Abstract
Background
Periodontitis is a chronic inflammatory disease characterized by the destruction of the components of the periodontium. It significantly impacts oral health and has been linked to systemic conditions like cardiovascular disease and diabetes. The critical role of neutrophils in the occurrence and development of chronic periodontitis has been paid increasing attention. The study aimed to explore the protective effects of D-mannose on chronic periodontitis and determine whether its underlying mechanisms is related to neutrophils.
Methods
To explore the protective effects of D-mannose on chronic periodontitis, the eight-week-old Sprague Dawley rat model of lipopolysaccharide (LPS)-induced periodontitis was established, followed by D-mannose treatment by oral gavage. To evaluate the protective effects of D-mannose against periodontal bone loss, methylene blue staining, hematoxylin and eosin (H&E) staining, and micro-CT scanning were utilized. Then, immunofluorescence (IF), Western Blot, and RT-PCR were applied to assess the expression levels of pro-inflammatory cytokines (IL-1β, IL-6, and IL-17), anti-inflammatory cytokine (IL-10), tumor necrosis factor-alpha (TNF-α), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), ten-eleven translocation 2 (TET2), and key glycolytic enzymes (HK1, HK2, PFKFB3), and to examine D-mannose’s impact on the recruitment and activation of neutrophils in the gingiva. Additionally, neutrophils isolated from the peripheral blood of healthy rats were treated with LPS and D-mannose, and changes in the expression levels of myeloperoxidase (MPO), IL-1β, IL-6, IL-17, IL-10, and TET2 were observed via IF.
Results
In vivo, D-mannose inhibited LPS-induced alveolar bone resorption in rats. After D-mannose treatment, the expression levels of IL-17 (p<0.01) and TET2 (p<0.01) were suppressed by IF, and the expression levels of IL-1β (p<0.05), IL-17 (p<0.05) and TET2 (p<0.01) were downregulated by WB. The results of qPCR showed that D-mannose reduced the expression levels of IL-1β (p<0.05), IL-6 (p<0.01), IL-17 (p<0.01), TNF-α (p<0.01), G-CSF (p<0.01), GM-CSF (p<0.01), TET2 (p<0.01), HK1 (p<0.01), HK2 (p<0.01), and PFKFB3 (p<0.01). D-mannose also inhibited the recruitment and activation of neutrophils in LPS-treated rat gingival tissues. In vitro, the results of IF showed that D-mannose inhibited the activation of neutrophils stimulated by LPS, downregulated the expression of IL-1β (p < 0.05), IL-6, IL-17 (p < 0.01), and TET2 (p < 0.01), and upregulated the expression of IL-10 (p < 0.01).
Conclusions
D-mannose can alleviate chronic periodontitis in rats by regulating the functions of neutrophils, potentially associated with the expression of TET2 and glycolysis, providing new insights into the potential application of D-mannose to chronic periodontitis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-024-05080-1.
Keywords: Chronic periodontitis, Mannose, Neutrophils
Introduction
Periodontitis is an alarming world public health problem [1]. Between 2011 and 2020, periodontitis in dentate adults was estimated to be around 62% and severe periodontitis 23.6%, unusually higher than the previous estimates from 1990 to 2010 [2]. More importantly, there is increased evidence of the epidemiologic associations between periodontitis and systemic diseases [3]. As a chronic and inflammatory disease, periodontitis is characterized by the destruction of the components of the periodontium, including gingiva, periodontal ligament, alveolar bone, and cementum [4]. Conventional non-surgical therapies for periodontitis focus mainly on plaque control and fail to achieve periodontal regeneration [5, 6]. Thus, it is high time to develop more effective preventive and therapeutic approaches for periodontitis.
Lots of natural substances have roles in the treatment of periodontitis. For instance, probiotics have been tested as effective adjuvants to non-surgical periodontal therapy, supporting the gold-standard treatment of periodontitis [7]. Besides, S-propargyl-cysteine (SPRC) can prevent the progression of periodontitis by regulating the Th17/Treg balance in vivo and in vitro [8]. As the C-2 epimer of glucose, D-mannose is a natural, safe, and multifunctional small molecule. D-mannose is a ubiquitous component of mammalian sera, whose average concentration is less than one-fiftieth of that of glucose [9]. When it increased up to 3- to 5-fold higher in human blood, no side effects were observed [10]. D-mannose exhibits strong anti-inflammatory properties, seen as a safe and promising novel strategy to suppress inflammatory diseases [11]. It can suppress inflammation by inducing regulatory T cells (Tregs), suppressing effector T cells and inflammatory macrophages, and increasing anti-inflammatory gut microbiome [11]. As for bone loss, it was found that D-mannose attenuated bone loss induced by senility and estrogen deficiency in mice, which may be mediated by D-mannose-induced proliferation of Tregs and gut microbiota-dependent anti-inflammatory effects [12]. Another study showed that D-mannose protected against bone loss in rats under weightlessness, which was mediated by inhibiting osteoclast cell fusion [13].
Recent studies have revealed a deeper insight into the critical role of neutrophils in the balanced regulation of chronic periodontitis. They are the most abundant leukocytes in periodontal pockets, the gingival crevice, and inflamed periodontal tissues [14], and the number of neutrophils increases with the severity of periodontal inflammation [15–18]. A recent study supported the pathogenic role of neutrophil extracellular traps (NETs) in the induction of IL-17/Th17 immunopathology in periodontitis [19]. Due to the pivotal role of neutrophils, neutrophil-modulating agents that can suppress hyper-responsive and poorly regulated neutrophils have been seen as potential therapeutic approaches in periodontitis [20, 21]. Importantly, neutrophil oxidative burst in inflammatory response to bacteria may be attenuated by D-mannose [22]. However, the effect of D-mannose on neutrophils in the development of periodontitis remains unclear. Therefore, we hypothesized that D-mannose could alleviate periodontitis by regulating the functions of neutrophils. To determine the effect of D-mannose on regulating neutrophils and alleviating periodontitis, we evaluated the degree of bone loss and gingival inflammation, and investigated the functions of neutrophils. Using the established model of LPS-induced periodontitis in vivo and treating primary neutrophils with LPS in vitro, the role of D-mannose on regulating the functions of neutrophils was evaluated by methylene blue staining, H&E staining, micro-CT scanning, IF, Western Blot, and RT-PCR, and so on. Hopefully, our work can provide new insights into the mechanism by which D-mannose treat periodontitis and suggest a new treatment target of periodontitis.
Materials and methods
Animals and treatments
Eighteen (half female, half male) eight-week-old Sprague Dawley rats were purchased from Huachuang Sino Co., Ltd. (Jiangsu, Taizhou). The sample size was determined using the resource equation method. All experimental equipment and procedures were examined and approved by the Institutional Animal Care and Use Committee of Shanghai Rat & Mouse Biotech Co., Ltd (No. SHDSYY-2022-3063-9). After adaptively fed for a week, they were anesthetized with 2% pentobarbital sodium (0.2 ml/100 g) and intralingual injected 2 µl of LPS (Salmonella typhimurium, Sigma, United States) (10 µg/µl/d) between the first molar and the second molar to induce periodontitis only one injection once a day for the first 2 days. The injection was made at the mesolateral side of the interdental papilla on both sides of the maxillary with a micro syringe (Hamilton, Switzerland). It was performed slowly, and the needle was kept in place for seconds after injection to guarantee that LPS was not lost after extraction. For D-mannose treatment, starting from the first day of the LPS challenge, rats were treated with 2 ml of 20% (w/v) D-mannose (Sigma-Aldrich, United States) by oral gavage twice a day for 14 days. The concentration of D-mannose is calculated based on our previous research [23] and the weight of rats and mice. In our previous research, mice were given 10% (m/v) D-mannose in drinking water and 200 uL 20% (m/v) D-mannose by oral gavage daily. In order to ensure that each rat receives the same amount of D-mannose, we simplified the administration method to simple gavage. The weight of rats is about ten times that of mice. So we finally decided to treat rats with 2 ml of 20% (w/v) D-mannose by oral gavage twice a day. According to Institutional Animal Care and Use Committee (IACUC), we euthanized rats with 150 mg/kg pentobarbital sodium. To evaluate periodontitis lesions, rats were sacrificed at 14 days (Fig. 1A). The eighteen rats were randomly divided into the following experimental groups (n = 6 for each group), using a computer-based random order generator. Only the investigator who administered the treatment based on the randomization table was aware of the treatment group allocation.
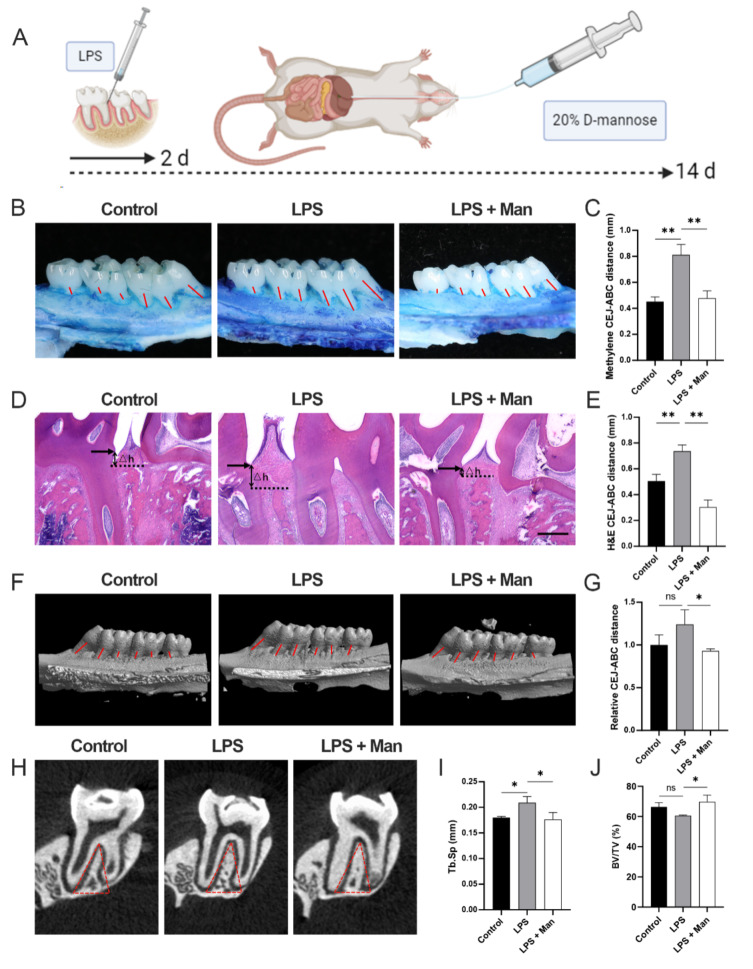
Fig. 1.
Effect of D-mannose on LPS-induced alveolar bone resorption in rats. (A) Schema chart; (B) Methylene staining of bone resorption of left maxilla; (C) Methylene staining data quantitative analysis; red lines indicate the measurement of the CEJ-ABC distance at each tooth root ; (D) H&E staining of bone resorption of maxilla sections; △h represents the measured CEJ-ABC distance (50 ×; Scale bars represent 100 μm); (E) H&E staining data quantitative analysis; (F) Sagittal 3D images; red lines indicate the measurement of the CEJ-ABC distance at each tooth root; (G) Relative CEJ-ABC distance assessed by micro-CT; (H) Coronal plane of the area under the second molar bifurcation (2D picture) red line indicate ROI; (I) Tb.Sp assessed by micro-CT; (J) BV/TV assessed by micro-CT. Data are shown as the means ± SD (n = 6 for methylene, H&E staining and micro-CT). *p < 0.05 vs. the LPS group, **p < 0.01 vs. the LPS group
−Control group: rats received a single intralingual injection of saline solution with an identical surgical procedure as described above.
−LPS group: rats were subjected to LPS-induced periodontitis as described above.
−LPS + Man group: rats were subjected to LPS-induced periodontitis as described above, and D-mannose was administered as described above.
Maxilla and soft tissue sample collection and treatment
The right maxillae and soft tissues from all samples were collected and fixed in 4% paraformaldehyde. The six fixed maxillae of each group were scanned and analyzed by micro-CT. All six samples of each group were then decalcified in 10% EDTA (EDTA 100 g, NaOH 11 g, Na2HPO4 6 g, NaH2PO4 9 g, ddH2O, mixed with 900 ml magnetic mixer, with ddH2O capacity to 1000 ml and PH to 7.2–7.4 and 4℃). After dehydration, the maxillae were embedded in paraffin for the paraffin section. The paraffin sections were stained with H&E. Meanwhile, IF staining proceeded in the next experiments. The left maxillae and gingival tissues from six samples in each group were collected. The six left gingival tissues were saved at − 80℃ for mRNA and protein extraction. The six left maxillae were stained by 1% methylene blue and then detected using the vernier caliper.
Micro-CT analysis
The right maxillae were selected as the tissue specimens. After fixing it in 4% paraformaldehyde for 24 h, the specimens were placed on the scanning bed parallel to the occlusal surface of the molar crown. Then, micro-CT (Skyscan1176, Bruker, United States) scanning was conducted (pixel resolution = 9 μm, 50 kV, 500 A).
The original data were reconstructed by the NRecon software (Skyscan, Kontich, Belgium) and transferred to the CTAnalyser and CTVox software (Skyscan, Kontich, Belgium) for further analysis. The triangular area below the fork root of the second molar root, whose width was 2 mm and depth was to the point at which the root forks until either root disappeared, was selected as the region of interest (ROI) (Fig. 1H, red line area). The cancellous bone in the ROI was selected and measured by an investigator thrice. The outcome measures included: percent bone volume (bone volume/tissue volume, BV/TV) and trabecular separation (Tb.Sp). To qualify bone loss, six-site (3 sites at the first molar, 2 sites at the second, and 1 at the third) cementoenamel junction and alveolar bone crest (CEJ-ABC) distance on the palatal side of each rat were selected and measured. All data of the CEJ -ABC distance at mentioned sites were analyzed by three blinded investigators. Mean values from the three measurements were calculated for further analysis.
IF staining and quantification
In vivo study, the maxillae were decalcified and embedded in paraffin, and then sliced into 5-mm-thick sections. In vitro study, after cell modeling, the cells were fixed in 4% paraformaldehyde at room temperature for 30 min and seeded on slides evenly.
For IF staining, after blocking (Thermo Fisher Scientific, United States), the slices were incubated with anti-IL-1β (1: 200, ABclonal, A22207, China), anti-IL-6 (1: 200, ABclonal, A22222, China), anti-IL-10 (1: 200, ABclonal, A12255, China), anti-IL-17 (1:1000, Abclonal, A10587, China), anti-MPO (1:200, Abmart, T62224, China), anti-TET2(1:200, Abclonal, A5682, China), anti-Ly6G (1:200, Abclonal, A22270, China) antibodies at 4℃ overnight. Then, after washing with PBS three times, the slices were incubated in the presence of Alexa Fluor 594 or Alexa Fluor 488-labeled secondary antibodies. If the sections were intended for co-localization staining, they were rinsed three times with PBS and incubated with a different secondary antibody for two hours at room temperature. The nucleus was visualized with DAPI and images were captured with a CarlZeiss LSM710 fluorescence microscope. The magnification of the objective lens is 10x, and the acquisition software is LAS V4.12 software. Images for each group were captured at the resolution of 1920 × 1440 pixels using uniform parameters (brightness, saturation, gamma adjustment, etc.). Brightness was consistently adjusted when processing photos.
For quantification, the relative fluorescence intensity was measured using ImageJ software. A fixed-area square ROI was drawn around the tissue and the Time Measurement analysis tool was used to measure the average fluorescent intensity within the ROI using measure function. Three individual fields were measured per tissue. Results are displayed as means ± SD. The ratio of positive cells was measured by ImageJ software as well. The number of cells displaying positive staining for the marker of interest versus the total number of cells was quantified and expressed as a percentage.
Western blot analysis
Protein samples were extracted from gingival tissues in RIPA lysis buffer (300 µl, BioSharp, China). BCA Protein Assay Kit (Reith Biotechnology, Shanghai, China), using BSA as a standard, was used to quantify the protein concentrations of the harvested lysate. Equal amounts of protein in each group mixed with 5× Sample Loading Buffer (BioSharp, China) were heated at 95℃ for 5 min, separated by electrophoresis on SDS-PAGE gels, and transferred onto polyvinylidene difluoride (PVDF) membranes (Merck Millipore, Temecula, CA, United States). After blocking in 5% milk in PBS for 1 h at room temperature, the membranes were incubated overnight at 4℃ with the following primary antibodies: GAPDH (1:10,000, Proteintech, 60004-1-Ig, United States), anti-IL-1β (1:1000, Cell Signal,12242 S, United States), anti-IL-6 (1:1000, Abclonal, A2222, China), anti-IL-10 (1:1000, Abclonal, A2171, China), anti-IL-17 (1:1000, Abclonal, A10587, China), anti-MPO (1:1000, Abcam, ab65871, United Kindom), anti-CD11b (1:1000, Abcam, ab133357, United Kingdom) and anti-TET2 (1:1000, Abclonal, A5682, China). The membranes were washed three times with PBST (PBS with 0.1% Tween-20), each lasting for 10 min, before applying secondary antibodies for 1 h at room temperature. Specific bands were visualized by Tanon-5200 Multi Chemiluminescent System (Tanon). Grayscale values were measured and counted using Image J software.
RT-PCR analysis
Total RNA was extracted from gingival tissues in each group by TRIzol (Thermo Fisher Scientific, United States). cDNAs were reversely transcribed with the PrimeScript RT Master Mix (Takara, Cat#RR036A, Japan) and subjected to RT-PCR using specific primers. The sequences of the forward and reverse primers were listed in Table 1.
Table 1.
The sequences of primers for RT-qPCR analysis
| Gene | Primer sequence (5’-3’) | |
|---|---|---|
| GAPDH | Forward primer | CAGGGCTGCCTTCTCTTGT |
| Reverse primer | TCCCGTTGATGACCAGCTTC | |
| IL-1β | Forward primer | CAACTGTCCCTGAACTCAACTGT |
| Reverse primer | GAGATGCTGCTGTGAGATTTGAA | |
| IL-6 | Forward primer | AAAGTCAACTCCATCTGCCCTTC |
| Reverse primer | TACTGGTCTGTTGTGGGTGGTAT | |
| IL-17 | Forward primer | GACTACCTCAACCGTTCCACTTC |
| Reverse primer | ACTTCTCAGGCTCCCTCTTCAG | |
| IL-10 | Forward primer | CTGCTATGTTGCCTGCTCTTACT |
| Reverse primer | GGCAACCCAAGTAACCCTTAAAG | |
| TNF-α | Forward primer | GACCCTCACACTCAGATCATCTT |
| Reverse primer | CCTTGAAGAGAACCTGGGAGTAG | |
| G-CSF | Forward primer | CCCAGAGCACCATGCCAATC |
| Reverse primer | TGCTCCAAGCAGGAGAACAAA | |
| GM-CSF | Forward primer | CACTACCAGACGAACTGCCC |
| Reverse primer | GGCTTCCAGCAGTCAAAAGG | |
| HK1 | Forward primer | CTCGCTAGGCAAGACCAGTA |
| Reverse primer | CTGGGTCTCCTGATCCTTGGG | |
| HK2 | Forward primer | TCAAAGAGAACAAGGGCGAG |
| Reverse primer | GAGGAAGCGGACATCACAG | |
| PFKFB3 | Forward primer | TGTAGAATCAGTGAGCACGC |
| Reverse primer | AAGTCAAGTTCGGTGTAAGAGG | |
| TET2 | Forward primer | CTTCTCTTGGCAGCTCCGAACAG |
| Reverse primer | GTGATGGTGGCGTCATGGTAGTG | |
Blood sample collection and neutrophil isolation
Twelve eight-week-old Sprague Dawley rats were purchased from Huachuang Sino Co., Ltd. (Jiangsu, Taizhou). All experimental equipment and procedures were examined and approved by the Institutional Animal Care and Use Committee of Shanghai Rat & Mouse Biotech Co., Ltd (No. SHDSYY-2022-3063-9). They were adaptively fed for a week. After anesthetized with 2% pentobarbital sodium (0.2 mL/100 g), rats blood samples from the abdominal aorta of all the rats were collected. Neutrophils were isolated from the blood sample with rat neutrophil isolation kit (Solarbio, P9200, China).
Flow cytometric analysis of neutrophils from the peripheral blood
Collected neutrophils (2 × 106 cells) were stained with certain antibodies against BV786 mouse anti-Rat RP-1 antigen (BD Biosciences, United States) to analyze the neutrophils frequency. The impacted cells were run with BD FACSCalibur and BD FACSAria III flow cytometers (BD Biosciences, United States). Using positive gates and gating controls by employing appropriate negative controls.
In vitro neutrophil treatments
The isolated neutrophils were randomly divided into three groups. All groups were placed in the incubator set to 37℃ with 5% CO₂ together. Only the investigator who administered the treatment based on the randomization table was aware of the treatment group allocation. After modeling, the neutrophils were centrifuged at 12,000 rpm for 2 min to remove the supernatant, resuspended and washed with 20 µl PBS, centrifuged again, and the supernatant removed. The cells were then fixed with 4% paraformaldehyde for 30 min, centrifuged, and the supernatant removed. After washing with PBS twice, the cells were spread on a marked culture plate and dried in an oven at 60℃ for about 5 min. Subsequent procedures followed the same protocol for tissue IF.
−Control group: neutrophils received no special treatment.
−LPS group: neutrophils were exposed to LPS for 4 h (100ng/ml, Porphyromonas gingivalis, Sigma-Aldrich, SMB00610, United States).
−LPS + Man group: before exposure to LPS at the same time with the LPS group, neutrophils were pretreated with a certain concentration of D-mannose for a certain period of time.
Statistical analysis
All data were analyzed using GraphPad Prism version 9.5.1 (733) software. Experimental data repeated at least three times are presented as the mean ± SD. Statistical comparisons were performed using one-way ANOVA followed by Dunnett’s post-hoc test, and p < 0.05 was considered statistically significant.
Results
D-Mannose suppresses LPS-induced alveolar bone resorption in rats
To investigate the potential of D-mannose in alleviating alveolar bone resorption in rats, a chronic periodontitis animal model was established (Fig. 1A). Compared to the control group, the CEJ-ABC distance revealed a increase in the LPS group by the methylene blue (Fig. 1B-C, p < 0.01), H&E staining (Fig. 1D-E, p < 0.01), and Micro-CT (Fig. 1F-G, p > 0.05), which showed that the rat periodontitis model was successfully constructed. Moreover, compared to the LPS group, the CEJ-ABC distance showed a significant decrease in the LPS + Man group in the study (Fig. 1B-G, p < 0.05). Quantitative analysis of the ROI confirmed that treatment with D-mannose led to a significant decrease in Tb. Sp and a significant increase in BV/TV compared to the LPS group (Fig. 1H-J). These results collectively showed that D-mannose inhibited the maxillary bone resorption in LPS-induced periodontitis rats.
D-mannose alleviates inflammation in gingival tissues of periodontitis rats
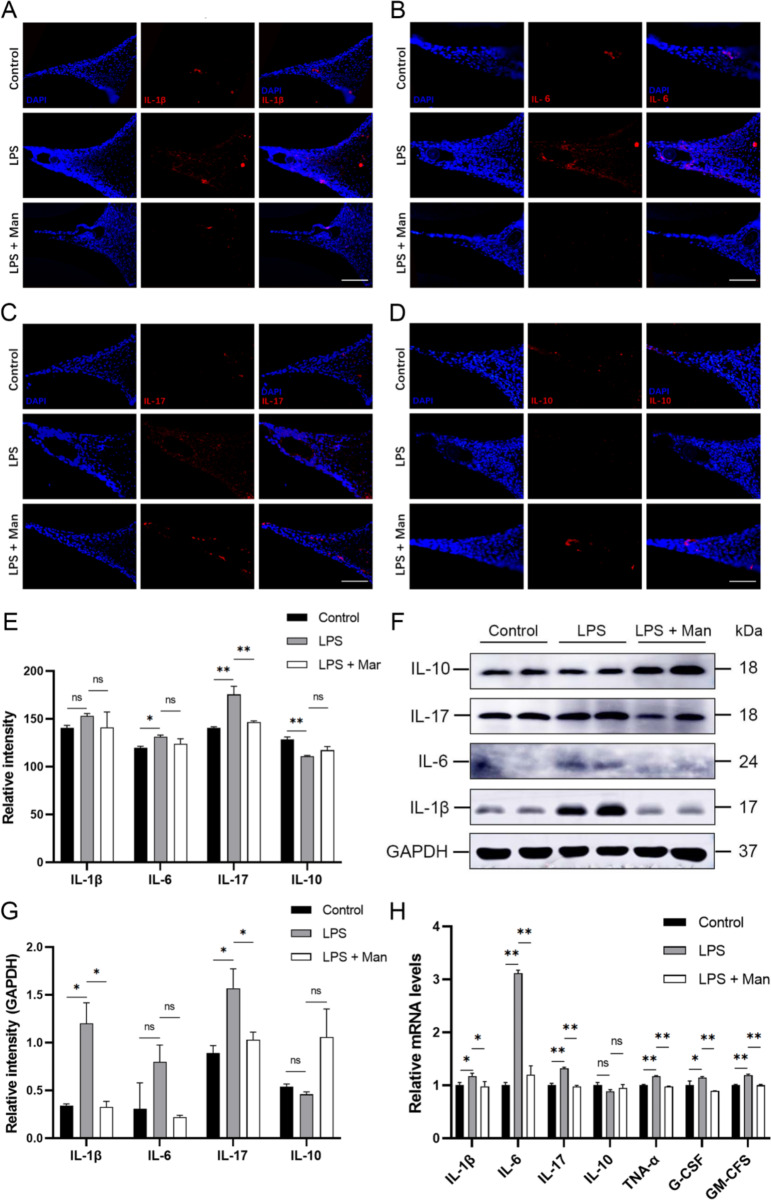
To validate the therapeutic effect of D-mannose on gingival inflammation in periodontitis rats, the expression levels of inflammatory factors in gingival tissues were determined. IF results demonstrated that, compared to the control group, there was an increase in the expression levels of proinflammatory factors, IL-6 and IL-17 (P < 0.05), accompanied by a significant decrease in the anti-inflammatory factor, IL-10 in the LPS group (p < 0.01). Compared to the LPS group, D-mannose treatment reduced the expression level of IL-17 (p < 0.01) and increased the expression level of IL-10, although it was not statistically significant (Fig. 2A-E). Western Blot analysis demonstrated the similar trend with IF in the expression levels of IL-1β, IL-17 (p < 0.05), IL-6 and IL-10 (p > 0.05) in the LPS + Man group compared to the LPS group (Fig. 2F-G). Besides, RT-PCR results revealed a significant decrease in the mRNA levels of IL-1β, IL-6, IL-17, TNF-α, G-CSF and GM-CSF in the gingival tissues after the D-mannose treatment compared with the LPS group (Fig. 2H, p < 0.05). These results showed that D-mannose downregulated the expression levels of inflammatory factors and upregulated the expression levels of anti-inflammatory factors in gingival tissues.
Fig. 2.
Effect of D-mannose on LPS-induced inflammation in gingival tissues in rats. (A-D) IF staining of IL-1β, IL-6, IL-17, and IL-10 (200×; Scale bars represent 100 μm); (E) IF staining data quantitative analysis; (F) The expression levels of IL-1β, IL-6, IL-17, and IL-10 were measured by Western Blot; (G) Western Blot data quantitative analysis; (H) The expression levels of IL-1β, IL-6, IL-17, IL-10, TNF-α, G-CSF and GM-CSF were measured by RT-qPCR. Data are shown as the means ± SD (n = 3). *p < 0.05 vs. the LPS group, **p < 0.01 vs. the LPS group. Note: The gels in F were cropped. The samples derived from the same experiment and gels were processed in parallel
D-mannose inhibits neutrophils recruitment, activation, and TET2 expression in gingival tissues of periodontitis rats
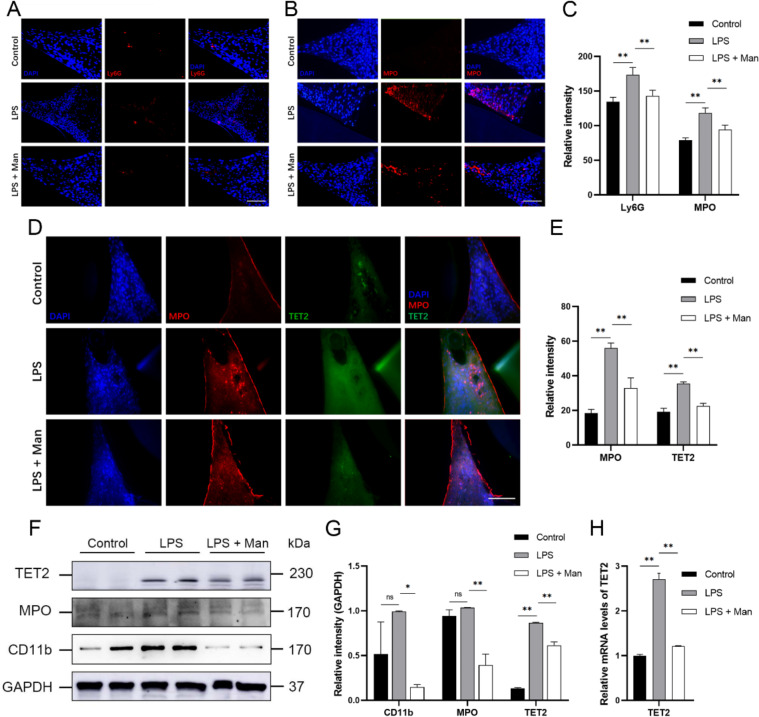
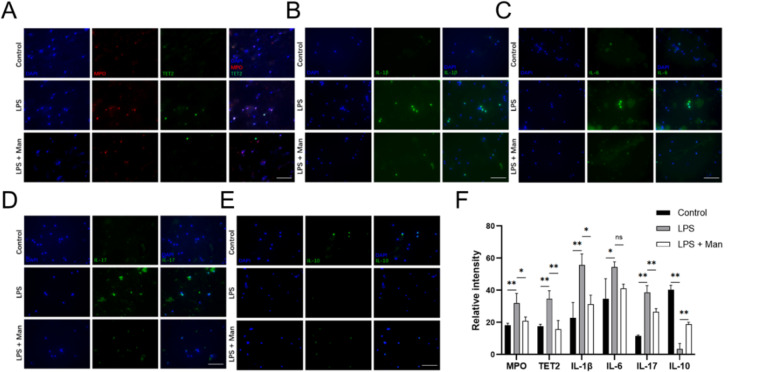
Given the importance of neutrophils in periodontitis [20, 24], our study evaluated the abundance and functional status of neutrophils in gingival tissues after D-mannose treatment. CD11b and Ly6G are markers of mature neutrophils, while MPO is a marker of neutrophil activation. IF analysis revealed a significant increase in the expression levels of Ly6G and MPO in the LPS group compared to the control group. In contrast, the D-mannose significantly decreased the expression levels of these markers in the gingival tissue in vivo (Fig. 3A-C, p < 0.01). Western Blot analysis showed the similar trends of MPO (p < 0.01) and CD11b (p < 0.01) with IF after the D-mannose treatment in vivo. These findings indicated that D-mannose inhibited the recruitment and activation of neutrophils in gingival tissues in periodontitis in vivo. Furthermore, since TET2 plays a crucial role in innate immune homeostasis [25], the expression level of TET2 in gingival tissues was analyzed. The results of TET2 levels in the gingival tissue showed the same trend with MPO in the three groups (p < 0.01) by IF staining (Fig. 3D-E), Western Blot (Fig. 3F-G), and RT-PCR (Fig. 3H) analyses. These results presented that D-mannose reduced the recruitment and activation of neutrophils, and downregulated the expression level of TET2, in the gingival tissues of rats with periodontitis.
Fig. 3.
Effect of D-mannose on neutrophils in gingival tissues in LPS-induced periodontal rats. (A-B) IF staining of Ly6G and MPO (200×; Scale bars represent 100 μm); (C) IF staining data quantitative analysis; (D) IF double staining of MPO and TET2 (200×; Scale bars represent 100 μm); (E) IF double staining data quantitative analysis; (F) The expression levels of CD11b, MPO, and TET2 were measured by Western Blot; (G) Western Blot data quantitative analysis; (H) The expression levels of TET2 was measured by RT-qPCR. Data are shown as the means ± SD (n = 3). *p < 0.05 vs. the LPS group, **p < 0.01 vs. the LPS group. Note: The gels in F were cropped. The samples derived from the same experiment and gels were processed in parallel
D-mannose inhibits LPS-induced neutrophils activation in vitro
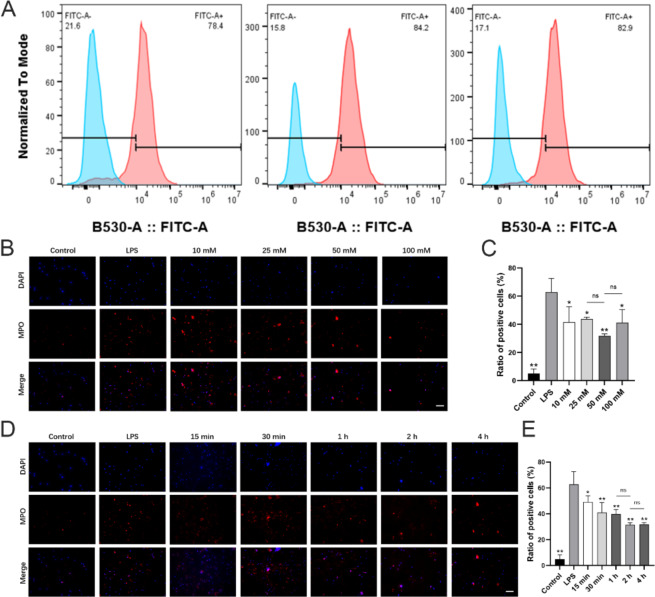
To validate the direct impact of D-mannose on neutrophils, LPS was used to stimulated the neutrophils in vitro. Neutrophils were isolated from rat peripheral blood, and their purity was assessed using flow cytometry with a high purity of neutrophils over 80% in total, which was used in further cellular experiments (Fig. 4A). To determine the effective concentration and duration of D-mannose treatment on neutrophils in vitro, IF was employed to observe changes in MPO expression. Initially, to determine the optimal concentration of D-mannose, neutrophils were pre-treated with varying concentrations of D-mannose. Results showed that compared with the LPS group, D-mannose inhibited the MPO expression from 25 mM to 100 mM (p < 0.05), while the concentration of 50 mM showed the lowest ratio of positive cells in vitro (Fig. 4B-C). Subsequently, D-mannose inhibited the neutrophil activation in a time-dependent manner, while the treatment duration of 2 h and 4 h exhibited no significant difference (p > 0.05) (Fig. 4D-E). Therefore, a concentration of 50 mM and a treatment duration of 4 h were chosen in the further experiments.
Fig. 4.
Effect of D-mannose on LPS-induced neutrophils activation in vitro. (A) The purity of the isolated neutrophils was analyzed by flow cytometry; (B) IF staining of MPO with the concentration of D-mannose pre-treatment varied and the duration constant (200×; Scale bars represent 100 μm); (C) IF staining data quantitative analysis of Fig. 4B; (D) IF staining of MPO with the duration of D-mannose pre-treatment varied and the concentration constant (200×; Scale bars represent 100 μm); (E) IF staining data quantitative analysis of Fig. 4D. Data are shown as the means ± SD (n = 3). *p < 0.05 vs. the LPS group, **p < 0.01 vs. the LPS group
D-Mannose suppresses LPS-induced neutrophils pro-inflammatory phenotype and TET2 expression in vitro
To further investigate the effects of D-mannose on neutrophil activation, we treated the neutrophils with 50 mM D-mannose for 4 h followed by LPS stimulation for 4 h. IF revealed that LPS significantly increased the expression levels of MPO, IL-1β, IL-6, IL-17 and TET2 (Fig. 5A-D, F, p < 0.05), and significantly decreased the expression level of IL-10 (Fig. 5E-F, p < 0.05). D-mannose treatment counteracted these trends (Fig. 5). Additionally, the co-localization of TET2 and MPO was observed by IF (Fig. 5A). These in vitro experimental results showed that D-mannose could inhibit the activation of neutrophils, suppress proinflammatory phenotype of neutrophils, and downregulate the expression level of TET2 in neutrophils.
Fig. 5.
Effect of D-mannose on LPS-stimulated neutrophils in vitro. (A) IF double staining of MPO and TET2 (400×; Scale bars represent 100 μm); (B-E) IF staining of IL-1β, IL-6, IL-17, and IL-10 (400×; Scale bars represent 100 μm); (F) IF (double) staining data quantitative analysis. Data are shown as the means ± SD (n = 3). *p < 0.05 vs. the LPS group, **p < 0.01 vs. the LPS group
D-mannose inhibits glycolysis in gingival tissues of periodontitis rats
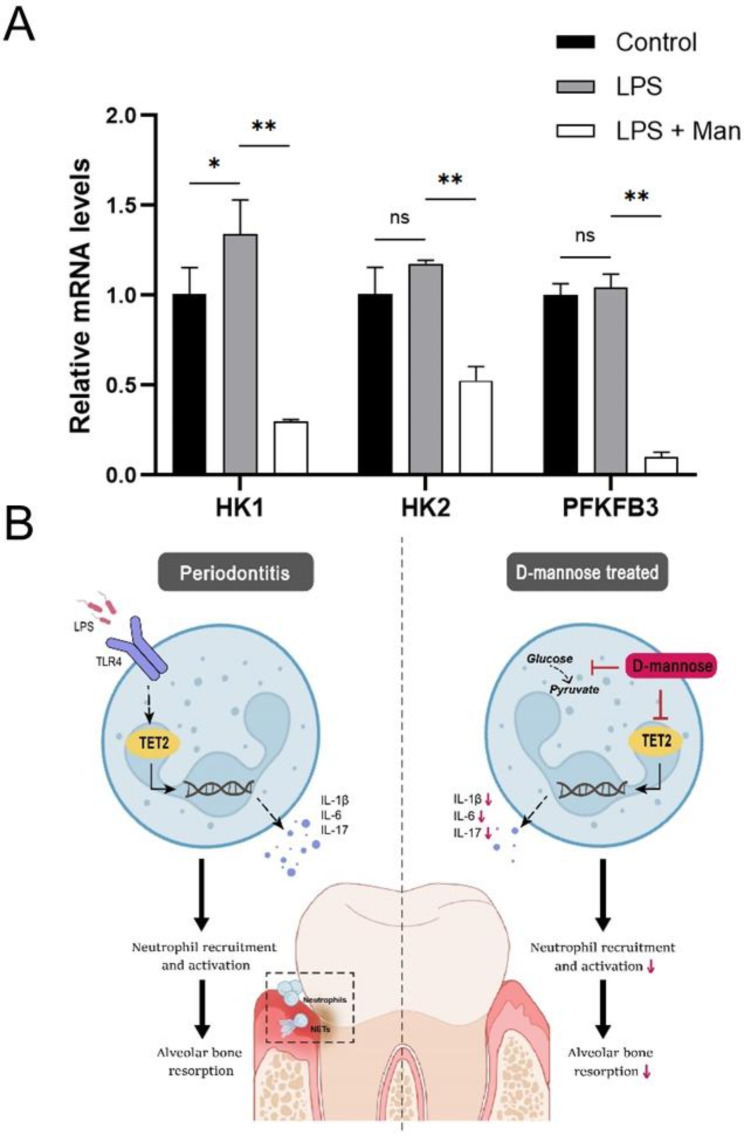
Considering the involvement of D-mannose in sugar metabolism [26] and the positive correlation between glycolysis and inflammatory responses [27], D-mannose might inhibit glycolysis in gingival tissues of periodontitis rats. RT-PCR analysis of key glycolytic enzymes (HK1, HK2, PFKFB3) in gingival tissues revealed that LPS significantly upregulated the mRNA levels of HK1 (p > 0.05), while D-mannose significantly downregulated the mRNA levels of these three enzymes (p < 0.01) (Fig. 6A). These results suggest that D-mannose may alleviate periodontitis by inhibiting glycolysis.
Fig. 6.
(A) The expression levels of the key glycolytic enzymes were measured by RT-qPCR. Data are shown as the means ± SD (n = 3). *p < 0.05 vs. the LPS group, **p < 0.01 vs. the LPS group; (B) Graphical abstract
Discussion
Our results showed that D-mannose inhibited the maxillary bone resorption in LPS-induced periodontitis rats. Different methods have been used to deliver periodontal pathogens to the oral cavity of experimental animals, such as with diet, bacteria-soaked ligature, gavage, or direct application of bacterial suspension to gingiva [28–32]. To achieve a site-specific infection and avoid gingival damage from ligature, we intralingual injected LPS between the first molar and the second molar to induce periodontitis, which has the advantages of good experimental controllability, time-saving, and reproducibility. However, the shortcomings are that the biochemical function of LPS is unstable. Besides, it is difficult to maintain the consistency of modeling associated with the injection dose and site. Moreover, we chose the LPS derived from Salmonella typhimurium, which is potent in stimulating periodontitis, but it should be noted that the model treated with Salmonella typhimurium-LPS could not provide comprehensive information about periodontitis compared to those with common periodontal pathogens. D-mannose inhibits macrophage activation by impairing IL-1β production [33]. D-mannose was reported to suppresses oxidative response and block phagocytosis in experimental neuroinflammation [34]. Meaningfully, D-mannose didn’t show obvious side effects on experimental animals [35] and humans [10]. Recently, it was reported that D-mannose could attenuate periodontal bone loss in mice with ligature-induced periodontitis [36], while the molecular mechanism was unclear. In this study, it is found that D-mannose attenuate the LPS-induced rats periodontitis progress by inhibition neutrophils activation, which maybe depend on TET2. These results suggested that D-mannose may be a potential application for patients with periodontitis, and encouraged us to research into the therapeutic effects natural substances.
Periodontitis is a chronic, inflammatory, and destructive disease caused by the imbalance of host immune response and dental biofilm. In periodontitis, dysregulated mucosal inflammation triggers the destruction of soft tissues and alveolar bone tissue, leading to tooth loss in severe cases [37]. The anti-inflammatory properties of D-mannose via reduction of IFN-γ, IL-17, and IL-4 expression in gingival tissues and intestinal tissues were shown in gingival tissues in vivo [36]. Our study determined the mRNA and protein expression levels of IL-1β, IL-6, IL-10, IL-17, TNF-α, G-CSF, and GM-CSF in gingival tissues after D-mannose treatment in periodontitis rats, which showed that D-mannose alleviated gingival inflammation in periodontitis rats in vivo and in vitro. Notably, the physiological concentration of D-mannose in human blood is about 50 µM, which shows the safety of the concentration of D-mannose in our study. Our findings added evidence to the opinion that D-mannose treatment is a promising novel strategy to suppress inflammation [11]. However, the appropriate dose of D-mannose for periodontitis patients is needed to be further confirmed. Researches prove that there are many immune cells involved in the periodontitis process, producing the inflammatory factors. The immune response in periodontitis involves both innate and adaptive immunity, with numerous immune cells and inflammatory pathways participating in a complex network of interactions, while the role of D-mannose on immune regulation is unclear.
The results of IF and WB showed that the expression levels of Ly6G and MPO were increased in LPS-induced periodontitis rats, which partly verified the vital role of neutrophils in periodontitis. By drafting the human oral mucosa cell atlas, researchers revealed that the over-activation of stromal cells in periodontitis is associated with excessive recruitment of immune cells, especially neutrophils, leading to further damage of the periodontal tissue [18]. Moreover, D-mannose reduced the recruitment and activation of neutrophils in gingival tissues. This result corresponded with that previous study suggesting that D-mannose could decrease the oxidative burst of neutrophils, and went a step further to explore the functions of neutrophils. We also exhibited an in vitro experiment to confirm the inhibition role of D-mannose on the activation of neutrophils, and to demonstrate its ability to suppress producing inflammatory cytokines in neutrophils. D-mannose facilitated immunotherapy and radiotherapy of triple-negative breast cancer via degradation of PD-L1 to activate T cells [23]. D-mannose treatment blocked macrophage phagocytosis in a dose-dependent manner in neurological diseases [34]. D-mannose promoted the differentiation of initial T cells into Tregs by reducing the level of IL-6 secreted by human periodontal ligament stem cells (hPDLSCs) [9]. Recent research suggested a feed-forward loop in which NETs trigger IL-17/Th17 response to promote immunopathology in periodontitis [19], stressing the important role of neutrophils in triggering periodontitis. Neutrophils from late-onset periodontitis patients have a higher cytokine reactivity with higher expression of chemokines and release of cytokines (IL-1β, IL-6, IL-8, and TNF-α) [38, 39]. Therefore, it is a quite meaningful question whether D-mannose can directly act on neutrophils or not. In our study, D-mannose suppressed neutrophil recruitment, activation, and pro-inflammatory phenotype in periodontitis rats in vivo and in vitro models. Since there are different neutrophil subsets [40, 41], it is an interesting question whether D-mannose plays an important role in the transition between neutrophil subsets. The NETs formation is the key factor in the neutrophil activation, it is worthy to detect the effects of D-mannose on NETs formation in future.
TET2 is an important epigenetic enzyme that plays a part in both initiation and resolution of inflammation [25]. DNA demethylation mediated by TET2 increases expression of genes related to neutrophil activation and function [42]. Therefore, TET2 may participate in the initiation of inflammation at least partially through neutrophils. Our study showed the significantly upregulated expression of TET2 in periodontitis lesions, which was downregulated by D-mannose in vivo. However, our study did not address the effect of D-mannose on the regulation of DNA methylation by TET2. Thus, further exploration is needed. The upregulated expression of TET2 was also found in separated rat LPS-stimulated neutrophils, which was inhibited by the D-mannose in vitro. Previous studies have suggested the diverse connection between glucose metabolism and TET2. A study found that increased glucose levels impeded AMPK-mediated phosphorylation, which resulted in the destabilization of TET2 followed by dysregulation of both 5-hydroxymethylcytosine (5hmC) and the tumor suppressive function of TET2 [43]. TET2 suppressed glycolysis in nasopharyngeal carcinoma cells [44]. There is a wide variety of molecular mechanisms which explain the relationship between glycolytic metabolites and the pro-inflammatory phenotype [27]. As a monosaccharide, D-mannose is closely related to glycolysis. D-mannose is taken up by the same transporters as glucose (GLUT) [45] but accumulates as mannose-6-phosphate in cells, and impairs the further metabolism of glucose in glycolysis [46]. It has been unraveled that glycolysis can promote inflammation in endothelial cells [47]. The blockade of glycolysis suppressed the LPS-induced macrophage pyroptosis, resulting in an attenuation of the inflammatory response and bone resorption in periodontitis [48]. It was found that HK2-mediated glycolysis promoted inflammatory responses in human gingival fibroblasts in gingival tissues [49]. Specifically, this study determined that the mRNA expressions of glycolysis-related genes HK1, HK2, and PFKFB3, which were upregulated in periodontal tissues. However, D-mannose decreased the expression of HK1, HK2, and PFKFB3 in vivo. Thus, it is reasonable that D-mannose may somehow downregulate the expression of TET2 by suppressing glycolysis, thereby suppressing inflammation. However, further study is needed to confirm the molecular mechanism of D-mannose on glycolysis and TET2 in periodontitis treatment.
Conclusions
In conclusion, we found that D-mannose could alleviate chronic periodontitis in rats by regulating the immune response of neutrophils. These findings indicated that D-mannose may be effective to alleviate the chronic periodontitis. What’s more, it provided new insights into the therapeutic mechanism of D-mannose on periodontitis, which may be related to epigenetics and glycolysis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to express our gratitude to the members of Qi lab, Qian Peng, Chao Yao, Dongmei Lan, Yurong Chen, Enhua Mei, and Yinan Chen, for their technical assistance and valuable discussions. We would also like to acknowledge the experiment platform of the Tenth People’s Hospital of Tongji University.
Abbreviations
- LPS
Lipopolysaccharide
- H&E
Hematoxylin and eosin
- IF
Immunofluorescence
- RT-PCR
Reverse transcription-polymerase chain reaction
- IL
Interleukin
- TNF-α
Tumor necrosis factor-alpha
- G-CSF
Granulocyte colony-stimulating factor
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- TET2
Ten-eleven translocation 2
- HK
Hexokinase
- PFKFB3
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3
- MPO
Myeloperoxidase
- NET
Neutrophil extracellular trap
- Treg
Regulatory T cell
- PD-L1
Programmed cell death 1 ligand 1
- hPDLSC
Human periodontal ligament stem cell
- AMPK
AMP-activated protein kinase
- 5hmC
5-hydroxymethylcytosine
- GLUT
Glucose transporter
- FDUROP
Fudan’s Undergraduate Research Opportunities Program
Author contributions
XL: Methodology, Validation, Investigation, Writing-Review & Editing. XC: Methodology, Validation, Formal analysis, Investigation, Data Curation, Writing-Original Draft, Writing-Review & Editing, Visualization. QZ: Methodology, Validation, Formal analysis, Investigation, Data Curation, Writing-Review & Editing, Visualization. PZ: Methodology, Validation, Formal analysis, Investigation, Data Curation, Writing-Review & Editing, Visualization. SN: Investigation. LL: Conceptualization, Resources, Writing-Review & Editing, Supervision. SQ: Conceptualization, Methodology, Resources, Writing-Review & Editing, Supervision, Funding acquisition. XL and XC contributed equally. All authors gave final approval and agreed to be accountable for this work.
Funding
This research was financially supported by the Nature Science Foundation of Shanghai, Grant Nos 20ZR1443100,21140904500, Shanghai Stomatological Hospital Science and Technology Innovation Talent Training Program Talent Project Nos SSH-2022-KJCX-B05, Fudan’s Undergraduate Research Opportunities Program (FDUROP, 22676), and Fudan University Shanghai Medical College Undergraduate Innovation Program “Qingfeng” Scholar Program (QF2319).
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
The animal experiment was conducted according to ARRIVE guidelines. All experimental equipment and procedures were examined and approved by the Institutional Animal Care and Use Committee of Shanghai Rat & Mouse Biotech Co., Ltd (No. SHDSYY-2022-3063-9).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xue Li and Xueting Chen contributed equally to this work.
Change history
1/28/2025
A Correction to this paper has been published: 10.1186/s12903-024-05374-4
Contributor Information
Lei Lv, Email: lvlei@fudan.edu.cn.
Shengcai Qi, Email: qishengcai@fudan.edu.cn.
References
- 1.Chen MX, Zhong YJ, Dong QQ, Wong HM, Wen YF. Global, regional, and national burden of severe periodontitis, 1990–2019: an analysis of the global burden of Disease Study 2019. J Clin Periodontol. 2021;48:1165–88. [DOI] [PubMed] [Google Scholar]
- 2.Trindade D, Carvalho R, Machado V, Chambrone L, Mendes JJ, Botelho J. Prevalence of periodontitis in dentate people between 2011 and 2020: a systematic review and meta-analysis of epidemiological studies. J Clin Periodontol. 2023;50:604–26. [DOI] [PubMed] [Google Scholar]
- 3.Genco RJ, Sanz M. Clinical and public health implications of periodontal and systemic diseases: an overview. Periodontol 2000. 2020;83:7–13. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Chen Y, Cai G, Ni Q, Geng Y, Wang T, et al. Roles of trained immunity in the pathogenesis of periodontitis. J Periodontal Res. 2023;58:864–73. [DOI] [PubMed] [Google Scholar]
- 5.Kornman KS, Giannobile WV, Duff GW. Quo vadis: what is the future of periodontics? How will we get there? Periodontol 2000. 2017;75:353–71. [DOI] [PubMed]
- 6.Zhang Z, Yu Y, Zhu G, Zeng L, Xu S, Cheng H, et al. The emerging role of plant-derived exosomes-Like nanoparticles in Immune Regulation and Periodontitis Treatment. Front Immunol. 2022;13:896745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butera A, Gallo S, Pascadopoli M, Taccardi D, Scribante A. Home oral care of Periodontal patients using Antimicrobial Gel with Postbiotics, Lactoferrin, and Aloe Barbadensis Leaf Juice Powder vs. Conventional Chlorhexidine Gel: a Split-Mouth Randomized Clinical Trial. Antibiot (Basel). 2022;11:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng Q, Zhao B, Lin J, Liu H, Zhou R, Lan D, et al. SPRC suppresses experimental periodontitis by modulating Th17/Treg Imbalance. Front Bioeng Biotechnol. 2021;9:737334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo L, Hou Y, Song L, Zhu S, Lin F, Bai Y. D-Mannose enhanced Immunomodulation of Periodontal ligament stem cells via inhibiting IL-6 secretion. Stem Cells Int. 2018;2018:7168231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alton G, Kjaergaard S, Etchison JR, Skovby F, Freeze HH. Oral ingestion of mannose elevates blood mannose levels: a first step toward a potential therapy for carbohydrate-deficient glycoprotein syndrome type I. Biochem Mol Med. 1997;60:127–33. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Cheng H, Gui Y, Zhan Q, Li S, Qiao W, et al. Mannose treatment: a Promising Novel Strategy to suppress inflammation. Front Immunol. 2021;12:756920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu H, Gu R, Zhu Y, Lian X, Wang S, Liu X, et al. D-mannose attenuates bone loss in mice via Treg cell proliferation and gut microbiota-dependent anti-inflammatory effects. Ther Adv Chronic Dis. 2020;11:2040622320912661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu R, Liu H, Hu M, Zhu Y, Liu X, Wang F, et al. D-Mannose prevents bone loss under weightlessness. J Transl Med. 2023;21:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Q, Zhao Y, Shui Y, Zhou X, Cheng L, Ren B, et al. Interactions between neutrophils and Periodontal pathogens in late-onset Periodontitis. Front Cell Infect Microbiol. 2021;11:627328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delima AJ, Van Dyke TE. Origin and function of the cellular components in gingival crevice fluid. Periodontol 2000. 2003;31:55–76. [DOI] [PubMed] [Google Scholar]
- 16.Klinkhamer JM, Zimmerman S. The function and reliability of the orogranulocytic migratory rate as a measure of oral health. J Dent Res. 1969;48:709–15. [DOI] [PubMed] [Google Scholar]
- 17.Murray PA, Patters MR. Gingival crevice neutrophil function in periodontal lesions. J Periodontal Res. 1980;15:463–9. [DOI] [PubMed] [Google Scholar]
- 18.Williams DW, Greenwell-Wild T, Brenchley L, Dutzan N, Overmiller A, Sawaya AP, et al. Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell. 2021;184:4090–e410415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim TS, Silva LM, Theofilou VI, Greenwell-Wild T, Li L, Williams DW, et al. Neutrophil extracellular traps and extracellular histones potentiate IL-17 inflammation in periodontitis. J Exp Med. 2023;220:e20221751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicu EA, Loos BG. Polymorphonuclear neutrophils in periodontitis and their possible modulation as a therapeutic approach. Periodontol 2000. 2016;71:140–63. [DOI] [PubMed] [Google Scholar]
- 21.Slots J. Periodontitis: facts, fallacies and the future. Periodontol 2000. 2017;75:7–23. [DOI] [PubMed] [Google Scholar]
- 22.Rest RF, Farrell CF, Naids FL. Mannose inhibits the human neutrophil oxidative burst. J Leukoc Biol. 1988;43:158–64. [DOI] [PubMed] [Google Scholar]
- 23.Zhang R, Yang Y, Dong W, Lin M, He J, Zhang X, et al. D-mannose facilitates immunotherapy and radiotherapy of triple-negative breast cancer via degradation of PD-L1. Proc Natl Acad Sci U S A. 2022;119:e2114851119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrmann JM, Meyle J. Neutrophil activation and periodontal tissue injury. Periodontol 2000. 2015;69:111–27. [DOI] [PubMed] [Google Scholar]
- 25.Cong B, Zhang Q, Cao X. The function and regulation of TET2 in innate immunity and inflammation. Protein Cell. 2021;12:165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma V, Ichikawa M, Freeze HH. Mannose metabolism: more than meets the eye. Biochem Biophys Res Commun. 2014;453:220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soto-Heredero G, Gómez de Las Heras MM, Gabandé-Rodríguez E, Oller J, Mittelbrunn M. Glycolysis - a key player in the inflammatory response. FEBS J. 2020;287:3350–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker PJ, Evans RT, Roopenian DC. Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Arch Oral Biol. 1994;39:1035–40. [DOI] [PubMed] [Google Scholar]
- 29.Pathirana RD, O’Brien-Simpson NM, Brammar GC, Slakeski N, Reynolds EC. Kgp and RgpB, but not RgpA, are important for Porphyromonas gingivalis virulence in the murine periodontitis model. Infect Immun. 2007;75:1436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klausen B. Microbiological and immunological aspects of experimental periodontal disease in rats: a review article. J Periodontol. 1991;62:59–73. [DOI] [PubMed] [Google Scholar]
- 31.Kimura S, Nagai A, Onitsuka T, Koga T, Fujiwara T, Kaya H, et al. Induction of experimental periodontitis in mice with Porphyromonas gingivalis-adhered ligatures. J Periodontol. 2000;71:1167–73. [DOI] [PubMed] [Google Scholar]
- 32.Polak D, Wilensky A, Shapira L, Halabi A, Goldstein D, Weiss EI, et al. Mouse model of experimental periodontitis induced by Porphyromonas gingivalis/Fusobacterium nucleatum infection: bone loss and host response. J Clin Periodontol. 2009;36:406–10. [DOI] [PubMed] [Google Scholar]
- 33.Torretta S, Scagliola A, Ricci L, Mainini F, Di Marco S, Cuccovillo I, et al. D-mannose suppresses macrophage IL-1β production. Nat Commun. 2020;11:6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Jalali Motlagh N, Wang C, Wojtkiewicz GR, Schmidt S, Chau C, et al. d-mannose suppresses oxidative response and blocks phagocytosis in experimental neuroinflammation. Proc Natl Acad Sci U S A. 2021;118:e2107663118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis JA, Freeze HH. Studies of mannose metabolism and effects of long-term mannose ingestion in the mouse. Biochim Biophys Acta. 2001;1528:116–26. [DOI] [PubMed] [Google Scholar]
- 36.Yang H, Han N, Luo Z, Xu J, Guo L, Liu Y. D-mannose alleviated alveolar bone loss in mice with experimental periodontitis via regulating the anti-inflammatory effect of amino acids. J Periodontol. 2023;94:542–53. [DOI] [PubMed] [Google Scholar]
- 37.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lakschevitz FS, Aboodi GM, Glogauer M. Oral neutrophil transcriptome changes result in a pro-survival phenotype in periodontal diseases. PLoS ONE. 2013;8:e68983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ling MR, Chapple ILC, Matthews JB. Peripheral blood neutrophil cytokine hyper-reactivity in chronic periodontitis. Innate Immun. 2015;21:714–25. [DOI] [PubMed] [Google Scholar]
- 40.Sansores-España LD, Melgar-Rodríguez S, Vernal R, Carrillo-Ávila BA, Martínez-Aguilar VM, Díaz-Zúñiga J. Neutrophil N1 and N2 subsets and their possible association with Periodontitis: a scoping review. Int J Mol Sci. 2022;23:12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fine N, Hassanpour S, Borenstein A, Sima C, Oveisi M, Scholey J, et al. Distinct oral neutrophil subsets define Health and Periodontal Disease States. J Dent Res. 2016;95:931–8. [DOI] [PubMed] [Google Scholar]
- 42.Walsh SW, Al Dulaimi M, Archer KJ, Strauss JF. Patterns of maternal neutrophil gene expression at 30 weeks of Gestation, but not DNA methylation, distinguish mild from severe Preeclampsia. Int J Mol Sci. 2021;22:12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu D, Hu D, Chen H, Shi G, Fetahu IS, Wu F, et al. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature. 2018;559:637–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Yang J, Shi D, Cao Z. TET2 suppresses nasopharyngeal carcinoma progression by inhibiting glycolysis metabolism. Cancer Cell Int. 2020;20:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab. 2010;298:E141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez PS, O’Prey J, Cardaci S, Barthet VJA, Sakamaki J-I, Beaumatin F, et al. Mannose impairs tumour growth and enhances chemotherapy. Nature. 2018;563:719–23. [DOI] [PubMed] [Google Scholar]
- 47.Xiao W, Oldham WM, Priolo C, Pandey AK, Loscalzo J. Immunometabolic endothelial phenotypes: integrating inflammation and glucose metabolism. Circ Res. 2021;129:9–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He Y, Wang Y, Jia X, Li Y, Yang Y, Pan L, et al. Glycolytic reprogramming controls periodontitis-associated macrophage pyroptosis via AMPK/SIRT1/NF-κB signaling pathway. Int Immunopharmacol. 2023;119:110192. [DOI] [PubMed] [Google Scholar]
- 49.Su W, Li J, Jiang L, Lei L, Li H. Hexokinase 2-mediated glycolysis supports inflammatory responses to Porphyromonas gingivalis in gingival fibroblasts. BMC Oral Health. 2023;23:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.