Abstract
Background
Globally, sexually transmitted infections (STIs) collectively cause 2.3 million deaths and 1.2 million cases of cancer annually. However, the epidemiology of STIs in the Middle East and North Africa (MENA) is not well assessed because of various social and cultural factors.
Methods
A systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and covering 23 MENA countries, 19 STIs, and data from 20,435,971 participants. PubMed, Embase, regional and international databases, and country-level reports were searched up to May 2024.
Results
The analysis revealed significant regional variations in the prevalence of STIs within the MENA region. In North Africa, the most common STIs were bacterial vaginosis (31%), human papillomavirus (HPV, 23%), and Candida spp. (15%). In the Gulf Cooperation Council region and Yemen, Ureaplasma (25%), nongonococcal urethritis (NGU, 16%), and Mycoplasma spp. (12%) were the predominant infections. In the Levant region, the top STIs were HPV (20%), hepatitis B virus (HBV, 9%), and Candida spp. (9%). In Iran, Ureaplasma spp. (18%), HPV (17%), and cytomegalovirus (8%) were the most prevalent infections, whereas Ureaplasma spp. (20%), Candida spp. (18%), and HPV (16%) were most frequently detected in Türkiye. Gender-based disparities were observed, with a higher prevalence of Ureaplasma spp., Neisseria gonorrhoeae, and herpes in men and higher rates of Mycoplasma spp., HPV, HBV, and Candida spp. in women. Overall, high rates of nongonococcal urethritis (16.3%), Ureaplasma spp. (13.7%), HPV (12.7%), and Candida spp. (9.4%) were recorded in the MENA region.
Conclusions
Most MENA countries lack national STI screening programs, and the reported data are primarily from symptomatic individuals. Establishing robust surveillance systems, addressing stigma and barriers to healthcare access, and expanding STIs screening and vaccination programs are crucial for accurately capturing the true burden of STIs in MENA countries.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-10153-1.
Keywords: STIs, MENA, HIV, HPV, HCV, HBV, Candidiasis, Ureaplasma, Bacterial vaginosis (BV), Nongonococcal urethritis (NGU)
Background
Globally, sexually transmitted infections (STIs) collectively cause approximately 2.3 million deaths and 1.2 million cases of cancer annually, highlighting their significant impact on public health. According to the World Health Organization (WHO), STIs contribute to various health complications, including cancer, with human papillomavirus (HPV) infection alone being responsible for over 311,000 cervical cancer deaths each year. (STIs) remain a significant public health concern, as more than 1 million new curable STIs are acquired daily among people aged 15–49 years, the majority of which are asymptomatic. In 2020, the four most common curable STIs—chlamydia, gonorrhea, syphilis, and trichomoniasis—caused an estimated 374 million new infections. Moreover, 8 million adults age 15–49 were infected with syphilis in 2022, whereas more than 500 million people in the same age group were estimated to have a genital herpes simplex virus (HSV) infection [1]. Human papillomavirus (HPV) infections are also concerning, being associated with more than 620,000 new cancer cases in women and 70,000 in men in 2019 [2, 3]. Compounding the burden, 1.1 million pregnant women were estimated to be infected with syphilis in 2022, leading to more than 390,000 adverse birth outcomes. STIs have far-reaching effects on sexual and reproductive health, including stigmatization, infertility, cancer, and pregnancy complications, while simultaneously increasing the risk of human immunodeficiency virus (HIV) transmission. Addressing this global challenge is further complicated by the growing threat of drug resistance, underscoring the critical need for comprehensive strategies to reduce the STI burden worldwide [1].
More than 30 different bacteria, viruses, and parasites can be transmitted through sexual contact, and some STIs can also be passed from mother to child. Of the pathogens linked to the highest STI incidence, syphilis, gonorrhea, chlamydia, and trichomoniasis are curable, whereas four are viral infections (hepatitis B virus [HBV], HSV, HIV, and HPV). Emerging outbreaks of sexually acquired infections, such as mpox, Shigella, Neisseria, Ebola, and Zika, as well as the re-emergence of neglected STIs including lymphogranuloma venereum, are further complicating STI prevention and control efforts. This dynamic landscape underscores the need for robust and responsive public health measures to address the diverse range of sexually transmitted pathogens [4].
The World Health Organization (WHO) is leading a global effort to fight against HIV, viral hepatitis, and STIs for the period 2022–2030. This initiative aims to guide the health sector in implementing strategically focused responses to achieve the goals of eradicating AIDS, HBV, hepatitis C virus [HCV], and other STIs by 2030. WHO has published the 2022–2030 strategies, which recommend shared and disease-specific country actions, supported by actions from WHO and its partners. These strategies consider the epidemiological, technological, and contextual shifts of recent years; foster learnings across the disease areas; and create opportunities to leverage innovations and new knowledge for effective responses to HIV, viral hepatitis, and other STIs [5].
As the global community works to combat the STI burden, it is crucial that individual countries assess their local epidemiological contexts and health system capacities to mount appropriate, targeted responses. This comprehensive systematic review and meta-analysis aimed to rigorously evaluate the current status of STIs in the Middle East and North Africa (MENA) region. By synthesizing available evidence, we sought to quantify the regional burden of key STIs and shed light on the looming challenge of antimicrobial resistance (AMR) among these pathogens.
Methods
Search strategy and selection criteria
We conducted a systematic review and meta-analysis of cross-sectional and observational studies to determine the prevalence of STIs in the MENA region following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [6]. We performed a literature search covering the period of January 2024 to May 2024 using electronic databases such as Scopus, Embase, MEDLINE, and PubMed with integrated Medical Subject Headings (MeSH) terms.
Our search included various STIs such as bacterial vaginosis (BV), cytomegalovirus (CMV), Candida spp., chancroid, chlamydia spp., Epstein–Barr virus (EBV), genital herpes, HIV, HBV, HCV, HPV, human T-lymphotropic virus 1 (HTLV-1), Mycoplasma spp. (genitalium/hominis), Neisseria gonorrhoeae (N. gonorrhoeae), nongonococcal urethritis (NGU), syphilis, Trichomonas vaginalis, toxoplasmosis, and Ureaplasma spp. (urealyticum/parvum).
Geographically, we focused on Algeria, Bahrain, Egypt, Iran, Iraq, Jordan, Kuwait, Lebanon, Libya, Morocco, Oman, Palestine, Qatar, Saudi Arabia, Sudan, Syria, Tunisia, United Arab Emirates (UAE), Türkiye, and Yemen. The specific MeSH terms were developed and shared among three data collectors, and they are presented in the supplementary file (Sect. 2 Supplementary file). We categorized the data into five geographical regions: (1) North Africa (Algeria, Egypt, Tunisia, Libya, Morocco, Sudan), (2) Iran, (3) Gulf Cooperation Council (GCC) and Yemen (Bahrain, Kuwait, Qatar, Saudi Arabia, UAE, Oman, Yemen), (4) Levant area (Syria, Iraq, Palestine, Jordan, Lebanon), and (5) Türkiye.
Study selection and inclusion/exclusion criteria
The search results were imported into the reference manager and manually screened for relevance. The inclusion criteria included reporting the prevalence of any of the listed STIs using a standard diagnostic assay. Data were collected from studies published between 2000 and April 2024. Each study was validated and checked by two authors, and in case of dispute, a third reviewer was assigned. The data extracted from each study in the present meta-analysis were sample size, year of collection, population of interest, location of the study, study design, and sex if possible (Sect. 3 Supplementary file). The studies were sorted into four main populations: (i) screening population (screening studies, annual reports, pregnant women, infertility screening, and annual cervical screening programs); (ii) symptomatic population (attending the clinic with STI complications); (iii) clinical population (including HIV cohort, virus-induced lesion/carcinoma); and (iv) high risk population, including immunocompromised patients (patients with cancer, organ transplant recipients, patients with beta-thalassemia) and individuals at high risk because of social or environmental factors (drug users, men who have sex with men, sex workers, prisoners).
The exclusion criteria included case reports, reviews, editorials, letters to editors, commentaries, animal studies, and seroprevalence studies. We also excluded any papers reporting STIs in children (< 18 years old). Studies reporting genotypes/variants of the STIs were considered carefully, as the collection method could be biased. Studies of sexually transmitted organisms but no clear mode of transmission were excluded.
Statistical analysis
Meta-analyses were performed to assess the prevalence of STIs by clinical population, sex, and geographical area. The meta-analysis was conducted using R version 4.4 (R Foundation for Statistical Computing, Vienna, Austria) with the packages “meta” and “metafor” [7]. Pooled prevalence estimates were calculated using the DerSimonian and Laird method with a mixed-effects model. Heterogeneity was assessed using forest plots, Cochran’s Q, and I2. Low, moderate, and high levels of homogeneity were indicated by I2 = 0–49%, I2 = 50–74%, and I2 ≥ 75%, respectively. The pooled prevalence and confidence interval (95% CI) were used to compare the STI prevalence rates between subgroups. P < 0.05 indicated a statistically significant difference.
To address publication bias, we used funnel plots and performed Egger’s test to screen studies for STIs with more than 10 publications (Sect. 4 Supplementary file). Our preliminary analysis revealed some publication-biased results, as expected from subgroup analysis [8]. Meanwhile, no test for small study k (< 10) effects was conducted for meta-analysis with subgroups. As proportional meta-analysis does not adequately adjust for funnel and Egger tools, we assessed the studies qualitatively [9].
Results
Study selection and characteristics
Overall, the database searches identified 11,290 records. Initially, 1806 duplicated records and 896 review articles were removed using automation tools, and 8588 records were screened. During the screening phase, 6930 records were excluded for the following reasons: no standard assay of detection (3210 articles), study design (1913 articles), study type (817 articles), seroprevalence studies (908 articles), and mode of transmission (85 articles). Thus, the remaining 395 studies were included in the meta-analysis (Figure S1 and Sect. 1 Supplementary file).
The meta-analysis covered 19 STIs and included data from 20,435,971 participants, who were categorized by region and population as presented in Table 1. Among the studies that specified gender, a total of 295,134 participants were male, while 11 million were female. However, a significant number of studies either did not report participant gender or lacked sufficient data to calculate sample sizes based on gender. By pathogens, the 395 studies, had a total of 639 subgroups, for example one study can report multiple pathogens accounted for as a subgroup. The majority of the studies were reported in Iran (n = 262/639 [41%]) and the GCC region (n = 134/639 [21%]). Pathogen-wise, HPV was the most reported STI (23.2%), followed by chlamydia (11%) and HBV (10.9%). Similarly, the most common cause of infection was HPV (13 million cases), followed by HIV (3.47 million cases) and HBV (2.6 million cases).
Table 1.
Summary of the studies by geographical areas, pathogen, and population
| Reported STIs | A) Studies included in the meta-analysis (n) | B) By population (n) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GCC and Yemen | Iran | Levant | North Africa | Türkiye | Total | Participants (n) | Screening | Symptomatic | Clinical | High risk | |
| 1. BV | 0 | 5 | 2 | 2 | 0 | 9 | 8183 | 6 | 3 | 0 | 0 |
| 2. CMV | 2 | 6 | 0 | 3 | 3 | 14 | 134,634 | 3 | 0 | 7 | 4 |
| 3. Candidiasis | 4 | 4 | 2 | 5 | 1 | 16 | 240,145 | 13 | 1 | 1 | 1 |
| 4. Chancroid | 4 | 0 | 0 | 0 | 0 | 4 | 167,700 | 3 | 1 | 0 | 0 |
| 5. Chlamydia | 15 | 24 | 9 | 13 | 10 | 71 | 4137 | 45 | 17 | 6 | 3 |
| 6. EBV | 3 | 14 | 3 | 9 | 0 | 29 | 4664 | 1 | 0 | 11 | 17 |
| 7. Genital herpes | 6 | 21 | 4 | 4 | 3 | 38 | 376,211 | 16 | 5 | 10 | 7 |
| 8. HIV | 13 | 25 | 4 | 14 | 1 | 57 | 3,474,533 | 19 | 1 | 6 | 31 |
| 9. HPV | 31 | 61 | 12 | 24 | 20 | 148 | 13,078,465 | 63 | 14 | 51 | 20 |
| 10. HTLV-1 | 0 | 5 | 0 | 0 | 0 | 5 | 39,3542 | 3 | 1 | 1 | 0 |
| 11. HBV | 20 | 26 | 6 | 11 | 7 | 70 | 2,622,312 | 21 | 2 | 31 | 16 |
| 12. HCV | 7 | 20 | 1 | 9 | 2 | 30 | 852,221 | 7 | 0 | 18 | 14 |
| 13. Mycoplasma spp. | 4 | 8 | 4 | 3 | 2 | 21 | 5545 | 13 | 5 | 2 | 1 |
| 14. NG | 10 | 15 | 5 | 9 | 5 | 44 | 272,148 | 26 | 11 | 4 | 3 |
| 15. NGU | 4 | 0 | 0 | 0 | 0 | 4 | 240,145 | 3 | 1 | 0 | 0 |
| 16. Syphilis | 6 | 5 | 2 | 7 | 3 | 23 | 1,065,959 | 13 | 0 | 4 | 6 |
| 17. TV | 3 | 13 | 3 | 6 | 3 | 28 | 765 | 19 | 7 | 0 | 2 |
| 18. Toxoplasmosis | 0 | 6 | 0 | 0 | 0 | 6 | 280,903 | 0 | 0 | 0 | 6 |
| 19. Ureaplasma spp. | 2 | 4 | 4 | 1 | 2 | 13 | 3330 | 8 | 4 | 1 | 0 |
| Total | 134 | 262 | 61 | 120 | 62 | 639 | 20,435,971 | 282 | 73 | 159 | 152 |
BV, bacterial vaginosis; CMV, cytomegalovirus; EBV, Epstein–Barr virus; HIV, human immunodeficiency virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HPV, human papillomavirus; HTLV-1 -1, human T-lymphotropic virus 1; NG, Neisseria gonorrhoeae; NGU, nongonococcal urethritis; TV, Trichomonas vaginalis
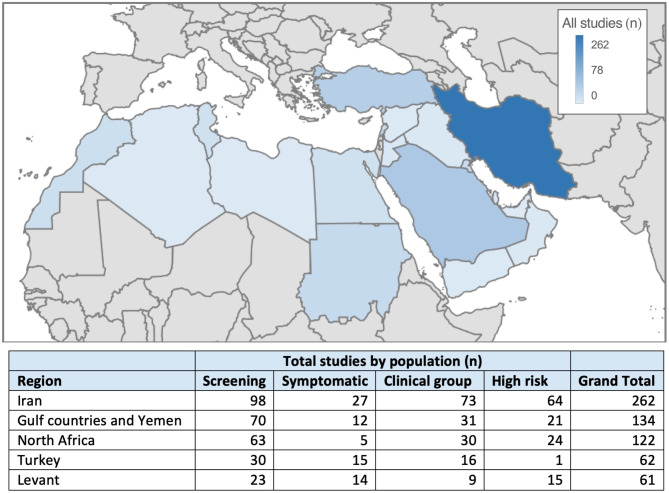
The data were stratified by population and geographical region, as presented in Fig. 1. In the MENA region, studies most frequently included a screening cohort (n = 282), followed by clinical (n = 159) and high risk cohorts (n = 150). For the screening population, the most reported STIs were HPV (n = 63), chlamydia (n = 71), and NG (n = 44). For the symptomatic population, the most reported STIs were chlamydia (n = 17), HPV (n = 14), and NG (n = 11). For the clinical population, HPV (n = 51), HBV (n = 31), and HCV (n = 18) were the most common STIs, whereas in high risk cohorts, HIV (n = 31) and HPV (n = 20) were most frequently reported.
Fig. 1.
Map of study enrollment by country and the general population. Iran reported the highest number of publications (n = 262), followed by Saudi Arabia (n = 78). Screening studies were most frequently reported in the entire Middle East and North Africa region
Common bacterial infections
The distribution of chlamydia spp. in the MENA region was examined across four populations, as presented in Fig. 2. Among these populations, the clinical population exhibited the highest crude prevalence (12%, 95% CI = 4–34), followed by symptomatic population (7%, 95% CI = 3–14), high risk population (6% 95% CI = 4–8), and screening population (5%, 95% CI = 3–8). Furthermore, the prevalence of chlamydia spp. varied across regions, with the highest prevalence observed in North Africa (10%, 95% CI = 4–33), whereas the lowest prevalence was recorded in the GCC, Levant, and Türkiye (4%). However, the differences between countries were not significant within the screening population (P = 0.40). Similarly, no significant differences were detected between men and women (5% vs. 4%, P = 0.82). Notably, within the symptomatic populations, significant differences were found by both sex and geographical location, indicating high heterogeneity. In the symptomatic cohort, the highest crude prevalence was observed in Iran (14%), whereas the lowest prevalence was noted in the GCC area (3%). Furthermore, although the prevalence was higher in men than in women (15% vs. 5%), the difference did not reach significance (P = 0.07).
Fig. 2.
Forest plots of studies reporting the prevalence of chlamydia in the Middle East and North Africa (MENA) by region and sex and categorized by population. Estimates were pooled using a random effects model, and p-values are provided for subgroups. Vertical ticks within the gray boxes and horizontal lines present the pooled prevalence effect and 95% confidence interval (CI) for each study. The gray diamond at the bottom denotes the cumulative effect with the 95% CI
The distribution of Mycoplasma spp. in the MENA region was analyzed in the symptomatic and screening cohorts, as presented in Fig. 3. The crude prevalence was 6% (95% CI = 4–8) in the symptomatic cohort and 5% (95% CI = 2–12) in the screening cohort. The prevalence of Mycoplasma spp. was the highest in the GCC region (12%, 95% CI = 1–80) and lowest in Türkiye (2%, 95% CI = 2–37). The difference by country was significant in the screening population (P < 0.01). Conversely, no sex-based difference was identified (P = 0.51), although the prevalence was higher in women than in men (7% vs. 4%). In the symptomatic population, a significant difference was detected by region with high heterogeneity. In the symptomatic population, the highest crude prevalence was detected in Türkiye (10%), and the lowest prevalence was detected in the Levant area (4%). Additionally, no significant difference was observed between men and women (7% vs. 6%, P = 0.43).
Fig. 3.
Forest plots of studies reporting the prevalence of Mycoplasma (M. genitalium or M. hominis) in the Middle East and North Africa (MENA) by region and sex and categorized by population. Estimates were pooled using a random effects model, and the p-value is presented for each subgroup. Vertical ticks within the gray boxes and horizontal lines denote the pooled prevalence effect and 95% confidence interval (CI) for each study. The gray diamond at the bottom denotes the cumulative effect and 95% CI
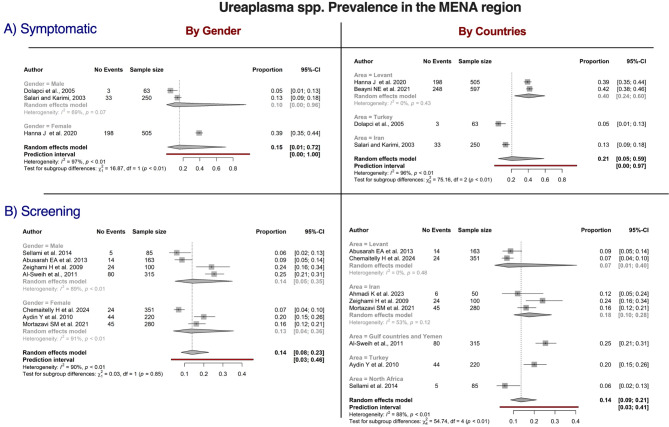
The distribution of Ureaplasma spp. within the MENA region was studied in the symptomatic and screening populations, as presented in Fig. 4. The crude prevalence was 21% (95% CI = 5–59) in the symptomatic population, versus 14% (95% CI = 9–21) in the screening population. In the screening population, the prevalence of Ureaplasma spp. was highest in the GCC area (25%, 95% CI = 21–31) and lowest in North Africa (6%, 95% CI = 9–21). The variation by country was significantly different in the screening population (P < 0.01). However, no significant difference was detected between men and women (14% vs. 13%, P = 0.85). In the symptomatic population, significant differences in prevalence were noted for sex and location with high heterogeneity. The Levant region had the highest crude prevalence (40%), whereas the prevalence was lowest in Türkiye (5%). In the symptomatic population, women exhibited a higher prevalence than men (39% vs. 10%, P < 0.01).
Fig. 4.
Forest plots of studies reporting the prevalence of Ureaplasma (U. urealyticum and U. parvum) in the Middle East and North Africa (MENA) by sex and gender and categorized by population. Estimates were pooled using a random effects model, and the p-value is provided for each subgroup. Vertical ticks within the gray boxes and horizontal lines denote the pooled prevalence effect and 95% confidence interval (CI) for each study. The gray diamond at the bottom denotes the cumulative effect and 95% CI
The distribution of N. gonorrhoeae within the MENA region was studied in symptomatic, screening, and clinical populations, as illustrated in Fig. 5. By population, the highest crude prevalence was observed in the symptomatic population (12%, 95% CI = 4–32), whereas the lowest prevalence was noted in the screening population (2%, 95% CI = 1–3). When examining the prevalence of N. gonorrhoeae by region in the screening population, the highest prevalence was found in the GCC region (3%, 95% CI = 1–21), whereas it was lowest in Türkiye (0%). Notably, the difference by country was not statistically significant in this population (P = 0.12). Conversely, the prevalence was significantly higher in men than in women (4% vs. 3%, P < 0.01). In the symptomatic populations, significant differences were detected by sex and region with high heterogeneity. The highest crude prevalence in the symptomatic cohort was observed in North Africa (40%, 95% CI = 24–60), whereas it was lowest in Türkiye (9%, 95% CI = 1–62). Meanwhile, the prevalence was higher in men than in women (27% vs. 2%, P < 0.01). Lastly, within the clinical population, the crude prevalence was highest in the GCC area (13%, 95% CI = 9–17) and lowest in Iran (7%, 95% CI = 2–26).
Fig. 5.
Forest plots of studies reporting the prevalence of Neisseria gonorrhoeae (NG) in the Middle East and North Africa (MENA) by region and gender and categorized by population. Estimates were pooled using a random effects-model, and the p-value is provided for each subgroup. Vertical ticks within the gray boxes and horizontal lines denote the pooled prevalence effect and 95% confidence interval (CI) for each study. The gray diamond at the bottom denotes the cumulative effect and 95% CI
The prevalence of syphilis, caused by the bacterium Treponema pallidum, in the MENA region was investigated across screening, clinical, and high risk populations, as illustrated in Figure S2. Among these populations, the crude prevalence was highest in the clinical population (7%, 95% CI = 1–30) and lowest in the screening population (1%, 95% CI = 0–35). Regionally, North Africa exhibited the highest prevalence in the screening population (2%, 95% CI = 0–8), whereas Iran had the lowest prevalence (0%). The differences in prevalence across regions were statistically significant (P < 0.01). In the clinical population, Iran had the highest crude prevalence (12%, 95% CI = 4–26), whereas Türkiye had the lowest prevalence (6%, 95% CI = 0–55). In the high risk population, North Africa had the highest crude prevalence (16%, 95% CI = 0–92), whereas Iran had the lowest prevalence (1%, 95% CI = 0–13). Furthermore, the distribution of syphilis in the high risk population was significantly different across countries (P < 0.01). When analyzing the prevalence by sex in the high risk population, no significant difference was observed between men and women (6% vs. 2%, P = 0.34).
Opportunistic and other infections
In our analysis, Candida spp. was exclusively observed in the screening population (Figure S3). We observed a borderline significant difference in its prevalence between women and men (11% vs. 2%, P = 0.05). Furthermore, significant variation was detected in different geographical locations with high heterogeneity (I2 = 99%). The highest prevalence was observed in North Africa (32%, 95% CI = 22–42), and the lowest prevalence was noted in the GCC region (5%, 95% CI = 5–6).
The prevalence of BV in the MENA region was studied in both screening and symptomatic populations (Figure S3). In the screening population, the crude prevalence was 6% (95% CI = 1–32), versus 32% (95% CI = 22–42) in the symptomatic population. The prevalence of BV in the screening population was highest in North Africa (31%) and the lowest in Iran (2%), and this difference was significant (P < 0.01). Furthermore, high heterogeneity was observed by region. In the symptomatic population, the highest prevalence was noted in the Levant region (32%, 95% CI = 8–72), followed by Iran (29%, 95% CI = 23–34), indicating a significant difference by region (Figure S3) with high heterogeneity (I2 = 79%).
CMV was prevalent in all populations in the MENA region excluding the symptomatic population (Figure S3). The crude prevalence in the screening cohort was 5% (95% CI = 0–85), versus 22% (95% CI = 3–72) in the high risk cohort. Stratification by region indicated that the prevalence of CMV was highest in Iran (8%) and lowest in Türkiye (1%), with no significant difference detected (P = 0.25). Additionally, no significant sex-based differences were observed in screening populations despite the higher prevalence in men (8% vs. 1%). In the clinical cohort, the highest CMV prevalence was reported in Iran (27%), whereas the GCC region had the lowest prevalence (2%). Notably, the highest prevalence in the high risk cohort was reported in Iran (32%), with the lowest prevalence detected in the GCC region (11%).
The prevalence of EBV in the MENA region was highest in the high risk population (24%, 95% CI = 14–37), followed by the screening population (17%, 95% CI = 0–100) and clinical population (16%, 95% CI = 6–35, Figure S3). Stratification by region did not reveal significant differences in the high risk cohort, but the crude prevalence was highest in the GCC region (33%) and lowest in North Africa (17%). Sex-based analysis in the high risk cohort did not yield significant differences, although the prevalence was numerically higher in men (34% vs. 17%, P = 0.09). In the clinical cohort, the highest prevalence of EBV was recorded in North Africa (33%), whereas the lowest value was observed in the GCC area (2%).
In the MENA region, the distribution of Trichomonas vaginalis was examined in the screening, symptomatic, and high risk cohorts. The highest crude prevalence was observed in the high risk population (12%, 95% CI = 6–22), followed by the symptomatic population (10%, 95% CI = 2–34) and screening population (3%, 95% CI = 2–6). Notably, the prevalence of Trichomonas vaginalis varied significantly by region in the screening population, being highest in the GCC area (7%, 95% CI = 0–84) and lowest in the Levant area (1%, 95% CI = 0–62, P < 0.01). In the symptomatic population, the highest crude prevalence was detected in Türkiye (24%, 95% CI = 19–31), and the lowest prevalence was recorded in the Levant area (3%, 95% CI = 0–97). The prevalence varied significantly by sex and region (P < 0.01). The high risk population in the MENA region was reported only in Iran, and the pooled prevalence was 12% (95% CI = 6–22).
Furthermore, the distribution of genital herpes in the MENA region was studied across all populations (Fig. 6). The highest crude prevalence was observed in the symptomatic population (20%, 95% CI = 1–82), followed by the clinical population (14%, 95% CI = 3–46), high risk population (13%, 95% CI = 6–26), and screening population (2%, 95% CI = 1–5). Sex differences were notable, with an elevated prevalence in men (32% vs. 1%) in the screening population. Significant variations in the prevalence of herpes by region and sex were also observed in the screening population (P < 0.01). In the clinical population, the highest crude prevalence was reported in North Africa (39%, 95% CI = 26–54), and the lowest prevalence was observed in Iran (12%, 95% CI = 2–46), although the difference by country and sex was not significant (P = 0.07). In the high risk population, a higher prevalence was noted among women (12%, 95% CI = 1–63) than among men (2%, 95% CI = 1–4, P < 0.01). Additionally, a significant difference in the prevalence of herpes by geographical area was recorded in the symptomatic population, being highest in Türkiye (55%, 95% CI = 43–68) and lowest in the Levant region (2%, 95% CI = 1–4).
Fig. 6.
Forest plots of studies reporting the prevalence of genital herpes in the Middle East and North Africa (MENA) by region and sex and categorized by population. Estimates were pooled using a random effects model, and the p-value is provided for each subgroup. Vertical ticks within the gray boxes and horizontal lines denote the pooled prevalence effect and 95% confidence interval (CI) for each study. The gray diamond at the bottom denotes the cumulative effect and 95% CI
Hepatitis (types B and C)
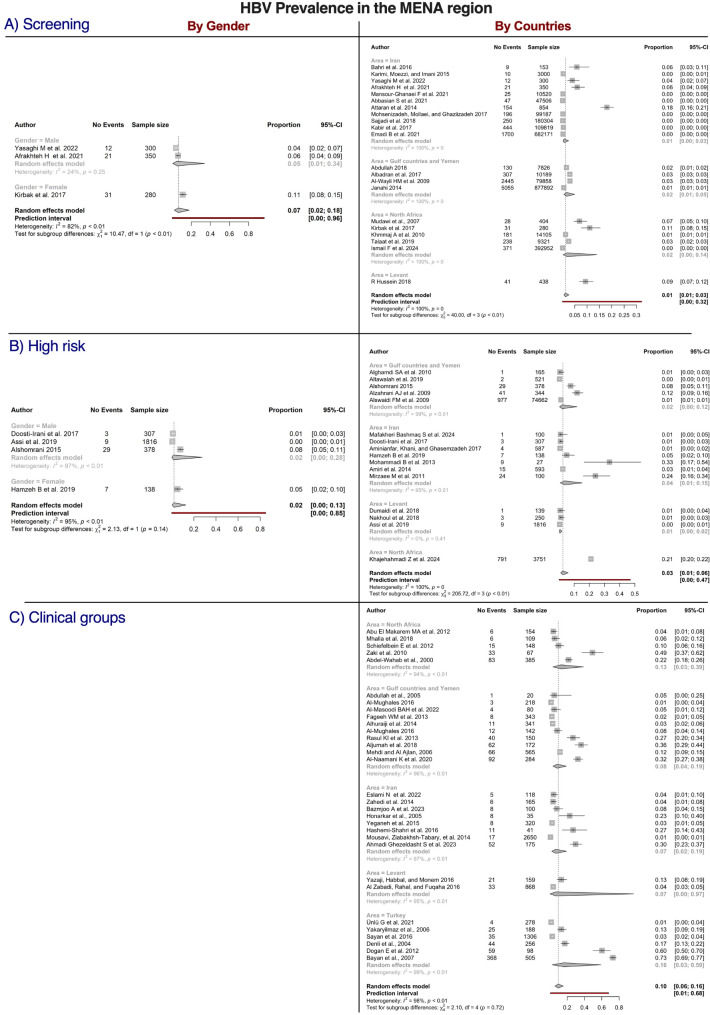
A comprehensive investigation of the distribution of HBV and HCV in the MENA region was conducted across the screening, clinical, and high risk populations, as illustrated in Figs. 7 and 8. The results revealed notable variations in prevalence across different populations and regions.
Fig. 7.
Forest plots of studies reporting the prevalence of hepatitis type C (HCV) in the Middle East and North Africa (MENA) by country and sex and categorized by population. Estimates were pooled using a random effects model, and the p-value was provided for each subgroup. Vertical ticks within the gray boxes and horizontal lines denote the pooled prevalence effect and 95% confidence interval (CI) for each study. The gray diamond at the bottom denotes the cumulative effect and 95% CI
Fig. 8.
Forest plots of studies reporting the prevalence of hepatitis type B (HBV) in the Middle East and North Africa (MENA) by country and gender and categorized by population. Estimates were pooled using a random effects model, and the p-value was provided for each subgroup. Vertical ticks within the gray boxes and horizontal lines denote the pooled prevalence effect and 95% confidence interval (CI) for each study. The gray diamond at the bottom denotes the cumulative effect and 95% CI
The highest crude prevalence of HCV was observed in the clinical population (18%, 95% CI = 6–33), followed by the high risk population (9%, 95% CI = 3–22) and screening population (0%, 95% CI = 0–2). The prevalence of HCV also varied by region, being highest in the GCC region (2%, 95% CI = 0–2) and lowest in Iran and North Africa (both 0%). Statistically significant differences in prevalence across countries were observed in the screening population (P = 0.03). In the clinical population, the highest crude prevalence was found in North Africa (31%, 95% CI = 5–78), whereas the lowest prevalence was recorded in the GCC (12%, 95% CI = 1–72). However, no statistically significant differences were observed across countries in the clinical population (P = 0.45). Significant differences were noted by region in the high risk population, with the highest crude prevalence observed in the Levant region (53%, 95% CI = 43–63), whereas the prevalence was 3% (95% CI = 3–4) in North Africa and 4% (95% CI = 0–90) in the GCC region. The prevalence of HCV was significantly higher in men than in women in the high risk population (21% vs. 2%, P = 0.04).
The distribution of HBV in the MENA region was reported for the screening, clinical, and high risk populations (Fig. 8). The highest crude prevalence was observed in the clinical population (10% 95% CI = 6–16), followed by the high risk population (3%, 95% CI = 1–6) and screening population (1%, 95% CI = 1–3). Significant differences in prevalence were observed across countries in the screening population (P < 0.01), and the prevalence was significantly higher in women (11%, 95% CI = 8–15) than in men (5%, 95% CI = 1–34, P < 0.01). No significant difference by geographical region was observed in the clinical population (P = 0.72), with the highest prevalence reported in Türkiye (16%, 95% CI = 3–59) and the lowest prevalence observed in the Levant region (7%, 95% CI = 0–97). The highest crude prevalence in the high risk population was found in North Africa (21%, 95% CI = 20–22), and the lowest prevalence was observed in Iran and the Levant region (both 1%). Significant differences in prevalence were found across countries in the high risk population (P < 0.01), whereas no significant difference in prevalence was observed between women (5%, 95% CI = 2–10) and men 2% (2%, 95% CI = 0–28, P = 0.14).
Detailed subgroup analyses of patients with HCV and HBV revealed further insights (Figure S5). Among patients with HCV, the prevalence was highest in patients with hepatocellular carcinoma (HCC; 49%, 95% CI = 12–87), whereas the prevalence among patients with HIV/AIDS was 24% (95% CI = 4–70). Regarding HBV, the highest crude prevalence was noted in patients with HCC (29%, 95% CI = 16–48), whereas its prevalence was 5% (95% CI = 2–11) in patients with HIV/AIDS.
Distribution of HIV in the MENA region
The distribution of HIV in the MENA region was investigated across screening, clinical, and high risk populations, as illustrated in Fig. 9. The highest crude prevalence was observed in the high risk population (5%, 95% CI = 3–10), followed by the clinical population (2%, 95% CI = 0–80) and screening population (0%). Statistical analysis revealed significant regional differences in the prevalence of HIV in the screening population (P < 0.01). Furthermore, both men and women in the screening population exhibited prevalence rates lower than 1%, and no significant difference was observed by sex (p > 0.99). Regarding geographical locations, no significant difference was noted in the clinical population, with the highest prevalence reported in the GCC region (3%) and the lowest prevalence recorded in Iran (2%). In the high risk population, the Levant region had the highest crude prevalence (14%, 95% CI = 0–87), whereas the GCC region exhibited the lowest prevalence (1%, 95% CI = 0–31). Statistical analysis demonstrated significant differences among regions in the high risk population (P = 0.04). Conversely, the prevalence did not differ between men and women (7% vs. 5%, P = 0.43).
Fig. 9.
Forest plots of studies reporting the prevalence of human immunodeficiency virus (HIV) in the Middle East and North Africa (MENA) by region and sex and categorized by population. Estimates were pooled using a random effects model, and the p-value is given for each subgroup. Vertical ticks within the gray boxes and horizontal lines denote the pooled prevalence effect and 95% confidence interval (CI) for each study. The gray diamond at the bottom denotes the cumulative effect and 95% CI
Distribution of HPV in the MENA region
The distribution of HPV in the MENA region was examined across four populations (Fig. 10). The highest crude prevalence was observed in the clinical population (47%, 95% CI = 36–59), followed by the symptomatic population (37%, 95% CI = 20–58), high risk population (26%, 95% CI = 17–36), and screening population (13%, 95% CI = 8–19). Geographically, the prevalence of HPV in the screening population was highest in North Africa (24%, 95% CI = 14–37) and lowest in the GCC region (3%, 95% CI = 0–16). The differences among countries within this population were not significant (P = 0.12). No significant variation by sex was detected (P = 0.46) despite the higher prevalence in women (14%, 95% CI = 9–22) than in men (8%, 95% CI = 1–57).
Fig. 10.
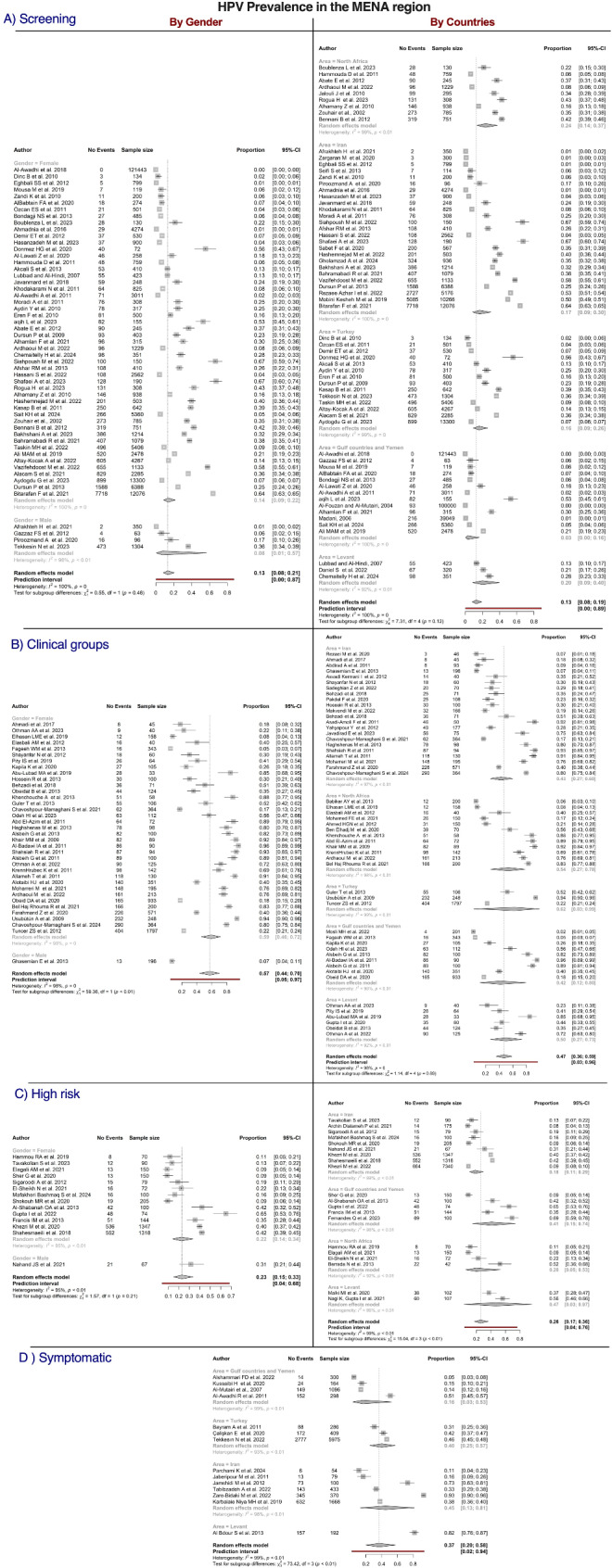
Forest plots of studies reporting the prevalence of human papillomavirus (HPV) in the Middle East and North Africa (MENA) by country and sex and categorized by population. Estimates were pooled using a random effects model, and the p-value is provided for each subgroup. Vertical ticks within the gray boxes and horizontal lines denote the pooled prevalence effect and 95% confidence interval (CI) for each study. The gray diamond at the bottom denotes the cumulative effect and 95% CI
No difference was observed among geographical regions in the clinical population (P = 0.89), with the highest prevalence reported in Türkiye (62%, 95% CI = 3–99) and lowest prevalence observed in Iran (7%, 95% CI = 27–60). The prevalence was significantly higher in women than in men (59% vs. 7%, P < 0.01). In the symptomatic population, significant differences were found among locations, with the highest prevalence noted in the Levant region (82%, 95% CI = 76–87) and the lowest prevalence recorded in the GCC region (16%, 95% CI = 3–53). In the high risk population, the highest crude prevalence was found in the Levant region (47%, 95% CI = 17–36), and the lowest prevalence was reported in Iran (18%, 95% CI = 11–29). The differences among countries within this population were significant (P < 0.01). No significance was detected between men and women (31% vs. 22%, P = 0.21).
Furthermore, we conducted detailed analyses of subgroups within the MENA region, as presented in Figure S6. Overall, the crude prevalence of HPV among patients with HPV-related lesions (oral or cervical) was 51% (95% CI = 39–63). Moreover, the prevalence of cervical lesions was 62% (95% CI = 49–73), while a significantly lower prevalence of 22% (95% CI = 13–34) was observed for oral lesions (P < 0.0001). No significant differences were detected in cervical lesions by geographical location; however, significant variations were observed in oral lesions. Additional detailed group data can be found in the supplementary files (Figures S6 and S7).
Overall STI distribution by country
An extensive analysis of the prevalence of STIs was conducted in the MENA region, as depicted in Fig. 11A. In North Africa, the predominant STIs were BV (31%), HPV (23%), and Candida spp. (15%). In the GCC and Yemen, Ureaplasma spp. (25%), nongonococcal urethritis (16%), and Mycoplasma spp. (12%) were the most prevalent. In the Levant region, the most common STIs were HPV (20%), HBV (9%), and Candida spp. (9%). In Iran, Ureaplasma spp. (18%), HPV (17%), and CMV (8%) were the most prevalent STIs. In Türkiye, Ureaplasma spp. (20%), Candida spp. (18%), and HPV (16%) were the most frequently detected STIs.
Fig. 11.
The distribution of sexually transmitted infections (STIs) in the screening population in the Middle East and North Africa (MENA) region, including Afghanistan, Algeria, Bahrain, Djibouti, Egypt, Iran, Iraq, Jordan, Kuwait, Lebanon, Libya, Morocco, Oman, Occupied Palestinian Territories, Pakistan, Qatar, Saudi Arabia, Somalia, Sudan, Syria, Tunisia, UAE, and Yemen. (A) Pooled prevalence of STIs in the MENA region by geographical area. Ureaplasma was the most common pathogen in the GCC area, Iran, and Türkiye; BV was the most common pathogen in North Africa; and HPV was the common pathogen in the Levant region. (B) Distribution of STIs by sex using data from studies that reported rates for both sexes. Ureaplasma, Neisseria gonorrhoeae, and herpes were the most common STIs in men, whereas Candida spp., hepatitis B virus (HBV), Mycoplasma spp., and human papillomavirus (HPV) were the most common STIs in women. (C) The overall distribution of STIs in the MENA region. The highest prevalence was noted for nongonococcal urethritis (NGU, 16.3%) followed by Ureaplasma (13.7%), HPV (12.7%), and Candida spp. (9.4%)
Additionally, our analysis investigated sex-based disparities. As presented in Fig. 11B, the prevalence of Ureaplasma spp., N. gonorrhoeae, and herpes was higher in men, whereas women exhibited higher rates of Mycoplasma spp., HPV, HBV, and Candida spp.. Importantly, nongonococcal urethritis (16.3%), Ureaplasma spp. (13.7%), HPV (12.7%), and Candida spp. (9.4%) were identified as the most prevalent STIs, as illustrated in Fig. 11C.
Discussion
Globally, HIV, viral hepatitis, and other STIs collectively cause 2.3 million deaths and 1.2 million cases of cancer annually, representing a major public health burden worldwide. More than 1 million people are newly infected with STIs daily, and an additional 4.5 million are newly infected with HIV, HBV, and HCV every year. These staggering statistics highlight the significant scale and impact of these communicable diseases, which disproportionately affect vulnerable populations and contribute to substantial morbidity and mortality globally [5].
The current meta-analysis comprehensively assessed the epidemiology of 19 distinct STIs in the MENA region by drawing data from 20,435,971 participants. The meta-analysis revealed that HPV was the most commonly reported STI in the region. The most commonly investigated STI was HPV, with 13,000,000 individuals included in the analysis. This finding aligns with the epidemiology of HPV, which is recognized as the most prevalent viral STI globally. However, our results suggest a lower prevalence of HPV in the MENA region than reported in other low- and middle-income countries [10]. This apparent lower burden of HPV in MENA might be attributable to several methodological and sociocultural factors rather than reflecting the true epidemiology of the virus. First, the available evidence on the prevalence of HPV in this region is largely derived from hospital-based studies, which might not be representative of the general population. These facility-based data sources tend to capture individuals seeking healthcare, potentially skewing the estimates toward higher-risk groups and those with symptomatic HPV-related conditions. By contrast, population-based national surveillance programs, which are lacking in many MENA countries, would provide a more accurate and generalizable assessment of the epidemiology of HPV. Additionally, cultural norms surrounding sexual health in some MENA societies might contribute to underreporting and underdiagnosis of HPV. Stigmas associated with STIs can deter individuals from seeking testing and care, leading to an underestimation of the true disease burden. Furthermore, the absence of robust national HPV screening programs in many MENA nations means that subclinical HPV infections might go undetected, further obscuring the accurate prevalence of the virus. Another factor that potentially influenced the reported HPV prevalence in the MENA region is the relatively high rate of male circumcision, with some studies suggesting that circumcision was associated with a potential reduction in HPV acquisition [11].
In our analysis, we distinguished significant differences in HPV prevalence by lesion type (oral: 22% vs. cervical: 62%). The geographical variation was significant only for oral lesions. This area of research is still lacking in the region, as reported in another review where HPV crude prevalence was 16% [12]. This result is consistent with a study conducted in Northern Sardinia, which reported a prevalence of 14.5% [13]. Indeed, the study on HPV-induced oropharyngeal squamous cell carcinoma (OPSCC) in North Sardinia highlights significant findings regarding the prevalence and diagnostic methods for HPV-related cancers, which can be compared to our systematic review of sexually transmitted infections (STIs) in the MENA region. In the Sardinian study, approximately 14% of OPSCC cases were found to be positive for HPV-DNA, with p16 immunohistochemistry (IHC) demonstrating high sensitivity but moderate specificity as a standalone diagnostic tool. This contrasts sharply with our findings, where HPV prevalence among cervical cancer patients in the MENA region was reported at 81%, with regional variations indicating a substantial burden of HPV-related diseases. In another study, the Sardinian research highlighted the importance of understanding HPV genotype dynamics, particularly in relation to cervical cancer prevention strategies [14]. These findings underscore the need for tailored public health initiatives in different regions. For instance, while our MENA review indicates a lack of national STI screening programs, the Sardinian study emphasizes the role of HPV vaccination in reducing the prevalence of high-risk genotypes such as HPV-16 and HPV-31. Furthermore, gender-based disparities were noted in our findings, with higher rates of HPV and Candida spp. observed in women, paralleling the Sardinian data that identified demographic factors influencing HPV risk. Additionally, our published meta-analysis on HPV epidemiology in the MENA region highlighted the substantial burden of HPV as a leading cause of cervical cancer, with a pooled prevalence rate of 81% among cervical cancer patients. While our study primarily focused on overall HPV prevalence, we recognize the importance of identifying high-risk HPV genotypes for early detection and prevention of cervical lesions. Future research should specifically assess high-risk HPV types to enhance prevention strategies and support effective vaccination programs [10].
Research published by Alhamlan et al. provided concerning insights into the cervical cancer landscape in GCC countries. The retrospective analysis of the Gulf Center for Cancer Control and Prevention registry over a 14-year period (1998–2012) revealed that most cases of cervical cancer in the GCC region were diagnosed at advanced stages. Indeed, the study found that nearly half of the patients presented with regional or distant metastasis, significantly reducing their chances of survival. Furthermore, the researchers reported that only 28% of patients in these countries sought medical care for early-stage cervical cancer [15]. This is especially troubling given that cervical cancer is one of the most preventable cancers, and it has a high survival rate when detected early. These findings underscore the urgent need to implement comprehensive cervical cancer screening programs and awareness campaigns in GCC countries. Such efforts would educate girls, women, and healthcare providers about the importance of early detection and promote timely access to diagnostic and treatment services.
It is worth mentioning that WHO is working globally, regionally, and nationally to increase political commitment, provide technical assistance, develop norms and standards, and coordinate the broader health ecosystem to achieve the ambitious goal of making cervical cancer a rare disease globally. WHO has adopted a global strategy to accelerate the elimination of cervical cancer called [90:70:90], which aims to fully vaccine 90% of girls with the HPV vaccine by age 15, screen 70% of women with a high-performance test by age 35 and again by age 45, and identify 90% of women receiving treatment for cervical diseases [2]. Despite WHO recommendations, not all MENA countries have implemented HPV cervical screening programs, and many rely on cervical cytology screening alone without specifically testing for HPV infection.
Vaginal self-sampling has emerged as a promising tool for cervical cancer screening, particularly in the MENA region, where traditional screening methods often fail to reach underrepresented women due to social, cultural, and geographical barriers. This approach has been adopted by 48 countries, demonstrating its potential to significantly boost participation in screening programs. Self-sampling allows women to collect specimens in the privacy of their own homes, thus addressing common concerns such as discomfort and stigma associated with clinical examinations. Evidence supports that self-sampling can enhance screening uptake, especially among women who may not engage with conventional healthcare services [16]. Additionally, this method can optimize resource allocation by streamlining the screening process and focusing healthcare efforts on follow-up care for those who test positive. By facilitating large-scale monitoring of HPV infection prevalence, self-sampling not only aids in evaluating the impact of HPV vaccination programs but also provides critical insights into the epigenetic mechanisms underlying cervical cancer development [17]. In summary, the available evidence suggests a significant burden of HPV infection in MENA countries, underscoring the need for public health interventions, including the introduction of HPV-based screening strategies and HPV vaccination programs. Given the established association between HPV and various types of human cancers, addressing the HPV burden in the MENA region should be a public health priority [18].
The second most reported STI infection in this meta-analysis was chlamydia (11.0%), which is caused by the bacterium Chlamydia trachomatis. According to recent global estimates, in 2020, approximately 128.5 million new C. trachomatis infections occurred globally among people aged 15–49 years. The global prevalence of chlamydia was estimated to be 4.0% for women and 2.5% for men in the 15–49 age group during the same year. These figures highlight the significant burden of chlamydia, particularly among young people, as these infections tend to be more common in this demographic [19]. The analysis of the prevalence of chlamydia across different populations in the MENA region provides valuable insights into the burden of this STI. The data reveal a concerning trend, with the highest crude prevalence observed in the clinical population (12%), followed by the symptomatic (7%), high risk (6%), and screening populations (5%). This suggests that chlamydia is frequently detected among individuals seeking medical care or exhibiting symptoms, underscoring the importance of comprehensive screening and early detection efforts. Additionally, the study found regional variations in the prevalence of chlamydia, with the highest rates reported in North Africa (10%) and the lowest in GCC countries, Levant, and Türkiye (4%). Although these differences were not statistically significant within the screening population, the heterogeneity observed in the symptomatic cohort highlights the need for tailored public health interventions that address the unique epidemiological patterns across the MENA region. Understanding these regional disparities can inform the development of targeted strategies to enhance chlamydia control and prevention efforts.
The study also examined the role of sex in the prevalence of chlamydia, revealing a slightly higher rate among men (5%) than among women (4%) in the screening population. By contrast, the rate was significantly higher in men (15%) than in women (5%) in the symptomatic cohort. This suggests that sex is a contributing factor to chlamydia transmission and healthcare-seeking behavior, particularly among individuals presenting with symptoms. These findings underscore the need for sex-sensitive approaches to chlamydia screening, diagnosis, and treatment in the MENA region. Addressing potential barriers and biases in healthcare access and utilization, as well as promoting gender-equitable sexual and reproductive health education, could be crucial for improving chlamydia control and reducing the burden of this infection across diverse populations. These findings highlight the significant burden of chlamydia across the MENA region and the need for urgent public health action. Chlamydial infections can have far-reaching consequences, potentially leading to a range of serious complications, including urethritis, cervicitis, pelvic inflammatory disease, ectopic pregnancy, tubal factor infertility, epididymitis, and prostatitis. Furthermore, chlamydia has been linked to an increased risk of HIV and cervical cancer [20]. The importance of chlamydia screening is emphasized by the fact that a significant proportion of infections are asymptomatic. Thus, screening has a key role in detecting and managing these asymptomatic infections. For example, the US Preventive Services Task Force (USPSTF) recently reviewed the evidence and updated its recommendations on chlamydia screening. According to the USPSTF’s evidence report, findings from four randomized controlled trials of chlamydia screening interventions demonstrated that screening for asymptomatic genital chlamydia was significantly associated with a lower risk of pelvic inflammatory disease in young women [21, 22]. Given that chlamydia is the second most prevalent STI in the MENA region, the implementation of effective screening policies and programs is crucial.
The meta-analysis indicated that HBV caused 10.9% of reported STIs in the MENA region, being responsible for an estimated 2,600,000 reported cases. The meta-analysis findings provide valuable insights into the distribution and prevalence of HBV infection across different populations in MENA. The results highlight the significant burden of HBV, with substantial variations observed in the reported prevalence across the screening, clinical, and high risk populations. The highest crude prevalence of HBV was observed in the clinical group, reaching 10% (95% CI = 6–16), followed by the high risk population (3%, 95% CI = 1–6) and screening population (1%, 95% CI = 1–3). These findings underscore the importance of targeted screening and surveillance efforts to identify and manage HBV infections, particularly within clinical settings and among high risk groups. Interestingly, the meta-analysis revealed significant differences in the prevalence of HBV across countries in the screening population (P < 0.01), and the rate was significantly higher in women (11%, 95% CI = 8–15) than in men (5%, 95% CI = 1–34). These disparities suggest the need for tailored public health interventions that address the unique epidemiological patterns and social determinants of HBV infection in the MENA region.
Furthermore, the subgroup analyses provided additional insights into the burden of HBV in specific clinical conditions. The highest crude prevalence of HBV was observed among patients with HCC (29%, 95% CI = 16–48), and its prevalence was also elevated in people with HIV/AIDS (5%, 95% CI = 2–11). These findings highlight the critical need for integrated care and management strategies that address the complex interplay between HBV and other comorbidities, ensuring comprehensive and coordinated patient-centered approaches. Collectively, the current results underscore the substantial public health challenge posed by HBV in the MENA region. Tailored, evidence-based interventions targeting high risk populations, improving access to screening and treatment services, and strengthening cross-border collaboration and data sharing will be essential to mitigate the burden of HBV and improve health outcomes in the region.
Viral hepatitis has attracted increasing attention, as the global 2020 target for reducing hepatitis B incidence was met through infant vaccination and prevention efforts and a nearly 10-fold increase in the number of people receiving treatment for chronic hepatitis C. Although hepatitis B and C-related mortality has declined, nearly 80% of infected people remain undiagnosed, and access to affordable treatments is limited. The response to the persistent global burden of STIs has severely lagged, including a lack of visibility, funding, and implementation support, resulting in no global 2020 targets being met and the incidence of most STIs plateauing [23]. Mahmud et al. found that HCV infection remains a major public health concern among people who inject drugs (PWID) in the MENA region. Based on data from 1989 to 2018, the pooled mean HCV antibody prevalence among PWID across MENA was 49.3%, with substantial variations recorded across countries. It is estimated that more than 221,000 PWID are chronically infected with HCV in the MENA region, with the highest numbers in Iran and Pakistan. Concerningly, the prevalence of HCV has not declined over time, indicating a persistent high burden. The genotype diversity was moderate, with genotypes 3 and 1 being predominant. These findings underscore the urgent need to dramatically increase harm reduction services and ensure accessible HCV testing and treatment for PWID in the MENA region to address this significant public health challenge [24].
During the WHO 2016–2021 implementation period, major advancements in antiviral drugs and vaccines led to significant global progress in responding to HIV and viral hepatitis. The HIV epidemic has been transformed through the large-scale expansion of antiretroviral therapy, reducing HIV-related deaths to their lowest level since 1994. However, despite these advancements, HIV-related mortality remains unacceptably high, with more than 1.5 million new infections occurring annually, and access to services is insufficient among children and adolescents. Key populations at high risk of HIV face substantial barriers to care [23]. The MENA region has experienced concerning increases in the incidence of HIV and AIDS-related mortality in recent years. Between 2000 and 2015, new HIV infections climbed by more than 33%, and AIDS deaths more than tripled [25]. Despite progress in HIV research and surveillance in MENA, much of the data remain unpublished or inaccessible. Since 2007, the “MENA HIV/AIDS Epidemiology Synthesis Project” has maintained a regional HIV database. Previous systematic reviews documented concentrated and emerging HIV epidemics among men who have sex with men and PWID in MENA, with most of these epidemics emerging within the past two decades [26]. Few meta-analyses examining the status of HIV in the MENA region have been published. One study investigated the epidemiology of HIV among female sex workers (FSWs) and their clients in MENA region. The pooled mean HIV prevalence was 1.4% among FSWs and 0.4% among clients, with substantial variation noted across countries. the prevalence was highest in the Horn of Africa (17.9%) and South Sudan (17.3%), but it was lower than 1% in most countries. The epidemic has been growing since the early 2000s, with the risk of infection climbing by 15% annually. However, HIV testing levels among FSWs remain far below UNAIDS targets. The findings highlight the need to increase HIV testing and prevention services for FSWs and their clients in the MENA region to address this emerging public health challenge [27].
HSV type 1 (HSV-1) is a highly infectious virus and the primary cause of orolabial herpes globally. Following initial infection, the virus establishes a lifelong latent infection in nerve cells. HSV-1 is commonly transmitted through nonsexual contact, such as oral secretions, and most infections are asymptomatic. When symptomatic, HSV-1 can lead to a range of clinical manifestations, including oral, cutaneous, ocular, and central nervous system disorders.
Genital herpes is a highly prevalent STI that affects millions worldwide. Caused by HSV-1 and HSV-2, this chronic condition has no known cure. In the US, genital herpes is the second most common STI, with an estimated 18.6 million people infected with HSV-2 alone [28]. Despite its widespread impact, diagnostic tools and treatment options for genital herpes remain suboptimal. Crucially, no vaccine has been developed to prevent this lifelong infection. Whereas HSV-1 is primarily transmitted nonsexually, the virus can also be transmitted through oral sex or sexual intercourse, resulting in genital herpes. Recent evidence from Western countries indicates a growing role of HSV-1 as an STI and a leading cause of genital herpes [29]. The epidemiology of HSV-1 appears to be shifting, with its seroprevalence declining in Western countries, particularly among younger age groups [30]. This suggests a shift from predominantly oral to increasingly genital HSV-1 infections, which has significant clinical and psychosocial implications. A study conducted in Qatar aimed to measure the seroprevalence and age distribution of HSV-1 among select populations in the MENA region. The results revealed a HSV-1 seroprevalence, ranging from 77.0% among Pakistanis to 97.5% among Egyptians. Its seroprevalence generally increased with age, although there were some variations across countries [31]. These findings suggest that HSV-1 remains highly prevalent in the MENA region, with the potential for ongoing transmission and clinical burden. The observed epidemiological trends underscore the importance of continued surveillance and public health interventions to address this persistent viral infection.
HSV-2 is a prevalent and lifelong STI that affects millions globally. In 2012, it was estimated that more than 400 million people worldwide were infected with HSV-2, with an annual incidence of nearly 20 million new cases. HSV-2 is a leading cause of genital ulcer disease, a condition affecting both developed and developing countries. Interestingly, the seroprevalence of HSV-2 can serve as a collective measure of sexual risk behavior and the potential for HIV transmission. A strong statistical association has been observed between HSV-2 and HIV seroprevalence with a Spearman rank correlation of approximately 0.7. This finding highlights the value of HSV-2 surveillance in identifying populations at risk of future HIV expansion [32]. Despite the growing research focus on STIs in MENA, our understanding of the epidemiology of HSV-2 in this context remains limited. A recent study in Qatar examined the seroprevalence of HSV-2 among male blood donors, providing insights into the local burden of this infection. The study found that the overall HSV-2 seroprevalence ranged from 0.5 to 6.0% across different nationalities, with some evidence of higher seroprevalence in older men. These findings add to our knowledge of HSV-2 epidemiology in the MENA region and underscore the persistent need for improved sexual health services and STI control measures [33].
The analysis of sexually transmitted infections (STIs) in the MENA region also revealed a notable prevalence of Candida infections, particularly in North Africa and the Levant region, where Candida spp. accounted for 15% and 9% of cases, respectively. This finding suggests that Candida infections are a significant concern within the screening populations, despite their association with clear symptoms in women. The higher prevalence rates in these regions may be influenced by various factors, including hormonal changes, antibiotic use, and underlying health conditions that predispose individuals to Candida colonization [34, 35].
The prevalence of other genital pathogens was not well reported in the MENA region. Mycoplasma and Ureaplasma species, particularly M. hominis, U. parvum, and U. urealyticum, are recognized as important causes of urogenital infections. Globally, the role of microorganisms such as Mycoplasma hominis and Ureaplasma spp. as pathogens remains a topic of considerable debate within the medical community. Historically, these bacteria were viewed as commensals of the urogenital tract, with low virulence; however, emerging evidence suggests they may contribute to various reproductive health issues under specific conditions. Because of the emergence of antibiotic resistance and a continuous rise in resistance, the treatment options are limited, making treatment more challenging and expensive. A recent meta-analysis estimated the worldwide resistance rates of genital Mycoplasma and Ureaplasma species to fluoroquinolones (ciprofloxacin, ofloxacin, moxifloxacin, and levofloxacin). The analysis included 30 studies from 16 countries, and the pooled ciprofloxacin, ofloxacin, moxifloxacin, and levofloxacin resistance rates among Mycoplasma and Ureaplasma urogenital isolates were 59.8% (95% CI = 49.6–69.1), 31.2% (95% CI = 23–40), 7.3% (95% CI = 1–31), and 5.3% (95% CI = 1–2), respectively. Meta-regression illustrated that the resistance rates to these fluoroquinolones increased over time, and the fluoroquinolone resistance rates significantly differed between continents/countries (P < 0.05) [36]. These findings suggested a concerning trend of widespread AMR among Mycoplasma and Ureaplasma species, which is likely to persist and potentially worsen in the future. This can be attributed to various factors, including the high prevalence of these infections, the extensive and unregulated use of antimicrobials, the limited surveillance of AMR and clinical failures, and the exceptional ability of these bacteria to develop resistance. It is worth mentioning the STIs can occur simultaneously. One study from Saudi Arabia investigated the association between HPV/STI coinfection and cervical dysplasia among women in Saudi Arabia. The results illustrated that 27% of the 351 samples were positive for STIs. Among the HPV-positive samples, 25% were also positive for STIs, with U. urealyticum/U. parvum (13%) and M. hominis (6%) being the most common pathogens. A significant correlation was found between HPV and STIs with the progression of abnormal cervical cytology (χ2 = 176, P < 0.0001). Additionally, the associations of the cervical cytology diagnosis with the HPV status, STI type (opportunistic and pathogenic), and presence of Ureaplasma spp. and M. hominis were statistically significant (P < 0.05) [37].
Another prevalent STI is syphilis, a sexually and vertically transmitted infection caused by the bacterium T. pallidum. Its prevalence remains high in low- and middle-income countries, and its incidence has increased in high-income countries in recent decades, particularly among men who have sex with men. This infection is a significant contributor to adverse pregnancy outcomes in resource-limited settings. The clinical presentation of syphilis includes a primary chance at the point of inoculation, followed weeks later by the characteristic rash of secondary syphilis. This can be followed by a latent period, and in some cases, involvement of the eyes, central nervous system, and cardiovascular system. Diagnosis is made through serological testing [38]. Control strategies involve routine screening and treatment of all pregnant women, as well as targeted interventions for high risk populations; however, not all countries report their findings. In the MENA region, limited data are shared. One study aimed to determine the prevalence of T. pallidum and HIV infections among expatriate workers undergoing mandatory pre-employment testing in Sharjah, UAE. The study population consisted of 20,670 expatriate workers of both sexes. Serological testing was conducted to detect specific antibodies against T. pallidum and HIV antigens and antibodies. The results indicated that 105 (0.51%) of the samples were positive for syphilis. The highest infection rates were observed among expatriates from India (30.5%), Pakistan (25.7%), and Bangladesh (15.2%). Three age groups were most affected by syphilis, and a significant correlation was found between age and T. pallidum infection. Additionally, an association was observed between sex and T. pallidum infection [39]. Of the 20,670 samples screened for HIV, 3 (0.014%) were positive for HIV antibodies and antigen, and infection was subsequently confirmed by Western blotting. This study provided the first-ever data on the prevalence of T. pallidum and HIV infections among expatriates in Sharjah, UAE. The findings can inform policymakers in developing appropriate prevention and control strategies for these STIs. In another meta-analysis, the prevalence of syphilis and chlamydia among FSWs in the MENA region was comparable to global levels. However, the prevalence of N. gonorrhoeae and Trichomonas vaginalis tended to be on the lower end of the global range [40]. Although the risk environment among FSWs in the MENA region might be less conducive to STI transmission compared with other regions, the prevalence levels of STIs in this population remain substantial. This might be attributable to poor access to healthcare and prevention interventions, as well as the absence of enabling environments for this vulnerable group, in a context of criminalization and stigma.
Limitations
Our study on sexually transmitted infections (STIs) in the MENA region has several limitations that must be acknowledged. One significant limitation is the lack of national screening programs and sexual health clinics, which may lead to selection bias, as the data primarily reflects individuals who sought care at healthcare facilities. This may not accurately represent the broader population, particularly those who are asymptomatic or avoid seeking medical attention due to stigma or cultural barriers. Additionally, social desirability bias may affect self-reported data, causing participants to underreport risky behaviors or symptoms associated with STIs. The diverse cultural and social norms across the MENA region can also influence STI prevalence and reporting. Furthermore, the study highlights challenges in STI diagnosis, noting that molecular and culture assays offer higher accuracy than serological methods, which may lead to underestimation of true infection rates and complicate public health efforts. An example of diagnostic challenges associated with Ureaplasma spp. and Mycoplasma hominis further complicate their classification as pathogens. Traditional culture methods may fail to detect them due to their fastidious nature, leading to reliance on molecular techniques such as PCR for accurate identification. This shift in diagnostic approach underscores the necessity for a nuanced understanding of these organisms’ roles in human health and disease. Also, the high cost of laboratory testing in some regions further complicates this issue, as many individuals may lack access to necessary diagnostic services. Additionally, there is a lack of robust reporting systems for STIs in many countries within the region. In conclusion, while our study provides valuable insights into the prevalence of STIs in the MENA region, these limitations highlight the need for improved surveillance systems and culturally sensitive interventions to accurately assess and address the burden of STIs across diverse populations.
Conclusions
STIs have been a persistent challenge for humanity, affecting individuals and communities globally for centuries. These infections, caused by a diverse array of pathogens, often lead to severe symptoms and significant morbidity, including cancers, infertility, and chronic pelvic pain. Socioeconomic factors and evolving behavioral trends have significantly influenced the prevalence of STIs globally. Although antimicrobial treatments have offered some relief, the growing threat of AMR, particularly in N. gonorrhoeae, M. genitalium, T. pallidum, and Chlamydia trachomatis (chlamydia) infections, as well as antiviral drug resistance in HIV and HSV infections, poses a grave public health concern, especially in low- and middle-income countries.
Our meta-analysis addressed critical gaps in the MENA region, underscoring the urgent need for a comprehensive, multipronged approach to effectively combat the challenges posed by STIs. A key priority is addressing the pervasive stigma and discrimination associated with STIs, which is vital to ensuring equitable access to healthcare services for all populations. This is particularly crucial because complex social, cultural, and religious factors have significantly affected the diagnosis, treatment, and prevention of STIs in the region. To address these multifaceted challenges, the analysis emphasizes the importance of strengthening STI prevention efforts through enhanced screening and counseling while simultaneously monitoring post-exposure prophylaxis. Accelerating vaccine development and improving diagnostic and predictive tools are crucial to enable timely and appropriate treatment decisions. Simultaneously, prioritizing new antimicrobial research, repurposing existing medications, and implementing holistic treatment strategies are essential for maintaining the treatability of these infections.
It is worth mentioning that several sexually transmitted infections (STIs) are vaccine-preventable, including Human Papillomavirus (HPV), Hepatitis A, and Hepatitis B. The HPV vaccine protects against HPV types that can lead to cervical cancer and genital warts, effectively reducing the incidence of these conditions. Hepatitis A, which can be transmitted through oral-fecal contact during sexual activity, is also preventable through vaccination. Additionally, the Hepatitis B vaccine protects against a virus that can be transmitted through infected body fluids and may lead to chronic liver disease and cancer. These vaccines are essential components of sexual health strategies, significantly reducing the burden of these infections and their associated complications.
This comprehensive approach holds the promise for improving overall sexual and reproductive health outcomes in the MENA region. By embracing a rights-based, people-centered strategy, policymakers, healthcare providers, and public health stakeholders can work collaboratively to address the unique challenges faced in the MENA region. Through concerted efforts, we can reduce the global burden of these communicable diseases and safeguard the health and well-being of individuals and communities worldwide.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We gratefully acknowledge the support of the Scientific Information Office, led by Dr. Khaled S. Alhadyan, at King Faisal Specialist Hospital and Research Centre for the editing service.
Abbreviations
- AMR
Antimicrobial resistance
- BV
Bacterial vaginosis
- CI
Confidence interval
- FSW
Female sex workers
- GCC
Gulf Cooperation Council
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HIV
Human immunodeficiency virus
- HSV
Herpes simplex virus
- MENA
Middle East and North Africa
- MeSH
Medical Subject Headings
- MJ
McKenzie JE
- NG
Neisseria gonorrhoeae
- NGU
Nongonococcal urethritis
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PWID
People who inject drugs
- STI
Sexually transmitted infections
- TV
Trichomonas vaginalis
- WHO
World Health Organization
Author contributions
D.O.: Designed the study, conducted data analysis, wrote the initial draft, and finalized the manuscript. F.A.: Collected data and reviewed relevant articles. R.A.: Collected data and reviewed relevant articles. A.A.: Provided intellectual input and reviewed the manuscript. W.K.: Reviewed the manuscript and provided clinical insights. M.A.: Contributed to the study design, reviewed the manuscript, and assisted with editing. M.S.A: Collected data. M.M.A.: Collected data. L.A.: Collected data. F.A.: Conceptualized the project, wrote the manuscript, and supervised the entire project.
Funding
This work is supported by King Faisal Specialist Hospital and Research Centre.
Data availability
Data available upon reasonable request from the authors.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Sexually transmitted infections (STIs). 2024.
- 2.WHO. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention [Internet]. 2nd edition. 2nd edition. Geneva: World Health Organization. 2021. [PubMed]
- 3.IRAC/WHO. Incidence and Mortality in Female Cancers. 2021.
- 4.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 5.WHO. Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022–2030. 2024.
- 6.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viechtbauer W. Conducting Meta-analyses in R with the metafor Package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 8.Song F, et al. Extent of publication bias in different categories of research cohorts: a meta-analysis of empirical studies. BMC Med Res Methodol. 2009;9(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker TH, et al. Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Med Res Methodol. 2021;21(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obeid DA, et al. Human papillomavirus epidemiology in populations with normal or abnormal cervical cytology or cervical cancer in the Middle East and North Africa: a systematic review and meta-analysis. J Infect Public Health. 2020;13(9):1304–13. [DOI] [PubMed] [Google Scholar]
- 11.Albero G, et al. Male circumcision and genital human papillomavirus: a systematic review and meta-analysis. Sex Transm Dis. 2012;39(2):104–13. [DOI] [PubMed] [Google Scholar]
- 12.Asiri SS, Obeid DA, Alhamlan FS. Human Papillomavirus Associated with Head and Neck Cancer in the Middle East and North Africa: a systematic review and Meta-analysis. 2020. 3(3): pp. 170–81.
- 13.Bussu F, et al. Low prevalence of HPV related Oropharyngeal Carcinogenesis in Northern Sardinia. Cancers. 2022;14. 10.3390/cancers14174205 [DOI] [PMC free article] [PubMed]
- 14.Muresu N et al. Cervical screening in North Sardinia (Italy): genotype distribution and prevalence of HPV among women with ASC-US Cytology. Int J Environ Res Public Health, 2022;19(2). [DOI] [PMC free article] [PubMed]
- 15.Alhamlan FS, et al. Human papillomaviruses: the cervical cancer saga in developing countries. J Infect Dev Ctries. 2017;11(11):819–25. [DOI] [PubMed] [Google Scholar]
- 16.Mekuria SF, et al. HPV self-sampling versus healthcare provider collection on the effect of cervical cancer screening uptake and costs in LMIC: a systematic review and meta-analysis. Syst Rev. 2023;12(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sechi I et al. Comparison of different self-sampling devices for Molecular Detection of Human Papillomavirus (HPV) and other sexually transmitted infections (STIs): a pilot study. 2022. 10(3): p. 459. [DOI] [PMC free article] [PubMed]
- 18.Fernandes Q et al. Human papillomaviruses-related cancers: an update on the Presence and Prevention Strategies in the Middle East and North African regions. Pathogens, 2022. 11(11). [DOI] [PMC free article] [PubMed]
- 19.WHO. Chlamydia. 2023.
- 20.Spiliopoulou A, et al. Chlamydia trachomatis: time for screening? Clin Microbiol Infect. 2005;11(9):687–9. [DOI] [PubMed] [Google Scholar]
- 21.Hocking JS, Geisler WM, Kong FYS. Update on the Epidemiology, Screening, and management of Chlamydia trachomatis infection. Infect Dis Clin North Am. 2023;37(2):267–88. [DOI] [PubMed] [Google Scholar]
- 22.Force USPST, et al. Screening for Chlamydia and Gonorrhea: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326(10):949–56. [DOI] [PubMed] [Google Scholar]
- 23.WHO. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021: accountability for the global health sector strategies 2016–2021: actions for impact. Geneva. 2021.
- 24.Mahmud S, et al. The status of hepatitis C virus infection among people who inject drugs in the Middle East and North Africa. Addiction. 2020;115(7):1244–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.UNAIDS. The Joint United Nations Programme on HIV/AIDS. Global AIDS update 2018. UNAIDS. Geneva 2018.
- 26.Abu-Raddad LJ, et al. Epidemiology of HIV infection in the Middle East and North Africa. AIDS. 2010;24(Suppl 2):S5–23. [DOI] [PubMed] [Google Scholar]
- 27.Chemaitelly H, et al. HIV epidemiology among female sex workers and their clients in the Middle East and North Africa: systematic review, meta-analyses, and meta-regressions. BMC Med. 2019;17(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Connolly KL, et al. Summary of the Centers for Disease Control and Prevention/National Institute of Allergy and Infectious Diseases Joint Workshop on genital herpes: 3–4 November 2022. Open Forum Infect Dis. 2024;11(5):ofae230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu F, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296(8):964–73. [DOI] [PubMed] [Google Scholar]
- 30.Bernstein DI, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis. 2013;56(3):344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nasrallah GK, et al. Estimating seroprevalence of herpes simplex virus type 1 among different Middle East and North African male populations residing in Qatar. J Med Virol. 2018;90(1):184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Looker KJ, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis. 2017;17(12):1303–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dargham SR, et al. Herpes Simplex Virus Type 2 seroprevalence among different national populations of Middle East and North African men. Sex Transm Dis. 2018;45(7):482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kao AS, et al. The epidemiology of candidemia in two United States cities: results of a population-based active surveillance. Clin Infect Dis. 1999;29(5):1164–70. [DOI] [PubMed] [Google Scholar]
- 35.Etienne M, Caron F. Management of fungal urinary tract infections]. Presse Med. 2007;36(12 Pt 3):1899–906. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y, et al. Trends of fluoroquinolones resistance in Mycoplasma and Ureaplasma urogenital isolates: systematic review and meta-analysis. J Glob Antimicrob Resist. 2024;36:13–25. [DOI] [PubMed] [Google Scholar]
- 37.Alotaibi HJ, et al. Association of sexually transmitted infections and human papillomavirus co-infection with abnormal cervical cytology among women in Saudi Arabia. Saudi J Biol Sci. 2020;27(6):1587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peeling RW, et al. Syphilis Lancet. 2023;402(10398):336–46. [DOI] [PubMed] [Google Scholar]
- 39.Eihab A, Abdel Aziz MA, Adam D, Abakar, Bakri YM, Nour. Ra’ed AbuOdeh, Ali ElBakri, Prevalence of Syphilis and Human Immunodeficiency Virus in expatriates in Sharjah, United Arab Emirates. Trop Biomed. 2016;33(4):613–8. [PubMed] [Google Scholar]
- 40.Chemaitelly H, et al. Epidemiology of Treponema pallidum, Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas Vaginalis, and herpes simplex virus type 2 among female sex workers in the Middle East and North Africa: systematic review and meta-analytics. J Glob Health. 2019;9(2):020408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available upon reasonable request from the authors.