Abstract
The present study aimed to investigate the effect of swimming training on the angiogenesis of endothelial progenitor cells (EPCs) in type 2 diabetes mellitus (T2DM) rats by upregulating the insulin-like growth factor 1 (IGF1) expression and to reveal its potential mechanism of action. Male Sprague-Dawley rats were divided into the Control, Model, Model train, Model train + short interfering (si)-NC and Model train + si-IGF1 groups. Serum glucose levels were measured using the oral glucose tolerance test. EPCs were isolated from the bone marrow cavity and identified through morphological observation and immunofluorescence staining. The expression of IGF-1 mRNA in rat serum and EPCs was analyzed by reverse transcription-quantitative PCR. The fasting insulin levels in serum were assessed by ELISA. Cell Counting Kit-8, scratch assay and tube formation assay were used to determine the cell viability, migration and tube formation of rat EPCs, and western blotting was employed to measure the expression levels of IGF1, phosphoinositide 3-kinase (PI3K), phosphorylated-PI3K, protein kinase B (AKT) and phosphorylated-AKT. The present study demonstrated that swimming training significantly decreased the glucose levels and homeostatic model assessment of insulin resistance scores, but increased the fasting insulin levels and IGF1 mRNA expression. Microscopic observation and immunofluorescence identification suggested that EPCs were successfully isolated. In addition, swimming training markedly elevated the levels of IGF1 and promoted cell viability, migration and tube formation in rat EPCs. Furthermore, IGF1 knockdown experiments indicated that swimming training might play a regulatory role by elevating the IGF1 expression to activate the PI3K/AKT pathway. Overall, swimming training promoted the angiogenesis of EPCs in T2DM rats and its potential mechanism may be related to the upregulation of IGF1 expression and the activation of the PI3K/AKT pathway.
Keywords: swimming training, type 2 diabetes mellitus, phosphoinositide 3-kinase/protein kinase B pathway, insulin-like growth factor 1, angiogenesis
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disease characterized by elevated blood glucose levels due to insulin resistance and pancreatic β-cell insufficiency (1). The number of adults with diabetes is rising rapidly and is predicted to reach 700 million by 2045 from the current 463 million, making it a serious global public health concern (2). As 80% of patients with diabetes die from cardiovascular disease, T2DM is widely recognized as an independent risk factor for cardiovascular disease, which is the leading cause of death in patients with diabetes (3,4). This condition is closely linked to vascular endothelial cell dysfunction and impaired angiogenesis. Notably, the endothelial progenitor cells (EPC) function is primarily responsible for angiogenic ability (5). The number of EPCs is reduced in patients with diabetes and the function of these cells is severely impaired, thereby hindering angiogenesis and increasing the risk of cardiovascular disease and other vascular complications (6). Therefore, in order to prevent and treat diabetes-related cardiovascular diseases, it is imperative to identify therapeutic strategies to enhance EPC function in patients with T2DM and elucidate potential mechanisms.
Swimming training, an aerobic exercise therapy, has been found to be effective in reducing insulin resistance and lowering blood glucose levels in T2DM rats (7,8). Currently, swimming training is considered a method for the clinical treatment of T2DM (9). In addition, aerobic exercise training has been reported to improve the proliferation, migration and angiogenesis of EPCs in healthy individuals and patients with metabolic syndrome, congestive heart failure, coronary artery disease and prediabetes, thereby maintaining the integrity of the endothelial monolayer (10). However, it is still unclear whether swimming training regulates the angiogenesis of EPCs in T2DM rats and what its mechanism of action might be.
The phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway is involved in numerous cellular physiological processes, such as cell survival and proliferation (11). Li et al (8) discovered that aerobic training could regulate blood glucose and improve insulin indexes by promoting the phosphorylation of the PI3K/AKT signaling pathway in T2DM rats. In addition, aerobic training has been discovered to effectively improve the migration and angiogenesis of EPCs in T2DM rats by activating the PI3K/AKT signaling pathway (12). Insulin-like growth factor 1 (IGF1) is a peptide hormone important for cell growth, differentiation and tissue repair (13). IGF1 plays a crucial role in angiogenesis, especially in the growth and angiogenesis of endothelial cells. Specifically, IGF1 can promote endothelial cell proliferation and migration, as well as vascular regeneration and repair (14). Swimming exercise has been demonstrated to stimulate IGF1/PI3K/AKT signaling to ameliorate senescence and inflammation and inhibit apoptosis (15). However, research has yet to be conducted on whether swimming training can activate the IGF1/PI3K/AKT signaling to improve angiogenesis in EPCs of T2DM rats. Therefore, the purpose of the present study was to investigate the potential mechanism by which swimming training affected the angiogenesis of EPCs in T2DM rats.
Materials and methods
Animal modeling and grouping
A total of 30 male Sprague-Dawley rats (8–10 weeks old, weight 200–220 g) were housed in a specific pathogen-free laboratory animal room (22±2°C; humidity 45–55%) with a 12-h light/dark cycle. Animals had free access to food and water during the experiment. All experiments were performed in accordance with the Ethics Committee of the Experimental Animal Center of Shenzhen People's Hospital (license no. AUP-220516-LL-0290-01).
The T2DM rat model was established according to the method described by Yang et al (9). Briefly, 24 rats were treated with a high-fat diet (10% cooked lard, 20% sucrose, 2.5% cholesterol, 1.0% cholate and 66.5% normal diet) for 4 weeks and then fasted for 12 h. The rats were intraperitoneally injected with a low-dose of streptozotocin (25 mg/kg) (16) once a day for two days to establish the T2DM rat model. On the third day, the fasting blood glucose (FBG) value was determined using a glucometer. Approximately 20–50 µl of blood was collected from the tail vein using a sterile needle and syringe. Rats with FBG levels >13.9 mmol/l were considered to be diabetic. All 24 rats were successfully modeled as T2DM.
The 30 rats were divided into the following groups (n=6 per group): i) The Control group: Rats were fed with a normal diet (24.6% protein, 12.5% fat and 62.9% carbohydrates). After 12 weeks, the rats were fasted for 12 h, followed by intraperitoneal injecting saline (25 mg/kg, once a day for two consecutive days); ii) the Model group (the T2DM rat model): The diabetic mice did not undergo any additional treatments; iii) the Model train group: After successful modeling, the rats were trained to swim in a 200 cm container filled with water to a depth of 50 cm and maintained at 30°C. In addition, the rats were forced to swim for 30 min once a day for 7 days and training applied for 8 weeks (9); iv) the Model train + short interfering (si)-negative control (NC) group: Following successful modeling, the rats were injected with recombinant adeno-associated virus 2 (AAV2)-Ctrl (1×1011 TU/l) in the tail vein. Afterward, the rats were subjected to the same swimming training protocol as the Model train group. v) The Model train + si-IGF1 group: Upon successful modeling, the rats were injected with recombinant adeno-associated virus 2-IGF1 RNAi (1×1011 TU/l) in the tail vein (17). Following injection, the rats were treated with the same swimming training protocol as the Model train group. The si-RNA sequences were as follows: si-IGF1: sense (5′-GCACCUCCAAUAAAGAUACACAUCA-3′) and anti-sense (5′-UGAUGUGUAUCUUUAUUGGAGGUGC-3′); si-NC: sense (5′-GCACUACUAAAGAUACACAACCUCA-3′) and anti-sense (5′-UGAGGUUGUGUAUCUUUAGUAGUGC-3′).
The total duration of the animal study was 12 weeks. During the study, the rats were monitored daily for health and behavior, including checks for signs of distress, changes in weight and overall well-being. Humane endpoints, such as severe weight loss (>20% of body weight), inability to feed, severe pain or distress unresponsive to analgesia and moribund state, were established to determine whether animals should be euthanized before the end of the study. No animals reached these humane endpoints and none were found dead during the study.
Oral glucose tolerance test
The rats were fasted for 12 h before the experiment (from 20:00 on the first day to 8:00 on the second day). On the second day, blood was drawn from the tail vein using a sterile blood collection tube and the fasting blood glucose level was detected using a glucometer in each group of rats. Subsequently, the rats were injected with 50% dextrose (2 g/kg) for an oral glucose tolerance test and the serum glucose levels in the tail vein were measured at 30, 60 and 120 min post-administration (17). Approximately 100 µl blood was collected at each time point.
Serum blood collection and the isolation of endothelial progenitor cells
Rats were anesthetized with 50 mg/kg pentobarbital sodium (MilliporeSigma) administered via intraperitoneal injection. Following anesthesia, samples of blood was collected from the abdominal aorta, with ~5 ml collected per rat. The serum was separated by centrifugation at 3,000 × g for 10 min at 4°C and stored at −80°C for further reverse transcription-quantitative (RT-qPCR) or ELISA analysis.
The medullary cavities of the tibia and femur of each group were dissected and rinsed with 10 ml of phosphate-buffered saline (PBS). The cell suspensions were collected by filtration and supplemented with lymphocyte separation solution at a ratio of 1:1. Then, the cell suspensions were centrifuged at 400 × g for 30 min at 4°C. The precipitates were subsequently cultured in a basic culture medium containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and vascular endothelial growth factor (R&D Systems, Inc.) (10). Following the collection of the required samples, the rats were euthanized by cervical dislocation. Animal death was verified by the absence of a heartbeat and lack of response to toe pinch reflex.
RT-qPCR
Total RNA was extracted from rat serum and EPCs using TRIzol® (Thermo Fisher Scientific, Inc.) following the manufacturer's protocol. The RNA concentration was quantified to ensure quality and purity by a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc.). The RNA was reverse transcribed into cDNA using a reverse transcription kit (Takara Bio, Inc.) according to the manufacturer's instructions. The obtained cDNA was used for reverse transcription-quantitative polymerase chain reaction with an SYBR Premix Ex Taq II kit (Takara Bio, Inc.) under the following cycling conditions: initial denaturation at 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec, 60°C for 30 sec and 72°C for 30 sec. The mRNA expression level of the target gene was calculated by the 2−ΔΔCq method (18), with GAPDH serving as the internal control. The primer sequences of IGF1 and the internal control gene (GAPDH) were as follows: IGF1 5′-GCTCTTCAGTTCGTGTGTGGA-3′, 5′-CGACTGCTGGAGCCATACC-3′ and GAPDH 5′-GTGGGCATCAATGGATTT-3′, 5′-ACACCATGTATTCCGGGTCAAT-3′.
ELISA
Fasting serum samples were collected before euthanasia and the serum was examined by the ELISA. The treated serum reacted with an insulin detection reagent at a certain concentration and then the absorbance of the samples was detected by an ELISA reader. The standard curve was generated with absorbance as the horizontal coordinate and insulin concentration as the vertical coordinate. The fasting insulin level of the rats was measured by calculating the absorbance of the samples. Ultimately, the homeostatic model assessment of insulin resistance (HOMA-IR) score was obtained: HOMA-IR=(fasting blood glucose level × fasting insulin level)/22.5. All procedures were performed in accordance with the instructions of the kit (cat. no. CSB-E08346r; Cusabio Technology, LLC).
Characterization and identification of EPCs
An inverted microscope was used to observe the morphology of the cells on days 4, 8 and 14. Cells were defined as bone marrow-derived EPCs if they were spindle-shaped (10). Immunofluorescence staining was performed using 1,1′-dioctadecyl-3,3,3′, 3′-tetramethylindocarbocyanine perchlorate-labeled acetylated low-density lipoprotein (Dil-ac-LDL; cat. no. MP6013500UG; MK Bio Science Co., Inc.) and fluorescein isothiocyanate-conjugated Ulex europaeus agglutinin I (FITC-UEA I; cat. no. MP6308-500UG; MK Bio Science Co., Inc.). Sterile cell slides were placed in 24-well culture plates. After digestion with trypsin (Gibco; Thermo Fisher Scientific, Inc.), EPCs were counted by a microscope. The EPCs stained with DiI-ac-LDL were red, those stained with FITC-UEA I were green and those stained with both were yellow. A total of 10 randomly selected areas were observed and images captured using a fluorescence microscope and the proportion of yellow EPCs to the total EPCs was calculated.
Subsequently, phenotypic identification of the EPCs was performed before washing with PBS. The cells were fixed with 4% paraformaldehyde at room temperature for 20 min, washed with PBS and permeabilized with a mixture of 10% goat serum. Next, primary antibodies were added for incubation overnight at 4°C. The primary antibodies were: CD31 (1:100; Proteintech Group; cat. no. CL488-66065), CD34 (1:100; BIOSS; cat. no. bsm-41197M) and CD133 (1:100; Proteintech Group; cat. no. 18470-1-AP). After washing with PBS (5 min/time), cells were incubated with CoraLite Plus 488-conjugated ACE2 Monoclonal antibody (Proteintech Group; cat. no. CL488-66699) and Alexa Fluor® 594 secondary antibody (Thermo Fisher Scientific; cat. no. R37121) in the dark at room temperature for 2 h. Upon a staining of cellular DNA with 4′,6-diamidino-2-phenylindole (Beijing Solarbio Science & Technology Co., Ltd.), images of the cells were captured using a fluorescence microscope.
Cell Counting Kit-8 assay for cell viability
The EPCs were seeded in 96-well plates (4×103 cells/well) and incubated for 24 h at 37°C. Cells were supplemented with 10 µl of Cell Counting Kit-8 solution (MilliporeSigma) for 4 h of incubation. The optical density was measured at a wavelength of 450 nm using a microplate reader.
Scratch assay for cell migration
EPCs were inoculated in 24-well plates (4×106 cells/well) and incubated for 24 h at 37°C. A straight line was scratched in each well with a 10 µl pipette tip. Then, the detached cells and debris were washed away with PBS and the culture medium was replaced with a fresh serum-free medium. Images were captured at 0 and 24 h in the marked area. The experiment was repeated three times and the scratched area was measured and counted using ImageJ software (version 1.54; National Institutes of Health). Ultimately, the cell migration rate was calculated as follows: cell migration rate=(0 h scratch width-24 h scratch width)/0 h scratch width ×100%.
Tube formation assay
EPCs were seeded in plates (1×105 cells/well) coated with Matrigel (R&D Systems, Inc.) and incubated for 18 h at 37°C with 5% CO2 (12). Then, the morphological images of the tubes were captured using an inverted microscope. Capillaries were defined as tubes with a length that was four times their width. The average number of capillaries was determined and images were analyzed using ImageJ software (version 1.54; National Institutes of Health).
Western blotting
After rat EPCs were lysed using a radioimmunoprecipitation assay buffer (RIPA buffer; Thermo Fisher Scientific, Inc.), the protein concentration was detected using a bicinchoninic acid kit (Thermo Fisher Scientific, Inc.). Upon denaturation, the proteins (20 µg) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Then, the proteins were transferred to polyvinylidene fluoride membranes. Afterward, the membranes were blocked with 5% skimmed milk for 2 h at room temperature. Subsequently, primary antibodies were added for incubation overnight at 4°C. The primary antibodies included IGF1 (cat. no. ab40657; 1:2,000), phosphorylated-PI3K (p-PI3K, ab302958, 1:1,000), PI3K (cat. no. ab283852; 1:2,000), phosphorylated-AKT (p-PI3K, ab8805, 1:1,000), AKT (cat. no. ab38449, 1:1,000) and GAPDH (cat. no. ab8245; 1:10,000; all from Abcam). On the next day, the membranes were incubated with goat anti-rabbit IgG secondary antibody (cat. no. ab6721; 1:2,000) or goat anti-mouse IgG secondary antibody (cat. no. ab205719; 1:20,000; both from Abcam) for 2 h at room temperature. The protein bands were visualized using ECL luminescent solution (MilliporeSigma) and the images were captured using an imaging system (Thermo Fisher Scientific, Inc.). The band intensities were semi-quantitatively analyzed using ImageJ software (version 1.54).
Statistical analysis
Statistical analysis of the experimental data was performed using SPSS software (version 24.0; IBM Corp.). A one-way analysis of variance was used to compare the differences between the two groups. The differences were compared among multiple groups by a Tukey's post hoc test. The results were expressed as mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of swimming training on IGF1 expression and metabolic parameters in T2DM rats
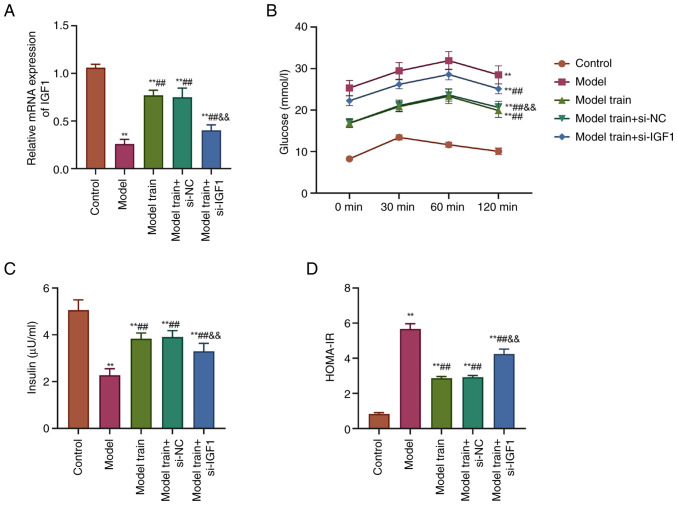
The mRNA expression level of IGF1 in the serum of rats was detected by RT-qPCR. The detection results showed that the mRNA expression level of IGF1 was markedly decreased in the serum of T2DM rats compared with the Control group. By contrast, the mRNA expression level of IGF1 was markedly increased in the Model train group as opposed to the Model group. After knocking down IGF1 expression, the Model train + si-IGF1 group exhibited a marked reduction in the mRNA expression level of IGF1 relative to the Model train + si-NC group (P<0.01; Fig. 1A).
Figure 1.
Effects of swimming training on IGF1 expression and metabolic parameters in rats with T2DM. (A) RT-qPCR to detect the mRNA expression level of IGF1 in the serum of rats in all groups. (B) Glucometer detection results of the glucose levels in the serum of rats in all groups. (C) Biochemical assays of serum insulin levels in rats in all groups. (D) Calculation of serum HOMA-IR scores in rats in all groups; **P<0.01 vs. Control group; ##P<0.01, vs. Model group; &&P<0.01, vs. Model train + si-NC group. IGF1, insulin-like growth factor 1; T2DM, type 2 diabetes mellitus; RT-qPCR, reverse transcription-quantitative PCR; HOMA-IR, homeostatic model assessment of insulin resistance; si, short interfering; NC, negative control.
The glucose level in the serum of T2DM rats was increased from 0–60 min, but decreased from 60–120 min. Nevertheless, swimming training resulted in a notable decrease in serum glucose levels. In contrast to the Model train + si-NC group, the glucose level in the serum of T2DM rats was raised after knocking down the expression of IGF1 (P<0.01; Fig. 1B).
The fasting insulin levels in serum were notably lower and the HOMA-IR scores were significantly higher in T2DM rats compared with those in the Control group. After swimming training, there was a marked upregulation in the serum fasting insulin levels and a marked downregulation in the HOMA-IR scores in rats. In comparison with the Model train + si-NC group, the Model train + si-IGF1 group had lower fasting insulin levels and significantly higher HOMA-IR scores (P<0.01; Fig. 1C and D). These results suggested that swimming training could improve the metabolic parameters of T2DM potentially through the upregulation of IGF1 expression.
Morphology and identification of EPCs
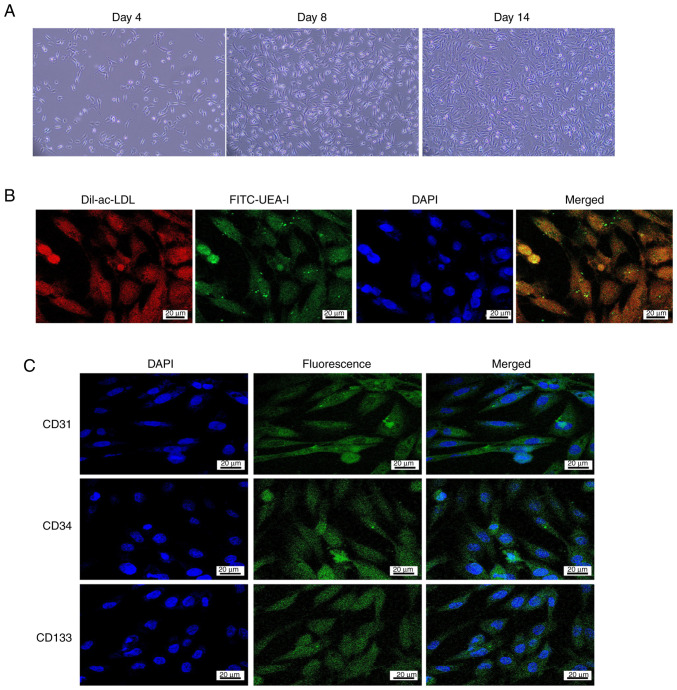
Subsequently, the EPCs were isolated and identified from the bone marrow cavities of rats in each treatment group. The results showed that most of the EPCs were round or oval with a few being spindle-shaped on day 4; on day 8, they were spindle-shaped and round; and on day 14, EPCs were well-grown and adhered to the bottom of the culture flasks (Fig. 2A). Immunofluorescence through the inverted microscope indicated that EPCs were red after cytoplasmic uptake of DiI-ac-LDL and green after cytomembrane uptake of FITC-UEA-I. Cells that were yellow after double-staining with DiI-ac-LDL and FITC-UEA-I were identified as differentiated EPCs (Fig. 2B). These results confirmed the successful isolation of EPCs.
Figure 2.
Morphology and identification of EPCs. (A) Characterization of EPCs from rats grown in vitro (magnification, ×100). (B) Immunofluorescence staining with Dil-ac-LDL and/or FITC-UEA-I (scale bar, 20 µm). (C) Fluorescence microscopy detection of phenotypes CD31, CD34 and CD133 (scale bar, 20 µm). EPC, endothelial progenitor cell; Dil-ac-LDL, 1,1′-dioctadecyl-3,3,3′, 3′-tetramethylindocarbocyanine perchlorate-labeled acetylated low-density lipoprotein; FITC-UEA-I, fluorescein isothiocyanate-conjugated Ulex europaeus agglutinin-I.
Effects of swimming training on the IGF1 expression in EPCs of T2DM rats
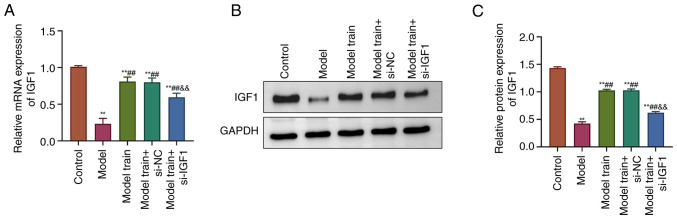
The effects of swimming training on the expression of IGF1 in EPCs of T2DM rats was observed. The results showed that the mRNA and protein expression levels of IGF1 in EPCs of T2DM rats were notably decreased compared with the Control group. By contrast, the mRNA and protein expression levels of IGF1 were markedly higher in the Model train group compared with the Model group. The Model train + si-IGF1 group exhibited markedly lower mRNA and protein expression levels of IGF1 compared with the Model train + si-NC group (P<0.01; Fig. 3). In summary, the aforementioned results indicated that swimming training notably upregulated the IGF1 expression in EPCs of T2DM rats.
Figure 3.
Effects of swimming training on the IGF1 expression in EPCs of T2DM rats. (A) RT-qPCR to measure the mRNA expression levels of IGF1 in EPCs of rats in all groups. (B) Western blotting detection of the IGF1 protein expression levels in rat EPCs in all groups. (C) Calculation of the IGF1 protein expression levels in all groups. **P<0.01, vs. Control group; ##P<0.01, vs. Model group; &&P<0.01, vs. Model train + si-NC group. IGF1, insulin-like growth factor 1; EPC, endothelial progenitor cell; T2DM, type 2 diabetes mellitus; RT-qPCR, reverse transcription-quantitative PCR; si, short interfering; NC, negative control.
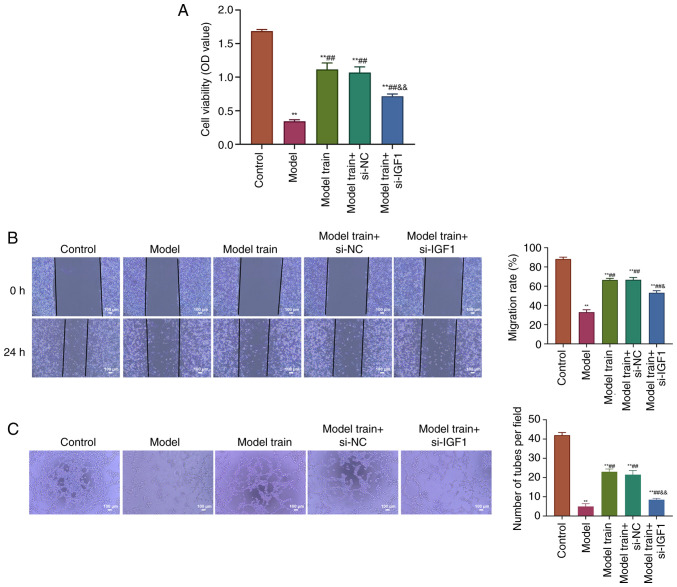
Swimming training promotes cell viability, migration and angiogenesis in EPCs of T2DM rats through upregulation of IGF1 expression. The present study analyzed the effects of swimming training on cell viability, migration and angiogenesis of EPCs in T2DM rats after knocking down IGF1. The results showed that there was a significant decrease in EPC viability, cell migration and tube formation in T2DM rats compared with the Control group. However, these parameters were much higher in the Model train group than those of the Model group. Knockdown of IGF1 expression could clearly decrease EPC viability, cell migration and tube formation in comparison with the Model train + si-NC group (P<0.01; Fig. 4). Overall, swimming training promoted cell viability, migration and angiogenesis of EPCs in T2DM rats through upregulation of the IGF1 expression.
Figure 4.
Swimming training promotes EPC viability, migration and angiogenesis in T2DM rats through upregulation of IGF1 expression. (A) CCK-8 assay to determine EPC viability in rats of the Control, Model, Model train, Model train + si-NC and Model train + si-IGF1 groups. (B) Scratch assay to analyze the EPC migration of rats in all groups (scale bar, 100 µm). (C) Tube formation assay to measure the EPC migration of rats in all groups (scale bar, 100 µm). **P<0.01, vs. Control group; ##P<0.01, vs. Model group; &&P<0.01, vs. Model train + si-NC group. EPC, endothelial progenitor cell; T2DM, type 2 diabetes mellitus; IGF1, insulin-like growth factor 1; CCK-8, Cell Counting Kit-8; si, short interfering; NC, negative control.
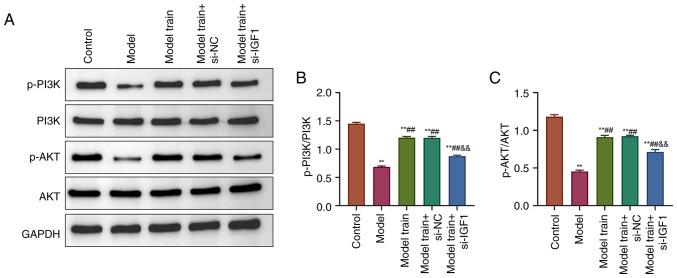
Swimming training activates the PI3K/AKT pathway in EPCs of T2DM rats by upregulating IGF1 expression
Finally, the effects of swimming training on PI3K/AKT pathway-related proteins in EPCs of T2DM rats after knocking down IGF1 were explored. The results revealed that, relative to the Control group, the phosphorylation levels of PI3K and AKT were significantly reduced in EPCs of T2DM rats (P<0.01). However, there was no significant difference in the total expression of PI3K and AKT (P>0.05). Compared with the Model group, rats in the Model train group displayed higher expression levels of p-PI3K and p-AKT (P<0.01). As opposed to the Model train + si-NC group, the expression of p-PI3K and p-AKT was considerably reduced in EPCs of the Model train + si-IGF1 group (P<0.01; Fig. 5). These findings suggested that the PI3K/AKT pathway was inhibited after knocking down IGF1 expression and swimming training could reverse this inhibitory effect by upregulating the IGF1 expression.
Figure 5.
Swimming training activates the PI3K/AKT pathway in EPCs of T2DM rats by upregulating the IGF1 expression. (A) Western blotting detected the expression levels of p-PI3K, PI3K, p-AKT and AKT in rat EPCs of all groups. (B) Calculation of p-PI3K/PI3K ratio of rat EPCs in all groups. (C) Calculation of p-AKT/AKT ratio of rat EPCs in all groups. **P<0.01, vs. Control group; ##P<0.01, vs. Model group; &&P<0.01, vs. Model train + si-NC group. PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; EPC, endothelial progenitor cell; T2DM, type 2 diabetes mellitus; IGF1, insulin-like growth factor 1; p-, phosphorylated; si, short interfering; NC, negative control.
Discussion
Atherosclerosis-induced cardiovascular disease is a complication of diabetes mellitus and, at present, a major cause of death for patients with diabetes (19). EPCs are essential for maintaining the integrity of vascular endothelium, especially in cases of atherosclerosis, impaired angiogenesis and vascular repair defects (20,21). Diabetes mellitus has been reported to impair the angiogenesis of EPCs and increase the morbidity and mortality of cardiovascular diseases (12). By contrast, aerobic exercise can reduce the incidence and mortality of diabetes-related vascular diseases by noticeably increasing the number of EPCs (10). Therefore, it is of great significance to investigate the mechanism by which aerobic exercise improves the function of EPCs in patients with T2DM. If the investigation is successful, it can provide a feasible targeted therapy to enhance endothelial function and reduce diabetes-related vascular diseases.
In the present study, a T2DM rat model was first constructed using a classical method, which involved feeding the rats with a high-fat diet for 4 weeks and then injecting low-dose streptozotocin intraperitoneally (9). Then EPCs were isolated to explore the role of swimming training in the angiogenesis of EPCs in T2DM rats in vivo and in vitro. The experimental results demonstrated that swimming training lowered serum glucose levels and HOMA-IR scores while elevating fasting insulin levels in T2DM rats.
Aerobic exercise, such as swimming, can enhance the insulin sensitivity in target organs or target tissues, reduce insulin resistance, increase the number and sensitivity of insulin receptors and improve the glycemic as well as lipid metabolism indexes of patients with diabetes (22). These findings align with the present study, confirming that swimming exercise indeed improves the glucose levels and insulin indices of patients with diabetes.
High glucose levels in patients with T2DM may lead to varying degrees of EPC dysfunction, such as decreased migration capacity and re-endothelialization. More specifically, the decreased migration capacity affects the adhesion ability of EPCs, thereby attenuating endothelial repair and further inducing re-endothelialization (23). Current evidence from animal or human models demonstrates the effects of physical activity on endothelial function and EPC activity (24). In the present study, EPCs isolated from the bone marrow of rats subjected to one week of swimming training exhibited higher levels of cell viability, cell migration and tube formation. This demonstrated that swimming training enhanced the EPC function, subsequently improving cardiovascular endothelium in rats with T2DM.
IGF1 is one of the most important regulators related to cell growth, differentiation and apoptosis. IGF1 has a metabolic effect similar to that of insulin, lowering blood glucose and blood lipids as well as dilating blood vessels. This regulatory factor plays a crucial role in the pathophysiological process of cardiovascular diseases, endocrine diseases and tumors (25). According to preliminary research, IGF1 can protect diabetic cardiovascular disease by preventing vascular endothelial dysfunction in various ways (26). In the present study, the IGF1 expression levels in serum and EPCs of T2DM rats were found to be markedly suppressed, whereas swimming training elevated the IGF1 expression levels. Notably, knocking down IGF1 expression reversed the benefits of swimming training. This suggested that IGF1 was involved in the regulation of diabetic cardiovascular disease and that swimming training enhanced the EPC function in T2DM rats by upregulating the IGF1 expression.
Additionally, the present study revealed that swimming training might enhance the EPC function by activating PI3K/AKT signaling. The PI3K/AKT activation promotes EPC function and exercise-induced increases in PI3K/AKT further enhance the EPC function (10), which is consistent with the findings of the present study. Moreover, in the present study, IGF1 knockdown could interfere with the enhancement of EPC function induced by the PI3K/AKT activation. This implied that swimming training might enhance the EPC function by upregulating the IGF1 expression to activate the PI3K/AKT signaling. Similar to the findings of the present study, IGF1 has been shown to increase insulin sensitivity, enhance EPC function and slow the development of vascular complications in diabetes by activating the PI3K/AKT pathway (27).
To further elucidate the mechanisms involved in EPC function and angiogenesis, it is important to investigate the roles of key angiogenic molecules, such as vascular endothelial growth factors (VEGF), platelet-derived growth factors (PDGF), matrix metalloproteinases (MMP) and transforming growth factor-β (TGF-β). The present study focused on the upregulation of IGF1 and the activation of the PI3K/AKT pathway, both of which are known for their roles in cellular growth, survival and angiogenesis. Due to resource constraints, VEGF, PDGF, MMP and TGF-β were not included in the present study. Future studies should include these angiogenic molecules and performing functional assays related to proliferation and cell cycle analysis to build a more comprehensive understanding of the angiogenesis in EPCs influenced by swimming training.
The novelty of the present study lay in its pioneering investigation of the effects of swimming training on EPC angiogenesis in T2DM rats. Unlike previous studies on aerobic exercise, the present study specifically explored how swimming enhanced the EPC function in T2DM. the present study demonstrated that swimming training significantly improved EPC viability, migration and tube formation by upregulating the IGF1 expression and activating the PI3K/AKT pathway. The present study provided new insights into the molecular mechanisms underlying exercise-induced angiogenesis in patients with diabetes, providing valuable knowledge for developing exercise-based therapies for diabetic vascular complications.
In conclusion, swimming training improves insulin resistance and vascular endothelial dysfunction in T2DM rats. This finding may be related to the stimulation of EPC angiogenesis by upregulating the IGF1 expression, thereby activating the PI3K/AKT pathway. The present study provides a valuable reference for selecting exercise regimens and encourages the creation of cell-based treatments for cardiovascular disease associated with diabetes. However, the present study was limited by the absence of key angiogenic molecules and functional assays, therefore further studies are required to include these angiogenic molecules and conduct functional validation experiments involving the PI3K/AKT pathway.
Acknowledgements
Not applicable.
Funding Statement
The present study was supported by the Shenzhen Key Medical Discipline Construction Fund (grant no. SZXK012).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
LL and XYM conceived and designed the experiments and wrote the manuscript. KWJ, YZ and YRC performed the experiments and analyzed the data. YRC made substantial contributions to manuscript revision and supervision. LL and XYM confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The use of laboratory animals in the present study was reviewed and approved by the institutional animal care and use committee of the Laboratory Animal Center of Shenzhen People's Hospital (approval no. AUP-220516-LL-0290-01). Consent to participate was not applicable for the present study as it involved no human participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Su J, Luo Y, Hu S, Tang L, Ouyang S. Advances in research on type 2 diabetes mellitus targets and therapeutic agents. Int J Mol Sci. 2023;24:13381. doi: 10.3390/ijms241713381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nandula SR, Kundu N, Awal HB, Brichacek B, Fakhri M, Aimalla N, Elzarki A, Amdur RL, Sen S. Role of canagliflozin on function of CD34+ve endothelial progenitor cells (EPC) in patients with type 2 diabetes. Cardiovasc Diabetol. 2021;20:44. doi: 10.1186/s12933-021-01235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu TJ, Wu DA, Hsu BG. Serum leptin level is positively correlated with aortic stiffness in patients with type 2 diabetes mellitus. Front Biosci (Landmark Ed) 2023;28:128. doi: 10.31083/j.fbl2806128. [DOI] [PubMed] [Google Scholar]
- 4.Sun H, Men C, Deng H, Wang C, Li R, Sun Y, Yang H, Wu M, Zheng W, Zhan Y. Construction of a nomogram model to identify atherosclerotic cardiovascular disease in patients with type 2 diabetes mellitus. Discov Med. 2023;35:1114–1122. doi: 10.24976/Discov.Med.202335179.108. [DOI] [PubMed] [Google Scholar]
- 5.Yiu KH, Tse HF. Specific role of impaired glucose metabolism and diabetes mellitus in endothelial progenitor cell characteristics and function. Arterioscler Thromb Vasc Biol. 2014;34:1136–1143. doi: 10.1161/ATVBAHA.114.302192. [DOI] [PubMed] [Google Scholar]
- 6.Altabas V, Marinkovic Radosevic J, Spoljarec L, Uremovic S, Bulum T. The impact of modern anti-diabetic treatment on endothelial progenitor cells. Biomedicines. 2023;11:3051. doi: 10.3390/biomedicines11113051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z, Cui D, Zhang T, Sun Y, Ding S. Swimming differentially affects T2DM-induced skeletal muscle ER stress and mitochondrial dysfunction related to MAM. Diabetes Metab Syndr Obes. 2020;13:1417–1428. doi: 10.2147/DMSO.S243024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, He D, Jiang K, Zhao Y. Effects of forced swimming stress on expression and phosphorylation of PI3K/Akt signal pathway in pancreas of type 2 diabetic rats. Ann Transl Med. 2020;8:1006. doi: 10.21037/atm-20-5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Q, Wang WW, Ma P, Ma ZX, Hao M, Adelusi TI, Lei-Du Yin XX, Lu Q. Swimming training alleviated insulin resistance through Wnt3a/β-catenin signaling in type 2 diabetic rats. Iran J Basic Med Sci. 2017;20:1220–1226. doi: 10.22038/IJBMS.2017.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhai L, Liu Y, Zhao W, Chen Q, Guo T, Wei W, Luo Z, Huang Y, Ma C, Huang F, Dai X. Aerobic and resistance training enhances endothelial progenitor cell function via upregulation of caveolin-1 in mice with type 2 diabetes. Stem Cell Res Ther. 2020;11:10. doi: 10.1186/s13287-019-1527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian Y, Li L, Lin G, Wang Y, Wang L, Zhao Q, Hu Y, Yong H, Wan Y, Zhang Y. lncRNA SNHG14 promotes oncogenesis and immune evasion in diffuse large-B-cell lymphoma by sequestering miR-152-3p. Leuk Lymphoma. 2021;62:1574–1584. doi: 10.1080/10428194.2021.1876866. [DOI] [PubMed] [Google Scholar]
- 12.Dai X, Zhai L, Su Q, Luo B, Wei C, Liu Y, Huang Y, Ma C, Ying Y. Effect of aerobic and resistance training on endothelial progenitor cells in mice with type 2 diabetes. Cell Reprogram. 2020;22:189–197. doi: 10.1089/cell.2019.0063. [DOI] [PubMed] [Google Scholar]
- 13.Caputo M, Pigni S, Agosti E, Daffara T, Ferrero A, Filigheddu N, Prodam F. Regulation of GH and GH signaling by nutrients. Cells. 2021;10:1376. doi: 10.3390/cells10061376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo SH, Hwang SY, Hwang S, Han S, Park H, Lee YS, Rho SB, Kwon Y. Hypoxia-induced ELF3 promotes tumor angiogenesis through IGF1/IGF1R. EMBO Rep. 2022;23:e52977. doi: 10.15252/embr.202152977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin JY, Kuo WW, Baskaran R, Kuo CH, Chen YA, Chen WS, Ho TJ, Day CH, Mahalakshmi B, Huang CY. Swimming exercise stimulates IGF1/PI3K/Akt and AMPK/SIRT1/PGC1α survival signaling to suppress apoptosis and inflammation in aging hippocampus. Aging (Albany NY) 2020;12:6852–6864. doi: 10.18632/aging.103046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salido EM, de Zavalía N, Schreier L, De Laurentiis A, Rettori V, Chianelli M, Keller Sarmiento MI, Arias P, Rosenstein RE. Retinal changes in an experimental model of early type 2 diabetes in rats characterized by non-fasting hyperglycemia. Exp Neurol. 2012;236:151–160. doi: 10.1016/j.expneurol.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Ding X, Jing N, Shen A, Guo F, Song Y, Pan M, Ma X, Zhao L, Zhang H, Wu L, et al. MiR-21-5p in macrophage-derived extracellular vesicles affects podocyte pyroptosis in diabetic nephropathy by regulating A20. J Endocrinol Invest. 2021;44:1175–1184. doi: 10.1007/s40618-020-01401-7. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17:83. doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang PH, Chen CY, Lin CP, Wang CH, Tsai HY, Lo WY, Leu HB, Chen JW, Lin SJ, Chu PH. Deletion of FHL2 gene impaired ischemia-induced blood flow recovery by modulating circulating proangiogenic cells. Arterioscler Thromb Vasc Biol. 2013;33:709–717. doi: 10.1161/ATVBAHA.112.300318. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Ma Y, Miao XH, Guo JD, Li DW. Neovascularization and tissue regeneration by endothelial progenitor cells in ischemic stroke. Neurol Sci. 2021;42:3585–3593. doi: 10.1007/s10072-021-05428-3. [DOI] [PubMed] [Google Scholar]
- 22.Pezhman L, Sheikhzadeh Hesari F, Ghiasi R, Alipour MR. The impact of forced swimming on expression of RANKL and OPG in a type 2 diabetes mellitus rat model. Arch Physiol Biochem. 2019;125:195–200. doi: 10.1080/13813455.2018.1446178. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh A, Gao L, Thakur A, Siu PM, Lai CWK. Role of free fatty acids in endothelial dysfunction. J Biomed Sci. 2017;24:50. doi: 10.1186/s12929-017-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao J, Pan X, Li G, Chatterjee E, Xiao J. Physical exercise protects against endothelial dysfunction in cardiovascular and metabolic diseases. J Cardiovasc Transl Res. 2022;15:604–620. doi: 10.1007/s12265-021-10171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Jiang R, Yue Q, Peng H. MicroRNA-29 regulates myocardial microvascular endothelial cells proliferation and migration in association with IGF1 in type 2 diabetes. Biochem Biophys Res Commun. 2017;487:15–21. doi: 10.1016/j.bbrc.2017.03.055. [DOI] [PubMed] [Google Scholar]
- 26.Higashi Y, Gautam S, Delafontaine P, Sukhanov S. IGF-1 and cardiovascular disease. Growth Horm IGF Res. 2019;45:6–16. doi: 10.1016/j.ghir.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye H, He Y, Zheng C, Wang F, Yang M, Lin J, Xu R, Zhang D. Type 2 diabetes complicated with heart failure: research on therapeutic mechanism and potential drug development based on insulin signaling pathway. Front Pharmacol. 2022;13:816588. doi: 10.3389/fphar.2022.816588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.