Abstract
Background
Diet may influence susceptibility of human papillomavirus (HPV) infection by affecting inflammation and immunity. However, the association between HPV infection and the inflammatory potential of diet has not been investigated. The research aimed to examine the correlation between HPV status and the dietary inflammatory index (DII®).
Methods
We utilized data from the National Health and Nutrition Examination Survey (NHANES) 2003–2016 to investigate the correlation between DII and HPV status among 9,256 women aged 18–59 years. DII scores were calculated based on 24-hour dietary recall interviews. The association between HPV status and DII was analyzed using weighted logistic regression and restricted cubic spline (RCS).
Results
Women with HPV infection exhibited higher DII scores than those without HPV infection. An increased likelihood of HPV infection was found to be significantly associated with higher DII scores (OR = 1.05, 95% CI: 1.01–1.09, P = 0.021), after full multivariate adjustment. Compared with the lowest tertile of DII scores, the ORs (95% CIs) for HPV infection were 1.20 (1.01, 1.42) and 1.27 (1.07, 1.51) for the second and third tertiles, respectively (P for trend = 0.006). RCS analysis showed a U-shaped relationship between DII and HPV infection, with a breakpoint identified at 0.13.
Conclusions
Our findings suggest that a pro-inflammatory diet is associated with an increased likelihood of HPV infection among women in the United States. Dietary interventions to reduce inflammation may help prevent HPV infection and related diseases.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-20490-0.
Keywords: Diet, Inflammation, Dietary inflammatory index, Human papillomavirus infection, National health and nutrition examination survey (NHANES)
Introduction
Human papillomavirus (HPV) infection is the most common sexually transmitted disease (STD) worldwide, contributing to 4.5% of newly diagnosed cancer cases [1]. It is also one of the etiological factors for benign lesions such as genital warts [2]. Almost 100% of cervical cancers and a considerable portion of oropharyngeal, anal, penile, vulvar, and vaginal cancers are caused by HPV infection [3].
Previous studies indicate that chronic inflammation and oxidative stress are associated with the risk of HPV infection, the formation of precancerous lesions, and the progression to cervical cancer [4–6]. Diet is one of the most crucial factors in regulating the body’s inflammatory status. Previous studies have shown that an unhealthy dietary pattern of consuming large amounts of red and processed meats, dips, chips, and snacks, as well as small amounts of olive oil, is associated with a higher risk of HPV infection, and can lead to increased inflammation, decreased control of infections, and an increased risk of auto-inflammatory diseases [7, 8]. However, healthy dietary patterns through the consumption of specific nutritious foods (including vegetables and fruits) and intake of nutritional antioxidants (such as vitamins A, B2, C, D, E, and folic acid) can reduce the likelihood of HPV infection, to prevent cervical cancer [8–11].
The dietary inflammatory index (DII®) serves as an important epidemiological instrument for assessing the inflammatory potential of diets and predicting chronic diseases based on dietary patterns [12, 13]. It distinguishes between the anti-inflammatory and pro-inflammatory properties of diets. A higher DII score indicates that the dietary pattern has more pro-inflammatory components. Recent studies have shown the association between a high intake of proinflammatory diets and an increased risk of cervical cancer among women with cervical intraepithelial neoplasia (CIN) grades 2 or 3 [14, 15].
Despite the established links between diet, inflammation, and the progression of HPV-related conditions, the specific role of dietary inflammation—measured by the DII—in influencing HPV infection remains unclear. This study aims to address this gap by examining the association between dietary inflammation, as quantified by the DII, and HPV infection using data from the National Health and Nutrition Examination Survey (NHANES). By exploring this association, we hope to provide valuable insights that could contribute to developing more effective dietary-based preventive strategies for HPV-related diseases.
Methods
Study population
NHANES is a comprehensive cross-sectional survey using a stratified, multistage probabilistic methodology to generate population estimates concerning the nutritional and health status of adults and children in the United States. This is achieved through personal interviews, health screenings, and laboratory specimen analyses. Details of the NHANES are available through the NHANES website [16] (https://www.cdc.gov/nchs/nhanes/index.htm). In this study, we initially included 35,936 female participants from seven cycles of NHANES spanning from 2003 to 2016. Among them, 23,302 participants were excluded due to missing information on HPV vaginal swab testing. Additionally, 608 participants were excluded because they lacked dietary information, and 2770 participants were excluded due to no information on covariates of interest. Finally, 9,256 participants were included in the analysis (Supplementary Figure S1).
Human papillomavirus infection
DNA samples from self-collected vaginal swabs of women aged 18–59 in seven NHANES surveys (2003–2016) were typed for 37 genotypes. Genotyping of all samples was conducted using the HPV L1 consensus polymerase chain reaction (PCR). More detailed HPV testing methods are publicly available on the NHANES website. The study’s outcome variable was the overall HPV infection status, categorized into three groups: no HPV, low-risk (LR) HPV, and high-risk (HR) HPV infection. Negative results for all 37 HPV types were classified as the no HPV group. Positive results for any of the 12 specified HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59) were classified as the HR-HPV group [17–19], while positive results for the remaining HPV genotypes were considered as the LR-HPV group.
Calculation of the DII
The dietary data used in this study were collected from NHANES using 24-hour dietary recall interviews. This method is widely employed in large-scale epidemiological studies, and to ensure accuracy, bilingual dietary interviewers conducted in-person interviews using the USDA’s Automated Multiple-Pass Method (AMPM) [20]. The dietary data collected from NHANES serves as forms the foundation for calculating the DII, a validated tool for assessing the inflammatory potential of diets, as demonstrated in previous research [12, 13]. The method assesses the inflammatory effects of dietary intake based on 45 nutrients. However, in this study, we calculated DII scores based on 28 of the 45 food and nutrient components due to data availability from the NHANES dietary recall interviews. These components included energy, alcohol, protein, fiber, cholesterol, carbohydrates, total fat, saturated fat, polyunsaturated fatty acid (PUFA), monounsaturated fatty acids (MUFA), n-6 fatty acids, n-3 fatty acids, vitamins (A, B6, B12, C, D, and E), thiamin, riboflavin, niacin, iron, magnesium, zinc, folic acid, selenium, caffeine and beta-carotene.
The DII calculation was performed following the standard methodology proposed by Hébert et al. [13]. Briefly, each food and dietary component was assigned an inflammation score based on its association with specific inflammatory markers (IL-1 β, IL-4, IL-6, IL-10, TNF-α, and CRP), resulting in a pooled score that reflects the pro-inflammatory or anti-inflammatory potential of an individual’s daily diet. The DII calculation was carried out as follows [12]: First, the Z-score for each food parameter was calculated using the formula: (daily average intake - global daily average intake)/standard deviation. Next, the Z-score was converted to a percentile score. Each percentile score was multiplied by 2, and 1 was subtracted from the result. The DII score for each specific food parameter was then calculated by multiplying this value by its corresponding inflammatory effect score. Finally, the total DII score was obtained by summing the DII scores for all food parameters.
Covariables
Covariates were included in this study, including age, race or ethnicity, marital status, poverty income ratio, education level, body mass index (BMI), physical activity, smoking status, drinking status, hypertension status (HTN), cardiovascular status (CVD), type 2 diabetes status (T2D) and number of lifetime sexual partners. Physical activity was categorized into three groups: the inactive group (no leisure-time physical activity, the insufficiently active group (1 to 5 moderate activities or 1 to 3 vigorous activities per week during leisure time), or the active group (more moderate or vigorous activity than mentioned above). There were three categories for smoking status: non-smoker, former smoker, or current smoker [21]. Non-smokers were people who had either never smoked or smoked less than 100 cigarettes in their lifetime. Former smokers were those who had smoked a minimum of 100 cigarettes in their lifetime but had since quit. Current smokers were those who had smoked a minimum of 100 cigarettes in their lifetime and were actively smoking. Drinking status was categorized as non-drinker, light/moderate drinker (≤ 2 drinks per day for males and ≤ 1 drink per day for females), and heavier drinker (> 2 drinks per day for males and > 1 drink per day for females) [22]. Hypertension was characterized by a systolic blood pressure of ≥ 130 mm Hg and/or a diastolic blood pressure of ≥ 80 mm Hg, taking anti-hypertensive medication, or being diagnosed with hypertension [23]. Diabetes was characterized by a glycated hemoglobin A1c level of 6.5% or higher, fasting glucose exceeding 7 mmol/L, a 2-hour post 75 g glucose load (OGTT) result of 11.1 mmol/L or more, self-reported diabetes diagnosis, or self-reported usage of insulin or other diabetic treatment [22, 24]. The cardiovascular condition was assessed using self-reported information on stroke, heart attack, congestive heart failure, angina or coronary heart disease.
Statistical analysis
Due to the complex survey design of the NHANES, all analyses utilized sample weights. The median and interquartile range (IQR) were used to characterize continuous variables, whereas the unweighted frequency and weighted percentage were used to represent categorical variables. For the purposes of analysis, DII scores were categorized into tertiles. The highest tertile (T3) represents the most pro-inflammatory diet, with higher DII scores indicating a greater inflammatory potential of the diet. Conversely, the lowest tertile (T1) represents a more anti-inflammatory diet, characterized by lower DII scores. To determine differences among different groups, the weighted chi-square or Kruskal-Wallis H test was used. The association between DII scores and HPV infection was analyzed using weighted logistic regression models. Using the lowest tertile as the reference, odds ratios (ORs) and 95% confidence intervals (CIs) were computed for each tertile of DII scores. A restricted cubic spline (RCS) was applied to visualize the probable non-linear correlation between HPV infection and DII. Model 3 was stratified by age group, race or ethnicity, marital status, education level, poverty income ratio, BMI group, physical activity, smoking status, drinking status, T2D status, HTN status, CVD status and number of sexual partners to evaluate the association between DII scores and HPV infection. A statistical interaction test was then used to assess the robustness of the observed modifications. R software version 4.4.0 was used for all analyses, and a two-sided p-value < 0.05 was regarded as statistically significant.
Results
Study participants and baseline characteristics
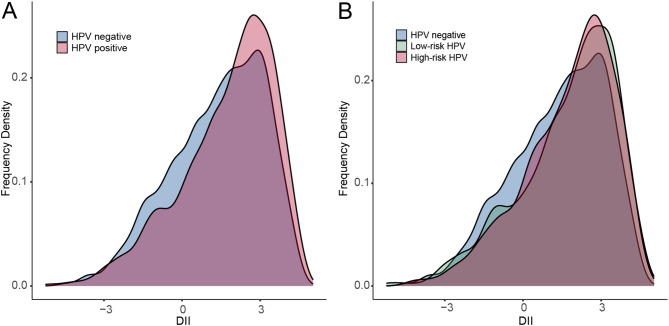
The baseline characteristics of the individuals based on their HPV infection status are shown in Table 1. There were 4100 participants infected with HPV, of which 1938 and 2162 were infected with HR-HPV and LR-HPV, respectively. Compared to the non-HPV infection group, participants in the HPV-positive group were younger, predominantly non-Hispanic black, less likely to be married, with lower levels of education and poverty income ratio, more likely to be current smokers and heavier drinkers, had 5 or more sexual partners during their lifetime and had a higher proportion of CVD status. Notably, participants with HPV infection tended to have a higher DII score than those without HPV (Fig. 1). Supplementary Table S1 displays the subject characteristics based on their DII score tertiles. Compared to participants in the lowest inflammation tertile (T1) of DII, those in the highest inflammation tertile (T3) were more likely to be younger, non-Hispanic black, never married, have lower physical activity levels, be smokers or drinkers, and have higher rates of obesity, T2D status, CVD status, and HPV positivity.
Table 1.
Baseline characteristics of participants with different HPV infection status
| Characteristic | Overall N = 92561 |
HPV negative N = 5156 (59%)1 |
HPV positive N = 4100 (41%)1 |
p-Value2 |
|---|---|---|---|---|
| Age | 41 (30, 50) | 42 (31, 51) | 38 (28, 48) | < 0.001 |
| Age group | < 0.001 | |||
| 20–34 years | 4,752 (48%) | 2,498 (44%) | 2,254 (52%) | |
| 35–49 years | 2,410 (26%) | 1,404 (27%) | 1,006 (25%) | |
| 50–59 years | 2,094 (26%) | 1,254 (29%) | 840 (22%) | |
| Race/ethnicity | < 0.001 | |||
| Hispanic | 2,414 (14%) | 1,408 (14%) | 1,006 (14%) | |
| Non-Hispanic White | 3,993 (67%) | 2,376 (71%) | 1,617 (62%) | |
| Non-Hispanic Black | 2,036 (12%) | 810 (8.0%) | 1,226 (19%) | |
| Other races/ethnicities | 813 (6.6%) | 562 (7.5%) | 251 (5.3%) | |
| Marital status | < 0.001 | |||
| Never married | 3,048 (29%) | 1,339 (23%) | 1,709 (39%) | |
| Married | 4,549 (54%) | 3,131 (65%) | 1,418 (37%) | |
| Widowed/Divorced/Separated | 1,659 (17%) | 686 (12%) | 973 (24%) | |
| Education level | < 0.001 | |||
| < High school graduate | 1,768 (13%) | 929 (12%) | 839 (15%) | |
| High school graduate/GED | 1,920 (20%) | 962 (19%) | 958 (23%) | |
| College Graduate or above | 5,568 (67%) | 3,265 (70%) | 2,303 (62%) | |
| Poverty income ratio | < 0.001 | |||
| < 1(poverty) | 2,150 (16%) | 990 (13%) | 1,160 (22%) | |
| ≥ 1(non-poverty) | 7,106 (84%) | 4,166 (87%) | 2,940 (78%) | |
| BMI group | 0.577 | |||
| Underweight (< 18.5) | 196 (2.2%) | 105 (2.0%) | 91 (2.5%) | |
| Normal (18.5 to < 25) | 2,812 (34%) | 1,596 (35%) | 1,216 (34%) | |
| Overweight (25 to < 30) | 2,468 (27%) | 1,379 (27%) | 1,089 (26%) | |
| Obese (30 or greater) | 3,780 (37%) | 2,076 (36%) | 1,704 (37%) | |
| Physical activity | 0.069 | |||
| Inactive | 2,026 (18%) | 1,084 (17%) | 942 (20%) | |
| Insufficient | 3,036 (34%) | 1,688 (35%) | 1,348 (33%) | |
| Recommended | 4,194 (48%) | 2,384 (48%) | 1,810 (47%) | |
| Smoking status | < 0.001 | |||
| Never smoker | 5,826 (60%) | 3,529 (65%) | 2,297 (53%) | |
| Former smoker | 1,429 (18%) | 820 (19%) | 609 (16%) | |
| Current smoker | 2,001 (23%) | 807 (17%) | 1,194 (31%) | |
| Drinking status | < 0.001 | |||
| Never drinker | 1,483 (12%) | 980 (15%) | 503 (8.3%) | |
| Light/moderate drinker | 7,015 (79%) | 3,878 (79%) | 3,137 (80%) | |
| Heavier drinker | 758 (8.4%) | 298 (6.4%) | 460 (11%) | |
| T2D status | 935 (8.3%) | 537 (8.9%) | 398 (7.5%) | 0.085 |
| HTN status | 3,186 (34%) | 1,748 (35%) | 1,438 (34%) | 0.379 |
| CVD status | 367 (3.7%) | 165 (2.8%) | 202 (4.9%) | < 0.001 |
| No. of sexual partners | < 0.001 | |||
| NO | 166 (1.3%) | 111 (1.7%) | 55 (0.7%) | |
| ≤ 5 | 5,356 (55%) | 3,467 (65%) | 1,889 (42%) | |
| > 5 | 3,734 (43%) | 1,578 (34%) | 2,156 (57%) | |
| DII score | 1.84 (0.38, 2.93) | 1.63 (0.15, 2.81) | 2.13 (0.68, 3.11) | < 0.001 |
| DII score categories3 | < 0.001 | |||
| T1 (-1.33, 0.38) | 2,832 (33%) | 1,765 (37%) | 1,067 (28%) | |
| T2 (1.39, 2.23) | 3,169 (33%) | 1,771 (33%) | 1,398 (34%) | |
| T3 (2.93, 3.70) | 3,255 (33%) | 1,620 (30%) | 1,635 (38%) |
1Median (IQR); n (unweighted) (%)
2Wilcoxon rank-sum test for complex survey samples; chi-squared test with Rao & Scott’s second-order correction
3DII tertile ranges. T1: low DII scores, T2: middle DII scores, T3: higher DII scores
Abbreviations: BMI, body mass index; T2D: type 2 diabetes; HTN: hypertension; CVD: cardiovascular disease; No.: number; DII: Dietary Inflammation Index
Fig. 1.
Distribution of DII score. (A) The distribution of DII in the population with non-HPV infection and HPV infection. (B) The distribution of DII in the population with different HPV genotypes
Association between DII and HPV infection
To investigate the relationship between DII and HPV infection, we performed multivariable logistic regression analysis (Table 2). In the unadjusted model, we found that the DII score was positively associated with HPV infection, with each unit increase in the DII score potentially leading to a 13% higher likelihood of HPV infection (OR = 1.13; 95% CI: 1.08, 1.17; P < 0.0001). After adjustment for age, race or ethnicity, marital status, education level and poverty income ratio in model 1, the relationship between DII and HPV infection remained significant and positive (OR = 1.06; 95% CI: 1.02–1.10; P = 0.006). In Model 2, which further adjusted for BMI group, physical activity, smoking status, drinking status, HTN status, CVD status, T2D status, and number of sexual partners, consistent results were observed (OR = 1.05; 95% CI: 1.01–1.09; P = 0.021). Similarly, participants in the intermediate (T2) and highest inflammation groups (T3) showed greater odds of HPV infection compared to those in the lowest inflammation group (T1 of DII scores), with ORs of 1.33 (95%CI: 1.13, 1.55) and 1.71 (95%CI: 1.47, 1.98), respectively, in the unadjusted model. After adjusting for all covariates in Model 2, the OR with 95% CI for HPV infection across the tertiles was 1.20 (1.01, 1.42) and 1.27 (1.07, 1.51), respectively. The study further examined the correlation between different HPV genotypes and DII scores. The results indicated that both low-risk HPV status and high-risk HPV status were significantly and positively associated with DII scores in the unadjusted model (Supplementary Figure S2). After adjusting for all covariates in Model 2, among those with low-risk HPV infection, the T3 group showed a significant positive association with DII scores compared to the T1 group (OR = 1.36; 95% CI: 1.12, 1.66; P = 0.003).
Table 2.
Odds ratios (ORs) and 95% confidence interval (CI) of the dietary inflammatory index for HPV infection
| DII | Model 0 | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Continuous | 1.13(1.08, 1.17) | < 0.001 | 1.06(1.02, 1.10) | 0.006 | 1.05(1.01, 1.09) | 0.021 |
| Categories | ||||||
| T1 | ||||||
| T2 | 1.33(1.13, 1.55) | < 0.001 | 1.18(1.00, 1.40) | 0.055 | 1.20(1.01, 1.42) | 0.039 |
| T3 | 1.71(1.47, 1.98) | < 0.001 | 1.32(1.12, 1.55) | 0.001 | 1.27(1.07, 1.51) | 0.006 |
| Trend test | < 0.001 | < 0.001 | 0.006 | |||
Model 0: no covariates were adjusted
Model 1: age, race or ethnicity, marital status, education level and poverty income ratio were adjusted
Model 2: adjusted for age, race or ethnicity, marital status, education level, poverty income ratio, BMI group, physical activity, smoking status, drinking status, T2D status, HTN status, CVD status and No. of sexual partners
Figure 2 illustrates a non-linear relationship between DII scores and HPV infection (P for nonlinearity = 0.0118). The likelihood of HPV infection decreased gradually with an increasing DII score until it reached around 0.13, after which it started to increase rapidly. In addition, restricted cubic spline revealed a U-shaped association with low-risk HPV status (P for nonlinearity = 0.0003, Supplementary Figure S3A), whereas a positive linear association between DII and high-risk HPV status (P for nonlinearity = 0.9177, Supplementary Figure S3B).
Fig. 2.
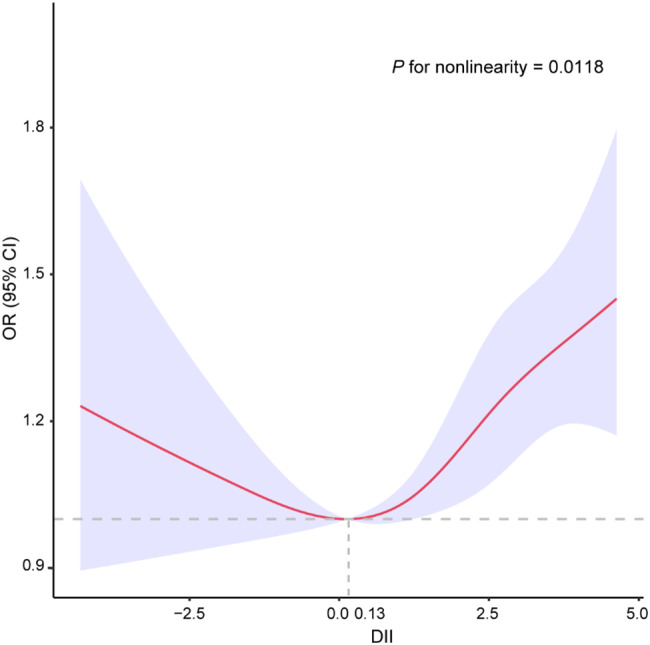
Restricted spline curve for the association between DII and HPV infection. The red represents estimates of odd ratios and dashed lines represent 95% confidence intervals
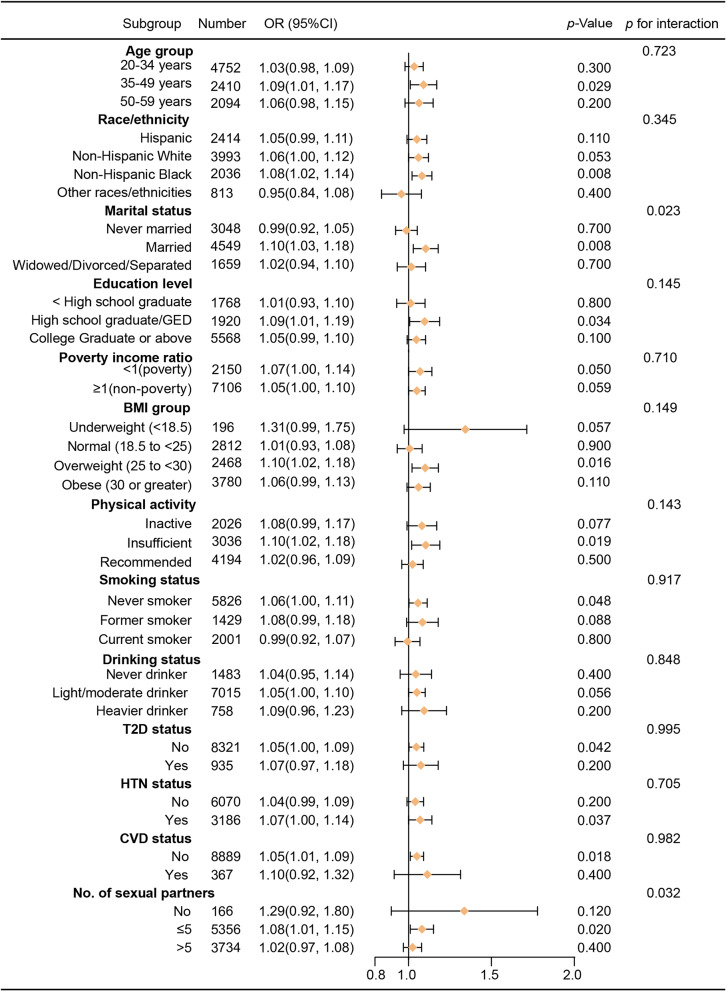
Subgroup analysis
As shown in Fig. 3, subgroup analysis revealed inconsistent associations between DII scores and HPV infection, despite a predominantly positive relationship in most categories. The results of the interaction test indicated that marital status and number of sexual partners modified the association between DII scores and HPV infection (P for interaction < 0.05).
Fig. 3.
Forest plots of stratified analysis of the associations between DII and HPV infection
Discussion
This study examined the association between HPV infection and the DII score utilizing data from NHANES 2003–2016. Our findings suggest a significant positive correlation between a pro-inflammatory diet and HPV infection. This association remained statistically significant even after full multivariable adjustment, with a significantly higher OR for HPV infection in those in the highest DII tertile (T3) compared with those in the lowest tertile (T1) at 1.27 (95% CI: 1.07, 1.51, P = 0.006). Restricted cubic spline demonstrated a significant U-shaped correlation between DII and HPV infection. Initially, the prevalence of HPV infection decreased as the DII score increased up to approximately 0.13. However, beyond this threshold, the prevalence of HPV infection rose sharply with higher DII scores, suggesting that a more pro-inflammatory diet may compromise immune function and increase susceptibility to HPV infection. Notably, there was a linear positive correlation between DII and high-risk HPV infection, emphasizing the impact of pro-inflammatory dietary patterns on increasing the risk of infection.
Over the period from 2003 to 2016 in the United States, there have been notable shifts in dietary patterns and food variety. While there has been a general trend towards healthier eating, with increased focus on whole foods and reduced consumption of processed foods, the intake of certain pro-inflammatory nutrients, particularly sugars and low-quality carbohydrates, has remained high [25–27]. Our study demonstrates that a higher DII score, indicative of a pro-inflammatory diet, is significantly associated with an increased risk of HPV infection. This finding aligns with previous research that emphasizes the role of diet-induced inflammation in modulating immune responses and disease progression [28].
Diets characterized by high levels of calories, salt, processed foods, red meats, and refined sugars —components that contribute to higher DII scores—are known to exacerbate systemic inflammation, thereby potentially weakening the immune system’s ability to clear HPV infections [29–31]. Conversely, a healthy diet that includes a regular intake of antioxidant nutrients, such as vitamins A, C, and E, has been shown to lower the risk of HPV infection and even cervical cancer [10, 32, 33]. For example, regular consumption of fruits and dark green vegetables has been shown to significantly reduce the risk of HR-HPV infection, highlighting the importance of diet in modulating inflammatory responses [9, 18]. Vitamin A is essential for the regulation of local immunity and the reduction of inflammatory responses [34], and its insufficient intake has been linked to HPV infection, HR-HPV infection, and cervical cancer [9, 35, 36]. Similarly, vitamin E has been shown to protect the integrity of immune cell membranes, suppress the production of prostaglandin E2 (PGE2), and inhibit the enzymatic activity of cyclooxygenase-2 (COX-2), which may enhance the body’s ability to clear HPV infection [37–39]. Folic acid also plays a crucial role in the production of erythrocytes, synthesis and repair of DNA, methylation processes, and cell proliferation [40]. Insufficient folic acid levels can elevate the risk of cervical cancer and HR-HPV infection [11, 41–43].
Furthermore, diet plays a crucial role in regulating the diversity, activity, and associated metabolites of human gut flora [44, 45]. Several studies have found that different nutrients in the diet and gut microbes can modulate the balance of pro-inflammatory and anti-inflammatory responses [46–48]. Existing studies suggest that bacterial strains can be transferred from the gut to the vagina [49–51]. The vaginal microbiota plays a crucial role in preventing inflammation and reducing susceptibility to microbial infections, including HPV infection [52–54]. For example, administering probiotic bacteria and yeast orally can hinder the progression and elimination of HPV infection by enabling probiotic microorganisms to travel from the gut to the vagina [55–57]. These mechanisms may underlie the connection between DII and HPV infection and warrant further exploration.
Notably, our study is the inaugural examination of the U-shaped correlation between DII and HPV infection in a representative sample of adult women in the United States. The likelihood of HPV infection significantly increased when the diet showed a pro-inflammatory pattern, highlighting the need for future longitudinal studies to confirm these findings and establish causative relationships. This finding is consistent with previous studies on the link between the inflammatory potential of diets and cervical carcinogenesis. For example, in a case-control study conducted by Sreeja et al. among Korean women, higher DII scores were significantly associated with increased odds of developing CIN2/3 and cervical cancer [14]. The study reported median DII scores of 1.1 (-0.7, 2.5) for women with CIN2/3 and 1.5 (-0.6, 3.0) for those with cervical cancer. Similarly, our study found that the prevalence of HPV infection increased in participants with DII scores above 0.13, indicating that even moderate shifts toward a pro-inflammatory diet can elevate infection risk. Furthermore, Maugeri et al. reported women with higher DII scores had elevated odds of developing high-grade CIN lesions in an Italian cohort [15]. These findings collectively suggest that a pro-inflammatory diets, as measured by DII, are linked to a higher risk of HPV infection and potentially cervical cancer.
However, this study has several potential limitations. First, being a cross-sectional study, it only provides a snapshot of data at a single point in time, which limits our ability to infer causality and may include unmeasured confounders. Second, the DII score was calculated based on a 24-hour dietary recall method. This method relies on participants’ memory and honesty, which can introduce recall bias and potentially inaccurate dietary assessments. The absence of long-term dietary data also limits our ability to capture habitual dietary patterns, which may affect the observed relationship between DII and HPV infection. Additionally, NHANES was designed to be primarily representative of the United States population, and certain population subgroups with different dietary habits or health conditions may be underrepresented, both of which may limit the generalization of our findings to more diverse or global populations.
Conclusion
In conclusion, our results suggest that among adult women in the United States, a pro-inflammatory diet is significantly and positively associated with HPV infection. The findings might offer useful insights for large-scale prospective studies to emphasize the significance of dietary health, especially for individuals following pro-inflammatory diets. Individuals with such dietary patterns are encouraged to increase their intake of anti-inflammatory foods to lower the likelihood of HPV infection.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to all those who contributed to the establishment and development of the NHANES database and all participants who responded to the survey. We also acknowledge the work of Dr. James Hébert and his team in developing the Dietary Inflammatory Index (DII®), which was crucial to the dietary inflammation assessment conducted in this study. The DII® was calculated based on their methodology.
Author contributions
Conception and design: Qian Li and Yu Chen; Collection and data curation: Yu Chen and Mei Yang; Data analysis and interpretation: Qian Li and Peibo Li; Manuscript writing: All authors; Final approval of manuscript: All authors.
Funding
This research was funded by 2023 key Disciplines on Public Health Construction in Chongqing, grant number 2023 − 00304.
Data availability
The datasets for this study can be found in the NHANES database (https://www.cdc.gov/nchs/nhanes/index.htm).
Declarations
Ethics approval and consent to participate
The studies involving humans were approved by the Centers for Disease Control and Prevention (CDC) National Increase for Health Statistics Research (NCHS) Ethics Review Board. Ethical review and approval were waived for this study because this study was a secondary analysis of public-use data available without personal identifiers. The patients/participants provided their written informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qian Li and Yu Chen contributed equally to this work and share first authorship.
Contributor Information
Mei Yang, Email: yangmeicqu@126.com.
Peibo Li, Email: 157318851@qq.com.
References
- 1.de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(664–70). 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed]
- 2.Doorbar J, Egawa N, Griffin H, Kranjec C, Murakami I. Human papillomavirus molecular biology and disease association. Rev Med Virol. 2015;25(Suppl 1):2–23. 10.1002/rmv.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jensen JE, Becker GL, Jackson JB, Rysavy MB. Human papillomavirus and Associated Cancers: a review. Viruses. 2024;16. 10.3390/v16050680. [DOI] [PMC free article] [PubMed]
- 4.Georgescu SR, Mitran CI, Mitran MI, Caruntu C, Sarbu MI, Matei C et al. New Insights in the Pathogenesis of HPV Infection and the Associated Carcinogenic Processes: The Role of Chronic Inflammation and Oxidative Stress. J Immunol Res. (2018) 2018,5315816. 10.1155/2018/5315816 [DOI] [PMC free article] [PubMed]
- 5.Hemmat N, Bannazadeh Baghi H. Association of human papillomavirus infection and inflammation in cervical cancer. Pathog Dis. 2019;77. 10.1093/femspd/ftz048. [DOI] [PubMed]
- 6.Liu Y, Ai H. Comprehensive insights into human papillomavirus and cervical cancer: pathophysiology, screening, and vaccination strategies. Biochim Biophys Acta Rev Cancer. 2024;1879(189192). 10.1016/j.bbcan.2024.189192. [DOI] [PubMed]
- 7.Myles IA. Fast food fever: reviewing the impacts of the Western diet on immunity. Nutr J. (2014) 13,61. 10.1186/1475-2891-13-61 [DOI] [PMC free article] [PubMed]
- 8.Barchitta M, Maugeri A, Quattrocchi A, Agrifoglio O, Scalisi A, Agodi A. The Association of Dietary Patterns with high-risk human papillomavirus infection and cervical Cancer: a cross-sectional study in Italy. Nutrients. 2018;10. 10.3390/nu10040469. [DOI] [PMC free article] [PubMed]
- 9.Barchitta M, Maugeri A, La Mastra C, Rosa MC, Favara G, Lio RMS, Agodi A. Dietary antioxidant intake and human papillomavirus infection: evidence from a cross-sectional study in Italy. Nutrients. 2020;12. 10.3390/nu12051384. [DOI] [PMC free article] [PubMed]
- 10.Ono A, Koshiyama M, Nakagawa M, Watanabe Y, Ikuta E, Seki K, Oowaki M. The Preventive Effect of Dietary antioxidants on Cervical Cancer Development. Medicina (Kaunas). (2020) 56. 10.3390/medicina56110604 [DOI] [PMC free article] [PubMed]
- 11.Lin HY, Fu Q, Kao YH, Tseng TS, Reiss K, Cameron JE, et al. Antioxidants Associated with Oncogenic Human Papillomavirus infection in women. J Infect Dis. 2021;2241520–8. 10.1093/infdis/jiab148. [DOI] [PMC free article] [PubMed]
- 12.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–96. 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hebert JR, Shivappa N, Wirth MD, Hussey JR, Hurley TG, Perspective. The Dietary Inflammatory Index (DII)-Lessons Learned, Improvements Made, and Future Directions. Adv Nutr. (2019) 10,185 – 95. 10.1093/advances/nmy071 [DOI] [PMC free article] [PubMed]
- 14.Sreeja SR, Lee HY, Kwon M, Shivappa N, Hebert JR, Kim MK. Dietary inflammatory index and its relationship with cervical carcinogenesis risk in Korean women: a case-control study. Cancers (Basel). (2019) 11. 10.3390/cancers11081108 [DOI] [PMC free article] [PubMed]
- 15.Maugeri A, Barchitta M, Magnano San Lio R, Scalisi A, Agodi A. Antioxidant and inflammatory potential of diet among women at risk of cervical cancer: findings from a cross-sectional study in Italy. Public Health Nutr. 2022;251577–85. 10.1017/S1368980021001944. [DOI] [PMC free article] [PubMed]
- 16.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2021. https://wwwn.cdc.gov/nchs/nhanes/Default.aspx. [Google Scholar]
- 17.de Sanjose S, Brotons M, Pavon MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol. 2018;47:2–13. 10.1016/j.bpobgyn.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Lin HY, Fu Q, Tseng TS, Zhu X, Reiss K, Joseph Su L, Hagensee ME. Impact of Dietary Quality on Genital Oncogenic Human Papillomavirus infection in women. J Infect Dis. 2023;2281385–93. 10.1093/infdis/jiad146. [DOI] [PMC free article] [PubMed]
- 19.Xia S, Li S, Li H. HPV-infection status and urinary incontinence: a population-based analysis of the NHANES 2005–2016. World J Urol. 2023;41:1597–603. 10.1007/s00345-023-04425-9. [DOI] [PubMed] [Google Scholar]
- 20.Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C. Update on NHANES Dietary Data: Focus on Collection, Release, Analytical considerations, and uses to inform Public Policy. Adv Nutr. 2016;7:121–34. 10.3945/an.115.009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuo R, Ge Y, Xu J, He L, Liu T, Wang B, et al. The association of female reproductive factors with risk of metabolic syndrome in women from NHANES 1999–2018. BMC Public Health. 2023;232306. 10.1186/s12889-023-17207-0. [DOI] [PMC free article] [PubMed]
- 22.Chen F, Song Y, Li W, Xu H, Dan H, Chen Q. Association between periodontitis and mortality of patients with cardiovascular diseases: a cohort study based on NHANES. J Periodontol. 2024;95:175–84. 10.1002/JPER.23-0276. [DOI] [PubMed] [Google Scholar]
- 23.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH et al. /ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. (2018) 138,e426-e83. 10.1161/CIR.0000000000000597 [DOI] [PubMed]
- 24.American Diabetes Association Professional Practice, C. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care. (2024) 47,S20-S42. 10.2337/dc24-S002 [DOI] [PMC free article] [PubMed]
- 25.Shan Z, Rehm CD, Rogers G, Ruan M, Wang DD, Hu FB, Mozaffarian D, Zhang FF, Bhupathiraju SN. Trends in Dietary Carbohydrate, protein, and Fat Intake and Diet Quality among US adults, 1999–2016. JAMA. 2019;3221178–87. 10.1001/jama.2019.13771. [DOI] [PMC free article] [PubMed]
- 26.Zeng L, Ruan M, Liu J, Wilde P, Naumova EN, Mozaffarian D, Zhang FF. Trends in Processed Meat, Unprocessed Red Meat, Poultry, and Fish Consumption in the United States, 1999–2016. J Acad Nutr Diet. 2019;119:1085–e9812. 10.1016/j.jand.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Rehm CD, Onopa J, Mozaffarian D. Trends in Diet Quality among Youth in the United States, 1999–2016. JAMA. 2020;323:1161–74. 10.1001/jama.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shivappa N, Hebert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, Marcos A, Huybrechts I. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br J Nutr. 2015;113665–71. 10.1017/S000711451400395X. [DOI] [PMC free article] [PubMed]
- 29.Sorensen LB, Vasilaras TH, Astrup A, Raben A. Sucrose compared with artificial sweeteners: a clinical intervention study of effects on energy intake, appetite, and energy expenditure after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2014;100:36–45. 10.3945/ajcn.113.081554. [DOI] [PubMed] [Google Scholar]
- 30.Binger KJ, Linker RA, Muller DN, Kleinewietfeld M. Sodium chloride, SGK1, and Th17 activation. Pflugers Arch. 2015;467:543–50. 10.1007/s00424-014-1659-z. [DOI] [PubMed] [Google Scholar]
- 31.Calder PC. n-3 PUFA and inflammation: from membrane to nucleus and from bench to bedside. Proc Nutr Soc. 2020;1–13. 10.1017/S0029665120007077. [DOI] [PubMed]
- 32.Guo L, Zhu H, Lin C, Che J, Tian X, Han S, Zhao H, Zhu Y, Mao D. Associations between antioxidant vitamins and the risk of invasive cervical cancer in Chinese women: a case-control study. Sci Rep. 2015;5:13607. 10.1038/srep13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preci DP, Almeida A, Weiler AL, Mukai Franciosi ML, Cardoso AM. Oxidative damage and antioxidants in cervical cancer. Int J Gynecol Cancer. 2021;31(265–71). 10.1136/ijgc-2020-001587. [DOI] [PubMed]
- 34.Gombart AF, Pierre A, Maggini S. A review of micronutrients and the Immune System-Working in Harmony to reduce the risk of infection. Nutrients. 2020;12. 10.3390/nu12010236. [DOI] [PMC free article] [PubMed]
- 35.Huang X, Chen C, Zhu F, Zhang Y, Feng Q, Li J et al. Association between Dietary Vitamin A and HPV Infection in American Women: Data from NHANES 2003–2016. Biomed Res Int. (2020) 2020,4317610. 10.1155/2020/4317610 [DOI] [PMC free article] [PubMed]
- 36.Ferrari FA, Magni F, Bosco M, Biancotto G, Zorzato PC, Lagana AS et al. The role of micronutrients in human papillomavirus infection, cervical dysplasia, and Neoplasm. Healthcare (Basel). (2023) 11. 10.3390/healthcare11111652 [DOI] [PMC free article] [PubMed]
- 37.Zhou Q, Fan M, Wang Y, Ma Y, Si H, Dai G. Association between Dietary Vitamin E Intake and human papillomavirus infection among US adults: a cross-sectional study from National Health and Nutrition Examination Survey. Nutrients. 2023;15. 10.3390/nu15173825. [DOI] [PMC free article] [PubMed]
- 38.Lewis ED, Meydani SN, Wu D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life. (2019) 71,487 – 94. 10.1002/iub.1976 [DOI] [PMC free article] [PubMed]
- 39.Alnouri MW, Roquid KA, Bonnavion R, Cho H, Heering J, Kwon J, et al. SPMs exert anti-inflammatory and pro-resolving effects through positive allosteric modulation of the prostaglandin EP4 receptor. Proc Natl Acad Sci U S A. 2024;121e2407130121. 10.1073/pnas.2407130121. [DOI] [PMC free article] [PubMed]
- 40.Guo X, Yang J. Advances in DNA methylation of imprinted genes and folic acid regulation of growth and development. Epigenomics. 2024;16:1117–27. 10.1080/17501911.2024.2384833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yenigul NN, Yazici Yilmaz F, Ayhan I. Can serum vitamin B12 and folate levels predict HPV penetration in patients with ASCUS? Nutr Cancer. 2021;73:602–8. 10.1080/01635581.2020.1807030. [DOI] [PubMed]
- 42.Lagana AS, Chiantera V, Gerli S, Proietti S, Lepore E, Unfer V, Carugno J, Favilli A. Preventing persistence of HPV infection with natural molecules. Pathogens. 2023;12. 10.3390/pathogens12030416. [DOI] [PMC free article] [PubMed]
- 43.Peitz JG, Adebamowo CA, Adebamowo SN. Association between serum folate and Vaginal High-Risk Human Papillomavirus infections in United States women. J Nutr. 2024;154:583–9. 10.1016/j.tjnut.2023.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB et al. Gut-microbiota-targeted diets modulate human immune status. Cell. (2021) 184,4137-53 e14. 10.1016/j.cell.2021.06.019 [DOI] [PMC free article] [PubMed]
- 45.Kimble R, Gouinguenet P, Ashor A, Stewart C, Deighton K, Matu J, et al. Effects of a mediterranean diet on the gut microbiota and microbial metabolites: a systematic review of randomized controlled trials and observational studies. Crit Rev Food Sci Nutr. 2023;63(8698–719). 10.1080/10408398.2022.2057416. [DOI] [PubMed]
- 46.Bolte LA, Vich Vila A, Imhann F, Collij V, Gacesa R, Peters V, et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut. 2021;701287–98. 10.1136/gutjnl-2020-322670. [DOI] [PMC free article] [PubMed]
- 47.Malesza IJ, Malesza M, Walkowiak J, Mussin N, Walkowiak D, Aringazina R, Bartkowiak-Wieczorek J, Madry E. High-Fat, Western-Style Diet, systemic inflammation, and gut microbiota: a narrative review. Cells. 2021;10. 10.3390/cells10113164. [DOI] [PMC free article] [PubMed]
- 48.Bilal M, Ashraf S, Zhao X. Dietary Component-Induced inflammation and its amelioration by Prebiotics, Probiotics, and Synbiotics. Front Nutr. 2022;9:931458. 10.3389/fnut.2022.931458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Decherf A, Dehay E, Boyer M, Clement-Ziza M, Rodriguez B, Legrain-Raspaud S. Recovery of Saccharomyces cerevisiae CNCM I-3856 in Vaginal samples of healthy women after oral administration. Nutrients. 2020;12. 10.3390/nu12082211. [DOI] [PMC free article] [PubMed]
- 50.Trifanescu OG, Trifanescu RA, Mitrica RI, Bran DM, Serbanescu GL, Valcauan L, et al. The Female Reproductive Tract Microbiome and Cancerogenesis: a Review story of Bacteria, hormones, and Disease. Diagnostics (Basel). 2023;13. 10.3390/diagnostics13050877. [DOI] [PMC free article] [PubMed]
- 51.Yefet E, Colodner R, Strauss M, Gam Ze Letova Y, Nachum Z. A randomized controlled open label crossover trial to Study Vaginal colonization of orally administered Lactobacillus Reuteri RC-14 and Rhamnosus GR-1 in pregnant women at high risk for Preterm Labor. Nutrients. 2020;12. 10.3390/nu12041141. [DOI] [PMC free article] [PubMed]
- 52.Mitra A, MacIntyre DA, Marchesi JR, Lee YS, Bennett PR, Kyrgiou M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome. (2016) 458. 10.1186/s40168-016-0203-0 [DOI] [PMC free article] [PubMed]
- 53.Seo SS, Oh HY, Lee JK, Kong JS, Lee DO, Kim MK. Combined effect of diet and cervical microbiome on the risk of cervical intraepithelial neoplasia. Clin Nutr. 2016;35:1434–41. 10.1016/j.clnu.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Yu T, Yan H, Li D, Yu T, Yuan T, et al. Vaginal microbiota and HPV infection: Novel mechanistic insights and therapeutic strategies. Infect Drug Resist. 2020;131213–20. 10.2147/IDR.S210615. [DOI] [PMC free article] [PubMed]
- 55.Ou YC, Fu HC, Tseng CW, Wu CH, Tsai CC, Lin H. The influence of probiotics on genital high-risk human papilloma virus clearance and quality of cervical smear: a randomized placebo-controlled trial. BMC Womens Health. 2019;19103. 10.1186/s12905-019-0798-y. [DOI] [PMC free article] [PubMed]
- 56.Nguyen HDT, Le TM, Lee E, Lee D, Choi Y, Cho J, et al. Relationship between human papillomavirus status and the Cervicovaginal Microbiome in Cervical Cancer. Microorganisms. 2023;11. 10.3390/microorganisms11061417. [DOI] [PMC free article] [PubMed]
- 57.Mazziotta C, Tognon M, Martini F, Torreggiani E, Rotondo JC. Probiotics mechanism of action on Immune cells and Beneficial Effects on Human Health. Cells. (2023) 12. 10.3390/cells12010184 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets for this study can be found in the NHANES database (https://www.cdc.gov/nchs/nhanes/index.htm).