Abstract
Venetoclax is a promising drug for patients with acute myeloid leukemia (AML) ineligible for anthracycline-based treatments. In rats, venetoclax is reported to cause myocardial injury. Our objectives were to report the frequency of cardiovascular (CV) events in patients treated with venetoclax, and, subsequently, to compare CV outcomes in matched patients treated with venetoclax or anthracyclines. Patients diagnosed with AML and treated with venetoclax or anthracyclines from January 2017 to July 2021 were identified. Major adverse cardiac events (MACE, including new-onset heart failure (HF), acute myocardial infarction, new onset atrial fibrillation (AF)) were recorded. Propensity-score method was then used to compare patients treated with venetoclax or anthracyclines. Patients treated with venetoclax (n=103) were older, with more hyperlipidemia than patients treated with anthracyclines (n=217). However, only 63% of patients treated with venetoclax underwent echocardiographic screening (vs. 93% of patients treated with anthracyclines, P< 0.001). Eighteen patients with venetoclax (17%) and 27 patients with anthracyclines (12%) developed MACE, including 10 % of new HF in each group. The median time to MACE was 8 days (interquartile range 5-98 days). In the matched cohort (n=132 patients), the cumulative incidence of MACE at one year was not different (17.5 % venetoclax, 9.2% anthracyclines, p =0.27). Thus, MACE incidence is similar in matched patients receiving venetoclax or anthracyclines. Close CV monitoring during the early phase of treatment may be helpful in patients treated with venetoclax.
Keywords: Venetoclax, Cardiotoxicity, Heart failure, Atrial fibrillation
Background
Venetoclax, a B-cell leukemia/lymphoma-2 inhibitor, is a promising novel drug that is currently used with hypomethylating agents (HMAs) in older patients with acute myeloid leukemia (AML) [1]. These patients are known to have a high burden of cardiovascular (CV) risk factors and comorbidities [2]. Additionally, venetoclax itself was reported to cause cardiac injury in a rat model [3]. The previous study revealed a cardiotoxicity of venetoclax [4, 5]. However, current guidelines do not provide appropriate strategy for preventing a cardiotoxicity by venetoclax as opposed to anthracyclines [6, 7].
Our aim was to characterize the prevalence of CV events in patients with AML treated with venetoclax, and by using propensity matching, compare the CV outcomes of venetoclax with those of anthracyclines in matched patients.
Methods
After approval by the local Institutional Review Board, adult patients with AML treated with venetoclax or anthracyclines between January 2017 and July 2021 at the Hospital of the University of Pennsylvania were retrospectively identified. Patients who received both anthracyclines and venetoclax were excluded. Patients with a history of atrial fibrillation (AF) or a history of symptomatic heart failure (HF) were also excluded to allow for diagnosis of new-onset events. The entry date was defined as the date of initiation of venetoclax or anthracyclines. Baseline clinical characteristics were extracted from the medical records. The endpoint of the study was the development of major adverse cardiac events (MACE), including new-onset symptomatic HF, acute myocardial infarction (AMI) or new onset AF. Heart failure was identified by using the American College of Cardiology/American Heart Association outcome definition for clinical trials. The MACE were adjudicated by two independent cardiologists (T.O. and Y.K.) with a third cardiologist (M.S-C.) in case of disagreement. Patients with sepsis or critical illness at the time of MACE were excluded. Baseline categorical variables are presented as frequencies, and continuous variables as mean ± SD or median with 25th to 75th percentile (Q1-Q3). Baseline continuous variables were compared using unpaired t-test or Mann-Whitney U test, differences between proportions assessed using chi-square analysis. To compare the occurrence of MACE in patients treated with venetoclax or anthracyclines, propensity score matching was performed. Logistic regression analysis was used to calculate a propensity score by examining all potential confounders of the association between venetoclax exposure and the outcomes of interest. Using the nearest neighbor matching with a caliper width of 0.2 SD, patients treated with venetoclax were matched with patients treated with anthracyclines. Patients were censored at the time of death or the date of last encounter (before November 31, 2022). Univariable Fine and Gray’s subdistribution hazard regression models were used to analyze the association between baseline variables and subsequent MACE. A p-value < 0.05 was considered significant. Statistical analyses were performed by R version ii386 3.5.0 (Vienna, Austria) and JMP 16.0 (SAS, Cany, NC).
Results
Of 320 consecutive patients with AML identified at the Hospital of the University of Pennsylvania, 103 patients (32%) received venetoclax (median age 64.0 [54.0-70.8] years). Baseline characteristics are summarized in the Table 1. Patients treated with venetoclax were older and had a higher prevalence of hyperlipidemia, and statin treatment. Compared to patients with anthracyclines, pre-treatment echocardiograms were less frequent (63% vs 93%, p < 0.001). There were no significant differences in left ventricular ejection fraction between 2 groups (61 [55-65]% vs 62.5 [60-65]%, p = 0.16). Eighteen patients treated with venetoclax and 27 patients treated with anthracyclines developed MACE (cumulative incidence of MACE within 1 year 19.2% and 7.3% respectively, p = 0.010). Ten patients treated with venetoclax and 20 patients treated with anthracyclines developed HF (cumulative incidence of HF within 1 year 11.4% and 6.4% respectively, p = 0.39). Twelve patients treated with venetoclax and 13 patients treated with anthracyclines developed AF (cumulative incidence of AF within 1 year 12.0% and 3.8% respectively, p = 0.033). Univariate Fine and Gray’s analysis in the patients treated with venetoclax demonstrated that female sex (hazard ratio (HR) 3.66 [1.32-10.0]) was associated with the incidence of MACE.
Table 1.
Baseline characteristics of patients treated with venetoclax or anthracyclines
| Variables | Venetoclax N = 103 |
Anthracyclines N = 217 |
p value | Standardized difference |
|---|---|---|---|---|
| Age, yrs | 71 [67-76] | 58 [47-66] | < 0.001 | 1.35 |
| Gender male/female, (%) |
56/47 (54/46) |
109/108 (50/50) |
0.49 | 0.08 |
| Body mass index, kg/m2 |
27.4 [24.3-31.7] |
27.1 [23.4-31.2] |
0.47 | 0.02 |
| ACEi/ARB, n (%) | 32 (31) | 51 (24) | 0.15 | 0.17 |
| β blockers, n (%) | 15 (15) | 33 (15) | 0.88 | 0.02 |
| Statins, n (%) | 51 (50) | 63 (29) | < 0.001 | 0.43 |
| Aspirin, n (%) | 22 (21) | 41 (19) | 0.60 | 0.06 |
| Hypertension, n (%) | 50 (48.5) | 115 (53) | 0.46 | 0.09 |
| Hyperlipidemia, n (%) | 57 (55) | 74 (34) | < 0.001 | 0.43 |
| Diabetes mellitus, n (%) | 25 (24) | 53 (24) | 0.98 | 0.00 |
| History of CAD, n (%) | 18 (17) | 25 (12) | 0.14 | 0.17 |
| History of CKD | 27 (26) | 57 (26) | 0.99 | 0.00 |
| History of AF | 0 (0) | 0 (0) | 1.0 | 0.00 |
| History of HF | 0 (0) | 0 (0) | 1.0 | 0.00 |
| Echo screening, n (%) | 65 (63) | 202 (93) | < 0.001 | 0.78 |
| LVEF, % | 61 [55-65] | 62.5 [60-65] | 0.16 | 0.47 |
| Event, n (%) | ||||
| MACE, n (%) | 18 (17) | 27 (12) | 0.23 | |
| HF, n(%) | 10 (10) | 20 (9) | 0.89 | |
| New AF, n(%) | 12 (12) | 13 (6) | 0.087 | |
| New myocardial infarction, n(%) | 1 (1) | 0 (0) | 0.13 | |
| Death, n (%) | 80 (78) | 89 (41) | < 0.001 | |
| Time to event (days) | ||||
| MACE | 8 [5-98] | 203 [30-1042] | < 0.001 | |
| HF | 12 [5-247] | 281 [28-995] | 0.004 | |
| New AF | 9 [5-30] | 85 [24-767] | 0.005 | |
| Death | 157 [64-444] | 264 [107-561] | 0.080 | |
| Follow-up period, days | 186 [75-491] | 491 [170-1074] | < 0.001 | |
| Cumulative incidence for 1 year, % | ||||
| MACE | 19.2 | 7.3 | 0.010 | |
| HF | 11.4 | 6.4 | 0.39 | |
| AF | 12.0 | 3.8 | 0.033 | |
Continuous data are expressed as median (25th to 75th percentile)
ACEi Angiotensin-converting enzyme inhibitors, ARB Angiotensin receptor blocker, BP Blood pressure, bpm beats per minute, CAD Coronary artery disease, CKD Chronic kidney disease, COPD Chronic obstructive pulmonary disease, CVD Cerebrovascular disease, Hx History, LVEF Left ventricular ejection fraction
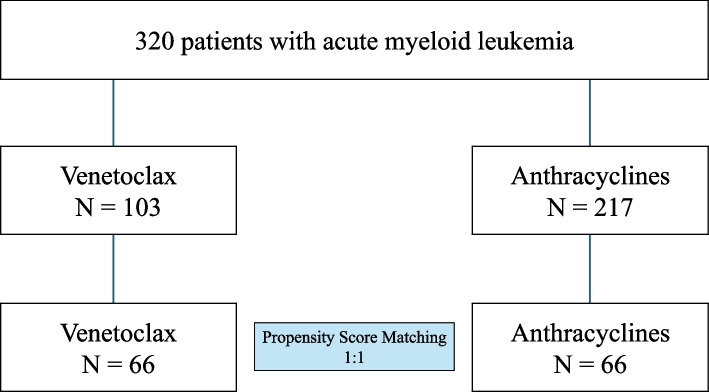
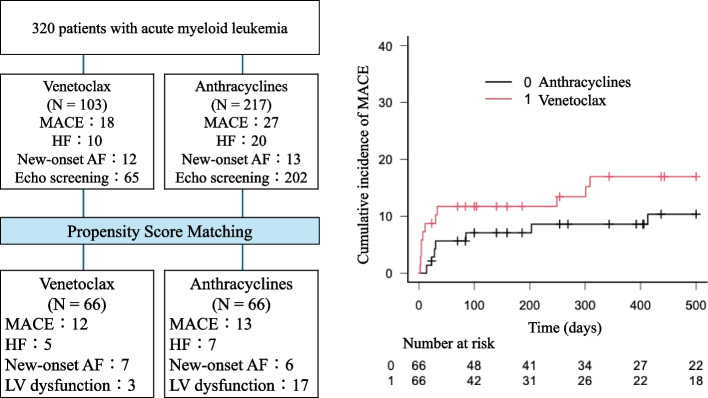
To generate the propensity score, age, gender, body mass index, hyperlipidemia, diabetes mellitus, history of coronary artery disease, history of chronic kidney disease, prescription of angiotensin-converting enzyme inhibitors or angiotensin receptor blocker, beta blockers, and statins were included. Sixty six patients treated with venetoclax were matched with 66 patients treated with anthracyclines (Fig. 1). All clinical variables were well balanced.
Fig. 1.
Flowchart of the Study
In the matched cohort, 10 patients in the venetoclax group (12 MACE: HF in 5 patients, new onset AF in 7 patients) and 10 patients in the anthracycline group (13 MACE: HF in 7 patients, new onset AF in 6 patients) developed MACE. The median time to MACE tended to be shorter in the venetoclax group (9 [4-262] days) than in the anthracycline group (58 [23-875] days, p = 0.064). Cumulative incidence of MACE within one year (17.5 % in the venetoclax group, 9.2 % in the anthracycline group, p = 0.27), and of HF (8.4% in venetoclax group, 7.6 % in anthracycline group, p = 0.78) were not different (Figs. 2 and 3). In the patients who underwent an echocardiographic examination before and after chemotherapy, 17 of 51 patients treated with anthracyclines (33%) and 3 of 25 patients treated with venetoclax (12%) developed a reduction in LVEF of ≥ 10% to a value < 50%. One patient developed HF, including one who developed HF concomitantly to tumor lysis syndrome.
Fig. 2.
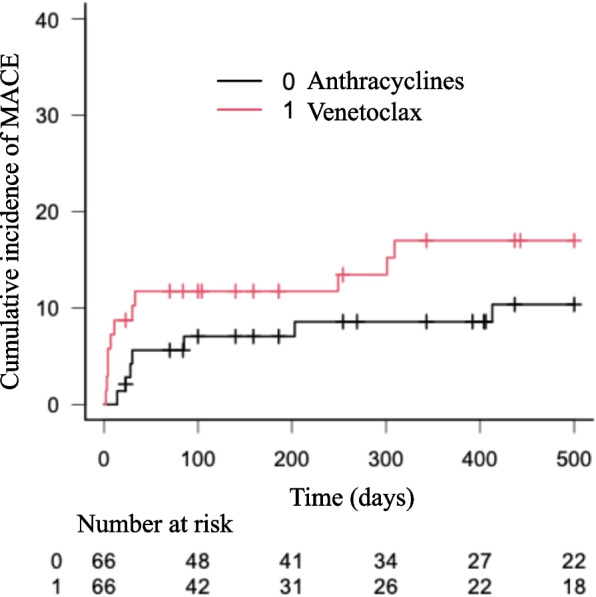
Cumulative incidence of Major Adverse Cardiac Events in a matched cohort of patients with AML treated with venetoclax or anthracyclines. Central Illustration. Major Adverse Cardiac Events in Patients with AML treated with anthracyclines or venetoclax. (Left) Flowchart and outcomes of the study. (Right) Cumulative incidence curve of MACE in a matched cohort of patients treated with venetoclax or anthracyclines
Fig. 3.
Central Illustration
Discussion
In the present retrospective study, patients treated with venetoclax had a cumulative incidence of MACE of 18.6% at one year, including 10.5% new symptomatic HF, higher to that of patients treated with anthracyclines during the same time period. This high occurrence in patients with venetoclax may be at least in part explained by the patient population itself. Patients treated with venetoclax were older and had more hyperlipidemia than patients treated with anthracyclines; however, when patients were matched for CV risk factors and history, the incidence of MACE remained similar between the venetoclax and anthracyclines groups.
Venetoclax could be cardiotoxic through several mechanisms. In experimental studies, treatment of rats with venetoclax increased the myocardial expression of hypertrophic markers such as β-Mhc and Bnp, and of pro-apoptotic proteins such as Bax while reducing the expression of the anti-apoptotic protein Bcl-2. Furthermore, venetoclax treatment increased reactive oxygen species production and activation of inflammation, as demonstrated by the decrease in the Sod-2 protein level and the increased gene and protein expression of Nf-κb-p-65 [3]. A previous retrospective study reported 20% MACE in patients with AML treated with venetoclax during a median follow-up period of 14 months [4]; although the present follow-up period was shorter (with a median of 6.3 months), the rate was comparable, underlining that most events occur early in this cohort.
Echocardiographic data and biomarkers were not systematically obtained in this retrospective study, preventing any conclusions on elevations in troponin levels or decreases in left ventricular EF in patients treated with anthracyclines or venetoclax [4, 5]. Although patients treated with anthracyclines and venetoclax were matched as thoroughly as possible, there may remain some potential confounders that prevent complete comparison of the two cohorts. Therefore, not all complications may be attributable to the venetoclax treatment itself.
Current cardio-oncology guidelines recommend evaluation of cardiac function before cardiotoxic chemotherapy; almost all patients treated with anthracyclines had an echocardiogram in our study [6, 7]. There are no guidelines for patients treated with venetoclax, and only 63 % of these patients had a baseline echocardiogram. The present results support a more careful cardiac evaluation and monitoring in patients treated with venetoclax. Prospective randomized longitudinal follow-up studies, using echocardiography, biomarkers and rhythm monitoring are needed to clarify the appropriate strategy for monitoring cardiotoxicity in patients treated with venetoclax.
Abbreviations
- AF
Atrial fibrillation
- AMI
Acute myocardial infarction
- AML
Acute myeloid leukemia
- EF
Ejection fraction
- HF
Heart failure
- HMAs
Hypomethylating agents
- MACE
Major adverse cardiac events
Authors’ contributions
T.O. and MS-C; design of the work and drafted the work AH. M and SR. M.; acquition of data J.C.; analysis of data A.V., Y.K., B.L, AM. S., and J.C.; interepretation of data
Funding
The study was supported by NIH-NHLBI R01 HL130539 (to MS-C).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was conducted at the Hospital of the University of Pennsylvania and was approved by the local Institutional Review Board. This study was a retrospective study a waiver of consent was obtained.
Consent for publication
This study was a retrospective study, a waiver of consent was obtained.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med. 2020;383:617–29. [DOI] [PubMed] [Google Scholar]
- 2.Tian F, Chen L, Qian ZM, et al. Ranking age-specific modifiable risk factors for cardiovascular disease and mortality: evidence from a population-based longitudinal study. EClinicalMedicine. 2023;64:102230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AlAsmari AF, Alghamdi A, Ali N, et al. Venetoclax Induces Cardiotoxicity through Modulation of Oxidative-Stress-Mediated Cardiac Inflammation and Apoptosis via NF-kappaB and BCL-2 Pathway. Int J Mol Sci. 2022;23(11):6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson IM, Bezerra ED, Farrukh F, et al. Cardiac events in patients with acute myeloid leukemia treated with venetoclax combined with hypomethylating agents. Blood Adv. 2022;6:5227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson IM, Karrar O, Rana M, et al. Cardiac events in newly diagnosed acute myeloid leukaemia during treatment with venetoclax + hypomethylating agents. Br J Haematol. 2024;204:1232–7. [DOI] [PubMed] [Google Scholar]
- 6.Curigliano G, Lenihan D, Fradley M, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31:171–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyon AR, Lopez-Fernandez T, Couch LS, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43(41):4229–361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.