Abstract
Objectives
The aim of the present study was to identify the incidence of common perioperative anaesthetic complications in cats undergoing anaesthesia for neutering in three UK first opinion practices.
Methods
A retrospective anaesthetic record analysis was performed on cats anaesthetised for neutering at practices 1 and 2 between 9 December 2017 and 2 February 2021 and practice 3 between 9 March 2020 and 7 January 2021. A search of the practice management system identified all cats that had undergone neutering in the selected timeframe. Data from 1019 cats were included in the study. Information relating to patient characteristics and data from the anaesthesia session were extracted from electronic patient records and anaesthesia record charts and entered into an Excel spreadsheet. A definition of the complications was created after reviewing the literature and their incidence determined from the data set. Comparisons between different groups of cats in the study were made using a χ2 test for homogeneity or Fisher’s exact tests to identify factors associated with increased incidence of complications.
Results
The anaesthetic-related mortality was 1/1019 (0.10%). The most common complications were hypotension (22.6%), bradycardia (16.7%) and hypothermia (13.8%). Less common complications were hypocapnia (12.7%), hypercapnia (8.7%), tachycardia (6.6%), apnoea (3.1%), hyperthermia (1.7%), hypertension (1.4%), endotracheal tube obstruction (1.1%), hypoxia (0.3%), undesirable recovery (0.6%) and cardiac arrhythmia (0.2%). Factors associated with increased risk of hypotension were acepromazine pre-anaesthetic medication, higher maximum isoflurane dose, longer anaesthetic duration and lower body weight. Factors associated with increased risk of bradycardia were medetomidine pre-anaesthetic medication, longer anaesthetic duration and higher body weight. Factors associated with increased risk of hypothermia were higher maximum isoflurane dose, increased anaesthetic duration and lower body weight.
Conclusions and relevance
This study showed that anaesthetic complications were frequently observed, with complications documented in 53.4% of the cats in the study. The information in this study may help to guide prioritisation of monitoring in feline anaesthesia.
Keywords: Anaesthesia, anaesthetic complications, anaesthetic mortality, hypotension, hypothermia, bradycardia
Introduction
Anaesthetic complications are unfavourable outcomes of an anaesthetic procedure, including hypotension, hypothermia, hyperthermia, hypoventilation and cardiac arrhythmias. Although several large-scale studies have looked at anaesthetic-related mortality in cats, less evidence exists about the likelihood of complications occurring in feline anaesthesia.
Early studies reported feline mortality rates in the range of 0.24–0.3%.1–3 More recent studies report lower mortality in primary care practice and spay-/neuter-based studies (range 0.048–0.11%).4–6 Studies from teaching or referral hospitals report higher mortality rates (0.43–5%) but also include a higher proportion of American Society of Anesthesiologists (ASA) grade III–IV cats.7–12
The objectives of the study were to establish the incidence of perioperative anaesthetic complications in cats undergoing anaesthesia for neutering in first opinion practice.
Materials and methods
Ethical approval for this study was granted by the University of Edinburgh Veterinary School Ethical Review Committee (reference number 115.20).
Anaesthetic records of cats anaesthetised for neutering at three primary care practices in the UK were studied retrospectively (sites 1 and 2: 19 December 2017 to 2 February 2021; site 3: 9 March 2020 to 7 January 2021). Records were checked to ensure the owners consented to the use of their data for research. Cats were anonymised at the point of data entry.
Recorded data fields were as follows: date of procedure; identification; signalment; ASA status; comorbidities; trap–neuter–return (TNR) status; anaesthetic protocol; administration of non-steroidal anti-inflammatory drugs (NSAIDs); anaesthetic monitoring parameters; and any additional relevant events. Missing data were excluded from the analysis for that complication. Complications were defined based on a literature review (Table 1),4,10,13–17 and the presence or absence of complications was recorded. For hypotension, cats with single blood pressure (BP) or multiple hypotensive readings (MHRs) were grouped separately.
Table 1.
Definitions of the complications used in this study
| Complication | Definition |
|---|---|
| Hypoxaemia | SpO2 <90%13,14 |
| Hypocapnia | ETCO2 <20 mmHg 13 |
| Hypercapnia | ETCO2 >45 mmHg10,14 |
| Hypothermia | Temperature <37°C4,15 |
| Severe hypothermia | Temperature <34°C 10 |
| Hyperthermia | Temperature >39.5°C13,17 |
| Bradycardia | HR <90 bpm 16 |
| Tachycardia | HR >180 bpm 13 |
| Hypotension | MAP <60 or SAP <80 mmHg13,14,16 |
| Hypertension | MAP >140 or SAP >180 mmHg 16 |
ETCO2 = end-tidal carbon dioxide; HR = heart rate; MAP = mean arterial pressure; SAP = systolic arterial pressure; SpO2 = oxygen saturation
Statistical analysis was performed using R studio. 18 Continuous variables were tested for normality using a Shapiro–Wilk test and visual inspection of histogram and Q–Q plots. Non-normally distributed data are reported as median (interquartile range [IQR]). Kendall rank correlation was used to assess relationships between variables, Wilcoxon or Kruskall–Wallis rank-sum test was used to compare non-normally distributed data, and χ2 test or Fisher’s exact test was used to compare categorical data. Odds ratios (ORs) were calculated where appropriate. Statistical significance was set at P <0.05.
Results
Data from 1019 anaesthetic records were included, with similar numbers of male (n = 514) and female (n = 505) cats. Age was known for 826 cats (range 3–144 months). Female cats were older than male cats (7 months [IQR 7] vs 5 months [IQR 4]; P <0.001). Of the 1019 cats, most were domestic crossbreeds (n = 875), with the most common pedigree breeds being British Shorthair (n = 28), Ragdoll (23), Bengal (15) and Siamese (12), with 57 cats of brachycephalic breeds (eg, Persian). Median body weight was 2.8 kg (range 0.82–8). Male cats were significantly heavier than female cats (3.1 kg [IQR 1.64] vs 2.6 kg [IQR 0.8]; P <0.001). Age was significantly correlated with weight (P <0.001).
Most cats were classified as ASA grade I–II (1008/1019). Comorbidities were recorded for 62 cats, including dental disease (n = 12), cardiac murmurs (n = 9), umbilical hernias (n = 9) and soft tissue wounds or infections (n = 7). Seven male cats were cryptorchid (n = 7/514). A single kidney and single ovary were discovered at the time of surgery in two related female Ragdoll cats. The 11 ASA grade III cats were all clinically stable and were included in the data analysis. No cats were ASA grade IV or V. Most cats were client owned (n = 817/1019), with 202/1019 in the care of charities, including 34 feral cats in a TNR programme.
Hospital-designed pre-anaesthetic checklists, adapted from the Association of Veterinary Anaesthetists (AVA) anaesthetic safety checklist, 19 were documented as completed in 294/1019 (28.9%) cases, with greater completion at site 1 compared with sites 2 and 3 (P <0.001) (Table 2).
Table 2.
Completion of pre-anaesthetic safety checklists at each practice site
| Practice site | Checklist completion (%) |
|---|---|
| 1 | 33.7* |
| 2 | 18.3 |
| 3 | 18.6 |
Significantly different from other sites
Neutering was performed under general anaesthesia in 1015/1019 cats, with four male cats castrated under sedation (opioid/medetomidine combination) and local anaesthesia. Pre-anaesthetic medication was a medetomidine/opioid combination in 929/1015 cats, 55/1015 received an acepromazine/opioid combination and 33 received a ketamine-based protocol (ketamine, medetomidine, an opioid and/or a benzodiazepine) (Table 3). Two cats received opioids only or opioid/benzodiazepine combinations. Intravenous (IV) and intramuscular (IM) pre-anaesthetic medication was administered in 479/1015 and 536/1015 cats, respectively.
Table 3.
The number of cats in each pre-anaesthetic medication group
| Pre-anaesthetic medication | Route | Number of cats | |
|---|---|---|---|
| ⩽0 µg/kg medetomidine | Methadone | IM | 395 |
| ⩽0 µg/kg medetomidine | Methadone | IV | 105 |
| ⩽0 µg/kg medetomidine | Buprenorphine | IM | 72 |
| ⩽0 µg/kg medetomidine | Buprenorphine | IV | 44 |
| >0 µg/kg medetomidine | Methadone | IM | 7 |
| >10 µg/kg medetomidine | Methadone | IV | 241 |
| >10 µg/kg medetomidine | Buprenorphine | IM | 2 |
| >10 µg/kg medetomidine | Buprenorphine | IV | 59 |
| Acepromazine | Buprenorphine | IM | 33 |
| Acepromazine | Buprenorphine | IV | 1 |
| Acepromazine | Methadone | IM | 21 |
| Ketamine-based protocols | Any opioid | IM | 33 |
| >10 µg/kg medetomidine | Butorphanol | IM | 4 |
| Benzodiazepine | Any opioid | IM or IV | 2 |
IM = intramuscular; IV = intravenous
Medetomidine doses were in the range of 5–100 μg/kg IM and 5–20 μg/kg IV. Methadone was the most common opioid (n = 779/1019), followed by buprenorphine (n = 234/1019) and butorphanol (n = 6/1019). All except four cats received NSAIDs (meloxicam in 943/1019 cats and robenacoxib in 72/1019 cats), administered with pre-anaesthetic medication in 32/1015 cats, during anaesthesia in 524/1015 cats, at the end of anaesthesia or in recovery in 371/1015 cats, and not documented in 88 cases. Timing of NSAID administration was similar in normotensive cats and cats with single hypotensive measurements (P = 0.34) or multiple low BP readings (P = 0.89). The dose of methadone in 779 cats was 0.1 mg/kg (n = 3), 0.2 mg/kg (n = 256), 0.3 mg/kg (n = 519) or 0.4 mg/kg (n = 1). The buprenorphine dose was always 0.02 mg/kg. The butorphanol dose was 0.3 mg/kg in two cats as part of a ketamine-based protocol and 0.5 mg/kg in four cats castrated under sedation.
An IV cannula was placed in 991/1019 (97.25%) cats. Of the 1015 cats, anaesthesia was induced with propofol in 968 cats, ketamine in 28, alfaxalone in 18 and isoflurane mask induction in one cat. Anaesthesia was maintained with isoflurane in 954 cats and with alfaxalone in four cats.
In most cats (958/1015), endotracheal intubation was performed. In the remaining 61 cats (including the four cats that were sedated), oxygen was provided via a facemask; of these cats, nine also received some isoflurane via the facemask. Endotracheal intubation varied according to site (site 1: 100%, site 2: 96%, site 3: 45%) and was more common in female cats (100%) than male cats (89.88%, P <0.001).
Propofol dose was recorded for 865/968 cats (median 4.17 mg/kg, range 0.71–12.63). The median induction dose of alfaxalone was 1.34 mg/kg (range 0.94–4.27).
Anaesthetic duration was recorded for 1018/1019 cats as being in the range of 5–205 mins. Most anaesthetic durations were short, but 41 were longer than 95 mins. Anaesthesia was significantly longer in female cats (45 mins, n = 30) than male cats (15 mins, n = 10; P <0.001).
All anaesthetics were monitored by a registered veterinary nurse (RVN) or a student veterinary nurse (SVN, under supervision) dedicated to this task with no concurrent responsibilities. In recovery, cats were monitored by a patient care assistant (PCA), SVN or RVN. Oscillometric BP monitoring was most common, with Doppler used in 43/1019 anaesthetics.
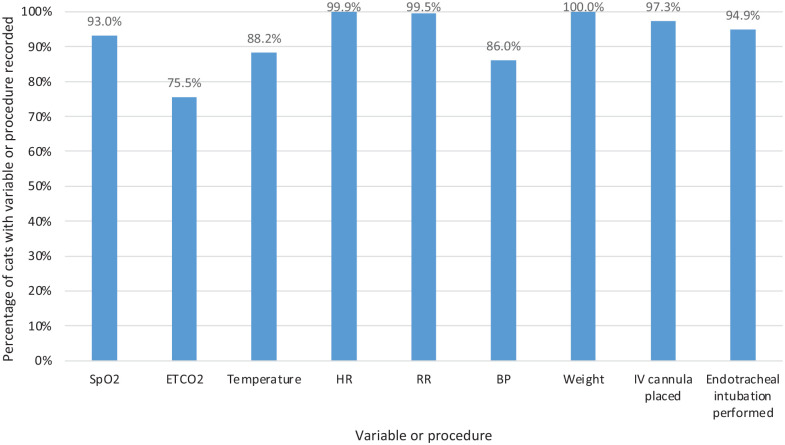
Figure 1 shows the distribution of recorded anaesthetic variables and the percentages of cats that had an IV cannula placed and that had endotracheal intubation performed.
Figure 1.
Graph showing the percentage of cats in the study that had each variable or procedure recorded
Mortality was 1/1019 (0.1%). The cat that died became dyspnoeic in recovery, then rapidly went into respiratory followed by cardiac arrest. Resuscitation attempts were unsuccessful. No complications were recorded during this anaesthetic and no comorbidities were known. Euthanasia was performed in one cat because of a positive feline immunodeficiency virus test.
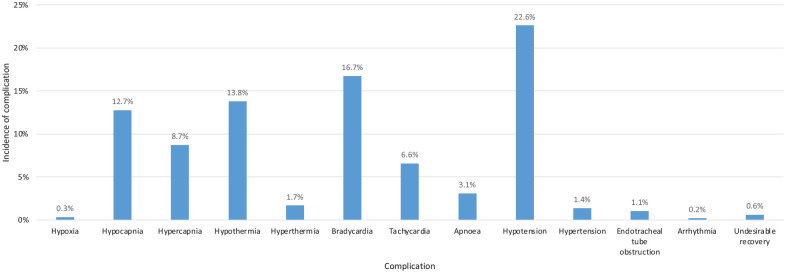
Anaesthetic-related complications were documented in 53.4% of cats (Figure 2), with hypotension, bradycardia and hypothermia being most common. No cats had severe hypothermia (<34°C). MHRs were documented in 11.9% of cats, with 7.7% having multiple consecutive low readings and 4.2% having recurrent low readings.
Figure 2.
Graph showing the incidence of complications reported in the study population
No significant association was demonstrated between bradycardia and hypothermia (P = 1) or bradycardia and hypotension (BP, P = 0.66; MHR, P = 1). Hypothermia was more common in cats with hypotension (24.3% vs 11.4%). Hypotension was documented in 20.96% of normothermic cats compared with 39.82% of hypothermic cats (P <0.001).
Endotracheal intubation was not associated with bradycardia (P = 0.91), hypotension (BP, P = 0.43; MHR, P = 0.29) or hypothermia (P = 0.43; female cats only, as male cats had shorter anaesthetic durations and were less likely to be intubated). No significant association was found between endotracheal intubation and apnoea (P = 0.41) or hypoxaemia (P = 0.10). Reintubation was required in 10 cats due to partial or complete tube obstruction.
Longer duration of anaesthesia was associated with an increased incidence of hypothermia (P <0.001, both overall and in female cats only) and an increased risk of bradycardia (P = 0.007). Duration of anaesthesia was associated with increased prevalence of hypotension overall (BP, P <0.001; MHR, P <0.001) but not in female cats only (BP, P = 0.12; MHR, P = 0.14).
Lower body weight was associated with an increased incidence of hypothermia (P = 0.009) and hypotension (BP, P = 0.005; MHR, P = 0.039). The incidence of complications was similar between pedigree and domestic cats: bradycardia (P = 0.73); hypothermia (P = 0.14); or hypotension (BP, P = 0.28; MHR, P = 0.38). The incidence of bradycardia (P = 0.99), hypothermia (P = 0.67) or hypotension (BP, P = 0.73; MHR, P = 0.61) was similar in brachycephalic and non-brachycephalic breeds.
There was no difference in the incidence of bradycardia (P = 0.29) (Table 4), hypothermia (P = 0.059) (Table 5) or hypotension (P = 0.99; MHR, P = 0.29) (Table 6) in the cats administered methadone compared with those receiving buprenorphine.
Table 4.
The incidence of bradycardia in cats receiving methadone compared with buprenorphine
| Opioid | Cats with bradycardia | Cats without bradycardia |
|---|---|---|
| Methadone | 137 (17.56) | 643 |
| Buprenorphine | 28 (14.07) | 171 |
Data are n or n (%)
Table 5.
The incidence of hypothermia in cats receiving methadone compared with buprenorphine
| Opioid | Cats with hypothermia | Cats without hypothermia |
|---|---|---|
| Methadone | 88 (12.68) | 606 |
| Buprenorphine | 32 (18.60) | 140 |
Data are n or n (%)
Table 6.
The incidence of hypotension in cats receiving methadone compared with buprenorphine
| Opioid | Cats with hypotension | Cats without hypotension | Cats with MHR |
|---|---|---|---|
| Methadone | 158 (22.60) | 541 | 69 (9.87) |
| Buprenorphine | 35 (22.15) | 123 | 21 (13.29) |
Data are n or n (%)
MHR = multiple hypotensive reading
The incidence of bradycardia was different across pre-anaesthetic medication groups (P <0.001), being significantly more common with medetomidine compared with acepromazine (OR 3.56, 95% CI 1.21–14.40; P = 0.04). Pre-anaesthetic medication significantly affected the incidence of hypotension, being more common in cats receiving acepromazine, with an OR of 7.05 (95% CI 3.27–16.17; P <0.001) for MHR and 17.07 (95% CI 7.28–42.56; P <0.001) for BP. There was no significant association between pre-anaesthetic medication and incidence of hypothermia (P = 0.52).
Propofol dose for induction was higher in cats receiving acepromazine than medetomidine (5.88 mg/kg [IQR 1.54] compared with 4.03 mg/kg [IQR 2.37]; P <0.001). There was a small but statistically significant difference in propofol dose required for cats premedicated with buprenorphine (4.55 mg/kg [IQR 2.6]) and methadone (4.12 mg/kg [IQR 2.77]; P = 0.04). Cats receiving medetomidine required significantly less isoflurane than those receiving acepromazine (P <0.001). Cats receiving methadone also required a lower maximum isoflurane dose compared with buprenorphine (P <0.001).
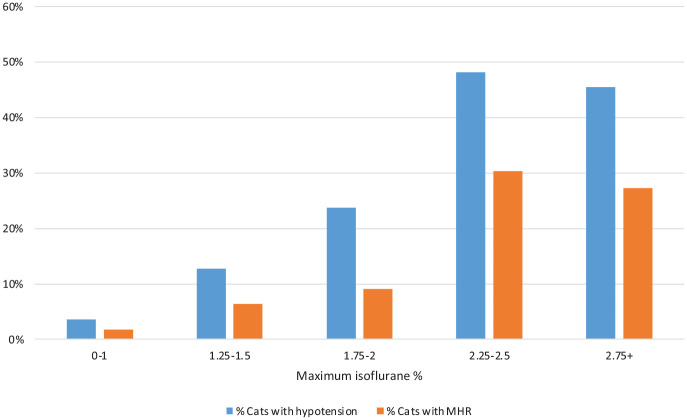
Both hypotension (BP, P <0.001; MHR, P <0.001) and hypothermia (P = 0.001) occurred more often in cats receiving higher maximum isoflurane, both overall and within medetomidine/opioid premedicated cats (BP, P <0.001; MHR, P <0.001) (Figures 3 and 4). Maximum isoflurane percentage was not associated with bradycardia (P = 0.08).
Figure 3.
Graph showing the incidence of hypotension in cats receiving a medetomidine pre-anaesthetic medication, grouped by maximum isoflurane percentage
Figure 4.
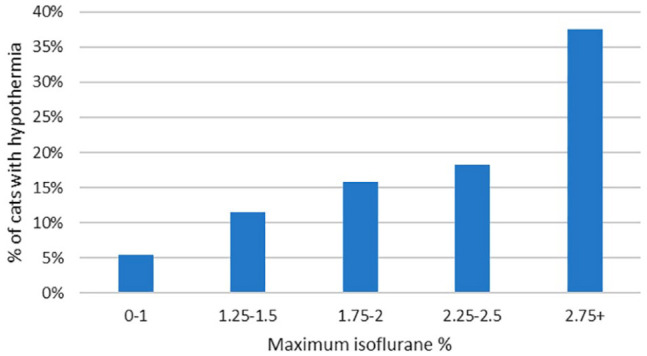
Graph showing the incidence of hypothermia in cats grouped by maximum isoflurane percentage
Discussion
This study aimed to document the incidence of perioperative anaesthetic complications in a healthy population of cats. Anaesthetic monitoring is important for patient safety and mortality;13,20 however, there is little evidence as to which variables are most affected by anaesthesia in cats in first opinion practice, and hence where to target monitoring. Cats undergoing neutering were selected as an apparently healthy population, although there were some inherent pre-existing feline clinical diseases present.
This study showed a pleasing comparison with early UK studies looking at anaesthetic management. Dodman 1 made four recommendations: routine weighing; routine premedication; endotracheal intubation for all but the shortest surgical procedures; and record keeping. All cats in this study had a weight recorded and received pre-anaesthetic medication. Endotracheal intubation was performed in all female cats and many male cats. In very few cases, no anaesthetic record was found, and it is likely that for some of the missing cases, it was mislaid rather than not produced. Pulse oximetry monitoring was more commonly used (in 93% of cases) compared with previous UK studies reporting 72% 3 and 85%. 21 This was a pleasing finding given its association with a decrease in anaesthetic mortality, both in the studies by Brodbelt et al 3 and Matthews et al. 5 Pulse oximetry provides an audio signal that has real value, especially given the low use of Doppler monitoring in this study population.
Increasingly affordable technology has enhanced the ability to monitor multiple variables, as demonstrated by the high levels of monitoring in this study. Lower use of capnography at site 3 was probably related to less frequent intubation compared with the other sites. Capnography is valuable to confirm endotracheal intubation, provide real-time feedback on cardiac output and to assess for possible cardiac arrest. This is an area for further study to generate evidence to support its more consistent use.
Reintubation was documented in 10 cats in this study, due to partial or complete endotracheal tube obstruction, making a convincing case for routine capnography to aid detection. The one anaesthetic-related death in this study was in the postoperative period and appeared to be caused by airway obstruction or spasm, reinforcing the importance of vigilant monitoring of end-tidal carbon dioxide to detect endotracheal tube obstruction. This is consistent with the studies by Brodbelt et al, 3 which identified recovery as the riskiest time, and by Clarke and Hall, 2 which identified airway obstruction as a cause of post-anaesthetic fatalities in cats.
In this study, recovery monitoring was often by PCAs rather than RVNs. It has been suggested that more highly trained staff may notice problems sooner, and it is recommended that postoperative monitoring is as vigilant as during anaesthetic maintenance. 22 Limited availability of veterinary nurses and time pressure to proceed with the next anaesthetic may require a change in staff; therefore, it is our duty to ensure the handovers are clear and that the receiving PCA has received training in recognising and calling for help in cases of respiratory and cardiac complications, if we wish to continue to reduce mortality in the postoperative period.
Endotracheal intubation has been associated with increased mortality in cats.3,20 Dyson et al 4 reported that 10% of complications documented in cats were related to intubation, and intubation was shown to increase the odds of a complication. Notably, in the study showing the lowest feline peri-anaesthetic mortality rates, 6 not all cats were intubated. Cats have an incredibly reactive trachea, 23 making them prone to laryngospasm as well as damage to their delicate arytenoids. The inflammation may not be evident until the postoperative period, which is indeed where much of feline mortality occurs. 20 It has been well known for many years that careful intubation techniques, including the use of lidocaine, a laryngoscope, proper tube length/measurement and patience, are a requirement for this species.
Patient monitoring in this study was consistent with the Guidelines for Safer Anaesthesia published by the Association of Veterinary Anaesthetists, 24 except for the low use of safety checklists. In human healthcare, safety checklists have been shown to decrease mortality and morbidity. 25 Human factors have been implicated in 60% of human anaesthetic-related deaths as a result of inadequate monitoring. 26 Although time pressure or a lack of understanding of their benefits may impede checklist usage, these simple and effective tools are one easy, accessible method to enhance patient safety. 27
This study was not designed or powered to investigate mortality, but the 0.1% mortality rate is between the mortality rates reported by Brodbelt et al 3 and Levy et al. 6 Less frequent use of mask inductions may be one factor reducing the mortality rate.
In this study, at least one anaesthetic-related complication was recorded in 53% of cats, with hypotension, bradycardia and hypothermia being most common. Not all complications would have necessitated intervention; in particular, a single low reading may not reflect genuine hypotension. However, multiple low BP readings were detected in 12% of cats, higher than the incidence of hypotension of 8.5% as reported by Gaynor et al. 7 In both studies, hypotension was the most commonly reported complication. McMillan and Darcy 11 used requirement for intervention (rather than a set boundary) to define adverse events and reported a prevalence of hypotension (3%), suggesting mild hypotension may not trigger intervention. In people, hypotension is seen as a dynamic event, with interventions based on the duration and magnitude. 28 However, in a survey of American College of Veterinary Anesthesia and Analgesia and European College of Veterinary Anaesthesia and Analgesia diplomates, the values reported for definition of hypotension and the threshold for intervention were very similar. 29 This discrepancy may be partly explained by the consideration that a single measurement of hypotension may not be believed, especially when using indirect methods of measurement.
The prevalence of hypercapnia reported in this study (8.7%) is similar to the relative frequency of hypoventilation requiring intervention in 9.7% reported by McMillan and Darcy. 11
Bradycardia was more common in this study than previously reported (16.7% vs 11.3% reported by Hosgood and Scholl). 8 This may be related to the use of medetomidine, in some cases at a relatively high dose, as well as opioid usage. 30
Electrocardiogram monitoring was not used in this study; therefore, cardiac arrhythmias were only documented if they were audible on thoracic auscultation, explaining the very low rate compared with previous studies.7,8,11
The incidence of hypothermia of 13.8% was lower than that reported in other studies (eg, 51.4% at <35°C in Hosgood and Scholl, 8 70.9% at <36.5°C in Redondo et al 10 ) despite the definition of hypothermia in this study being more conservative at <37°C. The heat mats and warm air devices used in this study appear to have been effective. In addition, most cats were anaesthetised for relatively short times.
Lower body weight was associated with hypothermia, which is to be expected with a larger surface area:volume ratio. The higher incidence of hypotension with lower weight could be age related. Higher body weight was unexpectedly associated with a higher incidence of bradycardia in this study, possibly due to drugs being administered per kilogram of weight, rather than surface area.
Pre-anaesthetic medication significantly affected anaesthetic complications and anaesthetic agent requirements. IV or IM pre-anaesthetic medication was not significant in the incidence of complications. The slightly higher incidence of bradycardia with IM dosing is probably due to the higher dose of medetomidine used. This identifies selection of dosage as a potential area for training, to enable lower dosing while still achieving the desired effect, as we may see more bradycardia at those higher doses. The opioid component of the pre-anaesthetic medication did not seem to affect the incidence of complications. Acepromazine was associated with a higher propofol and isoflurane requirement. The higher propofol requirement seen in cats receiving buprenorphine may be related to acepromazine being more commonly used with this opioid.
There was an association between cats receiving acepromazine and hypotension, probably related to vasodilation. 31 Acepromazine enhances the hypotensive effect of isoflurane. 32 At least one low BP reading was documented in 65% of cats receiving acepromazine in this study, comparable with the 64–82% reported by Costa et al. 33 Cats in this study that received a higher maximum percentage of isoflurane had an increased incidence of both hypothermia and hypotension, highlighting the benefits of using multimodal analgesia to decrease inhalant settings. 13
As a retrospective study, there was no standardisation of pre-anaesthetic medications, which means it was not possible to separate the effect of the opioid and sedative components of the pre-anaesthetic medication. Owing to the retrospective design of this study, some data were missing from records and not all variables were monitored in all cats. There may have been underreporting of events, which may not have been written as free-text entries on the anaesthetic record.
Larger-scale, prospective studies are warranted to investigate anaesthetic complications in different populations of cats. Further investigation into the association of endotracheal intubation with complications and feline mortality is also warranted.
Conclusions
This study provides evidence of the prevalence of anaesthetic complications, with 53% of cats undergoing neutering in this study exhibiting one or more complication. These data provide an evidence-based selection of monitoring where resources are limited and targeted training of staff monitoring anaesthesia. The only mortality was postoperative, consistent with risk identified in previous studies.
Footnotes
Accepted: 2 September 2024
Author note: This work was submitted as a dissertation as part of a masters degree in Veterinary Anaesthesia and Analgesia through the University of Edinburgh. This paper was presented in part at the 2023 ISFM Feline Congress.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: The work described in this manuscript involved the use of non-experimental (owned or unowned) animals. Established internationally recognised high standards (‘best practice’) of veterinary clinical care for the individual patient were always followed and/or this work involved the use of cadavers. Ethical approval from a committee was therefore not specifically required for publication in JFMS. Although not required, where ethical approval was still obtained, it is stated in the manuscript.
Informed consent: Informed consent (verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (experimental or non-experimental animals, including cadavers, tissues and samples) for all procedure(s) undertaken (prospective or retrospective studies). No animals or people are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Jenny F Brown  https://orcid.org/0009-0007-8650-5104
https://orcid.org/0009-0007-8650-5104
References
- 1. Dodman NH. Feline anaesthesia survey. J Small Anim Pract 1977; 18: 653–658. [DOI] [PubMed] [Google Scholar]
- 2. Clarke KW, Hall LW. A survey of anaesthesia in small animal practice: AVA/BSAVA report. Vet Anaesth Analg 1990; 17: 4–10. [Google Scholar]
- 3. Brodbelt D, Blissitt K, Hammond R, et al. The risk of death: the confidential enquiry into perioperative small animal fatalities. Vet Anaesth Analg 2008; 35: 365–373. [DOI] [PubMed] [Google Scholar]
- 4. Dyson DH, Maxie MG, Schnurr D. Morbidity and mortality associated with anesthetic management in small animal veterinary practice in Ontario. J Am Anim Hosp Assoc 1998; 34: 325–335. [DOI] [PubMed] [Google Scholar]
- 5. Matthews NS, Mohn TJ, Yang M, et al. Factors associated with anesthetic-related death in dogs and cats in primary care veterinary hospitals. J Am Vet Med Assoc 2017; 250: 655–665. [DOI] [PubMed] [Google Scholar]
- 6. Levy JK, Bard KM, Tucker SJ, et al. Perioperative mortality in cats and dogs undergoing spay or castration at a high-volume clinic. Vet J 2017; 224: 11–15. [DOI] [PubMed] [Google Scholar]
- 7. Gaynor JS, Dunlop CI, Wagner AE, et al. Complications and mortality associated with anesthesia in dogs and cats. J Am Anim Hosp Assoc 1999; 35: 13–17. [DOI] [PubMed] [Google Scholar]
- 8. Hosgood G, Scholl DT. Evaluation of age and American Society of Anesthesiologists (ASA) physical status as risk factors for perianesthetic morbidity and mortality in the cat. J Vet Emerg Crit Care 2002; 12: 9–15. [Google Scholar]
- 9. Bille C, Auvigne V, Libermann S, et al. Risk of anaesthetic mortality in dogs and cats: an observational cohort study of 3546 cases. Vet Anaesth Analg 2012; 39: 59–68. [DOI] [PubMed] [Google Scholar]
- 10. Redondo JI, Suesta P, Gil L, et al. Retrospective study of the prevalence of postanaesthetic hypothermia in cats. Vet Rec 2012; 170: 206. DOI: 10.1136/vr.100184. [DOI] [PubMed] [Google Scholar]
- 11. McMillan M, Darcy H. Adverse event surveillance in small animal anaesthesia: an intervention-based, voluntary reporting audit. Vet Anaesth Analg 2016; 43: 128–135. [DOI] [PubMed] [Google Scholar]
- 12. Varkoulis K, Savvas I, Anagnostou T, et al. A retrospective study on canine and feline mortality during anaesthesia at a university clinic in Greece. Animals (Basel) 2023; 13. DOI: 10.3390/ani13152486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robertson SA, Gogolski SM, Pascoe P, et al. AAFP feline anesthesia guidelines. J Feline Med Surg 2018; 20: 602–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schauvliege S. Patient monitoring and monitoring equipment. In: Duke-Novarkovski T, de Vries M, Seymour C. (eds). BSAVA manual of canine and feline anaesthesia and analgesia. 3rd ed. Gloucester: British Small Animal Veterinary Association, 2016, pp 77–96. [Google Scholar]
- 15. Armstrong SR, Roberts BK, Aronsohn M. Perioperative hypothermia. J Vet Emerg Crit Care 2005; 15: 32–37. [Google Scholar]
- 16. Haskins SC. Monitoring anesthetized patients. In: Robertson S, Lamont L, Tranquilli WJ, et al. (eds). Veterinary anesthesia and analgesia: the 5th edition of Lumb and Jones. 5th edn. Chichester: John Wiley & Sons, Ltd, 2015, pp 86–113. [Google Scholar]
- 17. Posner LP, Pavuk AP, Rokshar JL, et al. Effects of opioids and anesthetic drugs on body temperature in cats. Vet Anaesth Analg 2010; 37: 35–43. [DOI] [PubMed] [Google Scholar]
- 18. R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. https://www.R-project.org/ (accessed 10 February 2021).
- 19. Association of Veterinary Anaesthetists. Anaesthetic safety checklist. https://ava.eu.com/wp-content/uploads/2022/12/AVA-Anaesthetic-Safety-Checklist-FINAL-UK-WEB-copy-2-CC.pdf (accessed 16 July 2024).
- 20. Brodbelt D, Pfeiffer D, Young L, et al. Risk factors for anaesthetic-related death in cats: results from the Confidential Enquiry into Perioperative Small Animal Fatalities (CEPSAF). Br J Anaesth 2007; 99: 617–623. [DOI] [PubMed] [Google Scholar]
- 21. Richardson EM, McMillan M. Survey on conduct of anaesthetic monitoring in small animal practice in the UK. Vet Rec 2019; 185: 570. DOI: 10.1136/vr.105444. [DOI] [PubMed] [Google Scholar]
- 22. Grubb T, Sager J, Gaynor JS, et al. 2020 AAHA anesthesia and monitoring guidelines for dogs and cats. J Am Anim Hosp Assoc 2020; 56: 59–82. [DOI] [PubMed] [Google Scholar]
- 23. Rex MAE. Laryngospasm and respiratory changes in the cat produced by mechanical stimulation of the pharynx and respiratory tract: problems of intubation in the cat. Br J Anaesth 1971; 43: 54–57. [DOI] [PubMed] [Google Scholar]
- 24. Taylor S, Benney H, Beaumont G, et al. Guidelines for safer anaesthesia. https://ava.eu.com/resources/anaesthesia-guidelines/ (2018, accessed 10 February 2021).
- 25. Haynes AB, Weiser TG, Berry WR, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med 2009; 360: 491–499. [DOI] [PubMed] [Google Scholar]
- 26. Arbous M, Grobbee D, Kleef J, et al. Mortality associated with anaesthesia: a qualitative analysis to identify risk factors. Anaesthesia 2002; 56: 1141–1153. [DOI] [PubMed] [Google Scholar]
- 27. Hawker WTG, Singh AA-O, DeForge T, et al. Attitudes towards surgical safety checklists among American College of Veterinary Surgeons diplomates. Vet Surg 2024; 53: 816–823. [DOI] [PubMed] [Google Scholar]
- 28. Nafiu OO, Voepel-Lewis T, Morris M, et al. How do pediatric anesthesiologists define intraoperative hypotension? Paediatr Anaesth 2009; 19: 1048–1053. [DOI] [PubMed] [Google Scholar]
- 29. Ruffato M, Novello L, Clark L. What is the definition of intraoperative hypotension in dogs? Results from a survey of diplomates of the ACVAA and ECVAA. Vet Anaesth Analg 2015; 42: 55–64. [DOI] [PubMed] [Google Scholar]
- 30. Grint NJ, Burford J, Dugdale AH. Investigating medetomidine-buprenorphine as preanaesthetic medication in cats. J Small Anim Pract 2009; 50: 73–81. [DOI] [PubMed] [Google Scholar]
- 31. Ward JL, Schober KE, Fuentes VL, et al. Effects of sedation on echocardiographic variables of left atrial and left ventricular function in healthy cats. J Feline Med Surg 2012; 14: 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sinclair MD, Dyson DH. The impact of acepromazine on the efficacy of crystalloid, dextran or ephedrine treatment in hypotensive dogs under isoflurane anesthesia. Vet Anaesth Analg 2012; 39: 563–573. [DOI] [PubMed] [Google Scholar]
- 33. Costa GP, Monteiro ER, Marques É, et al. Sedative effects of acepromazine in combination with nalbuphine or butorphanol, intramuscularly or intravenously, in healthy cats: a randomized, blinded clinical trial. J Feline Med Surg 2021; 23: 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]