Abstract
Objectives
Sedation before veterinary visits is advocated to help reduce fear and anxiety in cats and facilitate safe handling. The aim of this study was to evaluate the effectiveness of trazodone, gabapentin and a trazodone/gabapentin combination for oral sedation in healthy feline patients before blood donation.
Methods
A total of 21 cats were included in the study. Baseline sedation scores were obtained, and cats were randomly assigned to receive oral trazodone at 5 mg/kg (T), oral gabapentin at 10 mg/kg (G), their combination (TG) or placebo (control group). A sedation score was obtained 1 h after drug administration. A blood sample was obtained at the time of blood collection for quantification of drug plasma concentrations. Agreement between observers was tested with a Cohen’s Kappa test. Sign tests to compare change within treatment and a Skilling–Mack rank ANOVA to test for differences between groups were performed to compare pre- and post-sedation scores as well as a magnitude of differences over time between the groups. A Spearman’s rank correlation coefficient test was used to correlate sedation scores with drug plasma concentrations.
Results
Post-sedation final scores were significantly higher only in the T (P = 0.022) and TG groups (P <0.001). The magnitude of change between pre- and post-sedation scores was larger in the TG (P <0.0032) and T groups (P <0.038) compared with the control group. There were no other significant differences between the groups. There was no correlation between drug plasma concentrations and sedation scores in any of the groups.
Conclusions and relevance
Administration of oral trazodone alone at 5 mg/kg or in combination with gabapentin at 10 mg/kg resulted in significant sedation in healthy cats with no evident side effects. The degree of sedation was more profound when both drugs were combined, but a gabapentin dose of 10 mg/kg alone failed to provide significant sedation in this population.
Keywords: Oral, sedation, trazodone, gabapentin
Introduction
Sedation is used in the medical setting to produce a calm and cooperative, but not unresponsive, patient. 1 Sedatives may be utilized in veterinary patients to promote calm or facilitate procedures. Goal-directed administration of sedatives increases patient safety by decreasing patient morbidity and mortality since under- or oversedation can increase the rate of complications.2,3
Undersedation can lead to patient self-injury, increased catecholamine production, which may lead to increased oxygen consumption and hemodynamic instability, severe anxiety and behavioral changes. On the other hand, oversedation in human medicine has been linked to increased time on mechanical ventilation, a prolonged stay in the intensive care unit, and cerebral or cognitive complications.2,3 Cats are especially prone to stress during hospital visits, which presents additional challenges to receiving proper veterinary care compared with dogs. 4 For this reason, the use of ‘stress-free’ practices have gained popularity as an attempt to facilitate feline veterinary visits. Among these initiatives is the use of oral sedatives and anxiolytics before transportation and hospital visits to reduce animal and owner stress and increase provider safety.
Trazodone hydrochloride is a triazolopyridine derivative classified as a serotonin antagonist and reuptake inhibitor (SARI). Trazodone has multiple therapeutic applications in humans; however, in veterinary medicine, it is primarily utilized as a sedative and anxiolytic. 5 It has been reported to be effective in dogs for short-term use during hospitalization and for postoperative confinement,6–8 and in cats for short-term sedation to facilitate transportation and physical examination.9,10
Gabapentin is a structural analog of gamma-aminobutyric acid (GABA), with a specific affinity for the α2–∂1 subunit of pre-synaptic calcium channels.11–13 Its mechanism of action is poorly understood; it is mostly used in human and veterinary medicine as an anticonvulsant and analgesic to treat chronic and neuropathic pain. The most common side effect seen after administration of gabapentin in veterinary patients is a dose-dependent sedation, which is why it is also used off label as an oral sedative alone or sometimes in combination with trazodone. Gabapentin may reduce fear-based behaviors in veterinary settings for some cats and cause mild sedation.14,15
The pharmacokinetics of oral gabapentin in the cat have been published by multiple authors.12,14–16 A recent study by our research group also reported pharmacokinetic parameters of oral trazodone (5 mg/kg) alone or in combination with gabapentin (10 mg/kg) in healthy research cats and found that both protocols provided significant sedation from 45 mins to 8 h, with no apparent side effects. Trazodone was absorbed quickly after oral administration, and the addition of gabapentin resulted in lower trazodone bioavailability with similar sedation, although the sample size was small. 17 Both trazodone and gabapentin and their combination show promising sedative properties but no study to date has compared the three protocols in a prospective controlled manner.
The objectives of this study were to evaluate the effectiveness of trazodone, gabapentin and a trazodone/gabapentin combination as oral sedatives in healthy feline patients before blood donation. We hypothesized that oral administration of trazodone/gabapentin would have more profound sedative effects than gabapentin or trazodone alone in healthy blood donor cats. We also hypothesized that these drugs would be well-tolerated and cause no significant side effects in this population.
Materials and methods
Animals
A total of 21 cats, determined to be healthy through physical examination, complete blood count and biochemistry evaluation, were enrolled in this study. All cats were active participants in the feline blood donor program at Ontario Veterinary College. Cats in this program are sourced from the local humane society and remain in foster homes for the length of time that they are active blood donors. After a maximum of 12 blood collections, the cats are adopted out, with the vast majority remaining with their foster owners. Utilizing data obtained from previous studies,17,18 a sample of 12 cats per group was determined to be adequate to detect a difference of 2–3 points in the descriptive sedation score between groups with a power of 80% and a confidence interval of 95%. A second power analysis was performed utilizing our own data and accounting for the unbalanced design with a general linear model and random effect. The power to detect a difference of 2 units in change with an SD of 2.4 on paired differences would be 0.82 with 14 pairs. The study was carried out in accordance with the guidelines of the Canadian Council of Animal Care and was approved by the Institutional Animal Care Committee at the University of Guelph (AUP no. 4253). Consent from the foster owners was obtained before the study.
Cats were scheduled for participation in the study according to their blood donation timeline with a minimum of 30 days between donations. Cats were deemed ineligible from participation in the study if aggressive behaviors prevented the safe administration of oral drugs. Cats were fasted overnight and boarded in cages in a feline-only ward on study days. Water, clean bedding and litter boxes were always freely available, excluding limited periods of sedation.
Treatment groups and test article administration
Four treatment groups – a placebo control group, trazodone 5 mg/kg PO (T group), gabapentin 10 mg/kg PO (G group) and a combination of trazodone 5 mg/kg and gabapentin 10 mg/kg PO (TG group), containing 12 cats each, were assigned a number between 1 and 4 and a random integer set generator (random.org) was used to create 25 sets (extra sets were created as a precaution). As cats were enrolled in the study, each cat was assigned a set number in chronological order. All sedation assessors were blinded to the treatment groups. All treatments were performed by a separate treatment team. In addition, sedation assessors were not present when treatments were administered.
Oral suspensions of compounded trazodone (10 mg/ml) and gabapentin (100 mg/ml; Chiron) were formulated with a triple-fish water-based flavoring agent added by the compounding pharmacy to improve palatability. A blank oral suspension with the same flavoring agent but no active drugs was used for the control group.
Sedation scoring
A previously validated feline multiparametric sedation scale (FMSS) was utilized for this study. 18 The FMSS scoring system consists of four categories: posture score; behavior; response to sound (clapping); and response to restraint and/or IM injection and/or IV catheter. Each category was scored separately from 0 to 3, with 0 corresponding to no sedation and 3 corresponding to non-responsive. The categories were then totaled for a final score of 0–12, with 0 indicating no sedation and 12 indicating maximum sedation (see Appendix 1 in the supplementary material). Sedation assessors also assigned a word choice of none, mild, moderate or profound at the time of sedation to qualify the sedation depth. Assessment and any noticeable behaviors were also recorded.
A pre-treatment sedation score was performed by three blinded assessors. Because of the clinical nature of this study, the assessors vary among cases. The assessment team was always a combination of one American College of Veterinary Anesthesia and Analgesia diplomate and/or one anesthesiology resident and one or two anesthesia technicians. All assessors were familiar with the sedation score and completed their scores independently without discussion. After the initial sedation scoring was completed, the treatment team administered the assigned treatment and notified the assessment team of the time of administration. A second sedation score was completed in the same manner 1 h after sedation by the same assessment team. Intramuscular administration of sedative drugs before general anesthesia was performed during the restraint category of the second assessment. Sedation protocols varied among cats but was kept consistent for the duration of the study and included administration of butorphanol with a sedative (acepromazine or dexmedetomidine) combined in the same syringe and administered into the epaxial musculature.
Blood collection
At the time of blood donation, 1.5 ml of whole blood was collected from the jugular vein of each cat to measure the plasma concentrations of both trazodone and gabapentin. Blood samples were collected via a jugular catheter with sterile Luer-Lock syringes using a two-stage sampling technique. Blood was then transferred to a sterile blood tube containing lithium heparin and immediately placed on ice. Samples were centrifuged at 3000 g for 10 mins within 2 h of collection, and the plasma supernatant removed via pipette and placed into 1 ml cryogenic storage tubes, then immediately transferred to a −80°C freezer for storage.
Plasma trazodone and gabapentin quantification
Liquid chromatography (LC) analysis was performed on a Vanquish Flex Binary UHPLC system (Thermo Fisher Scientific). Gabapentin and trazodone were separated on an ACQUITY UPLC BEH C18 Column (1.7 μm, 2.1 mm × 100 mm; Waters Corp) connected with a VanGuard UPLC BEH C18 Pre-Column, (1.7 μm, 2.1mm × 5 mm; Waters Corp). The auto sampler was maintained at 4°C and the column temperature was set at 35°C. The mobile phase consisted of water, acetonitrile and formic acid (70:30:0.1, v/v/v) with a flow rate of 200 μl/min. A 5 μl sample was injected onto the column. Gabapentin and its deuterated internal standard gabapentin-D10 were eluted at 1.4 mins; trazodone and its deuterated internal standard trazodone-D6 were eluted at 2.4 mins. The total LC run time was 6 mins.
A Q Exactive Focus Orbitrap mass spectrometer (Thermo Fisher Scientific) equipped with an Ion Max source and a heated electrospray (HESI) source (Thermo Fisher Scientific) was used for mass spectrometry (MS) analysis. Data were acquired in parallel-reaction monitoring (PRM) positive ion mode. In this PRM mode, protonated [M+H]+ precursor ions were selected as mass/charge ratio (m/z) 172.133 for gabapentin, m/z 182.196 for gabapentin-D10, m/z 372.159 for trazodone and m/z 378.196 for trazodone-D6. The resulting MS/MS product ion spectrum was detected in the Orbitrap at a resolution of 17,500 (full width at half maximum at m/z 200) with automatic gain control target set at 1e. 5 The ionization conditions were optimized using Tee infusion (10 μl/min) of gabapentin (10 μg/ml) and trazodone (10 μg/ml) into LC flow (200 μl/min). The ion source parameters are optimized as follows: spray voltage 3500 V; capillary temperature 250°C; S-lens RF level 50.0; and Aux gas heater temp 400°C. Data were acquired and processed using TraceFinder software (Thermo Fisher Scientific).
Sample preparation and method validation
To extract gabapentin and trazodone from feline plasma, a simple protein precipitation was carried out. Calibration standards (1–1000 ng/ml) and quality controls (1.5 ng/ml and 1500 ng/ml for gabapentin, and 1.5 ng/ml and 800 ng/ml for trazodone) were prepared on the day of analysis. Plasma samples with concentrations above the calibration curve range were diluted with blank feline plasma to obtain values within the calibration range. A method validation procedure determined assay specificity, selectivity, linearity, accuracy, intra- and inter-day precision using the sample preparation procedure, and LC MS/MS instrument conditions described above.
The limit of quantitation (LOQ) was 1 ng/ml for both gabapentin and trazodone in this assay. LOQs were determined as the lowest concentrations that had a coefficient of variation no greater than 20%. The analytical method was repeatable and reproducible with intra- and inter-day precision within 15% for each calibration standard, except for the LOQ of gabapentin, which was within 20%. The accuracy for each calibration standard was within 15% coefficient of variation for gabapentin and trazodone. Single-time plasma concentrations for trazodone and gabapentin are reported in ng/ml.
Statistical analysis
Agreement between assessors was tested via a Cohen’s Kappa test. The magnitude of the pre- vs post-sedation score differences were calculated by subtracting the post-sedation score from the pre-sedation score. It was confirmed that the data were not normally distributed with a residual examination and Anderson–Darling test. A sign test was used to compare changes in final sedation scores over time within treatments for animals with both a before and after measurement during a treatment, which varied from 13 to 17 pairs per treatment. To compare the changes in final sedation between the groups, 17 animals were included in a Skilling–Mack rank ANOVA. Missing values were given the average rank of the non-missing ranks within an animal. Paired t-tests were performed to compare plasma concentrations of gabapentin between the G and TG groups, and plasma trazodone concentrations between the T and TG groups. A Spearman’s rank correlation coefficient test was used to correlate sedation scores with plasma concentrations of trazodone and gabapentin. P values <0.05 were considered significant.
Results
A total of 21 blood donor cats were enrolled in the study. At the time of the first blood collection, cats had a mean age of 2.06 years (range 1–4) and mean weight of 5.24 kg (range 4.1–9.2). In total, 66 blood draws were completed; 13 cats finished all four treatment groups, two cats finished three treatments, two cats finished two treatments and four cats finished one treatment.
Inter-observer agreement of final sedation scores was excellent for both pre-sedation final scores (0.89; P <0.001) and post-sedation final scores (0.83; P <0.001). For statistical purposes, assessor 1 was used for subsequent analysis.
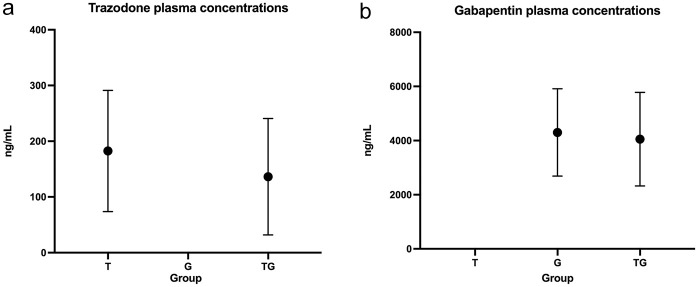
Post-sedation final scores (Figure 1) were significantly higher when compared with pre-sedation scores in the T (P = 0.022) and TG groups (P <0.001) but were not significantly different in the control (P = 0.99) or G groups (P = 0.387). The median differences between pre- and post-sedation scores are shown in Figure 2. The magnitude difference was only significantly larger in cats in the TG (P = 0.0032) and T groups (P = 0.038) compared with the control group, but not in the G group (P = 0.108) compared with the control group or when the treatment groups were compared with each other (P >0.05) (Table 1). Plasma concentrations of both trazodone and gabapentin are depicted in Figure 3. No differences were found in the plasma concentration of trazodone between the T and TG groups (P = 0.385), or in plasma gabapentin concentrations between the G and TG groups (P = 0.581).
Figure 1.
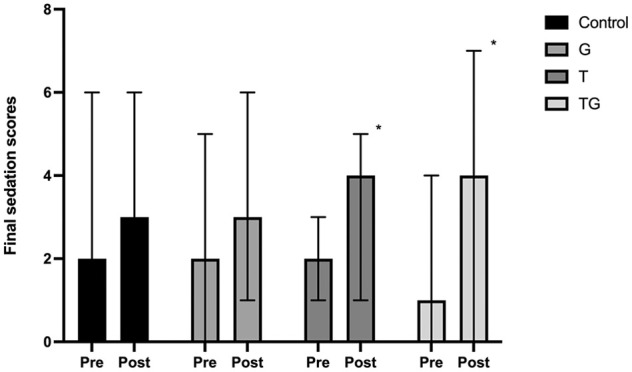
Sedation scores of cats before (Pre) and 1 h after (Post) administration of trazodone at 5 mg/kg PO (T), gabapentin at 10 mg/kg PO (G), their combination (TG) or placebo (control). The sedation score was calculated on a scale of 0–12, with 0 being no sedation and 12 being profound sedation. Data are expressed as median sedation scores (bars) and standard error (whiskers). Significant differences between Pre and Post sedation scores within the same group are indicated with * (P <0.05)
Figure 2.
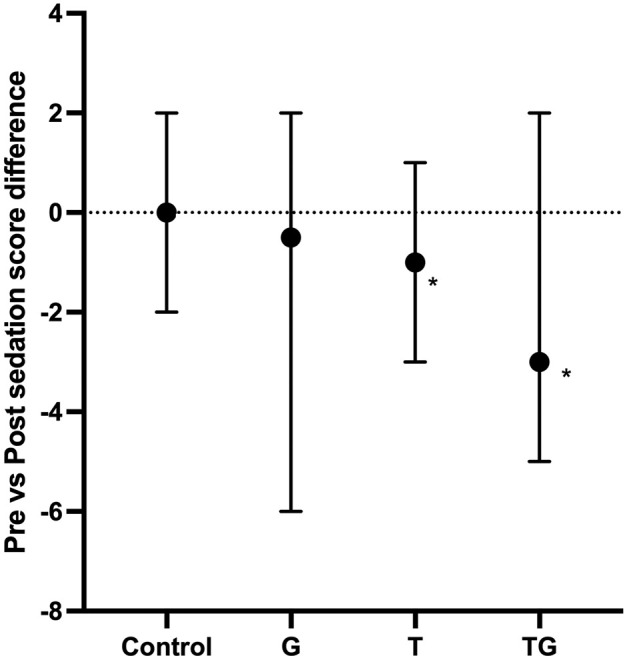
Differences between sedation scores before (Pre) and 1 h after (Post) administration of trazodone at 5 mg/kg PO (T), gabapentin at 10 mg/kg PO (G), their combination (TG) or placebo (control). The sedation score was calculated on a scale of 0–12, with 0 being no sedation and 12 being profound sedation. Data are expressed as median (solid black circles) and range (whiskers). Negative values signify higher sedation scores after drug administration compared with baseline values. Significant differences between the treatment and control groups are indicated with * (P <0.05)
Table 1.
Statistical results for Skilling–Mack (SM) rank ANOVA test for unbalance designed comparing differences between sedation scores before (Pre) and 1 h after (Post) administration of trazodone at 5 mg/kg PO (T), gabapentin at 10 mg/kg PO (G), their combination (TG) or placebo (control)
| Comparison | SM statistic | P value | DF | Total observations |
|---|---|---|---|---|
| Overall test | 11.96 | 0.0078 | 3 | 68 |
| C vs G | 2.57 | 0.108 | ||
| C vs T | 4.26 | 0.038 | ||
| C vs TG | 8.64 | 0.0032 | ||
| G vs T | 0.285 | 0.592 | ||
| T vs TG | 2.57 | 0.108 | ||
| G vs TG | 1.66 | 0.196 |
A P value <0.05 was considered statistically significant
DF = degree of freedom; G = gabapentin group; T = trazodone group; TG = combined gabapentin/trazodone group
Figure 3.
Plasma concentrations (ng/ml) for (a) trazodone and (b) gabapentin at the time of blood collection in cats receiving trazodone at 5 mg/kg PO (T), gabapentin at 10 mg/kg PO (G) or their combination (TG)
Spearman tests showed no correlation between trazodone plasma concentrations and final sedation scores in cats in the T (r = 0.165, P = 0.561) and TG groups (r = 0.408, P = 0.104) or correlation between gabapentin plasma concentrations and final sedation scores in the G (r = −0.059, P = 0.825) or TG (r = 0.367, P = 0.146) groups.
Discussion
Based on the results of this study, oral trazodone is an overall more consistent and reliable sedative for healthy cats than oral gabapentin alone. Administration of trazodone alone or in combination with gabapentin resulted in significantly higher sedation scores, while gabapentin failed to show the same effect at the doses tested. Results vary among published studies depending on the scale/method used to measure primary outcomes. Some authors have also found no differences in sedation scores after administration of oral gabapentin, 15 while most authors report decreased incidence of fear-based aggressive behaviors or stress scores.14,15,19–21 Cat population, gabapentin formulation and doses vary widely among studies, with doses in the range of 9.2–47.6 mg/kg. Primary outcomes, evaluation times and scores used also differ among studies, with most authors using simple numerical scales and evaluating sedation 1.5–3 h after oral administration. It is possible that higher doses of gabapentin administered earlier to allow for peak effect may be necessary to observe significant sedation in healthy cats.
Despite a few cats exhibiting mild and even moderate degrees of sedation after gabapentin administration, similar plasma concentrations of the drug resulted in no sedation in most cats. Oral gabapentin has a high bioavailability (88–94%), but a wide range of individual variation in peak plasma concentration after oral administration has been reported.12,16 The results from this study suggest that there is no correlation between plasma concentrations of gabapentin and sedation levels in healthy cats. No other studies have concurrently measured sedation and plasma concentration of gabapentin but, interestingly, one recent study found a positive correlation between gabapentin serum concentration and a simple numerical compliance score 3 h after administration of the same dose of gabapentin but not at 8 h. 22 The current study only correlated sedation and plasma concentrations of gabapentin 1 h after administration. Future studies with multiple sample times may be needed to clarify these findings.
The reason for the large interindividual variability in both plasma concentrations and sedative effects is not known; however, previous studies14,19,22 have also shown significant individual variation in response to gabapentin administration. This suggests that gabapentin may be useful as a single oral sedative for some cats, but the administration regimen should be tailored to the individual patient.
Interestingly, administration of oral trazodone alone at 5 mg/kg resulted in more consistent and noticeable sedation despite a high variability in plasma concentrations. Cats in the trazodone group had higher sedation scores when compared with baseline. Most cats exhibited mild to moderate sedation after trazodone administration. This is in agreement with previous studies where treatment with oral trazodone resulted in appreciable sedation at doses of 5–33.3 mg/kg.9,17 Oral trazodone administration at doses in the range of 7.7–15.2 mg/kg also resulted in fewer signs of anxiety during transport and examination. 10 It is worth noting that the drug formulations used in these studies varied; both studies from 2016 used a single tablet dose not adjusted for weight.
The magnitude of the change in sedation was only higher compared with the control group when trazodone was included in the sedation protocol, but the difference was more significant when trazodone and gabapentin were administered together, despite the lack of statistical differences when both groups were compared, suggesting synergistic effects. Trazodone and gabapentin act on different receptors and appear to have antinociceptive, synergistic effects in rodents and humans.23–25 As these two drugs also depress the central nervous system by different mechanisms of action, it is plausible that they have synergistic sedative effects that can explain the results from the current study. No side effects were observed in the study population and the combination appears safe and effective in healthy cats at the doses tested. Based on these observations there may be a clinical advantage to combining trazodone and gabapentin as oral sedatives in cats to facilitate restraint and intramuscular injections.
One of the limitations of this study is that plasma concentrations of trazodone and gabapentin were obtained approximately 30 mins after the post-sedation scores were recorded. This timing avoided additional blood sampling from the cats and unnecessary handling and stress during the pre-anesthetic period. We do not believe that this contributed to the lack of correlation between plasma concentrations of trazodone and gabapentin and sedation scores in any group since this finding was also noticed with trazodone in a prior study. 17 A lag between maximum plasma concentrations and maximum sedation was also observed. Because sedation requires action across the blood–brain barrier, it is likely that plasma blood concentrations alone are not predictive of sedation with trazodone and gabapentin. We hypothesize that measurement of central nervous system levels of trazodone and gabapentin both after administration and at the time of maximum sedation could help elucidate both the individual variability in clinical signs observed with these drugs, as well as the lack of correlation between plasma levels and sedation.
Another significant limitation of this study was the inability for all cats to complete all treatment groups. This study was designed as a prospective crossover study with four complete randomized blocks; however, hospital restrictions due to COVID-19 interfered with the ability for all blood collections to be observed. Despite this limitation, an adequate number of cats completed the study to find statistical significance and the appropriate statistical test was added to not only analyze the differences before and after within the same group, but to also compare the magnitude of the change among groups. This was added to the analysis in an attempt to compensate for incomplete treatment blocks.
Another limitation of this study was the variability in the makeup of the assessment team. Because of varying clinical schedules and the ad hoc nature of blood collection, the same three assessors could not be guaranteed throughout the study period. The FMSS has been previously validated, showing high inter-observer reliability in the current and prior studies, regardless of experience level of the assessor or degree of familiarity with the score system. Therefore, we do not believe that variation of observers significantly impacts study results when this scale is used.
A suspension formulation was chosen in this study over tablets to facilitate ease of administration. The authors recommend the addition of a flavoring additive owing to trazodone suspension’s poor palatability that may make administration difficult in some animals. All the cats in the present study were healthy young animals; additional research is needed to determine the safety of these drugs in cats with significant systemic disease and effectiveness in an aged population.
Conclusions
Oral trazodone at a dosage of 5 mg/kg appears to be a safe and reliable sedative for cats in a hospital setting. Based on our results, trazodone is preferential to gabapentin for oral sedation at the doses tested. The combination of oral trazodone at 5 mg/kg and gabapentin at 10 mg/kg appears to be a safe and effective combination that may provide better sedation than administration of both drugs separately in healthy cats.
Supplemental Material
Appendix 1. Feline multiparametric sedation score (FMSS).
Footnotes
Accepted: 16 August 2024
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was funded by a grant from the Ontario Veterinary College Pet Trust fund.
Supplementary material: The following file is available as supplementary material:
Appendix 1. Feline multiparametric sedation score (FMSS).
Ethical approval: The work described in this manuscript involved the use of non-experimental (owned or unowned) animals and procedures that differed from established internationally recognized high standards (‘best practice’) of veterinary clinical care for the individual patient. The study therefore had prior ethical approval from an established (or ad hoc) committee as stated in the manuscript.
Informed consent: Informed consent (verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (experimental or non-experimental animals, including cadavers, tissues and samples) for all procedure(s) undertaken (prospective or retrospective studies). No animals or people are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Andrea Sanchez  https://orcid.org/0000-0001-7565-1674
https://orcid.org/0000-0001-7565-1674
References
- 1. James K, Briggs S, Lewis R, et al. Introduction to specialist therapeutics. In: Tomlin M. (ed). Pharmacology and pharmacokinetics. London: Springer International Publishing, 2010, pp 53–66. [Google Scholar]
- 2. Ramsay MAE. Intensive care: problems of over- and undersedation. Best Pract Res Clin Anaesthesiol 2000; 14: 419–432. [Google Scholar]
- 3. De Jonghe B, Cook D, Appere-De-Vecchi C, et al. Using and understanding sedation scoring systems: a systematic review. Intensive Care Med 2000; 26: 275–285. [DOI] [PubMed] [Google Scholar]
- 4. Rodan I, Cannon M. Housing cats in the veterinary practice. In: Rodan I, Heath S. (eds). Feline behavioral health and welfare. St Louis, MO: WB Saunders, 2015, pp 122–136. [Google Scholar]
- 5. Chea B, Giorgi M. Trazodone: a review of its pharmacological properties and its off-label use in dogs and cats. Am J Anim Vet Sci 2017; 12: 188–194. [Google Scholar]
- 6. Gilbert-Gregory SE, Stull JW, Rice MR, et al. Effects of trazodone on behavioral signs of stress in hospitalized dogs. J Am Vet Med Assoc 2016; 249: 1281–1291. [DOI] [PubMed] [Google Scholar]
- 7. Gruen ME, Roe SC, Griffith E, et al. Use of trazodone to facilitate postsurgical confinement in dogs. J Am Vet Med Assoc 2014; 245: 296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jay AR, Krotscheck U, Parsley E, et al. Pharmacokinetics, bioavailability, and hemodynamic effects of trazodone after intravenous and oral administration of a single dose to dogs. Am J Vet Res 2013; 74: 1450–1456. [DOI] [PubMed] [Google Scholar]
- 9. Orlando JM, Case BC, Thomson AE, et al. Use of oral trazodone for sedation in cats: a pilot study. J Feline Med Surg 2015; 18: 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stevens BJ, Orlando JM, Frantz EM, et al. Efficacy of a single dose of trazodone hydrochloride given to cats prior to veterinary visits to reduce signs of transport- and examination-related anxiety. J Am Vet Med Assoc 2016; 249: 202–207. [DOI] [PubMed] [Google Scholar]
- 11. Eroglu Ç, Allen NJ, Susman MW, et al. The gabapentin receptor α2δ-1 is the neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 2009; 139: 380–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siao KT, Pypendop BH, Ilkiw JE, et al. Pharmacokinetics of gabapentin in cats. Am J Vet Res 2010; 71: 817–821. [DOI] [PubMed] [Google Scholar]
- 13. Sills GJ. The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol 2006; 6: 108–113. [DOI] [PubMed] [Google Scholar]
- 14. van Haaften KA, Forsythe LRE, Stelow EA, et al. Effects of a single preappointment dose of gabapentin on signs of stress in cats during transportation and veterinary examination. J Am Vet Med Assoc 2017; 251: 1175–1181. [DOI] [PubMed] [Google Scholar]
- 15. Pankratz KE, Ferris KK, Griffith EH, et al. Use of single-dose oral gabapentin to attenuate fear responses in cage-trap confined community cats: a double-blind, placebo-controlled field trial. J Feline Med Surg 2018; 20: 535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adrian D, Papich MG, Baynes R, et al. The pharmacokinetics of gabapentin in cats. J Vet Intern Med 2018; 32: 1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tucker LE, Sanchez A, Valverde A, et al. Pharmacokinetic, sedative, and physiological effects of oral compounded formulations of trazodone alone or in combination with gabapentin in male cats. J Vet Pharmacol Ther 2023; 46: 300–310. [DOI] [PubMed] [Google Scholar]
- 18. Rutherford AA, Sanchez A, Monteith G, et al. Description and validation of a new descriptive and multiparametric numeric rating scale to assess sedation in cats. Can Vet J 2022; 63: 603–608. [PMC free article] [PubMed] [Google Scholar]
- 19. Kruszka M, Graff E, Medam T, et al. Clinical evaluation of the effects of a single oral dose of gabapentin on fear-based aggressive behaviors in cats during veterinary examinations. J Am Vet Med Assoc 2021; 259: 1285–1291. [DOI] [PubMed] [Google Scholar]
- 20. Gurney M, Gower L. Randomised clinical trial evaluating the effect of a single preappointment dose of gabapentin on signs of stress in hyperthyroid cats. J Feline Med Surg 2022; 24: e85–e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crowe YC, Groth AD, Billson FM, et al. Gabapentin reduces stress and does not affect ocular parameters in clinically normal cats. Vet Ophtalmol 2022; 25: 493–498. [DOI] [PubMed] [Google Scholar]
- 22. Quimby JM, Lorbach SK, Saffire A, et al. Serum concentrations of gabapentin in cats with chronic kidney disease. J Feline Med Surg 2022; 24: 1260–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lipone P, Ehler E, Nastaj M, et al. Efficacy and safety of low doses of trazodone in patients affected by painful diabetic neuropathy and treated with gabapentin: a randomized controlled pilot study. CNS Drugs 2020; 34: 1177–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garrone B, di Matteo A, Amato A, et al. Synergistic interaction between trazodone and gabapentin in rodent models of neuropathic pain. PLoS One 2021; 16. DOI: 10.1371/journal.pone.0244649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oggianu L, Garrone B, Fiorentini F, et al. PK/PD analysis of trazodone and gabapentin in neuropathic pain rodent models: translational PK-PD modeling from nonclinical to clinical development. Clin Transl Sci 2023; 16: 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. Feline multiparametric sedation score (FMSS).