Abstract
Background
Developing interventions for older adults with subjective cognitive decline (SCD) has the potential to prevent dementia in this at-risk group. Preclinical models indicate that Citrus-derived phytochemicals could benefit cognition and inflammatory processes, but results from clinical trials are still preliminary. The aim of this study is to determine the effects of long-term supplementation with Citrus peel extract on cognitive performance and inflammation in individuals with SCD.
Methods
Eighty participants were randomly assigned to active treatment (400 mg of Citrus peel extract containing 3.0 mg of naringenin and 0.1 mg of auraptene) or placebo at 1:1 ratio for 36 weeks. The primary endpoint was the change in the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) total score across the 36-week trial period. Other cognitive outcomes included tests and scales evaluating verbal memory, attention, executive and visuospatial functions, and memory concerns. The secondary endpoint was the change of interleukin-8 (IL-8) levels over the 36-week trial period in a subsample of 60 consecutive participants. An Intention-to-treat approach with generalized linear mixed models was used for data analysis.
Results
The RBANS total score showed significant improvement in both Citrus peel extract and placebo groups at 36 weeks (p for time < .001, d = 0.36, p time x treatment = .910). Significant time effects were also found in cognitive domains of short- and long-term verbal memory (p < .001) and scales of subjective memory (p < .01), with no significant time x treatment interaction. The largest effect sizes were observed in verbal memory in the placebo group (d = 0.69 in short-term, and d = 0.78 in long-term verbal memory). Increased IL-8 levels were found at 36-week follow-up in both Citrus peel extract and placebo groups (p for time = .010, d = 0.21, p time x treatment = .772). Adverse events were balanced between groups.
Conclusions
In this randomized clinical trial, long-term Citrus peel extract supplementation did not show cognitive benefits over placebo in participants with SCD, possibly due to high placebo response. These findings might have specific implications for designing future nutraceutical trials in individuals experiencing SCD.
Trial registration
The trial has been registered at the United States National Library of Medicine at the National Institutes of Health Registry of Clinical Trials under the code NCT04744922 on February 9th, 2021 (https://www.clinicaltrials.gov/ct2/show/NCT04744922).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12937-024-01039-8.
Keywords: Subjective cognitive decline, Randomized clinical trial, Nutraceutical, Placebo response, Interleukin-8
Background
The concept of Alzheimer’s disease (AD) continuum recognized AD as a spectrum of clinical manifestations spanning from clinically asymptomatic to severely impaired [1]. Subjective cognitive decline (SCD) refers to individuals’ perceived decline in cognitive abilities compared to their previous performance, without objective cognitive deficits [2]. A recent systematic review and meta-analysis of prospective longitudinal studies estimated that SCD conferred a twofold excess risk for cognitive impairment and dementia [3], suggesting that some individuals with SCD may be in the preclinical phase of the AD continuum. A set of clinical features referred to as SCD plus [4] has been associated with AD pathology [5], enabling the identification of SCD individuals at high risk of cognitive progression. Implementing targeted treatments prior to the appearance of objective cognitive impairment has the potential to delay or prevent the progression across the continuum. Cognitive training or lifestyle interventions that could enhance cognition have been examined [6], but high-quality studies in this field are scanty [7].
There is an increased scientific interest in evaluating plant-derived nutraceuticals to promote cognitive function [8, 9], even in individuals with SCD [10, 11]. The use of over-the-counter supplements to boost memory is rapidly increasing among older adults [12, 13]. However, data about their efficacy are still controversial. A recent systematic review investigated the effect of herbal and nutritional medicines on cognitive function in older people with and without subjective cognitive impairment [14]. The quality assessment indicated high concern for the risk of bias across 21 randomized clinical trials. This finding prevented the authors from drawing firm conclusions about treatment efficacy in this population, claiming for future high-quality studies [14]. Common sources of bias regarded the domains of randomization, deviation from intended effect (i.e., no intention to treat analysis), treating of missing data and outcome measurement. Beside efficacy, issues concerning the quality of nutraceutical manufacturing and its impact on safety have been raised. Low quality of commercially available nutraceuticals can derive from the unknown origin of raw material, the presence of contaminants, the use of complex mixtures of plants with no accurate identification of the main active components, and the poor stability of the active components [15, 16]. In addition, chemically synthetized nutraceuticals may show different physiological properties than naturally occurring forms found in the whole plant [8].
To overcome the aforementioned shortcomings in the field, we conducted a randomized controlled trial investigating the cognitive and biological effects of a 36-week treatment with Citrus peel extract in older adults experiencing SCD [17]. The study was designed in accordance with the consolidated standards of reporting trials [18] and the recommendations of the International Academy on Nutrition and Aging Task Force [19]. The nutraceutical was manufactured according to good practice regulations in nutraceutical development [20].
We used Citrus peels as a source of nutraceutical because preliminary data from epidemiological and clinical studies in older adults showed a positive relationship between Citrus fruit intake and enhanced cognitive function, including global cognition and verbal memory [21, 22]. Concerning their phytochemical composition, Citrus peels are among the richest natural sources of flavonoids, specifically naringenin (NAR) [23], and auraptene (AUR), a phytochemical belonging to the coumarin class [24]. Naringenin and auraptene showed anti-inflammatory effects in pre-clinical models of ageing and AD, both as individual compounds and in combination [25–27]. Specifically, they have been shown to reduce the proinflammatory cytokine interleukin-8 levels (IL-8) [28, 29], which has been implicated in AD pathophysiology [30, 31].
In this paper, we report the results of the primary and secondary outcomes. We hypothesize that, in individuals taking Citrus peel extract relative to controls: (i) objective cognition, as rated by the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)—the primary endpoint—would be enhanced; and (ii) the levels of IL-8—the secondary endpoint—would be reduced.
Methods
Participants, study design and treatment
A 36-week, randomized, double-blind, parallel, monocentric, placebo-controlled clinical trial (NCT04744922) was conducted at the Laboratory of Alzheimer’s Neuroimaging and Epidemiology of the IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy, between March 2021 and August 2022. The full protocol was previously published [17]. Briefly, 80 older adults with SCD, aged 60–75 years, were recruited from the general community. Inclusion criteria were (i) the presence of SCD according to current research criteria [2] and (ii) performance within the normal range on standardized cognitive tests (see the paragraph below). The SCD criteria were operationalized to include features of the SCD plus category (i.e., the presence of a subjective decline in memory, rather than other domains of cognition, onset of SCD within the last 5 years, and worries associated with SCD expressed by the participant and/or an informant).
Exclusion criteria included: (i) cognitive performance below the normal range on two tests within a single cognitive domain (i.e., memory, executive function, or attention); (ii) the presence of current major neurological (including stroke, dementia or cognitive impairment, and cancer) or psychiatric (including major depressive disorder, bipolar disorder, and drug and alcohol dependence) disorders; (iii) severe depressive symptoms, as indicated by scores > 17 on the 30-item Geriatric Depression Scale [32] (current psychotropic therapy was allowed if at a stable dose over the previous 8 weeks); (iv) the presence of a chronic disease or acute unstable illness (respiratory, cardiovascular, digestive, renal, metabolic, hematologic, endocrine, infectious, or malignant) that would interfere with the aims of the study protocol; and (v) the use of supplements that could interfere with the study nutraceutical (e.g. cognitive enhancers). Current use of supplements was allowed if at a stable dose over the previous 8 weeks and maintained at a constant dose for the duration of the study.
Inclusion and exclusion criteria were preliminary ascertained by phone screening and thereafter by face-to-face assessment. Eligible participants were invited to the baseline visit (week 0) and randomly allocated at a 1:1 ratio to either 400 mg of Citrus limon (L.) Osbeck (Fam. Rutaceae) peel extract standardized in levels of AUR and NAR (n = 40) or placebo (n = 40). Details about randomization and sample size computation can be found in the full protocol [17]. The sample size was computed by using G*Power. The trial nutraceutical was manufactured at the Laboratory of Phytochemistry and Chemistry of Natural Products, Department of Pharmacy, University ‘G. D’Annunzio’ of Chieti-Pescara, Chieti, Italy. The description of the manufacturing process was reported in the full protocol [17]. Briefly, the plant material originated from fruits without any chemical and/or phytosanitary treatment planted in Barcellona Pozzo di Gotto (Sicily region, Italy). Fresh peels were homogenized and the semisolid material was lyophilized to obtain a fine powder. No solvents or other chemicals were used to obtain this whole dry extract. One capsule (daily dose) of active treatment contained 0.0892 ± 0.001 mg of AUR and 2.99 ± 0.004 mg of NAR. Total flavonoids and polyphenols concentrations per capsule were 69.5 ± 0.008 mg and 121.0 ± 0.012 mg, respectively. A blood sample for biomarker measurement was collected in a subsample of 60 consecutive subjects at weeks 0 and 36.
Participants attended follow-up visits at week 18 to assess cognition, compliance and safety and week 36 to assess cognition, compliance, safety, and to collect a second round of blood samples for biomarker measurement.
Outcome measures
The rationale for identifying objective cognitive performance as the main outcome of our study was based on the hypothesis that SCD individuals with preserved cognitive function had greater access to cognitive reserve, allowing them to enhance cognition or compensate for subtle cognitive deficits as a result of the intervention [6].
Primary endpoint
The primary endpoint of the study was the change from baseline to weeks 18 and 36 in the total index score on the RBANS [33]. The total index score ranges from 40 to 160, with higher scores indicating better cognition. Data on validity, reliability, and practice effects of this test were provided in the Supplementary Material. As reported in the study protocol [17], we selected the RBANS for the trial’s primary endpoint because it has been designed for clinical trial outcome measurement in early and prodromal AD; moreover, it has been recommended for drug trials and research in preclinical AD. Two Italian-validated forms (Forms A and B) were used to prevent a learning effect from serial assessments.
Secondary endpoint
The secondary endpoint of the study was the change in IL-8 levels over the 36-week trial period. These levels were measured by a multiplex immunoassay system using magnetic beads (Luminex Discovery Assay, Bio-techne). As reported in the study protocol [17], we selected IL-8 as the trial’s secondary endpoint because experimental studies showed that AUR and NAR exerted their anti-inflammatory properties mainly through IL-8 reduction; furthermore, clinical data in healthy elderly individuals suggested its potential role as a biological marker of early cognitive dysfunction.
Cognitive outcomes
Other cognitive outcomes included the mean score change in verbal memory (California Verbal Learning test, CVLT, alternate forms A and B [34]), attention and executive functions (Attentional Matrices [35]; Stroop test [36]; Trail Making Test, TMT [37]; Wisconsin Card Sorting test, WCST [38]), visuospatial function (Clock Drawing test, CDT [39]), and scales of memory concerns (Everyday Memory Questionnaire, EMQ [40]; Multifactorial Memory Questionnaire, MMQ [41]). The psychologists who administered the cognitive battery had experience as certified raters in pharmacological clinical trials and were blinded to the treatment.
Statistical analysis
Statistical analyses were performed using SPSS version 20 (SPSS Inc., Chicago, IL). P-values < 0.05 (two-tailed) were considered significant. The normality of each variable distribution by treatment group was examined with the Shapiro–Wilk test. Baseline sociodemographic, clinical, cognitive, and biological characteristics were compared between groups using the two-sample t-test (or Mann–Whitney test in the case of non-normal distribution) for continuous variables and the χ2 test for categorical variables.
We used the intention-to-treat approach. Missing data (i.e., the data from individuals lost to follow-up) were replaced using the mean-value imputation. The primary analysis was performed by using a linear mixed-effects model for repeated measures with treatment group, time (weeks 0, 18 and 36), treatment group–by–time interaction as fixed effects, while subject ID was treated as random. Moreover, a robust standard error estimator was used. A generalized linear mixed model with the same fixed and random effect specifications was run with IL-8 as the dependent variable and batch as the covariate. In both models, the addition of age, sex, education, and health-related behaviors (i.e., adherence to the Mediterranean diet and lemon consumption) as covariates did not change the results. Thus, we decided to focus on unadjusted models to prevent overfitting. Estimated means and their standard errors were reported. Each test was performed at an alpha level of 0.05 (two-sided).
For cognitive tests other than the primary endpoint, we computed composite scores to reduce the number of models. We transformed the raw score of each test into z-score using mean and standard deviation at baseline. Then, we averaged the z-scores to yield a composite score, namely: short-term verbal memory (CVLT immediate recall, and short delay free and cued recall), long-term verbal memory (CVLT long delay free and cued recall, and recognition), attention (Attentional matrices, and TMT B-A), and executive functions (WCST global score, Stroop test, and time interference). We multiplied the z-scores of TMT B-A and Stroop test by minus one to ensure that higher values of the composite scores consistently reflected improved cognitive performance. We also computed the z-score for the CDT (visuospatial function). Therefore, mixed-effects models with the same fixed and random effect specifications used for the primary endpoint were run with each composite score as the dependent variable.
Depending on the distribution of the outcome, linear mixed models or generalized linear mixed models were implemented. Since all outcome measures exhibited a nearly symmetrical, well-shaped distribution centered around a mean value, we applied the normal distribution with an identity link function and employed the maximum likelihood method for parameter estimation. To evaluate goodness-of-fit, we utilized a combination of the Akaike information criterion corrected in conjunction with the Bayesian information criterion. In the presence of outliers and/or imperfections in the shape of the distribution curve, we used a robust covariance matrix estimator.
When a cognitive outcome showed a significant change from baseline to 36 weeks, the effect size (ES) was calculated using Cohen’s d. Values of 0.20, 0.50, and 0.80 were indicative of small, medium, and large effect sizes, respectively.
Results
Study participants
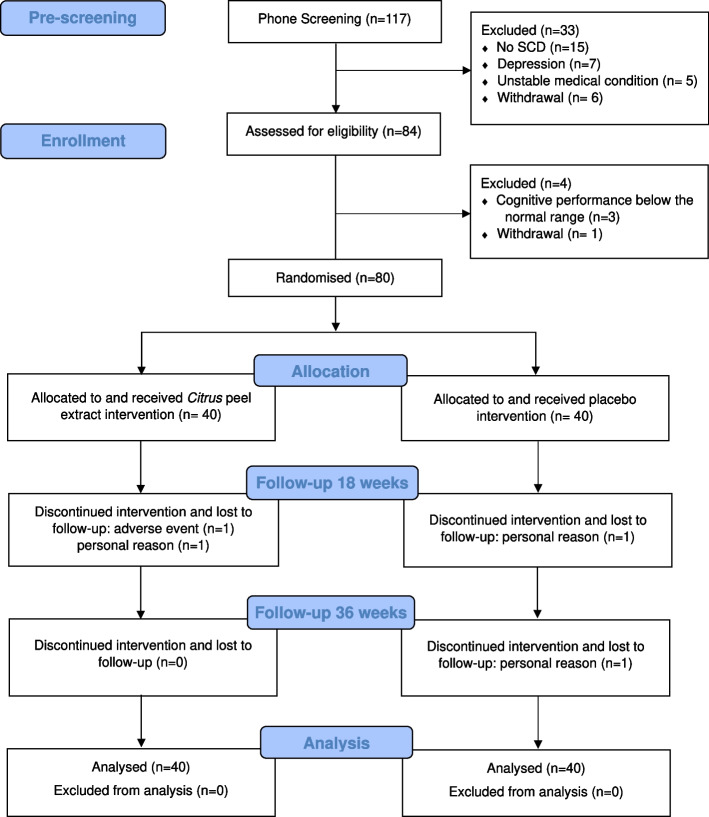
Figure 1 shows the participants’ flow in the study. A total of 80 participants (mean age 68.5 ± 4.2 years; 60 women, 75%; mean education 13.7 + 3.4 years) was randomly assigned to receive Citrus peel extract (n = 40) or placebo (n = 40). At the end of trial, 4 individuals discontinued the treatment and were lost to follow-up (n = 2 Citrus peel extract, n = 2 placebo). All participants remained in the assigned group for the intention-to-treat analysis. Both groups showed high compliance rates, as measured by the number of remaining capsules at 36 weeks (compliance rate in the total group: 98%).
Fig. 1.
CONSORT flowchart of the trial
At baseline, both groups demonstrated comparable sociodemographic and clinical characteristics (Table 1). Study participants were adherent to the Mediterranean diet (Mediterranean Diet Adherence Screener, mean score: 8.9 ± 1.8), and had low comorbidity (Cumulative Index Rating Scale, mean severity index: 1.5 ± 0.2). Prevalence of health-related behaviors (i.e., alcohol and coffee consumption, tobacco use) were similar between groups (Supplemental Table 1). The mean number of lemons consumed in a week was 2.1 ± 2.0 and 2.5 ± 2.0 in Citrus peel extract and placebo groups, respectively (p = 0.28). The most prevalent concomitant medications were drugs acting on the alimentary tract and metabolism, cardiovascular system, and supplements (60%, 56%, and 43%, respectively), with no differences between groups (Supplemental Table 2).
Table 1.
Baseline sociodemographic and clinical characteristics of the participants with SCD included in intention-to-treat analysis
| Citrus peel extract (n = 40) | Placebo (n = 40) |
|
|---|---|---|
| Sociodemographics | ||
| Age, years | 67.7 ± 4.0 | 69.3 ± 4.3 |
| Gender (female) | 32 (80%) | 28 (70%) |
| Education, years | 13.9 ± 3.7 | 13.5 ± 3.2 |
| Nutritional status and comorbidity | ||
| Body Mass Index | 25.1 ± 4.8 | 23.9 ± 3.9 |
| Mediterranean Diet Adherence Screener | 9.0 ± 1.9 | 8.8 ± 1.8 |
| Cumulative Illness Rating Scale | 1.5 ± 0.2 | 1.5 ± 0.2 |
| Depressive and anxious symptoms | ||
| Geriatric Depression Scale | 5.8 ± 4.2 | 6.6 ± 3.9 |
| State-Trait Anxiety Inventory—state | 34.1 ± 5.4 | 36.4 ± 7.3 |
| Global cognition | ||
| Mini Mental State Examination | 29.2 ± 0.8 | 29.2 ± 1.0 |
| Primary endpoint | ||
| RBANS Total Score | 103.6 ± 9.6 | 101.5 ± 11.6 |
| Secondary endpoint | ||
| Interleukin-8 (pg/mL) | 10.6 ± 4.5 | 11.0 ± 4.8 |
RBANS Repeatable Battery for the Assessment of Neuropsychological Status
Values denote mean ± SD or number (percentage)
Table 2.
Estimated mean change and effect size in cognitive outcomes showing a significant time effect from the mixed models, by treatment group
| Week 0 | Week 18 | Week 36 | Mean change (weeks 0–36) | Effect size change | p time | p time x treatment | |
|---|---|---|---|---|---|---|---|
| RBANS, total score | |||||||
| Citrus peel extract | 103.6 ± 8.6 | 100.5 ± 8.6 | 108.2 ± 8.6 | 4.63 ± 1.57 | 0.34 [0.11–0.56] | < .001 | .910 |
| Placebo | 101.8 ± 8.6 | 98.9 ± 8.6 | 107.4 ± 8.6 | 5.63 ± 1.57 | 0.38 [0.13–0.63] | - | - |
| Short-term verbal memory | |||||||
| Citrus peel extract | 0.14 ± 0.67 | 0.20 ± 0.67 | 0.54 ± 0.67 | 0.41 ± 0.11 | 0.43 [0.21–0.65] | < .001 | .246 |
| Placebo | -0.14 ± 0.67 | -0.06 ± 0.67 | 0.48 ± 0.67 | 0.62 ± 0.11 | 0.69 [0.46–0.92] | - | - |
| Long-term verbal memory | |||||||
| Citrus peel extract | 0.16 ± 0.59 | 0.19 ± 0.59 | 0.60 ± 0.58 | 0.44 ± 0.12 | 0.54 [0.24–0.83] | < .001 | .397 |
| Placebo | -0.16 ± 0.59 | -0.09 ± 0.59 | 0.48 ± 0.58 | 0.64 ± 0.12 | 0.78 [0.49–1.08] | - | - |
| Everyday Memory Questionnaire | |||||||
| Citrus peel extract | 66.6 ± 17.4 | 60.1 ± 17.2 | 62.4 ± 17.3 | 4.13 ± 3.11 | 0.18 [-0.05–0.42] | .012 | .864 |
| Placebo | 65.7 ± 17.4 | 60.7 ± 17.2 | 61.6 ± 17.3 | 4.05 ± 3.11 | 0.18 [-0.02–0.39] | - | - |
| MMQ, satisfaction | |||||||
| Citrus peel extract | 38.0 ± 6.5 | 40.2 ± 6.5 | 40.8 ± 6.5 | 3.88 ± 1.18 | 0.40 [0.16–0.63] | < .001 | .904 |
| Placebo | 36.3 ± 6.5 | 39.0 ± 6.5 | 40.1 ± 6.5 | 3.80 ± 1.18 | 0.44 [0.17–0.70] | - | - |
| MMQ, ability | |||||||
| Citrus peel extract | 43.8 ± 7.8 | 48.1 ± 7.8 | 47.6 ± 7.8 | 3.80 ± 1.14 | 0.34 [0.14–0.54] | < .001 | .948 |
| Placebo | 45.0 ± 7.8 | 49.2 ± 7.8 | 49.1 ± 7.8 | 4.08 ± 1.14 | 0.37 [0.17–0.57] | - | - |
Data are reported as mean ± SE. Effect sizes are reported as Cohen’s d [confidence intervals]. MMQ: Multifactorial Memory Questionnaire
Depressive and anxiety symptoms were below the clinical cut-off scores used for the Geriatric Depression Scale and the State-Trait Anxiety Inventory, without significant difference between the treatment groups (Table 1). In terms of cognitive functioning, participants scored within the normal range on general cognition, as measured by the Mini Mental State Examination (mean score 29.2 ± 0.9), as well as other cognitive domains. The raw scores of the cognitive tests were reported in Supplemental Table 3. There was no difference in the primary and secondary outcomes between the treatment groups at baseline (Table 1).
Table 3.
Adverse events
| Citrus peel extract (n = 40) | Placebo (n = 40) |
p | |
|---|---|---|---|
| AE, total number | 26 (65) | 32 (80) | .21 |
| AE, related to the intervention | 14 (35) | 8 (20) | .21 |
| AE, Type | |||
| Respiratory | 13 (33) | 15 (38) | .81 |
| Osteoarticular | 9 (23) | 14 (35) | .32 |
| Gastrointestinal | 9 (23) | 11 (28) | .78 |
| Dermatological | 1 (3) | 3 (8) | .62 |
| Cardiovascular | 2 (5) | 2 (5) | 1.0 |
| Others | 5 (13) | 9 (23) | .38 |
AE Adverse events. Values denote number (percentage %). AE type. Respiratory: COVID, bronchitis, flu, cough, cold. Osteoarticular: fracture, articular pain, discal hernia, knee prosthesis, Dupuytren disease. Gastrointestinal: stomach burning, abdominal pain, intestinal meteorism, intestinal polyp, stypsis, diarrhea. Dermatological: nevus, herpes zoster, eczema. Cardiovascular: hypertension, hypotension, coronary angioplasty. Others: cataract, hypovitaminosis B, benign prostatic hypertrophy, restless leg syndrome, depressive symptoms, dizziness
Endpoint results
Primary and secondary endpoints
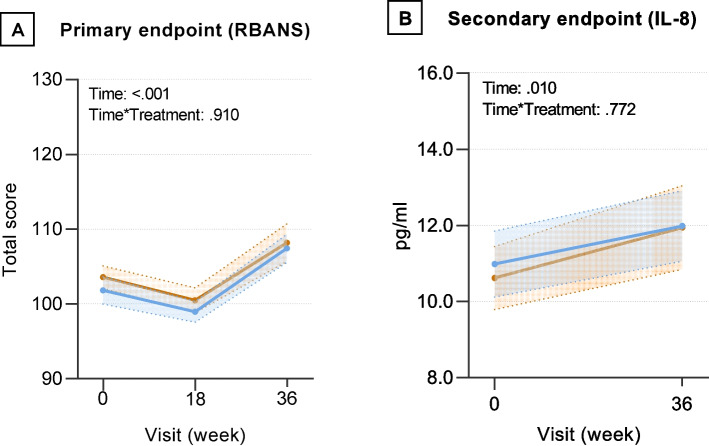
We observed a significant effect of time, but not time x treatment group interaction, on the RBANS, total score (p < 0.001) and IL-8 (p = 0.010) (Fig. 2), indicating that both Citrus peel extract and placebo groups showed better cognitive performance and higher IL-8 levels at 36-week follow-up. The effect size was small (d = 0.36, I.C. 0.19–0.53 for the primary endpoint, and d = 0.21, I.C. 0.006–0.41 for the secondary endpoint).
Fig. 2.
Estimated mean change and standard error in primary (panel A), and secondary (panel B) endpoint from baseline to 36 weeks. Orange and blue lines denote Citrus peel extract and placebo groups, respectively. RBANS: Repeatable Battery for the Assessment of Neuropsychological Status; IL: interleukin-8
Other cognitive outcomes
We found a significant effect of time, but not time x treatment interaction, on short- and long-term verbal memory (p < 0.001), indicating that both Citrus peel extract and placebo groups showed better memory performance at 36-week follow-up. Notably, the largest effect sizes, i.e., medium to large, were shown in the placebo group (Table 2). Furthermore, we observed a significant effect of time, but not time x treatment interaction, on satisfaction and ability dimensions of the MMQ (p < 0.001), as well as the EMQ (p = 0.012), indicating that both Citrus peel extract and placebo groups reported better subjective memory at 36-week follow-up. The effect sizes of these changes were small or small to medium, ranging from 0.18 to 0.42 (Table 2). There was no significant effect of either time or time x treatment interaction on attention and executive functions, nor on visuospatial function. Supplemental Fig. 1 shows the visual representation of the changes in all cognitive outcomes. We noted from data inspection that subjective measures ameliorated at 18 weeks and remained stable until 36 weeks, while objective measures were stable at 18 weeks and improved thereafter.
Safety
The overall incidence of adverse events was similar in the two groups (Table 3). There was no difference between the number of adverse events deemed as related to the treatment (35% vs 20% in the Citrus peel extract and placebo groups, respectively, p = 0.210). The most common adverse events (affecting > 10% of the participants) were respiratory, osteoarticular, or gastrointestinal, without significant differences between the two treatment groups. No serious adverse events occurred.
Discussion
This study investigated the effects of Citrus peel extract on the cognitive and biological characteristics of older adults experiencing SCD relative to placebo. The findings indicate that both Citrus peel extract and placebo groups improved in the primary endpoint (i.e., the RBANS) and in other cognitive outcomes, including short- and long-term verbal memory and subjective memory, at 36-week follow-up. Furthermore, both groups showed increased IL-8 levels at the follow-up.
In this study, we did not observe a significant difference in cognitive outcomes between the nutraceutical and placebo groups, notwithstanding significant improvements occurring in the Citrus peel extract, possibly due to high placebo response in the placebo group. Indeed, this latter group showed the largest effect sizes of changes, mainly in short-term and long-term verbal memory. The high placebo response has been related to failure in showing treatment benefits in pain and psychiatric disorders [42, 43]. However, the underlying mechanisms have not been fully understood, making it difficult to determine whether these clinical trials were unsuccessful because of methodological problems or because the treatment lacked efficacy [42].
The placebo research showed that the expectancy of positive treatment effects was the main mechanism of placebo responses [42, 44]. Participants in clinical trials can derive their expectancies from extrinsic and intrinsic factors [45]. Extrinsic factors are those related to the act of being recruited in a clinical trial, for example better attention received during the study visits [45, 46]. In our study, it is noteworthy that the research staff included trained personnel experienced in clinical trials. Therefore, main strategies to minimize a placebo response (e.g., avoiding positive information about the expected treatment benefits, limiting the time spent with study participants, and communicating in an impartial manner) were adopted. Furthermore, the limited number of follow-up visits prevented frequent interaction with the study staff. Factors specifically related to the treatment, such as the color and smell of capsules, were also taken into account to neutralize study participants’ expectancies [17].
Intrinsic factors are those related to participant characteristics [45, 47]. Our study participants were older adults worried about their subjective memory complaints. SCD is a heterogeneous condition: some individuals may have preclinical AD and are at increased risk for cognitive decline [4], while others (e.g., the ‘worried well’ people) may be affected by functional cognitive disorders and have non-progressive symptoms [48]. In these latter individuals, memory complaints are distressing and associated with emotional upset, prompting them to seek medical help. It has been suggested that, for the ‘worried well’ people, nutritional supplements can be viewed as a panacea to feel better and take control of their own health [49]. Furthermore, our study participants were mostly well-educated women: both high levels of education [50] and female gender [51] were recognized as predictors of a placebo response. Lastly, a proportion of our participants (55%) took part in previous research studies conducted at our Institute and expressed interest for future research. These characteristics may get these individuals more familiar with the study procedures and increased their positive perception about further research experience. Concerning the possibility that cognitive improvement in our participants was due to task familiarity related to test repetition, we acknowledge that test–retest studies in older adults observed practice effects on the RBANS in the short- (median time gap of 3.5 months) [52], medium- (one-year) [53], and long-term (four years) periods [54]. In particular, the immediate memory, delayed memory, and total score indices continued to increase with repeated assessments [54]. We cannot exclude that practice effects have contributed to cognitive and memory improvements in our trial, also considering that the form A was repeated at the timepoint where the improvements were evident. The use of more than two alternate forms – the form C is not yet validated in the Italian language – could be useful in designing future studies.
As regards the cognitive outcomes, the placebo group improved in both objective (RBANS, short- and long-term verbal memory) and subjective measures. The finding is in accord with research studies showing that young people were sensitive to the placebo response on subjective perception, but not on objective cognitive performance [55, 56], while older adults can also be sensitive on objective cognition, including verbal memory [57]. Notably, in our study the effect size of change for delayed verbal memory was quite large (d = 0.78). As a reference, an effect size of 0.40 has been reported for cognitive outcome measures as a clinically meaningful improvement of cognitive training interventions in healthy older adults [14]. The opposite pattern observed in objective and subjective improvements (i.e., subjective measures ameliorated at 18 weeks and remained stable until 36 weeks, while objective measures were stable at 18 weeks and improved thereafter) deserves additional comment. In placebo analgesia, subjective experience was the trigger of the placebo effect, thus activating brain systems and processes that resulted in pain relief [58]. Similarly, we speculate that better subjective memory in our participants activated brain pathways related to the subsequent improvement in objective cognition. Neuroimaging studies unrevealing the neural circuit basis of the placebo response are warranted.
As described above, some characteristics of our study participants can explain the high placebo response in the placebo group. It is more difficult to understand what happened in the nutraceutical group. Indeed, active treatments also have placebo components that contribute to their overall treatment effect [44, 59]. In randomized clinical trials, the classical additive model posited that the placebo response in the active treatment arm was equal to the placebo response in the placebo arm [60]. However, the non-additive model suggested that the former may be either smaller or greater than the latter, due to the complex interaction between drug-specific and placebo effects [44]. Unfortunately, the parallel design was not appropriate to answer this issue [44, 59]. Other study designs, such as three-armed clinical trials with placebo controls, might allow to better understand the placebo response mechanisms and reduce their effect [42, 43]. Another explanation for the inconclusive results on the treatment effect might be related to the small sample size [61]. As there were no comparable clinical trials, we determined the sample size of our study based on the effect of a different intervention (i.e., cognitive health program). To exclude this as a source of limitation, we computed a post-hoc power analysis, confirming that the sample size estimation was appropriate (see supplemental discussion for details).
In clinical trials showing high placebo responses, the low medication doses achieved in the active treatment may be a factor contributing to the failure to distinguish among treatment groups [62]. As reported in the study protocol [17], we established the trial nutraceutical dosage based on estimated data of flavonoid intake and major food sources of the elderly individuals. We acknowledge that this method may be subject to limitations, such as overlooking individual bioavailability and metabolism. Considering the complex chemical composition of a nutraceutical, which includes multiple bioactive compounds with varied physicochemical properties and complex pharmacokinetic traits, the importance of using appropriate pharmacokinetic modeling to optimize dosing regimens has been underlined [63]. Furthermore, as participants reported high adherence to the Mediterranean diet, the requirement for nutritional compounds supplied by Citrus peel extract might already be satisfied, thus undermining the possibility to detect an effect of the nutraceutical. No data on dietary intake were available in our study to test this hypothesis. Although we decided to manufacture the nutraceutical using a natural approach that avoided any chemical modification of the citrus peel extract, the use of whole foods provides a synergistic mixture of nutrients that could increase absorption and effectiveness [64, 65]. Future trials involving high intake of phytonutrient-rich whole food products could lead to more significant results.
The use of biological alongside clinical outcomes may provide insights into the physiological mechanisms that underlie the effect of a treatment. Contrary to our a-priori hypothesis, IL-8 did not decrease in the Citrus peel extract, but increased in both groups. Based on preclinical data about the anti-inflammatory effects exerted by NAR and AUR through reducing IL-8 levels, the finding was quite unexpected. However, contrasting results between the human and preclinical studies were frequently observed due to drug-disease interactions [66] or poor quality of animal researches [67]. The result was unforeseen also considering that elevated IL-8 levels have been associated with worse memory in older adults [68]. In contrast, a recent longitudinal study with AD biomarker collection showed the opposite finding: higher baseline IL-8 levels in the cerebrospinal fluid were associated with better memory performance, specifically in healthy older adults with lower load of AD pathology [69]. This study supported the concept that a neuroinflammatory response might be neuroprotective in aging and preclinical AD [70]. We speculate that increased IL-8 levels in our sample of older adults might represent an up-regulation of pro-inflammatory response associated with beneficial effects, possibly related to high placebo response. The use of surrogate biomarkers of the molecular response to placebo treatments is warranted to predict placebo responsiveness in clinical trials [71].
Limitations
The main limitation of this study is the lack of biological (AD biomarkers) and genetic (APOE4 genotype) characterization in our trial population. Since SCD individuals with AD pathology and/or APOE4 carriers were at increased risk of clinical progression to mild cognitive impairment or dementia [72, 73], the biomarker and genetic assessment might have identified individuals more prone to benefit from this trial. The absence of biological characterization might also have limited the generalization of our trial findings to other SCD samples. An enrichment strategy based on the selection of participants with high levels of inflammation at baseline might be an alternative to identify high responders to the treatment. Lastly, pharmacokinetic investigations to determine the optimal dosage ranges of nutraceutical and dietary intake assessment were not available.
Conclusions
Despite we did not find benefits of Citrus peel extract over placebo, the results of this study could be of significant interest for improving the design of future nutraceutical clinical trials aimed to test novel cognitive enhancers in SCD. Positing that SCD individuals, especially the ‘worried well’ people, might be more susceptible to a placebo response, the measurement of variables such as participants’ expectations, attitudes towards nutraceuticals and personality features could provide valuable insights into the accurate estimation of treatment effects. An improved understanding of the placebo response has the potential to increase the efficiency of future nutraceutical trials in SCD.
Supplementary Information
Supplementary Material 1: Supplemental methods. Data on validity and reliability of the RBANS. Supplemental table 1. Health-related behaviors of the participants with SCD included in intention-to-treat analysis. Supplemental table 2. Concomitant medications (Anatomical Therapeutic Chemical classification) of the participants with SCD included in intention-to-treat analysis. Supplemental table 3. Objective and subjective cognitive features of the participants with SCD included in intention-to-treat analysis. Supplemental figure 1. Estimated mean change and standard error in objective and subjective cognitive outcomes from baseline to 18 and 36 weeks. Supplemental Discussion. Post-hoc power analysis. Supplemental references.
Acknowledgements
The IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli acknowledges support from the Italian Ministry of Health (Ricerca Corrente).
Abbreviations
- AD
Alzheimer’s disease
- SCD
Subjective cognitive decline
- NAR
Naringenin
- AUR
Auraptene
- RBANS
Repeatable Battery for the Assessment of Neuropsychological Status
- IL-8
Interleukin-8
- APOE
Apolipoprotein E gene
Authors’ contributions
Conceptualisation: SGa, FE, SF; Methodology: SGa, MM, FE, SGe, SF; Formal analysis: EG, AG, NSB, CSS; Investigation: GS, LM, SF; data curation: SGa, LM, SF; writing - original draft: SGa; writing - review and editing: SGa, MM, EG, NSB, AC, FE, GBF, SGe, AG, LM, GS, CSS, MP, SF; supervision: SGa, MM, FE, MP. All authors approved the final version of the manuscript.
Funding
The study has been funded by the Wilhelm Doerenkamp-Foundation (‘Clinical and biological effects of Citrus-phytochemicals in subjective cognitive decline: a pilot randomized controlled trial’), NATVANTAGE GRANT 2020. This funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Data availability
The dataset analysed during the current study is available in the Mendeley repository (https://data.mendeley.com/datasets/m99btm5s7r/1).
Declarations
Ethics approval and consent to participate
The study protocol was approved by local ethical committee (Ethics Committee of the IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy, reference number 270–2020). All the participants provided written informed consent for their clinical data to be used for research purposes.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aisen PS, Cummings J, Jack CRJ, Morris JC, Sperling R, Frölich L, et al. On the path to 2025: understanding the Alzheimer’s disease continuum. Alzheimers Res Ther. 2017;9:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, et al. Subjective Cognitive Decline Initiative (SCD-I) Working Group: A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10:844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Wang Z, Hu H, Qu Y, Wang M, Shen X, et al. Association of subjective cognitive decline with risk of cognitive impairment and dementia: a systematic review and meta-analysis of prospective longitudinal studies. J Prev Alzheimers Dis. 2021;8:277–85. [DOI] [PubMed] [Google Scholar]
- 4.Jessen F, Amariglio RE, Buckley RF, van der Flier WM, Han Y, Molinuevo JL, et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020;19:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H, Tan C, Tan L, Xu W. Predictors of cognitive deterioration in subjective cognitive decline: evidence from longitudinal studies and implications for SCD-plus criteria. J Neurol Neurosurg Psychiatry. 2023;94:844–54. [DOI] [PubMed] [Google Scholar]
- 6.Smart CM, Karr JE, Areshenkoff CN, Rabin LA, Hudon C, Gates N, et al. Non-pharmacologic interventions for older adults with subjective cognitive decline: systematic review, meta-analysis, and preliminary recommendations. Neuropsychol Rev. 2017;27:245–57. [DOI] [PubMed] [Google Scholar]
- 7.Bhome R, Berry AJ, Huntley JD, Howard RJ. Interventions for subjective cognitive decline: systematic review and meta-analysis. BMJ Open. 2018;8:e021610-021610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajaram S, Jones J, Lee GJ. Plant-based dietary patterns, plant foods, and age-related cognitive decline. Adv Nutr. 2019;10:S422–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guarnieri L, Bosco F, Leo A, Citraro R, Palma E, De Sarro G, et al. Impact of micronutrients and nutraceuticals on cognitive function and performance in Alzheimer’s disease. Ageing Res Rev. 2024;95: 102210. [DOI] [PubMed] [Google Scholar]
- 10.Krikorian R, Shidler MD, Summer SS. Early intervention in cognitive aging with strawberry supplementation. Nutrients. 2023;15:4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.do Rosario V, Lorzadeh E, Brodaty H, Anstey KJ, Chan K, Roodenrys S, et al. Assessing the effect of anthocyanins through diet and supplementation on cognitive function in older adults at risk for dementia: protocol for a randomised controlled trial. BMJ Open. 2024;14:e086435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler M, Nelson VA, Davila H, Ratner E, Fink HA, Hemmy LS, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical alzheimer-type dementia: a systematic review. Ann Intern Med. 2018;168:52–62. [DOI] [PubMed] [Google Scholar]
- 13.Fravel MA, Ernst ME, Gilmartin-Thomas J, Woods RL, Orchard SG, Owen AJ, ASPirin in Reducing Events in the Elderly Investigator Group. Dietary supplement and complementary and alternative medicine use among older adults in Australia and the United States. J Am Geriatr Soc. 2023;71:2219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cave AE, Chang DH, Münch GW, Steiner-Lim GZ. A systematic review of the safety and efficacy on cognitive function of herbal and nutritional medicines in older adults with and without subjective cognitive impairment. Syst Rev. 2023;12:143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa JG, Vidovic B, Saraiva N, do Céu Costa M, Del Favero G, et al. Contaminants: a dark side of food supplements? Free Radic Res. 2019;53:1113–35. [DOI] [PubMed] [Google Scholar]
- 16.Lockwood GB. The quality of commercially available nutraceutical supplements and food sources. J Pharm Pharmacol. 2011;63:3–10. [DOI] [PubMed] [Google Scholar]
- 17.Galluzzi S, Zanardini R, Ferrari C, Gipponi S, Passeggia I, Rampini M, et al. Cognitive and biological effects of citrus phytochemicals in subjective cognitive decline: a 36-week, randomized, placebo-controlled trial. Nutr J. 2022;21:64–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gagnier JJ, Boon H, Rochon P, Moher D, Barnes J, Bombardier C. CONSORT Group: Recommendations for reporting randomized controlled trials of herbal interventions: Explanation and elaboration. J Clin Epidemiol. 2006;59:1134–49. [DOI] [PubMed] [Google Scholar]
- 19.Ferry M, Coley N, Andrieu S, Bonhomme C, Caubère JP, Cesari M, et al. How to design nutritional intervention trials to slow cognitive decline in apparently healthy populations and apply for efficacy claims: a statement from the International Academy on Nutrition and Aging Task Force. J Nutr Health Aging. 2013;17:619–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walia A, Mehra R, Kumar N, Singh TP, Kumar H. Good manufacturing practices and safety issues in functional foods and nutraceuticals. In: Bioactive components. Edited by Thakur M, Belwal T; 2023.
- 21.Haskell-Ramsay CF, Docherty S. Role of fruit and vegetables in sustaining healthy cognitive function: evidence and issues. Proc Nutr Soc. 2023;82:305–14. [DOI] [PubMed] [Google Scholar]
- 22.Howes MR, Perry NSL, Vásquez-Londoño C, Perry EK. Role of phytochemicals as nutraceuticals for cognitive functions affected in ageing. Br J Pharmacol. 2020;177:1294–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uçar K, Göktaş Z. Biological activities of naringenin: A narrative review based on in vitro and in vivo studies. Nutr Res. 2023;119:43–55. [DOI] [PubMed] [Google Scholar]
- 24.Genovese S, Epifano F. Auraptene: a natural biologically active compound with multiple targets. Curr Drug Targets. 2011;12:381–6. [DOI] [PubMed] [Google Scholar]
- 25.Zhou T, Liu L, Wang Q, Gao Y. Naringenin alleviates cognition deficits in high-fat diet-fed SAMP8 mice. J Food Biochem. 2020;44:e13375. [DOI] [PubMed] [Google Scholar]
- 26.Ghanbarabadi M, Iranshahi M, Amoueian S, Mehri S, Motamedshariaty VS, Mohajeri SA. Neuroprotective and memory enhancing effects of auraptene in a rat model of vascular dementia: Experimental study and histopathological evaluation. Neurosci Lett. 2016;623:13–21. [DOI] [PubMed] [Google Scholar]
- 27.Okuyama S, Katoh M, Kanzaki T, Kotani Y, Amakura Y, Yoshimura M, et al. Auraptene/Naringin-Rich Fruit Juice of Citrus kawachiensis (Kawachi Bankan) Prevents Ischemia-Induced Neuronal Cell Death in Mouse Brain through Anti-Inflammatory Responses. J Nutr Sci Vitaminol (Tokyo). 2019;65:66–71. [DOI] [PubMed] [Google Scholar]
- 28.La VD, Zhao L, Epifano F, Genovese S, Grenier D. Anti-inflammatory and wound healing potential of citrus auraptene. J Med Food. 2013;16:961–4. [DOI] [PubMed] [Google Scholar]
- 29.Bodet C, La VD, Epifano F, Grenier D. Naringenin has anti-inflammatory properties in macrophage and ex vivo human whole-blood models. J Periodontal Res. 2008;43:400–7. [DOI] [PubMed] [Google Scholar]
- 30.Gahtan E, Overmier JB. Inflammatory pathogenesis in Alzheimer’s disease: biological mechanisms and cognitive sequeli. Neurosci Biobehav Rev. 1999;23:615–33. [DOI] [PubMed] [Google Scholar]
- 31.Galimberti D, Schoonenboom N, Scarpini E, Scheltens P. Dutch-Italian Alzheimer Research Group: Chemokines in serum and cerebrospinal fluid of Alzheimer’s disease patients. Ann Neurol. 2003;53:547–8. [DOI] [PubMed] [Google Scholar]
- 32.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. [DOI] [PubMed] [Google Scholar]
- 33.Ponteri M, Pioli R, Padovani A, Tunesi S, De Girolamo G. RBANS repeatable battery for the assessment of neuropsychological status. Edizione italiana. Firenze: Giunti O.S.; 2007.
- 34.Argento O, Pisani V, Incerti CC, Magistrale G, Caltagirone C, Nocentini U. The California verbal learning test-II: normative data for two Italian alternative forms. Clin Neuropsychol. 2015;1:42. [DOI] [PubMed] [Google Scholar]
- 35.Della Sala S, Laiacona M, Spinnler H, Ubezio C. A cancellation test: its reliability in assessing attentional deficits in Alzheimer’s disease. Psychol Med. 1992;22:885–901. [DOI] [PubMed] [Google Scholar]
- 36.Brugnolo A, De Carli F, Accardo J, Amore M, Bosia LE, Bruzzaniti C, et al. An updated Italian normative dataset for the Stroop color word test (SCWT). Neurol Sci. 2016;37:365–72. [DOI] [PubMed] [Google Scholar]
- 37.Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E. Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci. 1996;17:305–9. [DOI] [PubMed] [Google Scholar]
- 38.Nyhus E, Barceló F. The Wisconsin Card Sorting Test and the cognitive assessment of prefrontal executive functions: a critical update. Brain Cogn. 2009;71:437–51. [DOI] [PubMed] [Google Scholar]
- 39.Caffarra P, Gardini S, Zonato F, Concari L, Dieci F, Copelli S, et al. Italian norms for the Freedman version of the Clock Drawing Test. J Clin Exp Neuropsychol. 2011;33:982–8. [DOI] [PubMed] [Google Scholar]
- 40.Calabria M, Manenti R, Rosini S, Zanetti O, Miniussi C, Cotelli M. Objective and subjective memory impairment in elderly adults: a revised version of the Everyday Memory Questionnaire. Aging Clin Exp Res. 2011;23:67–73. [DOI] [PubMed] [Google Scholar]
- 41.Raimo S, Trojano L, Siciliano M, Cuoco S, D’Iorio A, Santangelo F, et al. Psychometric properties of the Italian version of the multifactorial memory questionnaire for adults and the elderly. Neurol Sci. 2016;37:681–91. [DOI] [PubMed] [Google Scholar]
- 42.Benedetti F, Carlino E, Piedimonte A. Increasing uncertainty in CNS clinical trials: the role of placebo, nocebo, and Hawthorne effects. Lancet Neurol. 2016;15:736–47. [DOI] [PubMed] [Google Scholar]
- 43.Bschor T, Nagel L, Unger J, Schwarzer G, Baethge C. Differential outcomes of placebo treatment across 9 psychiatric disorders: a systematic review and meta-analysis. JAMA Psychiatry 2024;e240994. [DOI] [PMC free article] [PubMed]
- 44.Kube T, Rief W. Are placebo and drug-specific effects additive? Questioning basic assumptions of double-blinded randomized clinical trials and presenting novel study designs. Drug Discov Today. 2017;22:729–35. [DOI] [PubMed] [Google Scholar]
- 45.Anderson S, Stebbins GT. Determinants of placebo effects. Int Rev Neurobiol. 2020;153:27–47. [DOI] [PubMed] [Google Scholar]
- 46.Ito K, Romero K. Placebo effect in subjects with cognitive impairment. Int Rev Neurobiol. 2020;153:213–30. [DOI] [PubMed] [Google Scholar]
- 47.Horing B, Weimer K, Muth ER, Enck P. Prediction of placebo responses: a systematic review of the literature. Front Psychol. 2014;5:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McWhirter L, Ritchie C, Stone J, Carson A. Functional cognitive disorders: a systematic review. Lancet Psychiatry. 2020;7:191–207. [DOI] [PubMed] [Google Scholar]
- 49.Read NW. Placebo and Panacea: The Healing Effect of Nutritional Supplements. In: Ransley JK, Donnelly J, Read NW, editors. Food and Nutritional Supplements Their Role in Health and Disease. Springer; 2001. p. 45–64. [Google Scholar]
- 50.Arnold R, Murphy-Smith J, Ng CH, Mischoulon D, Byrne GJ, Bousman CA, et al. Predictors of the placebo response in a nutraceutical randomized controlled trial for depression. J Integr Med. 2024;22:46–53. [DOI] [PubMed] [Google Scholar]
- 51.Hafliðadóttir SH, Juhl CB, Nielsen SM, Henriksen M, Harris IA, Bliddal H, et al. Placebo response and effect in randomized clinical trials: meta-research with focus on contextual effects. Trials. 2021;22:493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng B, Udeh-Momoh C, Watermeyer T, de Jager Loots CA, Ford JK, Robb CE, et al. Practice effect of repeated cognitive tests among older adults: associations with brain amyloid pathology and other influencing factors. Front Aging Neurosci. 2022;14:909614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duff K, Beglinger LJ, Schoenberg MR, Patton DE, Mold J, Scott JC, et al. Test-retest stability and practice effects of the RBANS in a community dwelling elderly sample. J Clin Exp Neuropsychol. 2005;27:565–75. [DOI] [PubMed] [Google Scholar]
- 54.Reed C, Calamia M, Sanderson-Cimino M, DeVito A, Toups R, Keller J. Four year practice effects on the RBANS in a longitudinal study of older adults. Appl Neuropsychol Adult. 2023;1–7. [DOI] [PubMed]
- 55.Schwarz KA, Büchel C. Cognition and the Placebo effect-dissociating subjective perception and actual performance. PLoS ONE. 2015;10:e0130492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blokland A. Can placebo or nocebo pills improve or impair cognition performance? Hum Psychopharmacol. 2023;38:e2869. [DOI] [PubMed] [Google Scholar]
- 57.Oken BS, Flegal K, Zajdel D, Kishiyama S, Haas M, Peters D. Expectancy effect: impact of pill administration on cognitive performance in healthy seniors. J Clin Exp Neuropsychol. 2008;30:7–17. [DOI] [PubMed] [Google Scholar]
- 58.Wager TD, Atlas LY. The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci. 2015;16:403–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frisaldi E, Shaibani A, Benedetti F, Pagnini F. Placebo and nocebo effects and mechanisms associated with pharmacological interventions: an umbrella review. BMJ Open. 2023;13:e077243-077243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Enck P, Klosterhalfen S, Weimer K, Horing B, Zipfel S. The placebo response in clinical trials: more questions than answers. Philos Trans R Soc Lond B Biol Sci. 2011;366:1889–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pocock SJ, Stone GW. The Primary Outcome Fails - What Next? N Engl J Med. 2016;375:861–70. [DOI] [PubMed] [Google Scholar]
- 62.Katz J, Finnerup NB, Dworkin RH. Clinical trial outcome in neuropathic pain: relationship to study characteristics. Neurology. 2008;70:263–72. [DOI] [PubMed] [Google Scholar]
- 63.Mittal S, Sawarkar S, Doshi G, Pimple P, Shah J, Bana T. Pharmacokinetics and bioavailability of nutraceuticals. In: C. Anandharamakrishnan and Parthasarathi Subramanian, editors. Industrial Application of Functional Foods, Ingredients and Nutraceuticals. Academic Press; 2023. p. 725–83.
- 64.Jenkins DJA, Spence JD, Giovannucci EL, Kim YI, Josse R, Vieth R, et al. Supplemental vitamins and minerals for CVD prevention and treatment. J Am Coll Cardiol. 2018;71:2570–84. [DOI] [PubMed] [Google Scholar]
- 65.Kumkum R, Aston-Mourney K, McNeill BA, Hernández D, Rivera LR. Bioavailability of anthocyanins: whole foods versus extracts. Nutrients. 2024;16:1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu H, Mach J, Gnjidic D, Naganathan V, Blyth FM, Waite LM, et al. Comparing effects of polypharmacy on inflammatory profiles in older adults and mice: implications for translational aging research. J Gerontol A Biol Sci Med Sci. 2022;77:1295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bracken MB. Why animal studies are often poor predictors of human reactions to exposure. J R Soc Med. 2009;102:120–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baune BT, Ponath G, Golledge J, Varga G, Arolt V, Rothermundt M, et al. Association between IL-8 cytokine and cognitive performance in an elderly general population–the MEMO-Study. Neurobiol Aging. 2008;29:937–44. [DOI] [PubMed] [Google Scholar]
- 69.Capogna E, Watne LO, Sørensen Ø, Guichelaar CJ, Idland AV, Halaas NB, et al. Associations of neuroinflammatory IL-6 and IL-8 with brain atrophy, memory decline, and core AD biomarkers - in cognitively unimpaired older adults. Brain Behav Immun. 2023;113:56–65. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Dong C, Han Y, Gu Z, Sun C. Immunosenescence, aging and successful aging. Front Immunol. 2022;13:942796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yetman H, Peciña M, Tiwari A, Vollert J, Hall K. Molecular mechanisms of placebo responses. From genes to pathways. In: Colloca L, Noel J, Franklin PD, Seneviratne C, editors. Placebo Effects Through the Lens of Translational Research. Oxford Academic; 2023. p. 76–89.
- 72.Rostamzadeh A, Bohr L, Wagner M, Baethge C, Jessen F. Progression of subjective cognitive decline to mci or dementia in relation to biomarkers for alzheimer disease: A Meta-analysis. Neurology. 2022;99:e1866–74. [DOI] [PubMed] [Google Scholar]
- 73.Perna L, Stocker H, Burow L, Beyer L, Trares K, Kurz C, et al. Subjective cognitive complaints and blood biomarkers of neurodegenerative diseases: a longitudinal cohort study. Alzheimers Res Ther. 2023;15:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Supplemental methods. Data on validity and reliability of the RBANS. Supplemental table 1. Health-related behaviors of the participants with SCD included in intention-to-treat analysis. Supplemental table 2. Concomitant medications (Anatomical Therapeutic Chemical classification) of the participants with SCD included in intention-to-treat analysis. Supplemental table 3. Objective and subjective cognitive features of the participants with SCD included in intention-to-treat analysis. Supplemental figure 1. Estimated mean change and standard error in objective and subjective cognitive outcomes from baseline to 18 and 36 weeks. Supplemental Discussion. Post-hoc power analysis. Supplemental references.
Data Availability Statement
The dataset analysed during the current study is available in the Mendeley repository (https://data.mendeley.com/datasets/m99btm5s7r/1).