Abstract
Introduction
Autism Spectrum Disorder (ASD) is a disorder that severely affects neurodevelopment, and its underlying causes are not yet entirely understood. Research suggests that there may be a connection between the occurrence of ASD and changes in immune responses. This study aims to know if some biochemical and inflammatory cytokines are promising biomarkers for ASD and whether they are involved in the pathogenesis of ASD.
Methods
The serum levels of CRP, TNF-α, TGF-β, IL-1β, IL-10, 1 L-8, and IL-6 were measured in all of the patients (n = 22) and in the healthy (n = 12) children using ELISA method.
Results
The serum concentrations of IL-10 and IL-8 were significantly lower in the ASD patients compared to the control group (p < 0.05) and there were not significant differences between CRP, TNF-α, TGF-β, IL-6 and IL-1β levels in two groups. There were positive correlations between CRP and IL-10, also CRP and IL-8, in ASD group. In contrast to the ASD patients, the correlations of IL-8, IL-10, and CRP were not significant in the control group.
Conclusion
In conclusion, this study highlights the potential role of certain biochemical markers and inflammatory cytokines in ASD. Specifically, the lower levels of IL-10 and IL-8 in ASD patients, along with the significant correlations between CRP and these cytokines, suggest an altered immune response in individuals with ASD. These findings support the hypothesis that immune dysregulation may be involved in ASD pathogenesis. Further research is needed to explore these biomarkers and their mechanistic links to ASD, which could lead to improved diagnostics or therapeutic strategies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12887-024-05182-3.
Keywords: Autism spectrum disorder, Biochemical marker, Inflammatory network abnormalities, Cytokine
Introduction
Autism spectrum disorder (ASD) is one of the most frequent neurodevelopmental disorders [1]. The current autism prevalence estimates are divergent. Some recent reports of autism prevalence are 0.62–0.70% worldwide and 1–2% according to a large-scale survey, while the incidence of events continues to rise. The epidemiology of autism is not highly affected by geographic region, ethnic/cultural, or socioeconomic factors, but males are affected two-to-five times more often than females. The most important symptoms of ASD include persistent defects in communication, low social interaction, and limited and repetitive patterns of behavior, interests, or activities [2]. Researchers have linked high levels of elevated inflammatory responses to the pathogenesis of ASD, and a dysregulated state of inflammatory responses is often associated with immune system dysfunction. Several types of cells are involved in starting and maintaining these processes. The development, maintenance, and progression of autism depend on essential factors such as neuroinflammation and neuro-immune abnormalities [3]. Due to the complexity of the behavior in autism patients, diagnosing, treating, and understanding of this disorder are challenging. By focusing on identifying specific biological abnormalities in ASD, researchers were able to provide good clues for its diagnosis and treatment. They use biomarkers as an objective method to better identify and study biological abnormalities with the goals of more accurate diagnosis and measurement of treatment-induced changes [4].
Extensive evidence from different studies shows that cytokines can be considered as biomarkers in ASD. Cytokines in the immune system are a group of small proteins that play essential roles in regulating inflammation and neurodevelopment as messengers that modulate the immune system and endocrine glands [5]. The brain can recognize pro-inflammatory cytokines such as IL-1α, IL-1β, TNF-α, and IL-6 as molecular signals of disease. In addition, the cytokines TNF-α, IL-6, and IL-1β cross the blood-brain barrier and act on the hypothalamus, promoting fever and sickness behavior [6]. One of the factors involved in neuron survival and neurodevelopmental functions is transforming growth factor beta (TGF-β), a group of multifunctional growth factors. Some studies have evaluated the association between TGF-β abnormalities and autism spectrum disorders. One of the goals of this study is to compare TGF-β levels between autistic and healthy people [7].
IL-10 is a crucial immune regulatory cytokine known for its significant anti-inflammatory functions [8] It exerts its effects on various cells within the immune system, playing a pivotal role in limiting excessive tissue disruption resulting from inflammation.
Another marker considered in this study is C-reactive protein (CRP), an acute phase reactant. This indicator is suitable for detecting low-grade inflammation from infectious and non-infectious exposure. During acute infection, its level increases significantly with the secretion of interleukin-6 (IL-6). It has been reported that the occurrence of autism symptoms is associated with increased abnormal immune responses [9]. Researchers have shown that the levels of cytokines in people with autism are increased in the blood, brain, and cerebrospinal fluid. The exact mechanisms of the role of IL-6 in pathogenesis are still not well understood. With the increase of IL-6 and changes in excitatory and inhibitory synaptic formations, the balance of excitatory/inhibitory synaptic transmission is disturbed. Increased IL-6 can also be associated with abnormal changes in dendritic spines’ shape, length, and distribution pattern. The findings show that the increased IL-6 in the brain plays a role in autistic behaviors through the imbalance of neural circuits and synaptic vulnerability [10].
Recent studies have further clarified the role of cytokines and immune dysregulation in the pathogenesis of ASD. A review highlighted the complexity of cytokine alterations, emphasizing their role in neuroinflammation and synaptic dysregulation, which contribute to ASD’s characteristic behavioral symptoms [11]. Another study demonstrated that cytokine profiles in children with ASD, particularly IL-6 and TNF-α, are critical markers for immune system abnormalities, which may disrupt neuronal signaling and development [8]. The interplay between pro-inflammatory and anti-inflammatory cytokines, especially the significant reduction in IL-10 observed in ASD patients, suggests that impaired immune regulation may be a key factor in ASD pathophysiology [5]. Moreover, new diagnostic models propose using cytokine panels, including IL-10 and IL-8, for early ASD detection, offering improved sensitivity and specificity compared to traditional methods [4]. In light of these findings, there is a growing consensus that ASD is not solely a neurodevelopmental disorder but also involves systemic immune dysfunction [12].
Our results demonstrated significantly lower levels of these cytokines in ASD patients compared to healthy controls. This discrepancy suggests that ASD may not be universally linked to pro-inflammatory states but might involve complex immune dysregulation, where both pro-inflammatory and anti-inflammatory responses are expected [13]. IL-10, a key anti-inflammatory cytokine, is known for regulating immune responses and limiting tissue damage during inflammation. Reduced IL-10 levels in ASD patients could indicate an impaired ability to counterbalance inflammation [8]. Similarly, lower IL-8 concentrations, a pro-inflammatory chemokine, suggest disruptions in immune cell recruitment and signaling pathways critical to inflammatory responses [5]. These findings align with recent reports that show heterogeneity in cytokine profiles depending on factors like age and ASD severity [14]. Furthermore, IL-6, a multifunctional cytokine involved in neurodevelopment and immune responses, also showed reduced levels, contradicting earlier studies reporting elevated IL-6 in ASD patients. Although the reduction is not significant this outcome may highlight the need for a more nuanced understanding of cytokine dynamics in ASD, where altered immune signaling might reflect an underlying imbalance rather than overt inflammation [15]. Such immune abnormalities may contribute to ASD’s neurodevelopmental disruptions by affecting synaptic plasticity and brain connectivity [10]. Cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) mediate the communication between the immune system and the brain [16]. By crossing the blood-brain barrier, environmental cytokines, play a role in signaling and brain development [17]. By evaluating brain biopsies and observing abnormal cytokine profiles in ASD patients compared to healthy individuals, it has been determined that there is a relationship between cytokines and psychiatric disorders such as depression [18], schizophrenia, and ASD. This study investigates the correlation of biochemical and inflammatory network abnormalities in ASD patients compared to the healthy control individuals by measuring the serum concentration of CRP and some inflammatory cytokines such as, TNF-α, TGF-β, IL-1β, IL-10, Il-8, and IL-6.
Materials and methods
Data collection
Data collection in this study was carried out in the pediatric clinics of Amir Kabir Hospital in Arak, Iran from November 2023 to March 2024. Thirty-four people participated in this study (12 healthy controls and 22 ASD patients) (Table 1). We identified 55 children with autism in Markazi province, Iran. 22 of ASD patients consented and participated for the study. We acknowledged individuals willing to collaborate in the study with their lab results. Diagnosis was conducted by board certified psychiatrists following the criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [19].
Table 1.
Demographic data of ASD and control groups
| Demographic data | ASD | Control | P-value |
|---|---|---|---|
| Subjects (n) | 22 | 12 | |
|
Age (years) (mean value ± SD) |
5.41 ± 14.1 | 5.42 ± 14.8 | 0.19 |
| Gender (n) | |||
| Male | 18 | 10 | 0.677 |
| Female | 4 | 2 | |
The inclusion criteria for the ASD group were: (a) ASD diagnosis confirmed by board certified psychiatrists, (b) patients and parents understanding Persian language, (c) having the motivation to participate in the study, (d) the absence of neurological and syndromic problems, (e) the absence of other major psychiatric disorders including bipolar disorder, schizophrenia, and psychotic disorders, (f) no personal or family history of autoimmune disorders, (g) no history of severe allergies or atopic diseases (e.g., asthma, eczema), (h) no chronic infections, (i) no recent vaccinations, (j) free from medications that affect the immune system (e.g., anti-inflammatories, psychotropics), (k) no premature birth or history of birth complications, (l) regularly visited outpatients of the psychiatric clinic of Amir Kabir Hospital in Arak, Iran from November 2023 to March 2024, and (m) 4–7 years old.
The healthy children matched for age and sex were recruited as a control group. The healthy children in the control group were included if they met the following criteria:
(a) Neurotypical individuals (b) with no diagnostic criteria for ASD or any other major psychiatric disorder, (c) patients and parents understanding Persian language, (d) having the motivation to participate in the study, (e) no personal of family history of autoimmune disorders, (f) no history of severe allergies or atopic diseases (e.g., asthma, eczema), (g) no chronic infections, (h) no recent vaccinations, (i) free from medications that affect the immune system (e.g., anti-inflammatories, psychotropics), (j) no premature birth or history of birth complications (k) regularly visited outpatients of the pediatric clinics of Amir Kabir Hospital in Arak, Iran from November 2023 to March 2024, and (l) 4–7 years old.
Forty-two interviews were conducted by the psychiatrists. Twenty participants were excluded, including: (A) 4 patients did not meet the entry criteria, (B) 8 people lacked the motivation, and (C) the remaining 8 people refused to participate without giving a reason. Twenty-four participants aged 4–7 years with a diagnosis of ASD were enrolled, but 2 dropped out before the start of training due to scheduling conflicts. Dropout participants were not replaced and data were not analyzed. Therefore, the final sample consisted of 22 participants.
Serum preparation and ELISSA assay
The blood samples from ASD patients (n = 22) and control group (n = 12) were collected with serum separator tubes and were stood for 2 h at room temperature. The samples were centrifuged for 20 min at 1000×g. Then, the supernatants were collected and stored at -20 °C for less than 6 months until we conducted the ELISA assay. Human ELISA Kits were used to measure CRP (Quantikine, R&D Systems, cat. DCRP00B, USA, Sensitivity: 0.022 ng/mL), TNF-α (Immunological Sciences, cat. IK4185, Sensitivity: 1 pg/ml), TGF-β1(Diaclone Human TGF-β1 ELISA kit, Sensitivity: 9 pg/mL), IL-1β (Quantikine, R&D Systems, cat. HSLB00D, USA, Sensitivity: 0.063 pg/mL), IL-10 (Quantikine, R&D Systems, cat. HS100C, USA, Sensitivity: 0.17 pg/m), Il-8 (MabTech Human IL-8 ELISA kit, Sensitivity: 2 pg/mL), and IL-6 (Quantikine, R&D Systems, cat.HS600C, USA, sensitivity: 0.09 pg/mL) in the serum samples of ASD patients and control group, according to the Kit manufacturer.
Statistical analysis
Statistical analysis was done using SPSS (Statistical Package for Social Sciences) version 23. We applied the unpaired t-test for parametric data (IL-10) and Mann-Whitney test for non-parametric data (IL-6, IL-8, IL-10, IL-1β, TNF-α, TGF-β1 and CRP) to compare the difference between serum levels of the mentioned inflammatory factors between the two groups. The Pearson correlation was done in ASD and healthy groups. The P values less than 0.05 were considered to be statistically significant. A summary of the cytokine analysis results is presented in Table 2.
Table 2.
The minimum and maximum values, the median, and the IQR for each marker. The IQR represents the middle 50% of the data, with the 25th percentile and the 75th percentile values
| Marker | ASD Group | Healthy group | ||||
|---|---|---|---|---|---|---|
| Range (min-max) | Median | Interquartile Range (IQR) | Range (min - max) | Median | Interquartile Range (IQR) | |
| IL6 | 0.0–0.7 | 0.1 | 0.0–0.1 | 0.0–1.2 | 0.1 | 0.0–0.4 |
| IL8 | 0.2–84.5 | 9.2 | 1.7–54.9 | 44.3–61.8 | 50.4 | 48.3–56.8 |
| IL10 | 6.1–12.0 | 9.6 | 8.4–9.9 | 10.6–23.2 | 13.8 | 12.5–16.5 |
| IL1B | 0.0–1.0 | 0.0 | 0.0–0.2 | 0.0–0.5 | 0.1 | 0.0–0.3 |
| TGFB | 5.1–137.2 | 11.1 | 7.3–31.2 | 3.4–77.7 | 17.6 | 9.3–64.7 |
| TNF | 0.0–2.9 | 0.2 | 0.0–0.4 | 0.0–0.7 | 0.0 | 0.0–0.5 |
| CRP | 1.6–6.6 | 3.4 | 2.0–5.7 | 1.0–6.6 | 3.9 | 2.4–5.9 |
Results
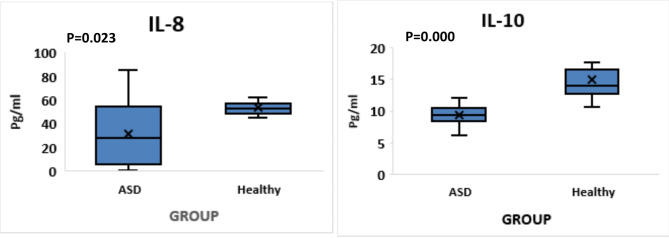
IL-10, and IL-8 were found to be significantly lower in the serum of ASD patients compared to the healthy control group (p = 0.000, and p = 0.023, respectively) (Fig. 1).
Fig. 1.
Comparing the serum concentrations of IL-10 and IL-8 in ASD and control groups
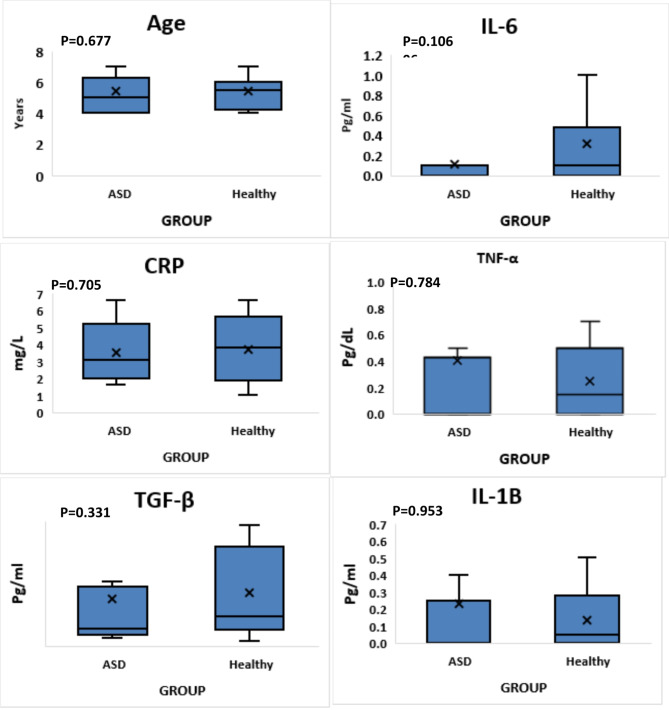
There was no significant difference between serum levels of CRP, TNF-α, TGF-β, IL-6 and IL-1β in the ASD and control groups (p = 0.705, p = 0.784, p = 0.331, P = 0.106 and p = 0.953, respectively) (Fig. 2).
Fig. 2.
Comparing the serum concentrations of CRP, TNF-α, TGF-β, IL-1β, and IL-6 between ASD patients and control individuals
The correlation between the biomarkers in the ASD group showed a positive correlation between CRP and IL-10 (p = 0.485), also CRP and IL-8 (p = 0.477) as shown in the green cells (Table 3). In contrast to the ASD patients, the correlation of CRP and IL-10 (p = 0.293), also CRP and IL-8 (p = 0.855) were not significant in the control group as shown in the Table 4.
Table 3.
Correlation matrix of inflammatory markers in the ASD patients
| Correlations | ||||||||
|---|---|---|---|---|---|---|---|---|
| IL6 | IL8 | IL10 | IL1B | TGFB | TNF | CRP | ||
| IL6 | Pearson Correlation | 1 | -0.345 | -0.145 | -0.150 | 0.112 | -0.190 | -0.096 |
| Sig. (2-tailed) | 0.116 | 0.521 | 0.504 | 0.619 | 0.398 | 0.670 | ||
| N | 22 | 22 | 22 | 22 | 22 | 22 | 22 | |
| IL8 | Pearson Correlation | − 0.0345 | 1 | 0.389 | 0.338 | -0.320 | 0.066 | 0.477* |
| Sig. (2-tailed) | 0.116 | 0.073 | 0.124 | 0.147 | 0.769 | 0.025 | ||
| N | 22 | 22 | 22 | 22 | 22 | 22 | 22 | |
| IL10 | Pearson Correlation | -0.145 | 0.389 | 1 | -0.108 | -0.046 | -0.123 | 0.485* |
| Sig. (2-tailed) | 0.521 | 0.073 | 0.633 | 0.840 | 0.585 | 0.022 | ||
| N | 22 | 22 | 22 | 22 | 22 | 22 | 22 | |
| IL1B | Pearson Correlation | -0.150 | 0.338 | -0.108 | 1 | 0.066 | 0.071 | 0.155 |
| Sig. (2-tailed) | 0.504 | 0.124 | 0.633 | 0.770 | 0.754 | 0.490 | ||
| N | 22 | 22 | 22 | 22 | 22 | 22 | 22 | |
| TGFB | Pearson Correlation | 0.112 | -0.320 | -0.046 | 0.066 | 1 | -0.191 | 0.139 |
| Sig. (2-tailed) | 0.619 | 0.147 | 0.840 | 0.770 | 0.395 | 0.539 | ||
| N | 22 | 22 | 22 | 22 | 22 | 22 | 22 | |
| TNF | Pearson Correlation | -0.190 | 0.066 | -0.123 | 0.071 | -0.191 | 1 | -0.223 |
| Sig. (2-tailed) | 0.398 | 0.769 | 0.585 | 0.754 | 0.395 | 0.318 | ||
| N | 22 | 22 | 22 | 22 | 22 | 22 | 22 | |
| CRP | Pearson Correlation | -0.096 | 0.477* | 0.485* | 0.155 | 0.139 | -0.223 | 1 |
| Sig. (2-tailed) | 0.670 | 0.025 | 0.022 | 0.490 | 0.539 | 0.318 | ||
| N | 22 | 22 | 22 | 22 | 22 | 22 | 22 | |
| *. Correlation is significant at the 0.05 level (2-tailed). | ||||||||
Table 4.
Correlation matrix of inflammatory markers in the control group
| Correlations | ||||||||
|---|---|---|---|---|---|---|---|---|
| IL6 | IL8 | IL10 | IL1B | TGFB | TNF | CRP | ||
| IL6 | Pearson Correlation | 1 | 0.073 | -0.160 | -0.169 | -0.074 | 0.097 | 0.114 |
| Sig. (2-tailed) | 0.822 | 0.619 | 0.600 | 0.820 | 0.764 | 0.724 | ||
| N | 12 | 12 | 12 | 12 | 12 | 12 | 12 | |
| IL8 | Pearson Correlation | 0.073 | 1 | 0.472 | -0.405 | 0.238 | 0.278 | -0.059 |
| Sig. (2-tailed) | 0.822 | 0.121 | 0.191 | 0.455 | 0.381 | 0.855 | ||
| N | 12 | 12 | 12 | 12 | 12 | 12 | 12 | |
| IL10 | Pearson Correlation | -0.160 | 0.472 | 1 | -0.103 | 0.077 | 0.413 | -0.331 |
| Sig. (2-tailed) | 0.619 | 0.121 | 0.751 | 0.811 | 0.183 | 0.293 | ||
| N | 12 | 12 | 12 | 12 | 12 | 12 | 12 | |
| IL1B | Pearson Correlation | -0.169 | -0.405 | -0.103 | 1 | -0.381 | 0.198 | 0.059 |
| Sig. (2-tailed) | 0.600 | 0.191 | 0.751 | 0.221 | 0.537 | 0.855 | ||
| N | 12 | 12 | 12 | 12 | 12 | 12 | 12 | |
| TGFB | Pearson Correlation | -0.074 | 0.238 | 0.077 | -0.381 | 1 | -0.379 | 0.008 |
| Sig. (2-tailed) | 0.820 | 0.455 | 0.811 | 0.221 | 0.224 | 0.981 | ||
| N | 12 | 12 | 12 | 12 | 12 | 12 | 12 | |
| TNF | Pearson Correlation | 0.097 | 0.278 | 0.413 | 0.198 | -0.379 | 1 | 0.122 |
| Sig. (2-tailed) | 0.764 | 0.381 | 0.183 | 0.537 | 0.224 | 0.706 | ||
| N | 12 | 12 | 12 | 12 | 12 | 12 | 12 | |
| CRP | Pearson Correlation | 0.114 | -0.059 | -0.331 | 0.059 | 0.008 | 0.122 | 1 |
| Sig. (2-tailed) | 0.724 | 0.855 | 0.293 | 0.855 | 0.981 | 0.706 | ||
| N | 12 | 12 | 12 | 12 | 12 | 12 | 12 | |
The control group shows no significant correlations between the cytokines and CRP or other inflammatory markers. Most correlations in this group are weak (close to 0) and non-significant (p-values > 0.05).
In the ASD group, significant positive correlations between IL8 and CRP, and IL10 and CRP suggest a relationship between these immune markers and inflammation in ASD. The strength of these correlations is moderate (near 0.5), and the p-values are less than 0.05, indicating statistical significance. The significant positive correlations between IL8/IL10 and CRP in ASD patients may indicate an active inflammatory response in individuals with ASD. IL8 is a known pro-inflammatory cytokine, and CRP is a marker of systemic inflammation. Their correlation could suggest heightened immune activation in ASD.
The absence of such correlations in the control group could imply that these relationships are specific to the ASD condition or due to differences in immune regulation. Identifying which correlations differ significantly between groups could lead to meaningful biological insights into ASD pathology. This analysis provides a basis for deeper investigation into how the correlation patterns may influence biological understanding of ASD versus control conditions.
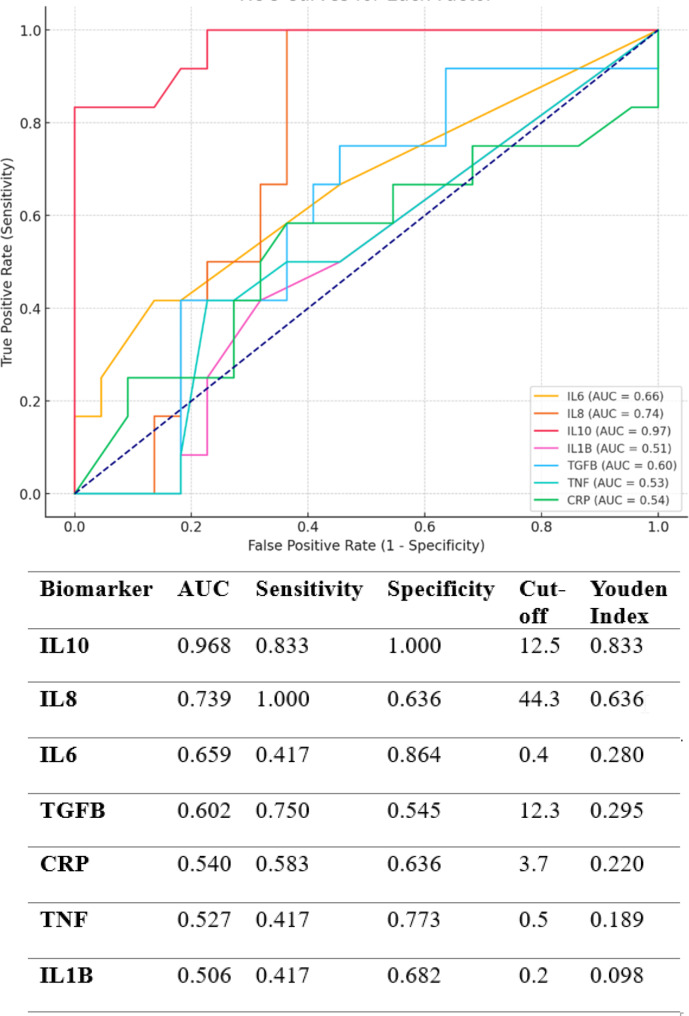
Diagnostic accuracy of the identified ASD biomarkers
We used ROC curves to study the performance of seven important cytokines remaining in the final logistic regression model (Fig. 3). The analysis of seven inflammatory biomarkers reveals a highly variable diagnostic performance for ASD. IL-10 emerges as the most promising biomarker, with a near-perfect AUC (0.968), high sensitivity (0.833), and perfect specificity (1.000), indicating strong discriminatory ability in distinguishing ASD from non-ASD cases. IL-8, while showing perfect sensitivity (1.000), suffers from reduced specificity (0.636), suggesting its potential for detecting ASD but also a higher risk of false positives. Other biomarkers, such as IL-6 (AUC 0.659) and TGF-B (AUC 0.602), exhibit moderate predictive value but have limitations in either sensitivity or specificity, which weakens their clinical reliability when used independently. On the contrary, biomarkers like CRP, TNF, and IL-1B show poor performance (AUCs below 0.55), with low sensitivity, specificity, and weak Youden Index values, indicating their minimal utility for ASD diagnostics. In fact, these markers approach random chance in their ability to distinguish between ASD and control groups. Their low AUCs and overlap with the ROC diagonal further undermine their relevance as individual diagnostic tools.
Fig. 3.
Receiver Operating Characteristic (ROC) curves illustrate the relationship between sensitivity and specificity in children with Autism Spectrum Disorder (ASD) compared to typically developing children. Certain parameter values are higher in autistic children than in their typically developing peers, while others are lower. Analyzing ROC curves that combine various parameters, even those with opposing effects, enhances both sensitivity and specificity. The ROC curve analyses utilized nonparametric methods, and the confidence intervals for the curves were established at 95%
Overall, the findings suggest that IL-10 holds the greatest potential for contributing to an ASD biomarker panel, while other markers, particularly CRP, TNF, and IL-1B, may add limited diagnostic value. A combined biomarker approach may be necessary to enhance sensitivity and specificity, addressing the limitations of individual markers and improving diagnostic accuracy in ASD. The integration of IL-10 as a key component in a multi-biomarker panel is recommended for future ASD-related diagnostic strategies.
Discussion
The immune dysregulation, particularly cytokine alterations, has been consistently observed in individuals with ASD, yet establishing a direct causal link remains challenging [20].
Recent reviews and studies underscore the complexity of immune involvement in ASD. For example, elevated levels of pro-inflammatory cytokines like IL-6, IL-17, and TNF-α have been detected in individuals with ASD, suggesting immune dysregulation. However, studies also reveal considerable heterogeneity in immune responses, making it difficult to draw firm conclusions about their role as causal agents in ASD pathology. The alterations could be secondary effects, correlating with ASD rather than directly causing [21]. In another study IL-6 and IL-17 are investigated in altering neuronal connectivity and excitability, potentially contributing to ASD-associated behaviors; but without direct experimental evidence of linking these immune changes to specific neural pathways, the findings remain correlative rather than causative [22].
While immune factors like cytokines may be involved in ASD, further mechanistic research is required to move beyond surface-level associations. This would provide more robust insights into how immune dysregulation contributes to the neurodevelopmental trajectory of ASD. The cytokines such as IL-6 and TNF-α have been shown to affect brain development and inflammation, the specific pathways by which they contribute to ASD are not fully understood. Gandal and colleagues found that broad transcriptomic dysregulation occurs across the cerebral cortex in ASD, which could be driven by both genetic and immune factors, yet this alone does not provide direct evidence of causality [23]. Furthermore, a systematic review by Parellada and colleagues stressed the need for biomarkers, like cytokines, to be studied in a way that accounts for their interaction with genetic and environmental factors, to better define their roles in ASD [24].
A study highlights that while immune mediators, including cytokines, are linked to ASD, the precise mechanisms by which they alter neurodevelopment remain elusive. The authors suggest that more sophisticated molecular and functional studies are needed to pinpoint how immune factors drive ASD symptomatology [25–27].
This study reported significantly lower serum concentrations of IL10, and IL-8 in the ASD patients compared to the control groups which was inconsistent with previous studies [5, 15, 28, 29]. In contrast, in a study by Bryn and colleagues [30], the report of a decrease in IL-10 was in line with our findings, while their report regarding the elevated IL-8 was inconsistent with this study. Also, the lower concentration of anti-inflammatory cytokines IL-10 is reported by Saghazadeh and colleagues [15].
Unfortunately, broad variations in the symptoms of people with ASD, can be further complicated by several co-occurring factors. ASD is a heterogeneous disorder with a wide range of symptoms and severity [31]. This diversity in ASD population can contribute to inconsistent findings. Differences in study design, such as sample size, participant selection criteria, and demographic characteristics can influence results. Varied populations with different genetic, environmental, or clinical factors may contribute to divergent outcomes [32].
Although the cause of ASD is not clearly defined, recent studies have shown that inflammation of the central nervous system affect the ASD pathogenesis [33]. Evidence regarding the changes in Central and peripheral immune system performance shows that there are subgroups of people with autism who have some dysregulation in the immune system specially in the cytokine concentrations [20]. Cytokine levels can vary over time, furthermore they can have alterations contributed to factors such as the stage of the disorder, medication use [34], or coexisting conditions [34].
There are different functions for CRP, IL-10, and IL-8. CRP is involved in the systemic inflammation and its expression is increased in response to various inflammatory stimuli [35]. IL-10 is known as an anti-inflammatory cytokine involved in the regulation immune responses such as suppressing the synthesize of pro-inflammatory cytokines [36]. A positive correlation with CRP could suggest a scenario where the body is attempting to counterbalance or regulate the inflammatory response by producing more IL-10. Due to its anti-inflammatory function to control and limit inflammatory processes, the lowered concentration shows deficits in anti-inflammatory response. Reduced IL-10 levels could contribute to a failure in controlling inflammation appropriately, leading to prolonged or excessive inflammatory reactions [13].
IL-8 is a pro-inflammatory chemokine and its function is attracting the immune cells to the site of inflammation [37]. The decreased concentrations of pro-inflammatory cytokines IL-8 and IL-6 in ASD patients suggest alterations in pro-inflammatory signaling pathways. IL-8 is involved in recruiting immune cells to the site of inflammation, while IL-6 is a multifunctional cytokine with roles in inflammation and immune response. Lower levels of these cytokines may indicate a disruption in the normal functioning of the pro-inflammatory arm of the immune system [37].
The control group shows no significant correlations between the cytokines and CRP or other inflammatory markers. Most correlations in this group are weak (close to 0) and non-significant (p-values > 0.05).
In the ASD group, significant positive correlations between IL8 and CRP, and IL10 and CRP suggest a relationship between these immune markers and inflammation in ASD. The strength of these correlations is moderate (near 0.5), and the p-values are less than 0.05, indicating statistical significance. The significant positive correlations between IL8/IL10 and CRP in ASD patients may indicate an active inflammatory response in individuals with ASD. IL8 is a known pro-inflammatory cytokine, and CRP is a marker of systemic inflammation. Their correlation could suggest heightened immune activation in ASD.
The absence of such correlations in the control group could imply that these relationships are specific to the ASD condition or due to differences in immune regulation. Identifying which correlations differ significantly between groups could lead to meaningful biological insights into ASD pathology. This analysis provides a basis for deeper investigation into how the correlation patterns may influence biological understanding of ASD versus control conditions.
A positive correlation with CRP may suggest that as systemic inflammation increases (as indicated by CRP) [35], there is a concurrent activation of immune cells, particularly neutrophils, which release IL-8 [37]. A positive correlation could indicate a scenario where inflammation and tissue damage are occurring simultaneously, leading to increased levels of both CRP and IL-8.
Our study suggests that the inflammatory network in ASD is characterized by a deficiency in anti-inflammatory regulation, altered pro-inflammatory signaling, and complex interactions within the immune system. In order to gain a better understanding of the factors contributing to increased inflammatory factors in autistic children, it is suggested that future studies explore these factors in-depth. More researches with larger number of participants, across various age groups (ranging from children to post-puberty), as well as investigation of other cytokines, including interleukin-2, interleukin-17, and interleukin-19 p42 in autistic children are recommended.
While previous studies often report elevated levels of pro-inflammatory cytokines like IL-6 and IL-8 in ASD patients, there is growing evidence suggesting that cytokine profiles may vary significantly depending on factors such as age, severity of the disorder, and other underlying conditions. Recent studies have shown mixed results regarding IL-6 and IL-8 levels. For example, while some report elevated levels, others, including our study, found lower concentrations. This suggests that ASD might not always be associated with a simple pro-inflammatory response, but rather, it could involve complex immune dysregulation, where both pro- and anti-inflammatory mechanisms are altered and there could be dissimilarities in certain ASD populations, particularly when considering the heterogeneity of the disorder. Lower IL-10 levels, an anti-inflammatory cytokine, further indicate a potential deficiency in anti-inflammatory responses, which could exacerbate ongoing inflammation or fail to regulate immune responses [14, 38, 39]. Recent evidence has highlighted the complexity of cytokine dysregulation in autism. For instance, some studies have reported a nuanced role of IL-10, IL-8, and IL-6 in ASD, with fluctuations in these cytokines depending on various factors, such as the age, gender, and specific characteristics of the ASD population studied. This includes reports of reduced anti-inflammatory cytokines like IL-10, which may reflect an impaired ability to regulate inflammation in some ASD subgroups [39].
Moreover, studies emphasize the heterogeneity of cytokine profiles in ASD. For example, IL-6 has been observed to both increase and decrease in different studies, potentially due to differences in sample type (e.g., serum vs. plasma), study design, or co-occurring medical conditions [40].
Recent studies have focused extensively on the signaling and molecular mechanisms behind cytokine changes in various disorders, particularly in relation to neurodevelopmental and immune-mediated conditions [12, 41]. In ASD, cytokines such as IL-6, TNF-α, and IL-10 have garnered attention due to their roles in immune dysregulation and neuroinflammation [12]. The molecular mechanisms underlying these cytokine alterations are multifaceted. IL-6, a pro-inflammatory cytokine, has been shown to contribute to synaptic imbalance and disrupted excitatory/inhibitory signaling, which is critical in neurodevelopmental processes. Studies highlight that elevated IL-6 levels can impair synaptic plasticity and connectivity, contributing to ASD-related behaviors. The pathway involves IL-6-mediated activation of the JAK/STAT signaling cascade, which leads to transcriptional changes that influence neurodevelopment [42, 43].
TNF-α, another pro-inflammatory cytokine, has been linked to neuronal death and inflammation via the NF-κB signaling pathway. This cytokine, when elevated in ASD, can exacerbate neuroinflammatory conditions, disrupting neuronal integrity. TNF-α can also interfere with the blood-brain barrier, allowing peripheral immune cells and cytokines to enter the brain, further amplifying inflammation [44].
IL-10, a key anti-inflammatory cytokine, plays a critical role in dampening immune responses. Its reduction in ASD suggests an impaired ability to regulate inflammation. IL-10 acts through the STAT3 signaling pathway, which suppresses the production of pro-inflammatory cytokines like IL-6 and TNF-α, and promotes tissue repair and immune homeostasis. A deficit in IL-10 may thus contribute to prolonged immune activation, exacerbating neuroinflammatory conditions [45]. Cytokines such as IL-6 and TNF-α interact with the central nervous system (CNS) through mechanisms like crossing the blood-brain barrier, triggering microglial activation, and initiating neuroinflammatory cascades. This cytokine-brain communication plays a significant role in the immune-to-brain signaling that underlies ASD and similar conditions [46]. These findings underscore the importance of understanding cytokine signaling mechanisms in ASD to unravel the broader implications of immune dysregulation in neurodevelopmental disorders. Further research into cytokine profiles and their signaling pathways could offer new therapeutic targets aimed at modulating immune responses in ASD [47]. As such, the lower cytokine levels observed in our study may represent a specific immunological signature in a subset of ASD patients, suggesting an alternative pathophysiological pathway involving impaired immune regulation rather than overt inflammation.
Conclusion
In conclusion, our study revealed significantly lower levels of IL-10, and IL-8 in individuals with ASD compared to the control group, suggesting potential immune dysregulation in ASD. The observed positive correlations between CRP and IL-10, also CRP and IL-8, indicate complex immune interactions in ASD patients. These altered cytokine profiles may contribute to the pathogenesis of ASD. Notably, the specificity of these findings to ASD is highlighted by the absence of significant correlations in the control group. Further research is needed to elucidate the underlying mechanisms and explore potential therapeutic implications of these immunological abnormalities.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank the caregivers for their consent to participate in the study. We also appreciate all the doctors and nurses working in the neonatal units of all the four hospitals where the study took place for their understanding and cooperation during the study. This project was carried out as a research program in the Department of.
Biology of Arak University, and acknowledge the authorities that financially helped us carry out this research.
Author contributions
A.N: Conceptualization, Methodology, Data collection, Formal analysis, Investigation, Writing – original draft. P.R: Formal analysis, Investigation, Data collection, Writing – original draft. J.S: Conceptualization, Writing – review & editing, Supervision, Data collection. A.A: Sample size, Formal analysis, Investigation, Data collection.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval
Ethical approval for our study was obtained from the Research Ethics Committees of Arak University for all the experimental procedures according to the guidelines set by the World Medical Association standards (IR.ARAKU.REC.1402.53).
Consent to participate
Written informed consent was obtained from the parents or legal guardians of the patients for the publication of this case report and any accompanying images. The consent includes permission to publish the patients’ clinical details and any accompanying images. All identifying information has been removed or anonymized.
Informed consent
Informed consent was obtained from the parents or legal guardians of all participants involved in the study. For participants under the age of 16, consent to participate was obtained from their parents or legal guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lord C, Brugha TS, Charman T, Cusack J, Dumas G, Frazier T, Jones EJ, Jones RM, Pickles A, State MW. Autism spectrum disorder. Nat Reviews Disease Primers. 2020;6(1):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Čorejová A, Fazekaš T, Jánošíková D, Repiský J, Pospíšilová V, Miková M, Rauová D, Ostatníková D, Kyselovič J, Hrabovská A. Improvement of the Clinical and Psychological Profile of patients with autism after Methylcobalamin Syrup Administration. Nutrients. 2022;14(10):2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siniscalco D, Schultz S, Brigida AL, Antonucci N. Inflammation and neuro-immune dysregulations in autism spectrum disorders. Pharmaceuticals. 2018;11(2):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen AR, Lane AL, Werner BA, McLees SE, Fletcher TS, Frye RE. Modern biomarkers for autism spectrum disorder: future directions. Mol Diagn Ther. 2022;26(5):483–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen Y, Li Y, Shi L, Liu M, Wu R, Xia K, Zhang F, Ou J, Zhao J. Autism spectrum disorder and severe social impairment associated with elevated plasma interleukin-8. Pediatr Res. 2021;89(3):591–7. [DOI] [PubMed] [Google Scholar]
- 6.Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun. 2001;15(1):7–24. [DOI] [PubMed] [Google Scholar]
- 7.Yousefi J, Khakzad MR, Hojati M, Ebrahimi SA, Hosseinpour M, Akhondian J. Is serum TGF-β1 and TGF-β2 levels correlated to children with Autism Intensity? Iran J Child Neurol. 2021;15(2):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nadeem A, Ahmad SF, Al-Harbi NO, Al-Ayadhi LY, Sarawi W, Attia SM, Bakheet SA, Alqarni SA, Ali N, AsSobeai HM. Imbalance in pro-inflammatory and anti-inflammatory cytokines milieu in B cells of children with autism. Mol Immunol. 2022;141:297–304. [DOI] [PubMed] [Google Scholar]
- 9.Mead J, Ashwood P. Evidence supporting an altered immune response in ASD. Immunol Lett. 2015;163(1):49–55. [DOI] [PubMed] [Google Scholar]
- 10.Wei H, Chadman KK, McCloskey DP, Sheikh AM, Malik M, Brown WT, Li X. Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochim et Biophys Acta (BBA)-Molecular Basis Disease. 2012;1822(6):831–42. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Wang B, Wu C, Wang J, Sun M. Autism spectrum disorder: neurodevelopmental risk factors, biological mechanism, and precision therapy. Int J Mol Sci. 2023;24(3):1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson-Agramonte MA, Noris García E, Fraga Guerra J, Vega Hurtado Y, Antonucci N, Semprún-Hernández N, Schultz S, Siniscalco D. Immune dysregulation in autism spectrum disorder: what do we know about it? Int J Mol Sci. 2022;23(6):3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masi A, Glozier N, Dale R, Guastella AJ. The immune system, cytokines, and biomarkers in autism spectrum disorder. Neurosci Bull. 2017;33:194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpita B, Massoni L, Battaglini S, Palego L, Cremone IM, Massimetti G, Betti L, Giannaccini G, Dell’Osso L. IL-6, homocysteine, and autism spectrum phenotypes: an investigation among adults with autism spectrum disorder and their first-degree relatives. CNS Spectr. 2023;28(5):620–8. [DOI] [PubMed] [Google Scholar]
- 15.Saghazadeh A, Ataeinia B, Keynejad K, Abdolalizadeh A, Hirbod-Mobarakeh A, Rezaei N. Anti-inflammatory cytokines in autism spectrum disorders: a systematic review and meta-analysis. Cytokine. 2019;123:154740. [DOI] [PubMed] [Google Scholar]
- 16.Hosoi T, Okuma Y, Nomura Y. The mechanisms of immune-to-brain communication in inflammation as a drug target. Curr Drug Targets-Inflammation Allergy. 2002;1(3):257–62. [DOI] [PubMed] [Google Scholar]
- 17.Banks WA. Blood–brain barrier transport of cytokines. NeuroImmune Biology. 2008;6:93–107. [Google Scholar]
- 18.Miller AH. Beyond depression: the expanding role of inflammation in psychiatric disorders. World Psychiatry. 2020;19(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grzadzinski R, Huerta M, Lord C. DSM-5 and autism spectrum disorders (ASDs): an opportunity for identifying ASD subtypes. Mol Autism. 2013;4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noriega DB, Savelkoul HF. Immune dysregulation in autism spectrum disorder. Eur J Pediatrics. 2014;173:33–43. [DOI] [PubMed] [Google Scholar]
- 21.Masi A, Quintana D, Glozier N, Lloyd A, Hickie I, Guastella A. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol Psychiatry. 2015;20(4):440–6. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez-Celis A, Van de Water J. Maternal immune dysregulation and autism spectrum disorder. Neural Engineering techniques for Autism Spectrum Disorder. edn.: Elsevier; 2023. pp. 21–61.
- 23.Gandal MJ, Haney JR, Wamsley B, Yap CX, Parhami S, Emani PS, Chang N, Chen GT, Hoftman GD, de Alba D. Broad transcriptomic dysregulation occurs across the cerebral cortex in ASD. Nature. 2022;611(7936):532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parellada M, Andreu-Bernabeu Á, Burdeus M, San José Cáceres A, Urbiola E, Carpenter LL, Kraguljac NV, McDonald WM, Nemeroff CB, Rodriguez CI. In search of biomarkers to guide interventions in autism spectrum disorder: a systematic review. Am J Psychiatry. 2023;180(1):23–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin H, Wang Z, Liu J, Li Y, Liu L, Huang P, Wang W, Shan Z, Sun R, Shen J. Dysregulation of immune and metabolism pathways in maternal immune activation induces an increased risk of autism spectrum disorders. Life Sci. 2023;324:121734. [DOI] [PubMed] [Google Scholar]
- 26.Cantando I, Centofanti C, D’Alessandro G, Limatola C, Bezzi P. Metabolic dynamics in astrocytes and microglia during post-natal development and their implications for autism spectrum disorders. Front Cell Neurosci. 2024;18:1354259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gevezova M, Sbirkov Y, Sarafian V, Plaimas K, Suratanee A, Maes M. Autistic spectrum disorder (ASD)–Gene, molecular and pathway signatures linking systemic inflammation, mitochondrial dysfunction, transsynaptic signalling, and neurodevelopment. Brain Behav immunity-health. 2023;30:100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyriklaki A, Margetaki K, Kampouri M, Koutra K, Bitsios P, Chalkiadaki G, Dermitzaki E, Venihaki M, Sarri K, Anousaki D. Association between high levels of inflammatory markers and cognitive outcomes at 4 years of age: the Rhea mother-child cohort study, Crete, Greece. Cytokine. 2019;117:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kordulewska NK, Kostyra E, Piskorz-Ogórek K, Moszyńska M, Cieślińska A, Fiedorowicz E, Jarmołowska B. Serum cytokine levels in children with spectrum autism disorder: differences in pro-and anti-inflammatory balance. J Neuroimmunol. 2019;337:577066. [DOI] [PubMed] [Google Scholar]
- 30.Bryn V, Aass HCD, Skjeldal OH, Isaksen J, Saugstad OD, Ormstad H. Cytokine profile in autism spectrum disorders in children. J Mol Neurosci. 2017;61:1–7. [DOI] [PubMed] [Google Scholar]
- 31.Mottron L, Bzdok D. Autism spectrum heterogeneity: fact or artifact? Mol Psychiatry. 2020;25(12):3178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YS, Leventhal BL. Genetic epidemiology and insights into interactive genetic and environmental effects in autism spectrum disorders. Biol Psychiatry. 2015;77(1):66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li X-M, Ji L, Brown T, Malik M. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207(1–2):111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011;36(12):2452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karadag F, Kirdar S, Karul AB, Ceylan E. The value of C-reactive protein as a marker of systemic inflammation in stable chronic obstructive pulmonary disease. Eur J Intern Med. 2008;19(2):104–8. [DOI] [PubMed] [Google Scholar]
- 36.Lobo-Silva D, Carriche GM, Castro AG, Roque S, Saraiva M. Balancing the immune response in the brain: IL-10 and its regulation. J Neuroinflamm. 2016;13:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsushima K, Yang D, Oppenheim JJ. Interleukin-8: an evolving chemokine. Cytokine. 2022;153:155828. [DOI] [PubMed] [Google Scholar]
- 38.Zhao H, Zhang H, Liu S, Luo W, Jiang Y, Gao J. Association of peripheral blood levels of cytokines with autism spectrum disorder: a meta-analysis. Front Psychiatry. 2021;12:670200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anastasescu CM, Gheorman V, Stoicanescu E-C, Popescu F, Gheorman V, Udriștoiu I. Immunological biomarkers in Autism Spectrum Disorder: the role of TNF-Alpha and dependent trends in serum IL-6 and CXCL8. Life. 2024;14(9):1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall MB, Willis DE, Rodriguez EL, Schwarz JM. Maternal immune activation as an epidemiological risk factor for neurodevelopmental disorders: considerations of timing, severity, individual differences, and sex in human and rodent studies. Front NeuroSci. 2023;17:1135559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim E, Huh JR, Choi GB. Prenatal and postnatal neuroimmune interactions in neurodevelopmental disorders. Nat Immunol. 2024;25(4):598–606. [DOI] [PubMed] [Google Scholar]
- 42.García-Juárez M, Camacho-Morales A. Defining the role of anti-and pro-inflammatory outcomes of interleukin-6 in mental health. Neuroscience. 2022;492:32–46. [DOI] [PubMed] [Google Scholar]
- 43.Gumusoglu SB. Maternal immune mechanisms and offspring neurodevelopment. The University of Iowa; 2020.
- 44.Anilkumar S, Wright-Jin E. NF-κB as an Inducible Regulator of Inflammation in the Central Nervous System. Cells. 2024;13(6):485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majerczyk D, Ayad EG, Brewton KL, Saing P, Hart PC. Systemic maternal inflammation promotes ASD via IL-6 and IFN-γ. Biosci Rep. 2022;42(11):BSR20220713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lampiasi N, Bonaventura R, Deidda I, Zito F, Russo R. Inflammation and the potential implication of macrophage-microglia polarization in human ASD: an overview. Int J Mol Sci. 2023;24(3):2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baranova J, Dragunas G, Botellho MC, Ayub ALP, Bueno-Alves R, Alencar RR, Papaiz DD, Sogayar MC, Ulrich H, Correa RG. Autism spectrum disorder: signaling pathways and prospective therapeutic targets. Cell Mol Neurobiol. 2021;41(4):619–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.