Abstract
Background
Access to inhaled nitric oxide (iNO) is limited in low resource settings due to non-availability and high cost. There is a need for research on low-cost alternative therapies for management of persistent pulmonary hypertension of the newborn (PPHN). We aimed to compare oral sildenafil and bosentan as monotherapy in the treatment of neonates with PPHN.
Study design
In this single-centre open-label randomized controlled trial (RCT), term and late preterm neonates with PPHN, defined as pulmonary arterial systolic pressure (PASP) > 35 mmHg and requiring fraction of inspired oxygen (FiO2) > 0.21, were randomized to receive oral sildenafil and bosentan. The primary outcome was reduction of PASP by 25% within 48 h after start of drug.
Results
Thirty-six neonates were analyzed (18 in each group). Initial PASPs were similar in both groups. The median (IQR) time for the primary outcome (PASP to reduce by 25% within 48 h) was 36 (24–48) h and 96 (48–120) h in sildenafil and bosentan groups respectively (p = 0.008). There was also a higher need to add other pulmonary vasodilators in bosentan group as compared to sildenafil group (p = 0.006).
Conclusion
Sildenafil was associated with quicker reduction of PASP and FiO2 in neonates with PPHN, as compared to bosentan. Large multicentre blinded trials to assess efficacy and safety of bosentan in comparison with other pulmonary vasodilators would help to get a clearer understanding of its role in the management of PPHN, particularly for use in resource-limited settings that lack iNO.
Clinical trial registration:
https://ctri.nic.in/Clinicaltrials/rmaindet.php? trialid=63997&EncHid=39716.16132&modid=1&compid=19[CTRI/2022/06/043328].
Supplementary Information
The online version contains supplementary material available at 10.1186/s12887-024-05107-0.
Keywords: Bosentan, Persistent pulmonary hypertension of the newborn, Pulmonary arterial pressure, Pulmonary vasodilator, Randomized controlled trial, Resource-limited setting, Sildenafil
Background
Persistent pulmonary hypertension of the newborn (PPHN) is characterized by inadequate circulatory adaptation at birth, with persistence of high pulmonary arterial pressures (PAP) and low pulmonary blood flow (PBF) in postnatal period. PPHN is associated with labile hypoxemia and associated with high mortality and morbidity, especially in low-and-middle-income countries (LMIC) [1–3]. Neonates with PPHN are born at term or late preterm; although about 2% of preterm infants < 34 weeks gestational age (GA) may have evidence of high PAP [4].
Supplemental oxygen, optimal ventilation and lung recruitment followed by pulmonary vasodilator therapy are the mainstay of treatment for PPHN [5]. Inhaled nitric oxide (iNO) is a potent and selective pulmonary vasodilator, without effect on systemic blood pressure and is used in term and early-term infants with PPHN and hypoxemic respiratory failure [6, 7]. iNO is expensive and unavailable in many resource-limited settings. In addition, as many as 30% of PPHN cases fail to respond to iNO [8]. Other low-cost pulmonary vasodilators, such as sildenafil, bosentan and milrinone play an essential role in treatment of PPHN in such settings [9].
Sildenafil is a phosphodiesterase-5 (PDE5) inhibitor and dilates pulmonary vasculature via the cGMP pathway [8]. In the 2017 Cochrane Review, the authors concluded that sildenafil has potential for reducing mortality and improving oxygenation in PPHN, especially in resource-limited settings where iNO is not available [10].
Bosentan is an oral endothelin-1 receptor antagonist. The primary action of bosentan is prevention of vasoconstriction, which may indirectly lead to pulmonary vasodilation [11]. Recently, trials have been undertaken to study efficacy of bosentan in PPHN, with mixed results. A retrospective study of 40 neonates with PPHN concluded that bosentan alone or in combination with iNO, was a safe and effective treatment to improve oxygenation index (OI) and oxygen saturation (SpO2) [12]. In a randomized placebo-controlled trial, bosentan monotherapy in a setting without iNO or ECMO showed significant improvement in OI and SpO2 [13]. However, in a multicentre randomized controlled trial (RCT), bosentan was well tolerated as an adjuvant therapy with iNO, but did not improve oxygenation or outcomes in patients with PPHN [14].
Although sildenafil and bosentan are being used for management of PPHN as independent drugs or as adjuvant therapy, there are few studies that compare the efficacy and safety of the two drugs [15, 16]. Access to iNO in Indian NICUs is limited due to non-availability or high cost [9]. There is therefore, a compelling need for research on less expensive alternative pulmonary vasodilators for the management of PPHN. In this RCT, we aimed to compare the effects of oral sildenafil and bosentan on reduction of pulmonary arterial pressure, in late preterm and term neonates with PPHN.
Materials and methods
This was a single-center, open-label, parallel RCT, conducted at a level III NICU of a tertiary care teaching hospital. The study was approved by the institutional ethics committee and registered in the clinical trials registry of India [CTRI/2022/06/043328] [17-06-2022]. The enrolment period was from July 2022 to January 2024.
Term and late preterm neonates (GA ≥ 34 weeks) who were diagnosed with PPHN, defined as pulmonary artery systolic pressure (PASP) more than 35 mmHg, as calculated by tricuspid regurgitant (TR) peak jet velocity by echocardiogram, and fraction of inspired oxygen (FiO2) requirement > 0.21, were enrolled. Neonates with congenital heart disease (except patent ductus arteriosus, single muscular ventricular septal defect < 4 mm, or atrial septal defect < 6 mm), congenital diaphragmatic hernia, and lethal congenital anomalies were excluded. Neonates with PPHN, who were on pulmonary vasodilators before randomization, including those on milrinone for cardiac dysfunction at the time of enrolment, were excluded.
During the study period, all eligible neonates were screened for PPHN by echocardiography. Neonatal physicians trained in functional echocardiography (FnECHO) measured PASP as calculated by TR peak jet velocity using modified Bernoulli’s equation (Phillips 50G, 12–4 MHz high-frequency phased array transducer probes). We enrolled only those patients in whom we were able to quantify PASP from a complete envelope of TR jet. Neonates meeting inclusion criteria were enrolled after obtaining informed consent from either of the parents. Patients were randomized into two groups - oral sildenafil and oral bosentan in a 1:1 ratio by computer-generated simple randomization. Details of baseline characteristics of the neonates were recorded. Neonates randomized into oral sildenafil group received 2 mg/kg per dose every 6 h. An oral tablet of Sildenafil (Penegra-Zydus Healthcare Ltd, Ahmedabad, Gujarat, India) of strength 20 mg was dissolved in 20 ml distilled water, and a calculated dose was given through the orogastric tube. Neonates randomized into oral bosentan group were given 1 mg/kg per dose every 12 h. An oral tablet of Bosentan (Sun Pharmaceutical Industries Ltd, Mumbai, Maharashtra, India) of strength 62.5 mg was dissolved in 30 ml distilled water, and a calculated dose was given through the orogastric tube. FnECHO was performed every 24 h or earlier if clinically indicated, until the PASP decreased below 30 mmHg, and the neonate did not require respiratory support. Any adverse effects after administration of sildenafil or bosentan were documented and the drug was stopped if a major event such as systemic hypotension, pulmonary hemorrhage, or liver dysfunction was observed. If PPHN persisted at time of stoppage of study drug, milrinone was used as alternative therapy. Once PASP decreased below 30 mmHg, and the FiO2 requirement was 0.21 (with or without respiratory support), drugs were tapered and stopped as per protocol. Oral sildenafil dosing interval was tapered from 6 h to 8 h to 12 h to 24 h regime every 24 h and then stopped. Oral bosentan was tapered from 12 h regime to 24 h regime for 24 h and then stopped.
Responders to therapy were defined as infants with reduction of PASP by 25% within 48 h after use of oral sildenafil or bosentan. Treatment failure was defined as need for use of any other pulmonary vasodilator in addition to intervention drug, or failure of reduction of PASP by 25% within 48 h after starting intervention drug. The secondary outcomes compared between both groups included changes in FiO2 and SpO2, duration of ventilation, hospital stay, respiratory support, mortality and time taken to achieve full feeds.
Sample size estimation
Our primary outcome was hours to reduce estimated PASP by 25% from baseline. Our preliminary data with sildenafil had shown that 39 ± 20 h were needed to reduce PASP by 25%. We planned a study of a continuous response variable from sildenafil and bosentan in a 1:1 ratio. If the true difference in the time for reduction of PASP by 25% between sildenafil and bosentan was 20 h, we had to study 17 experimental and 17 control subjects to be able to reject the null hypothesis that the population means of the experimental and control groups are equal with probability (power) 0.8. The Type I error probability associated with the test of this null hypothesis was 0.05 [17].
Statistical analysis
Statistical analysis was performed by SPSS version 25.0 (IBM SPSS Statistics). Categorical variables were expressed as percentages (analyzed using Chi-Square test). Continuous variables were described as mean ± SD (analyzed using independent t-test) if they followed a normal distribution; and as median (IQR) if they followed a non-normal distribution (analyzed using Mann Whitney U Test). Friedman test was used to compare before and after data of more than two dependent groups when data was not normally distributed. P-value < 0.05 was considered statistically significant. Intention to treat analysis was done for primary and secondary outcomes.
Results
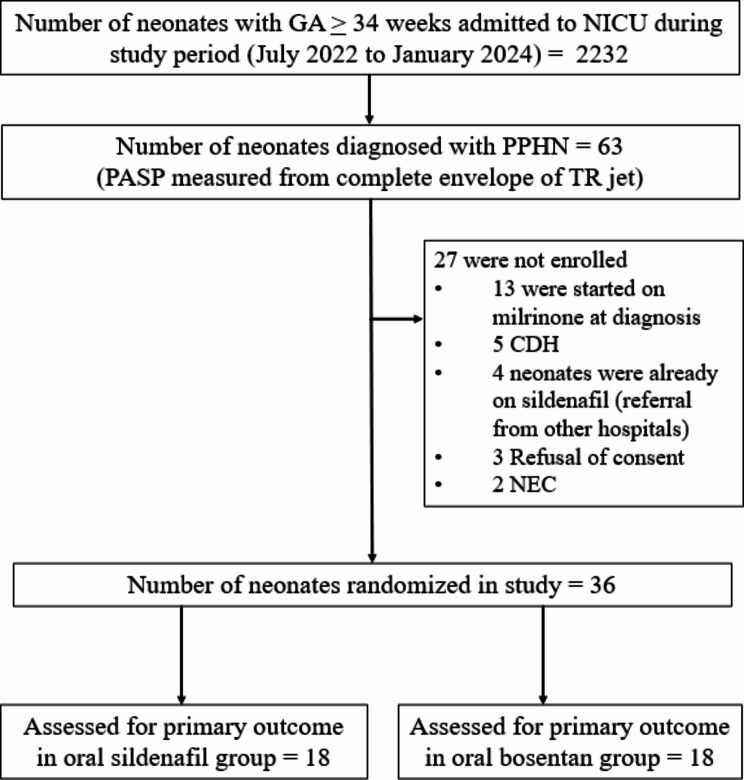
The flow chart of the study is illustrated in Fig. 1. Sixty-three neonates were diagnosed to have PPHN by using FnECHO, of which 36 were randomized to oral sildenafil or oral bosentan group (18 neonates in each arm).
Fig. 1.
Study flow diagram. (NICU, neonatal intensive care unit; GA, gestational age; PPHN, persistent pulmonary hypertension of the newborn; PASP, pulmonary artery systolic pressure; TR, tricuspid regurgitant; CDH, congenital diaphragmatic hernia; NEC, necrotising enterocolitis)
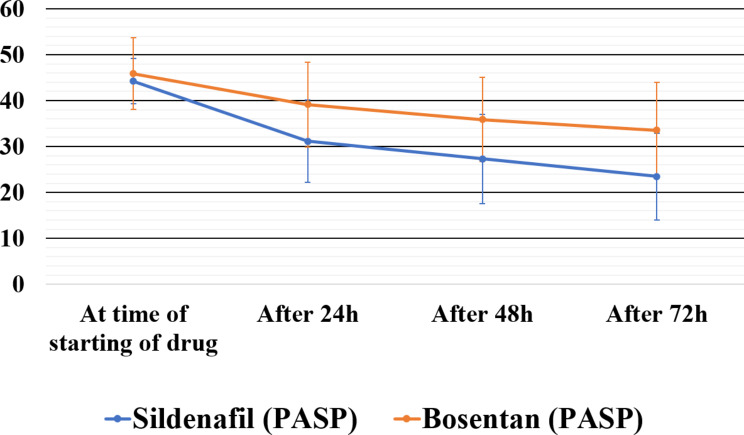
The baseline maternal and neonatal characteristics in both groups were similar (Table 1). The median (IQR) time taken for PASP to reduce by 25% was significantly shorter with sildenafil [36 (24–48) h] compared to bosentan [96 (42–120) h] (p-value 0.008, Table 2). In sildenafil group, 3 (16.6%) cases required addition of another pulmonary vasodilator due to lack of response, cardiac dysfunction, or hypotension. All three subjects received milrinone. Of the three neonates, one neonate was diagnosed to have Multisystem Inflammatory Syndrome Neonate (MIS-N), and developed catecholamine-resistant shock requiring adrenaline and noradrenaline, and another neonate was started on noradrenaline for systemic hypotension. In bosentan group, in 11 (61.1%) cases, additional pulmonary vasodilator therapy (milrinone or sildenafil depending on the presence or absence of cardiac dysfunction) was required due to lack of response or cardiac dysfunction or hypotension. Out of the five cases where milrinone was started, two cases required addition of noradrenaline for systemic hypotension. The details of these therapies are depicted in Table 2. As depicted in Fig. 2 we also observed a statistically significant decrease in PASP in sildenafil group at 24 h, 48 h, and 72 h as compared to bosentan group (p-value = 0.024, 0.008, and 0.001 respectively).
Table 1.
Comparison of maternal and neonatal characteristics
| Maternal characteristics | Oral Sildenafil n = 18 |
Oral Bosentan n = 18 |
|---|---|---|
|
Primigravida n (%) Hypothyroidism n (%) GDM n (%) Hypertensive disorder n (%) Abruptio placenta n (%) MSAF n (%) Cesarean section n (%) |
8 (44.4%) 3 (16.6%) 2 (11.1%) 6 (33.3%) 0 9 (50%) 13 (72.2%) |
9 (50%) 5 (27.7%) 3 (16.6%) 2 (11.1%) 1 (5.5%) 9 (50%) 15 (83.3%) |
| Neonatal characteristics | ||
|
Gestational age (weeks) * Birth weight (grams) * Female n (%) SGA n (%) AGA n (%) APGAR at 5 min ** Resuscitation required n (%) |
37.82 (2.27) 2720 (620) 8 (44.4%) 2 (11.1%) 16 (88.9%) 8.5 (7–9) 5 (27.2%) |
38.18 (1.93) 2710 (470) 11 (61.1%) 3 (16.6%) 15 (83.4%) 9 (7–9) 8 (44.4%) |
|
Etiology of PPHN n (%) MAS MIS-N RDS TTN Pneumonia EONS |
9 (50%) 3 (16.6%) 1 (5.5%) 1 (5.5%) 3 (16.6%) 0 |
9 (50%) 0 5 (27.2%) 0 2 (11.1%) 1 (5.5%) |
| Time of initiation of study drug (HOL)** | 13 (5.75–32.75) | 12 (5.75–40.50) |
| Initial PASP (mmHg)* | 46.50 (5.54) | 46.44 (6.96) |
*Mean (± SD), ** Median (IQR)
GDM, gestational diabetes mellitus; MSAF, meconium-stained amniotic fluid; SGA, small for gestational age; AGA, appropriate for gestational age; PPHN, persistent pulmonary hypertension of the newborn; MAS, meconium aspiration syndrome; MIS-N, multisystem inflammatory syndrome of the neonate; RDS, respiratory distress syndrome; TTN, transient tachypnoea of the newborn; EONS, early onset neonatal sepsis; HOL, hours of life; PASP, pulmonary arterial systolic pressure
Table 2.
Primary and secondary outcomes
| Outcome | Oral sildenafil | Oral bosentan | p-value |
|---|---|---|---|
|
Primary outcome Hours taken for pulmonary systolic arterial pressure to reduce by 25%, Median (IQR) |
36 (24–48) | 96 (42–120) | 0.008 α |
| Secondary outcomes | |||
| Failure rate, n (%) | 3 (16.6%) | 12 (66.6%) | 0.002 β |
|
Tapering of drug n (%) Tapering possible Changed as no response Stopped due to hypotension |
16 (88.8%) 1 (5.5%) 1 (5.5%) |
8 (44.4%) 9 (50%) 1 (5.5%) |
0.011 β |
| Requirement of other pulmonary vasodilators, n (%) | 3 (16.6%) | 11 (61.1%) | 0.006 β |
|
Requirement of vasopressor agent, n (%) Noradrenaline Adrenaline |
2 (11.1%) 1 (5.5%) |
2 (11.1%) 0 |
0.99 β |
|
Hours on invasive ventilation Median (IQR) |
82 (43–98) n = 13 |
67 (30-129.5) n = 13 |
0.793 α |
| Hours on non-invasive ventilation, Median (IQR) |
90 (46–124) n = 17 |
132 (79–167) n = 18 |
0.079 α |
|
Hours taken to reach room air (FiO2 0.21 and off respiratory support) Median (IQR) |
114 (85–200) | 179 (139.5–287) | 0.077 α |
|
Days taken to achieve full feeds, Median (IQR) |
5(4.5-7) n = 16 |
6.5 (5.75–7.25) n = 18 |
0.153 α |
|
Duration of hospital stay (days), Median (IQR) |
10.5 (7.75-14) | 18 (10.75–20.25) | 0.064 α |
|
Complications n (%) Hypotension |
1(5.5%) | 1(5.5%) | > 0.99 β |
| Mortality n (%) | 1 (5.5%) | 0 | 0.310 β |
|
α Mann-Whitney U-test β Chi-Square test | |||
Fig. 2.
Mean (± SD) pulmonary artery systolic pressures (PASP) in mmHg over the first 72 h after the start of intervention drug
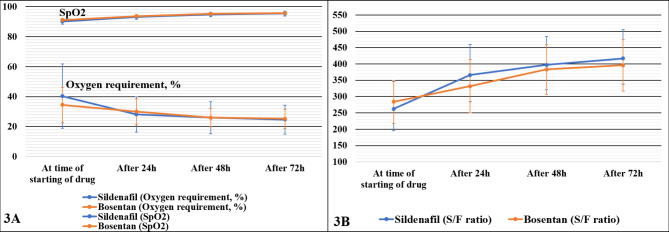
The mean (± SD) inspired oxygen requirement at start of intervention was similar in both groups (sildenafil 40.3% [21.4%], bosentan 34.4% [11.7%]) (p-value = 0.31). At 24 h, the decrease in inspired oxygen in the sildenafil group was more significant than for the bosentan group (p-value = 0.021 Fig. 3A). By 48 h and 72 h, there was a further reduction in inspired oxygen requirement in both groups, but the decrease was not statistically significant (p-value = 0.168 and 0.152 respectively). The mean (SD) oxygen saturation (SpO2) and SpO2/FiO2 (S/F) at time of intervention were similar in sildenafil and bosentan groups (p-value = 0.08 and 0.275 respectively). There was an increase in both parameters in both groups after intervention at 24 h, 48 h, and 72 h, but the difference was not statistically significant. (Figure 3A and B)
Fig. 3.
A: Mean (± SD) oxygen requirement in %, and oxygen saturation in % (SpO2) over the first 72 h after the start of intervention drug. B: Mean (± SD) SpO2/FiO2 (S/F) ratio over the first 72 h after the start of intervention drug
Treatment failure was significantly higher in bosentan group (66.6%) as compared to sildenafil group (16.6%, p-value 0.002). The need for additional pulmonary vasodilators was also higher in bosentan group (61.1%) as compared to the sildenafil group (16.6%) (p-value 0.006). There was no statistical difference in median (IQR) duration of invasive ventilation, non-invasive ventilation, number of days to reach full feeds, time taken to reach room air (FiO2 0.21 and weaned off respiratory support), duration of hospital stay (Table 2). There was only one death in the study, which was in the sildenafil group; a neonate diagnosed to have MIS-N who died due to catecholamine-resistant shock.
Discussion
In this RCT, we compared enteral monotherapy with sildenafil and bosentan in PPHN, confirmed with echocardiography. We found that although both sildenafil and bosentan were well tolerated, sildenafil was quicker in reducing PASP and FiO2 compared to bosentan in late preterm and term neonates with PPHN. There was also a higher need to add other pulmonary vasodilators in bosentan group compared to sildenafil group.
In a recent survey on management of PPHN in Indian NICUs, only 25% of respondents reported the availability of iNO in their units [9]. Most Indian NICUs therefore used alternative non-selective pulmonary vasodilators for the treatment of PPHN. Sildenafil was reported as the most commonly used pulmonary vasodilator followed by milrinone, and bosentan. Among these, bosentan was a relatively less preferred vasodilator of choice. However, this could be because bosentan as monotherapy has been less studied compared to sildenafil. Likewise, in our neonatal unit, iNO was not available for treatment of PPHN during the study period. Sildenafil and milrinone were commonly used and bosentan was occasionally used as an add-on pulmonary vasodilator. The use of bosentan in neonates for the management of PPHN has gained some interest over the last decade. We felt that research on safe and effective low-cost alternative pulmonary vasodilators is of key importance for management of PPHN in countries with limited access to iNO.
Few studies in the literature have compared bosentan with sildenafil in the management of PPHN. Maneenil et al. conducted a retrospective study of 31 neonates diagnosed with PPHN who did not receive iNO (16 bosentan and 15 sildenafil) in Thailand [15]. Oxygenation index improved within 24 h in both groups, and SpO2 significantly improved at 96 h of bosentan and 36 h of sildenafil treatment respectively, and authors concluded that both drugs were safe and effective in treatment of PPHN, especially in centres lacking iNO. However, this was a retrospective study with inherent bias related to lack of randomization. In our study, SpO2 increased in both groups at 24 h, 48 h and 72 h, but in sildenafil group, oxygen requirement reduced considerably at 24 h as compared to bosentan, although the reduction was similar at 48 h and 72 h. The other study comparing bosentan with sildenafil was an RCT by Farhangdoust and colleagues in Iran, in a unit without iNO availability [16]. Forty newborns with PPHN were analyzed to compare the efficacy and safety of bosentan (15 patients) and sildenafil (25 patients). Bosentan was found to be as effective as sildenafil in reducing PAP and improving cardiac output. However, bosentan reduced PAP over a shorter period as compared to sildenafil. Although both groups were matched, their study had a large number of preterm infants with a mean GA of 33 weeks. Our study comprised a more homogeneous population of late preterm and term neonates.
While Maneenil et al. used OI and SpO2 as outcome measures, Farhangdoust et al. included improvement in PAP, TR, and ejection fraction (EF) on echocardiogram repeated on day 6 and day 12 of starting study drug as outcomes [15, 16]. Since baseline PASP might be different in both groups, we used a reduction in PASP by 25% within 48 h as our primary outcome measure. Pathophysiology of PPHN is dynamic, and hence compared to the RCT by Farhangdoust et al., we measured the PASP more frequently, every 24 h after initiation of the study drug and compared the decrease in PASP in both groups over time. As some of our patients were on non-invasive ventilation, mean airway pressure could not be reliably measured. Also, since not all patients had arterial access, we used S/F ratio instead of OI as a surrogate marker of oxygenation in our study. In our investigation, baseline PASP for bosentan and sildenafil groups were similar, so it was easier to compare the decrease in pressure over time. However, in the study by Farhangdoust et al., the PASP was higher in bosentan group at baseline, and they found that all echo parameters (PAP, PI, EF) were similar in both groups at the 12 days echo [16]. Based on these findings, the authors concluded that by 12 days, the bosentan group having a higher baseline PASP, resulted in a higher reduction of PAP. In contrast to this, in our study, we found a consistent difference in the reduction of PASP at 24 h, 48 h, and 72 h, which were statistically significant in favour of sildenafil group at each time point; thus, indicating that sildenafil was more effective in reduction of PASP over time, as compared to bosentan. The pharmacokinetics of bosentan in neonates are still poorly understood and its enteral absorption is said to be slow during first 12 h of therapy. PPHN likely requires drugs with an earlier onset of action, which may explain the lack of efficacy of bosentan in this study [14].
Both study drugs were well tolerated in our RCT, and only one neonate in each group developed hypotension. Previous studies also demonstrated a good safety profile for both sildenafil and bosentan for PPHN [12–15, 18, 19].
In our study, milrinone was started for patients who developed cardiac dysfunction during course of management of PPHN (bosentan-28%, sildenafil-16%). There was no statistically significant difference between use of milrinone in either group. Maneenil et al. also reported the usage of milrinone (bosentan-25%, sildenafil-20%) in their retrospective study [15]. However, in the RCT by Farhangdoust et al., milrinone use was higher (sildenafil-70%, bosentan 30%). Additionally, this study utilized dopamine as an inotrope, (bosentan 71%, sildenafil 29%), with an unequal distribution of dopamine and milrinone in both groups [16]. It is unclear from the above studies whether milrinone was started before or after randomization. Since milrinone has pulmonary vasodilatory properties, apart from the inotropic effect, we excluded neonates who had cardiac dysfunction requiring milrinone infusion at time of enrolment, as its vasodilatory effect would have already started before randomization.
There was only one death in our study, a late preterm neonate in the sildenafil group diagnosed to have MIS-N, who died due to catecholamine-resistant shock. The lack of severe PPHN patients in our study may account for low mortality. Further research is needed to assess survival rates and determine long-term neurodevelopmental outcomes of neonates who have been treated with bosentan and sildenafil, as well as to evaluate their effectiveness in comparison to other pulmonary vasodilators.
Strengths and limitations
With limited access to iNO, research on low-cost alternative therapies for management of PPHN in LMIC countries is the need of the hour. There are very few prospective studies in the literature, and this is the first RCT from India comparing sildenafil and bosentan as monotherapy in PPHN. There are several limitations to our study however. The sample size was small, so there is a possibility of type II error. The typical frequency of echocardiograms was every 24 h and higher frequency of evaluation might have generated different results. As the dosage, dosing interval, and tapering protocol of the drugs were different in both groups, healthcare providers were not blinded to the intervention drugs in our study. Lack of blinding and allocation concealment can introduce bias into study results. OI was not included in our study and we used a non-invasive index (S/F ratio) as a surrogate marker of oxygenation. Since we excluded patients who had cardiac dysfunction at baseline and were started on milrinone before randomization, our results may not apply to such neonates, who may have had severe PPHN at the start of the study. In order to maintain uniformity in the measurement method, we used PASP calculated from TR peak jet velocity as a measure of PAP, and some cases of PPHN that get diagnosed by measurement of shunt pressures across a patent ductus arteriosus (PDA) might have been missed. Tricuspid valve regurgitation is not always observed in patients with PPHN. Similarly, we enrolled only neonates in whom PASP was quantifiable from a complete TR jet envelope. As a result, some cases of PPHN may have remained undetected at the time of enrolment.
Conclusion
In this study, we found that sildenafil was associated with a more rapid reduction of PASP and FiO2 in neonates with PPHN, as compared to bosentan. Although both oral sildenafil and bosentan were well tolerated, the need to add other pulmonary vasodilators was more frequent in bosentan group. Sildenafil is a well-studied and widely used medication for treatment of PPHN. However, the role of bosentan is relatively unclear due to limited research. Large multicentre blinded trials designed to assess efficacy and safety of bosentan in comparison with other pulmonary vasodilators would help to get a clearer understanding of its role in the management of PPHN.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the participating neonates and their parents, for providing consent, without which the study would not have been possible.
Abbreviations
- iNO
Inhaled nitric oxide
- PPHN
Persistent pulmonary hypertension of the newborn
- FiO<
Subscript>2</Subscript> – Fraction of inspired oxygen
- RCT–
Randomised controlled trial
- PASP –
Pulmonary arterial systolic pressure
- PAP–
Pulmonary artery pressure
- PBF –
Pulmonary blood flow
- LMIC –
Low- and middle-income countries
- GA –
Gestational age
- PDE-5 –
Phosphodiesterase inhibitor
- OI–
Oxygenation index
- SpO<Subscript>2</Subscript>–
Oxygen saturation
- FnECHO –
Functional echocardiography
- MIS-N –
Multisystem inflammatory syndrome-Neonate
- PDA –
Patent ductus arteriosus
Author contributions
P.S. (Pradeep Suryawanshi), S.D., and A.K. conceptualized and designed the study. A.K., P.S. (Pari Singh), R.G., S.D., and S.L. contributed to the acquisition, analysis, and interpretation of data. A.K. wrote the initial draft. S.D., S.L., R.M., and P.S. reviewed the draft. All authors reviewed the final draft of the study and approved it for publication. All authors agree to be personally accountable for the submitted literature.
Funding
No funding or sponsorship was received for this study.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was started after approval by the institutional ethics committee, Bharati Vidyapeeth Deemed University, vide letter number BVDUMC/IC/01 and registered in the clinical trials registry of India [CTRI/2022/06/043328] [17-06-2022]. Informed consent was obtained from legal guardians of the subjects enrolled in the study for study participation. This study was performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lakshminrusimha S, Keszler M. Persistent Pulmonary Hypertension of the Newborn Educational Gap [Internet]. http://neoreviews.aappublications.org/ [DOI] [PMC free article] [PubMed]
- 2.Sardar S, Pal S, Mishra R. A retrospective study on the profile of persistent pulmonary hypertension of newborn in a tertiary care unit of Eastern India. J Clin Neonatol. 2020;9(1):18. [Google Scholar]
- 3.Arshad MS, Adnan M, Anwar-Ul-haq HM, Zulqarnain A. Postnatal causes and severity of persistent pulmonary hypertension of newborn. Pak J Med Sci. 2021;37(5):1387–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathew B, Lakshminrusimha S. Review persistent pulmonary hypertension in the newborn. Volume 4. Children. MDPI; 2017. [DOI] [PMC free article] [PubMed]
- 5.Singh Y, Lakshminrusimha S. Pathophysiology and management of Persistent Pulmonary hypertension of the Newborn. Clinics in Perinatology. Volume 48. W.B. Saunders; 2021. pp. 595–618. [DOI] [PMC free article] [PubMed]
- 6.Barrington KJ, Finer N, Pennaforte T, Altit G. Nitric oxide for respiratory failure in infants born at or near term. Vol. 2017, Cochrane Database of Systematic Reviews. John Wiley and Sons Ltd; 2017. [DOI] [PMC free article] [PubMed]
- 7.Mitra S, Altit G. Inhaled nitric oxide use in newborns. Paediatr Child Health. 2023;28(2):119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Travadi JN, Patole SK. Phosphodiesterase Inhibitors for Persistent Pulmonary Hypertension of the Newborn: A Review. Vol. 36, Pediatric Pulmonology. 2003. pp. 529–35. [DOI] [PubMed]
- 9.Singh P, Deshpande S, Nagpal R, Garegrat R, Gupta S, Suryawanshi P. Management of neonatal pulmonary hypertension-a survey of neonatal intensive care units in India. BMC Pediatr. 2023;23(1). [DOI] [PMC free article] [PubMed]
- 10.Kelly LE, Ohlsson A, Shah PS. Sildenafil for pulmonary hypertension in neonates. Vol. 2017, Cochrane Database of Systematic Reviews. John Wiley and Sons Ltd; 2017. [DOI] [PMC free article] [PubMed]
- 11.Motte S, McEntee K, Naeije R. Endothelin receptor antagonists. Pharmacol Ther. 2006;110:386–414. [DOI] [PubMed] [Google Scholar]
- 12.Maneenil G, Thatrimontrichai A, Janjindamai W, Dissaneevate S. Effect of bosentan therapy in persistent pulmonary hypertension of the newborn. Pediatr Neonatol. 2018;59(1):58–64. [DOI] [PubMed] [Google Scholar]
- 13.Mohamed WA, Ismail M. A randomized, double-blind, placebo-controlled, prospective study of bosentan for the treatment of persistent pulmonary hypertension of the newborn. J Perinatol. 2012;32(8):608–13. [DOI] [PubMed] [Google Scholar]
- 14.Steinhorn RH, Fineman J, Kusic-Pajic A, Cornelisse P, Gehin M, Nowbakht P et al. Bosentan as Adjunctive Therapy for Persistent Pulmonary Hypertension of the Newborn: Results of the Randomized Multicenter Placebo-Controlled Exploratory Trial ARTICLE IN PRESS THE JOURNAL OF PEDIATRICS • www.jpeds.com. 2016; Available from: 10.1016/j.jpeds.2016.06.078. [DOI] [PubMed]
- 15.Maneenil G, Talek S, Thatrimontrichai A, Janjindamai W, Dissaneevate S. The use of bosentan and sildenafil as rescue therapy in persistent pulmonary hypertension of the newborn: a single center’s experience. Prog Pediatr Cardiol. 2022;67.
- 16.Farhangdoust S, Mehralizadeh S, Bordbar A. Comparison of the effects of bosentan and sildenafil in the treatment of persistent pulmonary arterial hypertension in infants. J Clin Neonatol. 2020;9(4):249. [Google Scholar]
- 17.Dupont WD, Plummer WD, Power. and Sample Size Calculations for Studies Involving Linear Regression [Internet]. Vol. 19, Controlled Clin Trials. 1998. http://www.mc.vanderbilt.edu/prevmed/psintro.htm [DOI] [PubMed]
- 18.Chetan C, Suryawanshi P, Patnaik S, Soni NB, Rath C, Pareek P et al. Oral versus intravenous sildenafil for pulmonary hypertension in neonates: a randomized trial. BMC Pediatr. 2022;22(1). [DOI] [PMC free article] [PubMed]
- 19.Vargas-Origel A, Gómez-Rodríguez G, Aldana-Valenzuela C, Vela-Huerta MM, Alarcón-Santos SB, Amador-Licona N. The use of sildenafil in persistent pulmonary hypertension of the newborn. Am J Perinatol. 2010;27(3):225–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.