul 6;129(2):225–228. doi: 10.1016/0014-5793(81)80170-6. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A., Tselentis B. S. Effects of thyroidectomy and starvation on the activity and properties of hepatic carnitine palmitoyltransferase. Biochem J. 1982 Dec 15;208(3):667–672. doi: 10.1042/bj2080667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stakkestad J. A., Bremer J. The outer carnitine palmitoyltransferase and regulation of fatty acid metabolism in rat liver in different thyroid states. Biochim Biophys Acta. 1983 Feb 7;750(2):244–252. doi: 10.1016/0005-2760(83)90025-5. [DOI] [PubMed] [Google Scholar]
- Stubbs C. D., Kinosita K., Jr, Munkonge F., Quinn P. J., Ikegami A. The dynamics of lipid motion in sarcoplasmic reticulum membranes determined by steady-state and time-resolved fluorescence measurements on 1,6-diphenyl-1,3,5-hexatriene and related molecules. Biochim Biophys Acta. 1984 Sep 5;775(3):374–380. doi: 10.1016/0005-2736(84)90193-7. [DOI] [PubMed] [Google Scholar]
- Vidal J. C., McIntyre J. O., Churchill P., Andrew J. A., Péhuet M., Fleischer S. Influence of diabetes on rat liver mitochondria: decreased unsaturation of phospholipid and D-beta-hydroxybutyrate dehydrogenase activity. Arch Biochem Biophys. 1983 Jul 15;224(2):643–658. doi: 10.1016/0003-9861(83)90252-7. [DOI] [PubMed] [Google Scholar]


