Abstract
Background
The presence of API2/MALT1 fusion in gastric mucosa-associated lymphoid tissue (MALT) lymphoma predicts poor response to Helicobacter pylori (Hp) eradication therapy. This study aimed to assess the correlation between endoscopic morphology of MALT lymphoma and API2/MALT1 fusion and evaluate treatment response to Hp eradication based on morphological subtypes.
Methods
A retrospective review was conducted on patients diagnosed with gastric MALT lymphoma between January 2011 and December 2022. Endoscopic morphology was categorized as superficial, non-superficial, or mixed type. The superficial type was further classified into gastritis superficial lesion and localized superficial lesion based on border clarity. Logistic regression models evaluated the impact of clinical and endoscopic characteristics on anti-Hp therapy effectiveness.
Results
Among the 114 patients included, 93 (81.6%) were Hp-positive, and API2/MALT1 fusion was detected in 58 (50.9%) cases, The superficial type was the predominate morphology (73/114, 64%). The regular arrangement of collecting venules (RAC) sign was noted in 21 (18.4%) cases. In superficial subtypes, the RAC signs were more frequently observed in localized lesion than gastritis lesion (35.6% vs. 7.1%, p = 0.01). and the superficial localized lesion was more common in individuals with positive API/MALT1 fusion than negative ones (76.9% vs. 44.1%, p = 0.01). Following Hp eradication, the remission rate for localized lesion was 34.3%, significantly lower than for gastritis lesion (66.7%, p = 0.01). Both endoscopic morphology (OR = 0.26, 95% CI 0.09–0.75) and API2-MALT1 fusion (OR = 14.29, 95% CI 4.19–48.67) impacted the efficacy of anti-Hp therapy. However, multivariate analysis identified API2-MALT1 fusion as the only independent predictor of treatment outcome (OR = 12.18, 95% CI 3.49–42.55, p < 0.001).
Conclusion
Gastric MALT lymphomas with superficial-type morphology, particularly those with defined borders resembling early gastric cancer, were associated with API2/MALT1 fusion and a lower remission rate after Hp eradication therapy. This suggests that endoscopic morphology, along with API2/MALT1 fusion status, could help predict the therapeutic response, with API2/MALT1 fusion serving as a critical indicator of treatment resistance.
Keywords: MALT, API2/MALT1 fusion, Helicobacter pylori, Endoscopy
Background
The incidence of primary gastric lymphomas has risen in recent years, with mucosa-associated lymphoid tissue (MALT) lymphoma being the most prevalent, accounting for approximately 60–75% of all extranodal MALT lymphomas. It is also the second most common gastric malignancy after gastric adenocarcinoma, representing about 5% of malignant gastric tumors [1]. Gastric MALT lymphoma is present in Helicobacter pylori (Hp) infection in 70-90% of cases, which shows a high remission rate (60–80%) following eradication therapy [2, 3]. However, its effectiveness significantly diminishes in cases without Hp infection. The abnormality of the API2/MALT1 fusion is considered one of the primary causes of Hp-negative MALT lymphoma, making it crucial for treatment decision-making [4, 5]. Radiotherapy is main as the optimal treatment for gastric MALT lymphoma with API2/MALT1 fusion instead of Hp eradication therapy [4, 6]. A recent study demonstrated a correlation between the endoscopic morphology of gastric MALT lymphoma and Hp infection status, with Hp-negative MALT lesions presenting as smaller, undifferentiated, discolored, and depressed lesions compared to Hp-positive cases [7]. However, there remains a gap in the literature regarding the relationship between endoscopic morphology and API2/MALT1 fusion status. Therefore, this study aims to explore the correlation between endoscopic features of MALT lymphomas and the status of API2/MALT1 fusion, and to analyze the prognosis of MALT lymphomas with different endoscopic morphologies after anti-Hp therapy.
Method
The study retrospectively reviewed patients who underwent gastroscopy and were diagnosed with gastric MALT lymphoma from January 2011 to December 2022 at West China Hospital of Sichuan University. Exclusion criteria included: (1) incomplete endoscopic images; (2) absence of Hp infection status at the time of diagnosis; (3) patients who were followed up without further treatment (watch and wait strategy) (4) absence of API2-MALT1 gene test. Data collected from the enrolled patients included Hp infection status, Lugano stage, endoscopic features, pathologic diagnosis, and treatment evaluation after anti-Hp therapy. Baseline information included age, sex, and clinical symptoms at diagnosis. This research protocol was approved by the Ethics Committee of the West China Hospital of Sichuan University (2023 − 1480, Aug.22, 2023).
Hp infection status was confirmed by either urease breath test (UBT) or histology. Lugano staging is based on radiologic findings, including contrast-enhanced computed tomography (CT) or Positron Emission Tomography-computed tomography (PET-CT) scans. Stage I lesions are confined to the gastrointestinal tract; Stage II lesions spread to abdominal lymph nodes and are further subdivided into perigastric (Stage II1) or distant lymph node involvement (Stage II2); Stage IIE lesions penetrate the serosa, involving adjacent tissues or organs; Stage IV lesions involve diffuse extranodal organs and supradiaphragmatic lymph nodes. Stage I and II1 lesions are recommended for Hp eradication as the first-line treatment, defined as localized stage, while Stage II2, IIE, and IV lesions are more extensive, defined as advanced stage.
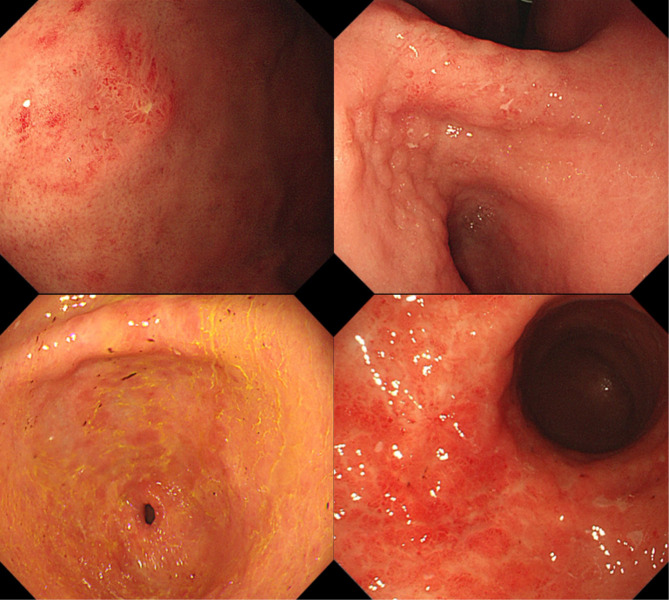
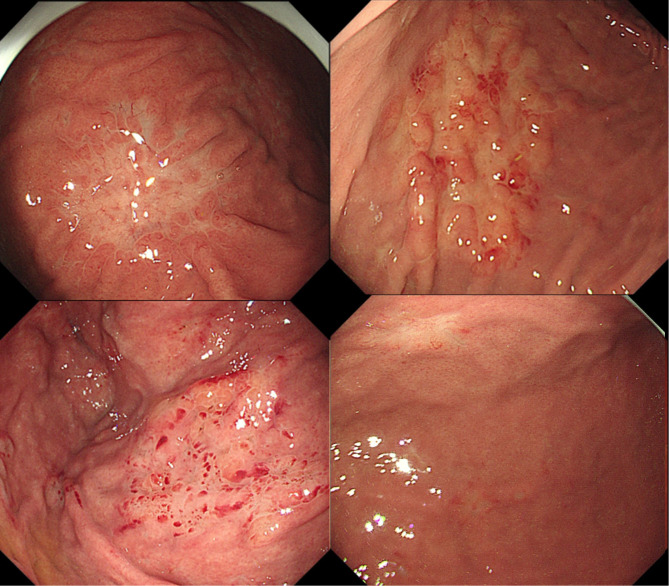
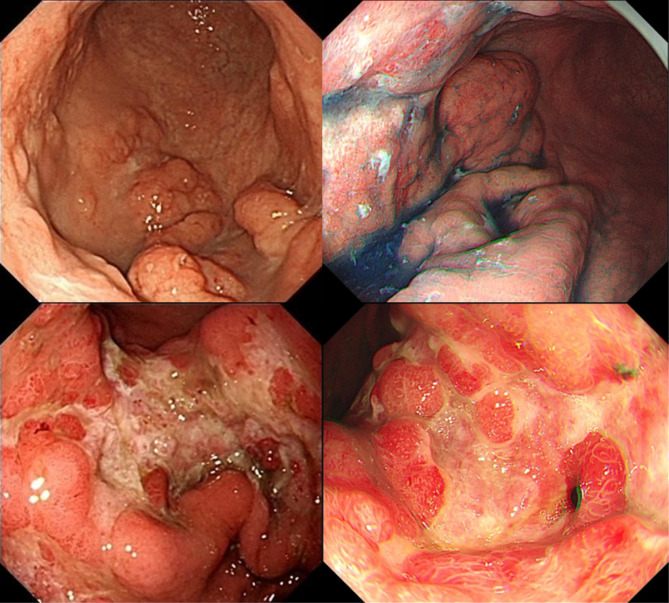
Endoscopic features were re-evaluated by two experienced endoscopists based on the original endoscopic images, with a third endoscopist added for decision-making in cases of disagreement. Recorded features included the presence of extensive atrophic gastritis, regular arrangement of collecting venules (RAC) sign, lesion site, number of lesions, and endoscopic morphology of the lesion. RAC refers to the regular arrangement of collecting venules in the lower portion of the gastric body glands and gastric horns. The site of the lesion was documented by dividing the stomach into three parts: upper, middle, and lower; involvement of more than two sites was recorded as multiple sites. Endoscopic morphology was categorized into superficial, non-superficial, and mixed types. Superficial types were defined as lesions confined to the mucosa or submucosa, further subdivided into gastritis superficial lesions and localized superficial lesions based on the clarity of borders and obvious inflammation of surrounding gastric mucosa. Gastritis superficial lesions exhibit mucosal congestion, redness, swelling, erosion, and hemorrhagic spots, similar to gastritis, making it difficult to recognize clear borders due to inflammation (Fig. 1). Localized superficial lesions exhibit manifestations similar to early gastric cancer with identifiable borders under white light endoscopy, almost discolored, or discolored predominantly (Fig. 2). Non-superficial types include: (1) ulcerated lesions, single or multiple, similar to Bormann type 2 or 3; (2) protruding lesions or folds thickening without surface ulceration (Fig. 3). Mixed lesions could not be singularly categorized into the above single subtypes.
Fig. 1.
Endoscopic feature of gastric MALT lymphomas presents as gastritis superficial lesion
Fig. 2.
Endoscopic feature of gastric MALT lymphomas presents as localized superficial lesion
Fig. 3.
Endoscopic features of gastric MALT lymphomas present as non-superficial lesions (ulcerated lesions and protruding lesions or folds thickening type)
Pathologic diagnosis was based on the Wotherspoon scoring system, graded according to the depth of infiltration of centrocyte-like and monocyte-like cells, plasma cell differentiation, and the formation of lymphoepithelial lesions (LELs), with cases scoring 4 or 5 diagnosed as MALT lymphoma. Initial pathological diagnosis and post-treatment pathological evaluation were performed by a pathologist specializing in lymphoma, documenting API2/MALT1 fusion status, IgH/IgK gene rearrangement, and Ki-67 proliferation index. The treatment response to anti-Hp therapy was evaluated based on endoscopic manifestations or combined with radiologic images. According to the consensus of the European Gastrointestinal Lymphoma Group for Gastric MALT Lymphoma in 2011, Complete remission (CR) is defined as no visible lesions during endoscopy and no lymphoma in biopsy tissue. Partial remission (PR) indicates endoscopic lesion shrinkage or disappearance and histologic lesion regression. Stable disease (SD) shows no evident endoscopic or histologic changes. Progressive disease (PD) shows endoscopic lesion enlargement, imaging spread, or histologic transformation to diffuse large B-cell lymphoma. Responders were defined as patients assessed as CR or PR, while non-responders were those classified as SD or PD.
Statistics.
Statistical analysis was performed using SPSS version 22.0. Categorical variables were expressed as frequencies, and continuous variables as mean ± standard deviation. The χ² test or Fisher’s exact test was used for categorical variables, and the Student’s t-test was used for continuous variables. The relationship between clinicopathologic factors and the effectiveness of anti-Hp therapy was assessed using logistic regression models. A multifactorial analytical model was developed using the Backward Wald method to avoid the influence of possible interacting factors on the effect of anti-Hp treatment. The prognostic power of covariates was documented by odds ratios (OR) and 95% confidence intervals (CI). A p-value < 0.05 was considered statistically significant.
Results
Clinicopathologic features of MALT
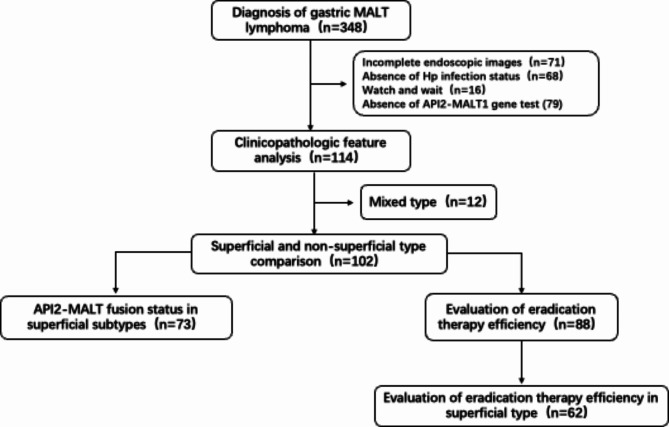
A total of 348 patients were diagnosed with gastric MALT lymphoma, and 114 patients were finally included in the analysis after applying exclusion criteria (Fig. 4). The mean follow-up duration was 641 ± 673 days, with a median follow-up time of 439 days. The duration between anti-Hp treatment and response assessment was 429 ± 581 days. The male to female ratio was 1.43:1. Most patients underwent esophagogastroduodenoscopy for non-specific gastrointestinal discomfort, with other manifestations including gastrointestinal bleeding in 19 cases and fever with weight loss in 1 case (Table 1). There were 93 (81.6%) cases coinfected with Hp. Lugano staging showed that72.8% (83 cases) were in the localized stage (stage I + II1). The API2/MALT1 fusion was detected in 58 (50.9%) of all included patients. As evaluation of endoscopic features, 73 cases (64%) presented with superficial type, which were the predominant type; 30 cases were identified with non-atrophic background mucosa, and 21 cases showed RAC sign. Lesions were mainly located in the middle third of the stomach, accounting for 46.5%. Histopathological, the Ki-67 index exceeded 5% in 67.5% (77/114) of patients; 92.1% (105 cases) were positive for IgH gene rearrangement, and 86.8% (99 cases) were positive for IgK gene rearrangement.
Fig. 4.
Flow chart of the study
Table 1.
Clinical characteristics of included gastric MALT patents
| Clinical features | No. of patients (n = 114) | |
|---|---|---|
| Age | 52.66 ± 14.48 | |
| Sex | Male | 67(58.8%) |
| Female | 47(41.2%) | |
| Clinical Manifestations | Asymptomatic | 9(7.9%) |
| GERD | 2(1.8%) | |
| Abdominal discomfort | 56(49%) | |
| Gastrointestinal bleeding | 19 (16.7%) | |
| Fever with wight loss | 1 (0.9%) | |
| Unknow | 27(23.7%) | |
| Hp infection | Presence | 93(81.6%) |
| Absence | 21(18.4%) | |
| Tumor location | Upper | 10(8.8%) |
| Middle | 53(46.5%) | |
| Lower | 24(21.1%) | |
| More than 2 parts | 27(23.7%) | |
| Endoscopic morphology | Superficial type | 73(64%) |
| Non-superficial type | 29(25.4%) | |
| Mixed type | 12(10.5%) | |
| Lugano stage | I | 64(56.1%) |
| II 1 | 19(16.7%) | |
| II 2 | 3(2.6%) | |
| II E | 4(3.5%) | |
| IV | 17(14. 9%) | |
| Unknow | 7(6.1%) | |
| API2/MALT1 fusion | Yes | 58(50.9%) |
| No | 56(49.1%) | |
| Extensive atrophic gastritis | Yes | 30(26.3%) |
| No | 54 (47.4%) | |
| Unknow | 30 (26.3%) | |
| RAC sign | Presence | 21 (18.4%) |
| Absence | 83 (72.8%) | |
| Unknown | 10 (8.8%) | |
| Number of lesions | Single | 49 (43%) |
| Multiple | 65 (57%) | |
| Ki-67 proliferation | < 5% | 37 (32.5%) |
| ≥ 5% | 77 (67.5%) | |
| Ig H rearrangement | Positive | 105 (92.1%) |
| Negative | 7 (6.1%) | |
| Unknow | 2(1.8%) | |
| Ig K rearrangement | Positive | 99 (86.8%) |
| Negative | 12(10.5%) | |
| Unknow | 3(2.6%) | |
Superficial and non-superficial type comparison
Excluding patients with mixed-type morphology, clinical, endoscopic, and pathological features of 102 patients were compared between superficial and non-superficial types (Table 2). The RAC sign is more common in superficial type than non-superficial type (24.7% vs. 3.4%, p = 0.03); more than half of the superficial type occurred in the middle third of the stomach compared to non-superficial type (53.4% vs. 37.9%, p = 0.02). No significant differences were observed in age, gender, Lugano staging, atrophic background mucosa, numbers of lesion and Ki-67 level between the two groups.
Table 2.
Features of superficial and non-superficial types
| Feature | Non-Superficial (n = 29) | Superficial (n = 73) | p-Value |
|---|---|---|---|
| Age | 48.83 ± 17.95 | 53.66 ± 13.56 | 0.198 |
| Gender | 12 (41.4%) | 31 (42.5%) | 1.00 |
| Female | 12 (41.4%) | 31 (42.5%) | |
| Male | 17 (58.6%) | 42 (57.5%) | |
| Hp Infection | |||
| Presence | 27 (93.1%) | 58 (79.5%) | 0.14 |
| Absence | 2 (6.9%) | 15 (20.5%) | |
| Lugano Stage | |||
| Localized stage (I, II1) | 20 (69.0%) | 54 (74.0%) | 0.80 |
| Advanced stage (II2-IV) | 7 (24.1%) | 15 (20.5%) | |
| Unknown | 2(6.9%) | 4(5.5%) | |
| API2-MALT1 fusion | |||
| Positive | 12 (41.4%) | 39 (53.4%) | 0.38 |
| Negative | 17 (58.6%) | 34 (46.6%) | |
| Extensive atrophic gastritis | |||
| Yes | 7 (24.1%) | 17 (23.3%) | 0.34 |
| No | 11 (37.9%) | 38 (52.1%) | |
| Unknown | 11(37.9%) | 18(24.7%) | |
| RAC sign | |||
| Presence | 1 (3.4%) | 18 (24.7%) | 0.03 |
| Absence | 24 (82.9%) | 49 (67.1%) | |
| Lesion Location | |||
| Upper | 0 (0%) | 10 (23.7%) | 0.02 |
| Middle | 11 (37.9%) | 39 (53.4%) | |
| Lower | 8 (27.6%) | 12 (16.4%) | |
| More than two | 10(34.5%) | 12(16.4%) | |
| Number of lesions | |||
| Single | 13 (44.8%) | 35 (47.9%) | 0.83 |
| Multiple | 16 (55.2%) | 38 (52.1%) | |
| Ki-67 proliferation | |||
| < 5% | 6 (20.7%) | 27 (37.0%) | 0.16 |
| ≥ 5% | 23 (79.3%) | 46 (63.0%) | |
| Ig H rearrangement | |||
| Presence | 2(6.9%) | 4(5.5%) | 0.77 |
| Absence | 26(89.7%) | 68(93.2%) | |
| Unknown | 1(3.4%) | 1(1.4%) | |
| Ig K rearrangement | 0.28 | ||
| Presence | 27(93.1%) | 64(87.7%) | |
| Absence | 1(3.4%) | 7(9.6%) | |
| Unknown | 1(3.4%) | 2(2.7%) |
API2-MALT1 fusion status in superficial subtypes
Among 73 cases with complete data on superficial type, 28 were classified as gastritis superficial lesion and 45 as localized lesion. The gastritis lesion had a higher likelihood of Hp infection than localized lesion (26/28 vs. 32/45, 92.9% vs. 71.1%, p = 0.04). RAC signs were more frequently observed in localized lesion with clear borders than gastritis lesion. (16/45 vs. 2/28, 35.6% vs. 7.1%, p = 0.01). The superficial localized morphology was more likely seen in individuals with positive API/MALT1 fusion than negative ones (76.9% vs. 44.1%, p = 0.01). No significant differences were found in age, gender, Hp infection status, Lugano staging, atrophic background mucosa, RAC sign, lesion location, numbers of lesion, Ki-67 level, or IgH/IgK gene rearrangement between the subtypes (Table 3).
Table 3.
Correlation of API2-MALT1 fusion and superficial morphology subtypes
| Feature | API2-MALT1 fusion positive (n = 39) | API2-MALT1 fusion Negative (n = 34) | p-Value |
|---|---|---|---|
| Age | 55.85 ± 13.16 | 51.15 ± 13.77 | 0.14 |
| Gender | |||
| Female | 15 (38.5%) | 16 (47.1%) | 0.47 |
| Male | 24 (61.5%) | 18 (52.9%) | |
| Hp Infection | |||
| Presence | 28 (71.8%) | 30 (88.2%) | 0.15 |
| Absence | 11 (28.2%) | 4 (11.8%) | |
| Lugano Stage | |||
| Localized stage (I, II1) | 27 (69.2%) | 27 (79.4%) | 0.16 |
| Advanced stage (II2-IV) | 11 (28.2%) | 4 (11.8%) | |
| Unknown | 1(2.6%) | 3(8.8%) | |
| Endoscopic Superficial Morphology | |||
| Gastritis lesions | 9 (23.1%) | 19 (55.9%) | 0.01 |
| Localized lesions | 30 (76.9%) | 15 (44.1%) | |
| Extensive atrophic gastritis | |||
| Yes | 8 (20.5%) | 9 (26.5%) | 0.86 |
| No | 21 (53.8%) | 17 (50%) | |
| Unknown | 10(25.6%) | 8(23.5%) | |
| RAC sign | |||
| Presence | 9 (23.1%) | 9 (26.5%) | 0.93 |
| Absence | 27 (69.2%) | 22 (64.7%) | |
| Unknown | 3(7.7%) | 3(8.8%) | |
| Lesion Location | |||
| Upper | 6 (15.4%) | 4 (11.8%) | 0.20 |
| Middle | 23 (59%) | 16 (47.1%) | |
| Lower | 3 (7.7%) | 9 (26.5%) | |
| More than two | 7(17.9%) | 5(14.7%) | |
| Number of lesions | |||
| Single | 20 (51.3%) | 15 (44.1%) | 0.64 |
| Multiple | 19 (48.7%) | 19 (55.9%) | |
| Ki-67 proliferation | |||
| < 5% | 14 (35.9%) | 13 (38.2%) | 1.00 |
| ≥ 5% | 25 (64.1%) | 21 (61.8%) | |
| Ig H rearrangement | |||
| Presence | 36(92.3%) | 32(94.1%) | 0.47 |
| Absence | 3(7.7%) | 1(2.9%) | |
| Unknown | 0(0%) | 1(2.9%) | |
| Ig K rearrangement | |||
| Presence | 34(87.2%) | 30(88.2%) | 0.58 |
| Absence | 5(12.8%) | 3(8.8%) | |
| Unknown | 0(0%) | 1(2.9%) |
Evaluation of eradication therapy efficiency
In the analysis of Hp eradication treatment outcomes, excluding patients with unknown Hp treatment history, and unknown Lugano staging, a total of 88 patients were included. Among Hp-infected patients, those patients with API2-MALT1 fusion had a significantly lower remission rate after anti-Hp therapy compared to those without (21.1% vs. 75.0%, p < 0.001) (Table 4). Among Hp-negative patients, none of the three API2-MALT1 fusion patients achieved remission after Hp eradication therapy, all presenting endoscopic morphology with localized superficial lesions. Additional, of the three Hp-positive cases but without API2-MALT1 fusion, only one achieved remission, presenting with non-superficial type that showed partial regression after Hp treatment but received additional radiotherapy thereafter.
Table 4.
Evaluation of Hp eradication therapy efficiency
| Hp infection | API2-MALT1 fusion | Numbers | Responders | Non-Responders | p-Value |
|---|---|---|---|---|---|
| Presence | Positive | 38 | 8 (21.1%) | 30 (78.9%) | < 0.001 |
| Negative | 44 | 33 (75.0%) | 11 (25.0%) | ||
| Absence | Positive | 3 | 0 (0%) | 3 (100%) | 1.00 |
| Negative | 3 | 1 (33.3%) | 2 (66.7%) |
Among the 88 patients who received anti-Hp therapy, 62 cases (70.5%) presented superficial type at diagnosis. Binary logistic regression analysis was used to assess factors potentially impacting the effectiveness of anti-Hp therapy, both in univariate and multivariate models. The analysis revealed that API2-MALT1 fusion was the only independent risk factor significantly affecting the outcome of anti-Hp therapy (OR = 8.89, 95% CI 3.13–25.28, p < 0.001) .
In the superficial type, 35 (56.5%) were localized lesion, with a 50.0% (31/62 cases) API2-MALT1 fusion rate. The localized lesion had a remission rate of only 34.3% (12/35 cases) after anti-Hp therapy, while gastritis lesion had a remission rate of 66.7% (18/27 cases), showing a statistically significant difference (34.3% vs. 66.7%, p = 0.01). Further analyses were conducted on patients with superficial subtypes (27 with gastritis lesion and 35 with localized lesion) using both univariate and multivariate approaches. While the endoscopic morphology (OR = 0.26, 95% CI 0.09–0.75, p = 0.013) and API2-MALT1 fusion (OR = 14.29, 95% CI 4.19–48.67, p < 0.001) were identified as factors affecting the efficacy of anti-Hp therapy. However, multivariate analysis indicated that API2-MALT1 fusion (OR = 12.18, 95% CI 3.49–42.55, p < 0.001) was the only independent risk factor influencing the treatment outcome (Table 5).
Table 5.
Factors affect the response of Hp eradication therapy in superficial subtypes
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |||
| Age | <50 | 0.442 | 0.180–1.085 | 0.075 | NA | NA | NA | |
| 50–70 | ||||||||
| ≥ 70 | ||||||||
| Gender | Female | 1.125 | 0.413–3.062 | 0.818 | NA | NA | NA | |
| Male | ||||||||
| Hp Infection | Presence | 0 | 0 | 0.999 | NA | NA | NA | |
| Absence | ||||||||
| Lugano stage | Localized stage | 3.522 | 0.851–14.571 | 0.082 | NA | NA | NA | |
| Advanced stage | ||||||||
| API2-MALT1 fusion | Positive | 14.286 | 4.193–48.673 | < 0.001 | 12.184 | 3.489–42.549 | < 0.001 | |
| Negative | ||||||||
| Endoscopic superficial morphology | Localized lesion | 0.261 | 0.090–0.754 | 0.013 | 0.382 | 0.108–1.346 | 0.134 | |
| Gastritis lesions | ||||||||
| Extensive atrophic gastritis | Yes | 0.645 | 0.189–2.199 | 0.483 | NA | NA | NA | |
| No | ||||||||
| RAC sign | Presence | 1.750 | 0.215–14.224 | 0.601 | NA | NA | NA | |
| Absence | ||||||||
| Lesion location | Upper | 0.371 | 0.076–1.821 | 0.371 | NA | NA | NA | |
| Middle | ||||||||
| Lower | ||||||||
| More than two | ||||||||
| Number of lesions | Single | 1.482 | 0.544–4.036 | 0.441 | NA | NA | NA | |
| Multiple | ||||||||
| Ki-67 proliferation | <5% | 0.955 | 0.333–2.735 | 0.931 | NA | NA | NA | |
| ≥ 5% | ||||||||
| Ig H rearrangement | Presence | 0.467 | 0.040–5.435 | 0.543 | NA | NA | NA | |
| Absence | ||||||||
| Ig K rearrangement | Presence | 0.208 | 0.022–1.986 | 0.173 | NA | NA | NA | |
| Absence | ||||||||
Discussion
Gastric MALT lymphoma is a common indolent B-cell lymphoma. Regardless of Lugano staging, Hp-infected gastric MALT lymphoma patients should undergo anti-Hp therapy [4]. However, Hp-negative patients and those positive for the API2/MALT1 fusion show poor response to eradication therapy. This study found that endoscopic morphology correlates with API2/MALT1 fusion status, with the most common endoscopic morphology of MALT being superficial lesions. If the superficial lesion discolored area has clear borders, resembling early gastric cancer, it is highly associated with API2/MALT1 fusion, potentially serving as a simpler predictor of response to Hp eradication therapy.
RAC sign is highly specific for diagnosing Hp-uninfected status and is an easily recognizable objective sign under endoscopy [8–11]. Hp-related MALT is associated with chronic Hp infection stimulating lymphocyte proliferation. If endoscopy can identify signs without chronic Hp infection, it suggests that MALT emergence is independent of Hp infection, and eradication therapy is unlikely to be effective. However, this study did not find RAC sign could predict failure of anti-Hp treatment as expected. Data shows that RAC sign is more common in superficial lesions, likely because non-superficial lesions are concentrated in the middle to lower thirds of the stomach, impacting RAC sign recognition. Additionally, the proportion of RAC sign in the superficial subgroup was lower than the proportion of Hp-uninfected status in the same group of patients, possibly due to the retrospective evaluation of the RAC sign have been overlooked. Prospective data is needed to verify this hypothesis regarding RAC sign’s role in predicting eradication efficacy.
Further, in the context of Hp-uninfected status with RAC, gastric MALT more commonly presents as localized superficial lesion, often distinguished from poorly differentiated carcinoma in non-atrophic mucosal background under endoscopy. This study defined such superficial MALT as localized superficial type, characterized by discolored area with identifiable borders and non-inflammatory surrounding mucosa. This type of gastric MALT is more likely to coexist with API2/MALT1 fusion, suggesting poor response to eradication therapy, thereby confirming that detailed endoscopic morphology descriptions can help predict treatment response to Hp eradication. This is not the first study to focus on the endoscopic morphology of gastric MALT lymphoma; previous studies have summarized characteristics of gastric MALT lymphoma under white light endoscopy and proposed various classifications, including slightly abnormal mucosa, congested mucosa, elevated or ulcerated types, etc [7, 12]. However, these classifications are numerous and not clearly related to prognosis, limiting their clinical adoption. This study, through retrospective analysis of endoscopic features in patients with gastric MALT lymphoma undergoing anti-Hp therapy, found a correlation between different MALT morphologies and response to eradication therapy. Previous studies indicated that ulcerative lesions and other non-superficial lesions have poorer response to eradication, but in this study, the difference in eradication response between superficial and non-superficial lesions was not statistically significant, possibly due to the high proportion of API2/MALT1 positivity in superficial lesions, leading to a lower CR rate even if lesions are superficial involved [13, 14]. Unlike previous studies, this study classified superficial type into gastritis-like superficial type and localized superficial type, with the latter showing clearer borders and more RAC sign, requiring differentiation from early poorly differentiated gastric cancer. Although superficial type did not show a higher remission rate compared to non-superficial type, the subtype of localized superficial lesion was associated with non-response to eradication. However, in multivariate analysis, this difference was not statistically significant, primarily due to the impact of API2/MALT1 fusion.
API2/MALT1, located at t(11;18)(q21;q21), is a specific chromosomal abnormality in MALT lymphoma that activates the nuclear factor-kB (NF-kB) pathway. For Hp-infected gastric MALT lymphoma without API2/MALT1 fusion, the remission rate after Hp therapy can reach 76.1%, but for API2/MALT1 fusion patients, the remission rate after Hp therapy is below 20% [4, 13, 15]. Guidelines suggest that gastric MALT lymphoma with API2/MALT1 fusion shows minimal response to Hp eradication and direct radiotherapy can be considered [16]. Given the limited availability of API2/MALT1 testing, particularly in resource-limited settings, our findings suggest that detailed endoscopic descriptions may serve as a useful adjunct in predicting treatment outcomes. Furthermore, the optimal timing for evaluating the efficacy of Hp eradication therapy remains variable, ranging from 3 to 18 months [4, 16]. Therefore, the correlation between endoscopic morphology and post eradication response is of clinical value, with a greater preference for the wait-and-watch strategy in asymptomatic patients of superficial gastritis lesion, waiting for an opportunity of delayed regression after 6 months or even longer.
In an earlier reported MALT morphology study, it was noted that lymphomas may be present in cases where the gastric mucosa appears normal. There is a high probability that these are superficial lesions observed on high-definition endoscopy. Such cases highlight the importance of mapping biopsies during treatment response evaluation. In our study, the inclusion of CR and PR as a combined responder category mitigates the potential overestimation of CR due to inactive biopsy results [17].
This retrospective study has inherent limitations associated with its design, with significant subjectivity in the endoscopic morphology classification posing a primary challenge. The assessment under endoscopy may vary among physicians, which might introduce variability in the interpretation of results. However, the localized superficial type, characterized by well-defined borders resembling early adenocarcinoma is easy to define. Additionally, the RAC sign, initially presumed to have a direct correlation with the response to eradication therapy, did not demonstrate the expected relationship. This discrepancy may stem from the underemphasis of RAC sign assessment during initial diagnoses, possibly leading to inadequate documentation in retrospective analyses. Furthermore, this study did not explore the long-term clinical and survival outcomes associated with watchful waiting, additional radiotherapy, chemotherapy, or surgical interventions. There is also a need to examine the potential for aggressive transformation and the correlation between endoscopic appearances and long-term transformation. This aspect underscores the necessity for longitudinal studies to validate the prognostic value of endoscopic morphology in managing gastric MALT lymphoma, particularly in predicting invasive transformations and guiding long-term treatment strategies.
Conclusion
This study demonstrated that localized superficial gastric MALT lymphoma is strongly associated with API2/MALT1 fusion, suggesting that endoscopic morphology has potential application value in predicting the response of gastric MALT lymphoma to eradication therapy.
Acknowledgements
None.
Abbreviations
- MALT
Mucosa-associated lymphoid tissue
- Hp
Helicobacter pylori
- UBT
Urease breath test
- CT
Computed tomography
- PET-CT
Positron Emission Tomography -computed tomography
- RAC
Regular arrangement of collecting venules
- LELs
Lymphoepithelial lesions
- CR
Complete remission
- PR
Partial remission
- SD
Stable disease
- PD
Progressive disease
Author contributions
Y.B.C. and Y.H.L. wrote the main manuscript text and L.X.Y. and L.Y.Q. collected the data. Y.J.L and W.Z designed the research. All authors reviewed the manuscript.”
Funding
This work is supported by National Key Research and Development Program of China (2022YFC3602101).
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The need for written informed consent to participate was waived by the Ethics Committee of the West China Hospital of Sichuan University (2023 − 1480, Aug.22, 2023) due to retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bing-Can Yang and Hai-Lin Yan contributed equally to this work.
References
- 1.Raderer M, Kiesewetter B, Ferreri AJ. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). CA Cancer J Clin. 2016;66(2):153–71. [DOI] [PubMed] [Google Scholar]
- 2.Ishikawa E, Nakamura M, Satou A, Shimada K, Nakamura S. Mucosa-Associated Lymphoid tissue (MALT) lymphoma in the gastrointestinal tract in the modern era. Cancers (Basel) 2022, 14(2). [DOI] [PMC free article] [PubMed]
- 3.Stolte M, Bayerdorffer E, Morgner A, Alpen B, Wundisch T, Thiede C, Neubauer A. Helicobacter and gastric MALT lymphoma. Gut. 2002;50(Suppl 3):III19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruskone-Fourmestraux A, Fischbach W, Aleman BM, Boot H, Du MQ, Megraud F, Montalban C, Raderer M, Savio A, Wotherspoon A, et al. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut. 2011;60(6):747–58. [DOI] [PubMed] [Google Scholar]
- 5.Dierlamm J, Baens M, Wlodarska I, Stefanova-Ouzounova M, Hernandez JM, Hossfeld DK, De Wolf-Peeters C, Hagemeijer A, Van den Berghe H, Marynen P. The apoptosis inhibitor gene API2 and a novel 18q gene, MLT, are recurrently rearranged in the t(11;18)(q21;q21) associated with mucosa-associated lymphoid tissue lymphomas. Blood. 1999;93(11):3601–9. [PubMed] [Google Scholar]
- 6.Zucca E, Arcaini L, Buske C, Johnson PW, Ponzoni M, Raderer M, Ricardi U, Salar A, Stamatopoulos K, Thieblemont C, et al. Marginal zone lymphomas: ESMO Clinical Practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(1):17–29. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe M, Nonaka K, Kishino M, Nagashima Y, Tokushige K. Endoscopic features of gastric mucosa-Associated Lymphoid tissue lymphoma without Helicobacter pylori. Diagnostics (Basel) 2024, 14(6). [DOI] [PMC free article] [PubMed]
- 8.Anagnostopoulos GK, Yao K, Kaye P, Fogden E, Fortun P, Shonde A, Foley S, Sunil S, Atherton JJ, Hawkey C, et al. High-resolution magnification endoscopy can reliably identify normal gastric mucosa, Helicobacter pylori-associated gastritis, and gastric atrophy. Endoscopy. 2007;39(3):202–7. [DOI] [PubMed] [Google Scholar]
- 9.Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan C, Lin XM, Ou Y, Cai L, Cheng Q, Zhou P, Liao J. Association between regular arrangement of collecting venules and Helicobacter pylori status in routine endoscopy. BMC Gastroenterol. 2021;21(1):389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glover B, Teare J, Ashrafian H, Patel N. The endoscopic predictors of Helicobacter pylori status: a meta-analysis of diagnostic performance. Ther Adv Gastrointest Endosc. 2020;13:2631774520950840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran QT, Nguyen Duy T, Nguyen-Tran BS, Nguyen-Thanh T, Ngo QT, Tran Thi NP, Le V, Dang-Cong T. Endoscopic and Histopathological Characteristics of Gastrointestinal Lymphoma: A Multicentric Study. Diagnostics (Basel) 2023, 13(17). [DOI] [PMC free article] [PubMed]
- 13.Nakamura S, Sugiyama T, Matsumoto T, Iijima K, Ono S, Tajika M, Tari A, Kitadai Y, Matsumoto H, Nagaya T, et al. Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow-up study of 420 patients in Japan. Gut. 2012;61(4):507–13. [DOI] [PubMed] [Google Scholar]
- 14.Zullo A, Hassan C, Cristofari F, Andriani A, De Francesco V, Ierardi E, Tomao S, Stolte M, Morini S, Vaira D. Effects of Helicobacter pylori eradication on early stage gastric mucosa-associated lymphoid tissue lymphoma. Clin Gastroenterol Hepatol. 2010;8(2):105–10. [DOI] [PubMed] [Google Scholar]
- 15.Inagaki H, Nakamura T, Li C, Sugiyama T, Asaka M, Kodaira J, Iwano M, Chiba T, Okazaki K, Kato A, et al. Gastric MALT lymphomas are divided into three groups based on responsiveness to Helicobacter Pylori eradication and detection of API2-MALT1 fusion. Am J Surg Pathol. 2004;28(12):1560–7. [DOI] [PubMed] [Google Scholar]
- 16.Lymphomas B-C. (Version 2.2024) [https://www.nccn.org/guidelines/guidelines]
- 17.Kolve M, Fischbach W, Greiner A, Wilms K. Differences in endoscopic and clinicopathological features of primary and secondary gastric non-hodgkin’s lymphoma. German gastrointestinal lymphoma Study Group. Gastrointest Endosc. 1999;49(3 Pt 1):307–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.