Abstract
We have recently established a cell-free system from human cells that initiates semi-conservative DNA replication in nuclei isolated from cells which are synchronised in late G1 phase of the cell division cycle. We now investigate origin specificity of initiation using this system. New DNA replication foci are established upon incubation of late G1 phase nuclei in a cytosolic extract from proliferating human cells. The intranuclear sites of replication foci initiated in vitro coincide with the sites of earliest replicating DNA sequences, where DNA replication had been initiated in these nuclei in vivo upon entry into S phase of the previous cell cycle. In contrast, intranuclear sites that replicate later in S phase in vivo do not initiate in vitro. DNA replication initiates in this cell-free system site-specifically at the lamin B2 DNA replication origin, which is also activated in vivo upon release of mimosine-arrested late G1 phase cells into early S phase. In contrast, in the later replicating ribosomal DNA locus (rDNA) we neither detected replicating rDNA in the human in vitro initiation system nor upon entry of intact mimosine-arrested cells into S phase in vivo. As a control, replicating rDNA was detected in vivo after progression into mid S phase. These data indicate that early origin activity is faithfully recapitulated in the in vitro system and that late origins are not activated under these conditions, suggesting that early and late origins may be subject to different mechanisms of control.
INTRODUCTION
Initiation of eukaryotic DNA replication is a tightly controlled process. In metazoa, αβουτ 30 000 replicons are coordinately activated along the chromosomes to ensure that the entire genome is replicated precisely once throughout S phase (1). This regulation requires the concerted action of cis-acting elements that constitute origins of DNA replication and soluble trans-acting factors interacting with them, eventually triggering replication fork establishment in a spatially and temporally controlled fashion.
In mammalian cells, only a few origins of DNA replication have been mapped on the chromosomal DNA (reviewed in 2–4). The origin of DNA replication that has been mapped with the highest degree of resolution to date in human cells is located at the 3′-end of the lamin B2 gene (5,6). The transition point from continuous to discontinuous replication along the DNA sequence at this origin has been determined at nucleotide resolution (7). This origin is activated at the very beginning of S phase (8). The DNA sequence specificity for initiation is not so clearly defined for other human origins. For instance, in the naturally amplified locus coding for rRNA, initiation of DNA replication occurs at multiple sites in a much broader region spanning the 31 kb spacer, which separates the tandemly repeated transcription units (9–11). However, preferential sites of initiation have been mapped by different techniques within an ∼10 kb region upstream of the rRNA gene promoter (9,10,12–14).
The establishment of origin specificity and timing has been addressed by experiments involving the transfer of Chinese hamster cell nuclei isolated at various points in the G1 phase of the cell cycle into replication-competent Xenopus egg extracts. It was found that the site specificity of initiation of DNA replication in the DHFR initiation zone is established at a discrete point in mid G1, the ‘origin decision point’ (ODP) (15). The ODP precedes the restriction point in late G1 phase, where cell cycle progression becomes independent of mitogen stimulation (16). Similarly, the defining point for replication timing of the DHFR replication origin in early S phase occurs at another discrete point in early G1, the ‘timing decision point’ (TDP) (17,18). The precise molecular events that constitute the ODP and TDP are still unknown (19).
Higher eukaryotic replicons are thought to be organised as functional clusters of five to ten synchronously activated origins (for a review see 20). Furthermore, DNA replication is observed to occur at discrete foci in the nucleus, as shown by incorporation of halogenated nucleotide precursors into the genomic DNA and detection by immunofluorescence microscopy (21,22). The patterns of replication foci are highly dynamic during S phase. During the first half of S phase foci are located in the transcriptionally active euchromatin, excluding the nucleoli and nuclear periphery. This pattern is collectively referred to as type I (22); this classification will be used throughout this paper. In mid to late S phase foci are located at the nuclear periphery and in perinucleolar and nucleolar regions, referred to as type II. In late S phase replication occurs within nucleoli and satellite heterochromatic regions, referred to as type III (22,23). Using different established cell lines, the same progression of replication foci patterns has been observed, but some authors subdivide the patterns into five stages of S phase and refer to types I–V (17,24,25). Activation of the first cohort of replication foci defines the onset of S phase, but further activation of new foci is asynchronous and occurs throughout the remainder of S phase (26–29). Individual foci are active for ∼45–60 min (26,29,30) and progression from earlier S phase to later stages depends on completion of the earlier events (28). The spatio-temporal patterns of chromosomal replication are essentially maintained from one cell generation to the next (17,29–31). The molecular mechanisms underlying these controls remain to be elucidated.
We have recently established a cell-free system from human cells that allows molecular studies of the initiation of DNA replication in isolated nuclei (32,33). For a preparation of active template nuclei, cells need to be synchronised in late G1 phase (32,33). This synchronisation is efficiently achieved by a block with the plant amino acid mimosine (34,35; and references therein), which needs to be added at concentrations of ≥0.5 mM to proliferating human cells for successful synchronisation in late G1 phase; lower concentrations fail to arrest cells before onset of S phase and result in their accumulation in S phase (35). Significantly reduced or no initiation in vitro is observed when template nuclei are prepared from early G1 or from G2 phase human cells, respectively (32).
In the human in vitro system, efficient initiation of semi-conservative DNA replication is triggered in nuclei isolated from mimosine-arrested cells upon addition of a cytosolic extract from proliferating human cells (33). Essential initiation factors have been identified in this system as G1/S phase-specific cyclin/Cdk complexes (32,33,36). These observations together demonstrate that cell cycle control for initiation is maintained in this human cell-free system.
The spatial and temporal regulation of the initiation of DNA replication in the human cell-free system has not been addressed so far. Such an analysis is warranted by results obtained from another model system, derived from the eggs of the frog Xenopus laevis (37; and references therein). In the Xenopus system, initiation is observed in an origin-specific manner only when intact post-ODP nuclei are used as a template (15,38). In contrast, protein-free DNA, sperm chromatin, pre-ODP nuclei or permeabilised post-ODP nuclei all initiate DNA replication with no requirement for specific DNA sequences in the Xenopus egg system (15,37–39). This in vitro behaviour mimics the sequence-independent selection of initiation sites characteristic for the early Xenopus embryo (40,41).
In this paper, we address the site specificity of initiation in the human system derived from proliferating somatic cells (32,33). We first demonstrate that the intranuclear sites of DNA replication initiated in vitro coincide with the sites of earliest replicating DNA sequences, where DNA replication had been initiated in the previous cell cycle in vivo. In contrast, chromosome domains that replicate later in S phase were not replicated in vitro. We next demonstrate by nascent strand analysis using quantitative real-time PCR that DNA replication initiates in this cell-free system site-specifically at the lamin B2 replication origin, which is also activated in vivo upon entry into S phase. In contrast, replication intermediates within the rDNA locus are not initiated in the cell-free system nor were they detected when intact cells entered S phase in vivo. However, they were detected in vivo following progression of cells into mid S phase, suggesting that origin usage in the human cell-free system follows the spatial and temporal pattern as seen in S phase in vivo.
MATERIALS AND METHODS
Cell culture and synchronisation
Human HeLa-S3 and EJ30 cells were cultured as proliferating monolayers on 145 mm plates in DMEM supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin and 0.1 mg/ml streptomycin (Gibco BRL). EJ30 cells were arrested in quiescence by culturing confluent plates for 7 days in DMEM supplemented with 0.5% FCS, 2.5 µg/ml amphotericin B (fungizone; Gibco BRL), 100 U/ml penicillin and 0.1 mg/ml streptomycin (Gibco BRL). These quiescent cultures were stimulated by washing and re-cultivating in DMEM containing 10% FCS.
Proliferating cells were arrested in late G1 phase by adding 0.5 mM mimosine (Sigma) from a 10 mM stock solution to the culture medium for 24 h (35). Serum-stimulated EJ30 cells were arrested at the G1/S border by adding 5 µg/ml aphidicolin (Sigma) to the culture medium 8 h post-stimulation and further cultivation for 20 h. Synchronisation was monitored by flow cytometry of isolated nuclei (35).
Labelling of nascent DNA with halogenated nucleotides
To label DNA replicated in vivo, 20 µM 5-chloro-2′-deoxyuridine (CldU; Sigma) was added to the culture medium of human HeLa or EJ30 cells. Earliest replicating DNA was pulse labelled in vivo by washing aphidicolin-arrested EJ30 cells with phosphate-buffered saline (PBS) and releasing them into fresh DMEM containing 20 µM CldU for 20 min, followed by replacing the culture medium with DMEM without label. DNA replicated in early S phase in vivo following release from a late G1 phase block imposed by 0.5 mM mimosine (35) was labelled by replacing the culture medium with DMEM containing 20 µM 5-iodo-2′-deoxyuridine (IdU; Sigma) for 3 h.
DNA replication in vitro
Template nuclei were prepared by hypotonic treatment of the cells, followed by Dounce homogenisation and centrifugation, exactly as described previously (35). Cytosolic extract was prepared from asynchronously proliferating HeLa cells exactly as described (33). Protein concentrations were determined with the Bio-Rad protein assay using bovine serum albumin as standard.
DNA replication initiation reactions were performed as described, with minor modifications (32,33). The reaction mixture contained HeLa cell cytosolic extract (100 µg protein); a buffered mix of rNTPs and dNTPs, including digoxigenin-11-dUTP (dig-dUTP; Roche), an ATP-regenerating system and 2–5 × 105 nuclei from mimosine-arrested HeLa cells. The final reaction volume of 50 µl was adjusted with replication elongation buffer (20 mM K–HEPES, pH 7.8, 100 mM potassium acetate, 1 mM MgCl2, 0.1 mM dithiothreitol). DNA replication elongation assays (35) contained the same components except for the initiating cytosolic extract. For analysis of nascent DNA strand abundance by real-time PCR and for 2D gel electrophoresis, these in vitro reactions were scaled up 800 times to a final volume of 4 ml. Incubation time was 3 h at 37°C.
Analysis of DNA replication products
Confocal immunofluorescence microscopy. For differential analysis of labelled nascent DNA by confocal fluorescence microscopy, the CldU- and IdU-specific antibody protocol (42,43) was adapted and integrated into the labelling protocol for DNA replicating in vitro (33,35). In detail, 2–5 × 105 isolated nuclei or an entire 50 µl replication reaction was resuspended in 1 ml of 2% paraformaldehyde in PBS, mixed well and fixed at room temperature for 5 min. Nuclei were spun onto poly(lysine)-coated coverslips and washed once in PBS and once in water. To allow immunostaining, the coverslips were incubated in 0.8 N HCl at room temperature for 30 min and washed in water, followed by PBS and PBS containing 0.1% Triton X-100, 0.02% SDS for 2 min each. Coverslips were blocked in blocking buffer (PBS, 2% non-fat dry milk, 0.1% Triton X-100, 0.02% SDS) for 30 min. Primary antibodies [rat anti-bromodeoxyuridine monoclonal antibody MAS250b (Harlan Sera Lab) for the detection of CldU and mouse anti-bromodeoxyuridine antibody 347580 (Beckton Dickinson) for the detection of IdU] were diluted 1:10 in blocking buffer and incubations were at 37°C for 1 h. Secondary antibodies [Texas Red-conjugated anti-rat IgG (ImmunoKontact) and fluorescein-conjugated anti-mouse IgG (Amersham)] were diluted 1:100 in blocking buffer and incubations were at 37°C for 1 h. For detection of DNA replicated in vitro, fluorescein-conjugated anti-digoxigenin Fab fragments (Boehringer Mannheim) were used at 1:100 dilution in blocking buffer during the secondary incubation. For quantitative analysis, total nuclear DNA was counterstained with 1 nM TOTO-3 (Molecular Probes) during the secondary incubation. Finally, coverslips were washed in PBS containing 0.1% Triton X-100, 0.02% SDS, followed by PBS, and mounted in 85% glycerol containing 2.5% n-propylgallate (Sigma).
Confocal immunofluorescence was performed on a Leica TCS microscope using the Leica Confocal software. For high resolution double labelling with fluorescein and Texas Red, excitation was with krypton and argon lasers at 488 and 568 nm, respectively, and recording of the fluorescence signal was set at 510–530 nm for the fluorescein channel and at 620–670 nm for the Texas Red channel. For a quantitative analysis at lower magnification, triple labelling with fluorescein, Texas Red and TOTO-3 was performed with an additional excitation by a helium/neon laser at 633 nm. The fluorescein channel was unchanged in this case, but the Texas Red channel was adjusted and recorded at 610–625 nm and TOTO-3 was recorded at 650–700 nm. Images were recorded as RGB files at 1024 × 1024 pixels at 8 bits/pixel. Eight averaging runs were performed per sample. Low resolution fields were recorded through a Leica PL APO 40× oil immersion objective and high resolution images through a Leica PL APO 63× oil immersion objective at 4× zoom. Images were processed via Adobe Photoshop 6.0 software using standardised brightness and contrast operations only. All panels shown per figure were processed identically.
Real-time PCR analysis of nascent DNA strands. Nascent DNA strands were prepared as detailed previously (44) with minor modifications. For preparation from intact cells, ∼1 × 108 HeLa cells were trypsinised and washed twice in ice-cold 1× PBS and once in RBS (10 mM Tris, pH 7.4, 10 mM NaCl, 3 mM MgCl2). Cells were resuspended at ∼2.5 × 107 cells/ml in RBS and left on ice for 5 min. Subsequently, the same volume of RBS containing 1% NP-40 was added and incubated on ice for a further 10 min. Then nuclei were pelleted at 2000 r.p.m. for 10 min in a swinging bucket (HB4) rotor, washed twice in RBS and finally resuspended in RBS buffer at 5 × 107 nuclei/ml. For preparation from isolated nuclei incubated in vitro, 0.5–1 × 108 nuclei were incubated in a scaled up in vitro assay for 2 h, which was stopped by adding an equal volume of ice-cold PBS. Nuclei were pelleted at 5000 r.p.m. in an SS34 rotor for 5 min, washed once in SuNaSpBSA, pelleted again and resuspended in RBS buffer at 5 × 107 nuclei/ml. The same volume of 2× lysis buffer (20 mM Tris, pH 8.0, 20 mM EDTA, 2% SDS, 500 µg/ml proteinase K) was added to the preparations and incubated overnight at 56°C. Total genomic DNA was carefully extracted with phenol/chloroform, precipitated with isopropanol and dissolved in TE buffer.
For denaturation, the DNA was incubated at 85°C for 10 min, followed by chilling on ice. Denatured DNA was then loaded onto linear neutral 5–30% sucrose gradients in TNE (10 mM Tris, pH 8.0, 100 mM NaCl, 1 mM EDTA, pH 8.0). In a parallel gradient, double-stranded size marker DNA (1 kb ladder; MBI Fermentas) was loaded as a reference for nascent DNA strand size selection. Samples were centrifuged at 20°C in a Beckman SW28 rotor for 20 h at 26 000 r.p.m. Fractions of 1 ml were collected from top to bottom. As a reference, fractions containing marker DNA were precipitated with ethanol, separated on a 1% agarose gel and stained with ethidium bromide. Fractions from the denatured genomic DNA corresponding to an average single-stranded reference size of 1 kb were collected and precipitated.
For quantitative analysis of nascent DNA strands in the lamin B2 origin region the following primer sets were synthesised and used: LB-forward (5′-ggc tgg cat gga ctt tca ttt cag-3′), LB-reverse (5′-gtg gag gga tct ttc tta gac atc-3′), LBC1-forward (5′-gtt aac agt cag gcg cat ggg cc-3′), LBC1-reverse (5′-cca tca ggg tca cct ctg gtt cc-3′), LBC2-forward (5′-cac agc atg cgg ctg ctg atc tg-3′) and LBC2-reverse (5′-cct ggt gcg tcc cat ctg cct gc-3′). We chose to generate these primer sets because other published primer sets used to amplify DNA sequences near the lamin B2 origin (44) did not give single PCR products using the settings for quantitative real-time PCR. For real-time PCR, annealing temperatures were adjusted to 66°C for the LBC1 and LB primer sets and 68°C for the LBC2 set. Real-time PCR was performed with a Light Cycler instrument (Roche) using a ready-to-use ‘hot start’ reaction mix (Light Cycler FastStart SYBR Green I Kit; Roche). The mix contains Taq DNA polymerase and the fluorescent SYBR Green I dye. PCR reactions contained 2.5 mM MgCl2 and 5 pmol of each primer and were transferred to specialised Light Cycler capillaries, which fit into the instrument’s adaptor. For construction of a standard curve, DNA samples of known concentrations were serially diluted containing 30 ng, 3 ng, 300 pg, 30 pg and 3 pg human genomic DNA. Nascent DNA strands were added using 1/20 from the respective fractions. Two reactions of each sample were set up in parallel for double determinations.
Quantifications by the Light Cycler instrument were performed at 50 PCR cycles using the settings of the standard experimental protocol recommended by Roche. PCR products were checked by melting curve analysis and gel electrophoresis. For quantitative analysis, the copy number in target molecules was calculated by plotting the logarithm of fluorescence versus the cycle number and setting a baseline x-axis. To produce the standard curve, the x-axis crossing point of each standard sample was determined and plotted against the logarithm of concentration. About 300 pg of DNA refer to 100 genomic equivalents. The amount of nascent DNA strands in copy numbers was determined by extrapolation from the standard curve. All calculations were carried out with the Light Cycler software.
Neutral/neutral 2D gel electrophoresis of replication intermediates. For analysis of rDNA replication in vivo, DNA replication intermediates from intact cells were isolated on a nuclear matrix essentially as described (45; method E), with minor modifications detailed below. For isolation from in vitro reactions, 0.5–1 × 108 nuclei were incubated in a scaled up in vitro assay for 3 h, which was stopped by adding an equal volume of ice-cold PBS. Nuclei were pelleted at 5000 r.p.m. in an SS34 rotor for 5 min, washed once in SuNaSpBSA and stored frozen. Thawed nuclei were washed once in cell wash buffer (CWB; 45) containing 0.1% digitonin and further processed as nuclei from intact cells (45; method E). Nuclear matrices isolated from 3–5 × 107 nuclei from both sources were digested with EcoRI and HindIIII, replication intermediates were isolated from the digested matrices and were further purified using one or two rounds of benzylnaphthyl-DEAE (BND)–cellulose chromatography, as previously described (45; method E). Additional rounds of EcoRI + HindIII digestion were performed after each BND–cellulose column to maximise digestion of replication intermediates. Neutral/neutral 2D gel electrophoresis was performed as described (46), with modifications for the analysis of large fragments (47): the first dimension gel was 0.3% (w/v) agarose, run at 0.3 V/cm for 3–5 days at room temperature; the second dimension was 0.6% (w/v) agarose, 0.3 µg/ml ethidium bromide, run at 1.0 V/cm for 2–3 days at room temperature. The gels were blotted in 0.4 M NaOH onto Hybond-N+, hybridised with probes CHB and CPE or a probe corresponding to the EcoRI fragment B of the human rDNA locus (9) and analysed on a Fuji BAS 1000 phosphorimager.
RESULTS
De novo formation of DNA replication foci in the human cell-free system
We first controlled for the specificity of initiation of DNA replication in the human cell-free system (33) by introducing a double label analysis using differently halogenated nucleotide precursors (42; and see below). We asked whether new replication foci are formed de novo in late G1 phase nuclei that did not replicate during the synchronisation treatment with mimosine. The results confirmed previous observations (33) demonstrating that new replication foci were formed in vitro in ∼40% of true G1 phase nuclei that had not synthesised DNA during the synchronisation treatment with mimosine in vivo (data in Supplementary Material). Results also confirmed that DNA replication elongates in vitro in contaminating S phase nuclei at existing forks in ∼10% of the nuclei (cf. 33), however, in these few S phase nuclei new foci were also initiated next to existing ones in vitro (data in Supplementary Material). Similar percentages of nuclei initiating DNA replication foci were obtained in control experiments where mimosine-arrested cells were released from the block and allowed to enter S phase in vivo (data in Supplementary Material).
Replication initiates in vitro at the earliest replicating chromatin domains of the previous cell cycle
We used a double labelling protocol (Fig. 1A) to ask whether DNA replication initiates in late G1 phase nuclei in vitro within the chromosome domains that replicate first upon entry of intact cells into S phase in vivo. Quiescent human EJ30 cells were stimulated to proliferate and were subsequently arrested during the first G1/S transition by addition of aphidicolin, an inhibitor of DNA polymerases. Earliest replicating DNA was pulse labelled with CldU for 20 min following release from the aphidicolin block, and sites of incorporation were detected with CldU-specific antibodies by confocal microscopy (42). Further transit through S, G2 and M phase was allowed in the absence of label and these cells were then arrested in the following late G1 phase by addition of mimosine (35).
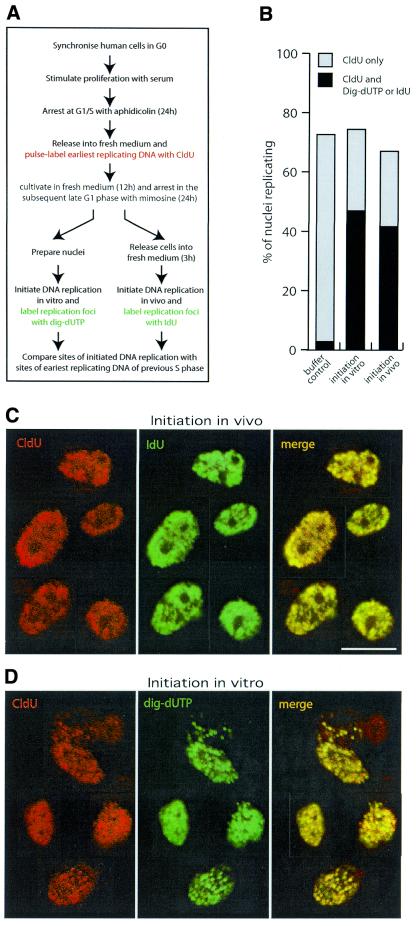
Figure 1.
Intranuclear sites of initiation of DNA replication in vitro co-localise with sites of earliest DNA replication in vivo. (A) Experimental protocol. See text for details. (B) Quantification of initiation of DNA replication in vivo and in vitro. As a negative control for the initiation of DNA replication in vitro, nuclei from CldU pulse-labelled, mimosine-arrested cells were incubated in vitro in replication elongation buffer (buffer control). The same nuclei were incubated in initiating cytosolic extract (initiation in vitro). As a control, nuclei released from the mimosine block in vivo were also analysed (initiation in vivo). The percentages of total nuclei that were only pulse labelled in early S phase with CldU (grey bars) and of those nuclei labelled with both CldU and digoxigenin-dUTP in vitro or CldU and IdU in vivo (black bars) were scored. Between 600 and 1000 nuclei were recorded per sample. (C and D) High resolution confocal laser immunofluorescence microscopy. Earliest replicating DNA was pulse labelled at the G1/S boundary with CldU in vivo and sites of incorporation were detected with anti-CldU antibodies (red images). (C) Sites of DNA replication initiated in vivo in the subsequent cell cycle following release from a mimosine block were labelled with IdU and detected with anti-IdU antibodies (green images). (D) Sites of DNA replication initiated in vitro in the nuclei isolated from mimosine-arrested cells were labelled with digoxigenin-dUTP and detected with anti-digoxigenin antibodies (green images). Signals of both channels were merged and co-localisation of red and green yields a yellow signal (merge). Representative images of nuclei are shown at identical magnification. Scale bar 10 µm.
To control for intranuclear sites of replication initiated in the intact cell in vivo, the mimosine-arrested cells were released into very early S phase using fresh culture medium for 3 h (35). Sites of DNA replication were labelled by inclusion of IdU and detected with IdU-specific antibodies (42). We found that 68% of the nuclei were labelled with CldU in the first S phase and 42% were labelled again with IdU upon entry into the second S phase in vivo (Fig. 1B, right column). Thirty-two per cent of the nuclei were not labelled at all and no nuclei were detected with only the IdU label. The intranuclear position of the replication foci formed in these two consecutive cell cycles in vivo was compared by high resolution confocal microscopy using fluorescent antibodies against CldU and IdU (Fig. 1C). Both labels co-localised at discrete sites in these nuclei (Fig. 1C), forming a pattern of replication foci typical for early S phase (type I; see ref. 22). These data establish that DNA replication in vivo initiates after release from a late G1 phase block by mimosine at the same intranuclear chromatin domains at which S phase had initiated in the previous cell cycle.
We then used the same approach to test whether sites of DNA replication initiated in the human cell-free system also co-localised with sites of earliest replicating DNA in vivo. Nuclei prepared from mimosine-arrested cells (72% were pre-labelled with CldU in vivo, Fig. 1B) were used as templates for initiation of DNA replication in vitro. Sites of DNA replication in vitro were labelled with digoxigenin-dUTP and detected with digoxigenin-specific antibodies (Fig. 1A). The percentage of active S phase contaminants in this preparation was 3%, as measured by the in vitro elongation control in buffer only (Fig. 1B, left column). Upon addition of cytosolic extract containing factors essential for replication initiation, an additional 45% initiated DNA replication in vitro (Fig. 1B, middle column; cf. 33). At high resolution, the replication foci initiated in this reaction in vitro clearly co-localised with sites of earliest replicating DNA of the previous cell cycle in vivo, forming patterns of replication foci typical for early S phase (Fig. 1D). These data indicate that the specificity for chromatin domains where DNA replication initiates in this human cell-free system recapitulates the spatial control observed in vivo and occurs at intranuclear sites that are conserved between subsequent cell cycles. Furthermore, the confinement of initiation in vitro to earliest replicating DNA sites suggests that the temporal control of S phase progression is maintained in vitro and late replicating sites do not initiate DNA replication prematurely in this system.
We tested this conclusion directly by pulse labelling asynchronous cells in vivo with CldU and looking for mid S phase nuclei replicating perinuclear and perinucleolar heterochromatin, and we then asked whether these sites overlap with sites of DNA replication initiated in the subsequent early S phase in vivo (Fig. 2A). Clearly, DNA replication foci were initiated after release from mimosine in vivo at sites that are adjacent to, and mostly exclude, perinuclear and perinucleolar heterochromatin that had been pulse labelled in the previous mid S phase (Fig. 2B). We then tested whether replication foci initiated in nuclei isolated from mimosine-arrested cells upon incubation in cytosolic extract in vitro also exclude later replicating heterochromatin (Fig. 2C). Again, replication foci initiated in vitro are located adjacent to, and mostly exclude, later replicating sites, forming separate patterns of incorporation (Fig. 2C). Taken together, these data confirm that the general order of replication initiation timing is recapitulated in the human cell-free initiation system and chromosome domains that replicate in mid S phase are not prematurely initiated when G1 phase nuclei are incubated in initiating cytosolic extract.
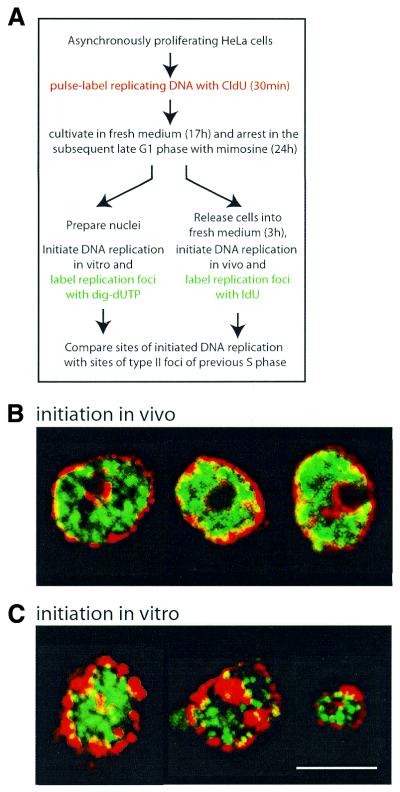
Figure 2.
Exclusion of de novo initiated DNA replication foci from perinuclear and perinucleolar heterochromatin replicating in mid S phase. (A) Experimental protocol. See text for details. (B and C) High resolution confocal laser immunofluorescence microscopy. Replicating DNA was pulse labelled with CldU in vivo, chased into the following G1 phase, subsequently arrested with mimosine and sites of incorporation detected by anti-CldU antibodies (red signal). (B) Sites of DNA replication initiated in vivo following release from the mimosine block were labelled with IdU and detected with anti-IdU antibodies (green signal). (C) Sites of DNA replication initiated in vitro in nuclei isolated from mimosine-arrested cells were labelled with digoxigenin-dUTP and detected with anti-digoxigenin antibodies (green signal). Signals of both channels were merged and representative nuclei identified by a type II pattern of CldU incorporation are shown. Scale bar 10 µm.
In vitro replication in isolated human cell nuclei initiates at specific origin sequences
To determine whether DNA replication initiates at specific DNA sequences in this human cell-free system, we first analysed the abundance of nascent DNA strands at an established cellular origin of DNA replication in human cell nuclei.
Lamin B2 origin. A cellular origin of DNA replication that initiates very early in S phase has been mapped with high resolution to a 474 bp DNA sequence ovelapping the 3′-end of the lamin B2 gene (LMB2) and the promoter of the PPV1 gene on chromosome 19 in human cells (5–7,48). We used real-time PCR to quantify the abundance of nascent DNA strands emanating from this origin sequence, using as reference two sites located 4 kb upstream and 3 kb downstream of the origin, respectively (Fig. 3A). Nascent DNA strands of ∼1 kb were first prepared from cells released from a mimosine block into early S phase in vivo and subjected to real-time PCR using the three primer sets outlined in Figure 3A. We found that the lamin B2 origin sequences were enriched ∼5-fold over the neighbouring sequences 3–4 kb away from the origin (Fig. 3B, black bars), indicating efficient activation of this origin upon entry into S phase. This result independently confirms earlier reports (5–7,48). As a control, we prepared DNA under identical conditions from cells arrested in late G1 phase by mimosine. In this preparation, the origin sequence amplified by primer set LB was <2-fold enriched over the two neighbouring sequences amplified by primer sets LBC1 and LBC2 (Fig. 3B, grey bars), indicating that no efficient initiation had occurred during the mimosine treatment in vivo. The small enrichment of the origin sequences in this sample is most likely due to the small percentage of S phase contaminants present in the population of nuclei in the preparation (cf. Fig. 1B; 33,35). Taken together, these results show that cells arrested in late G1 by mimosine enter S phase by activating specific origin sequences upon removal of mimosine in vivo, thus supporting the observation that the lamin B2 origin fires very early in S phase in vivo (Fig. 3B; cf. 5).
Figure 3.
Site-specific initation of DNA replication at the human lamin B2 origin in vivo and in vitro. (A) Map of the human lamin B2 origin and the primer sets used. Three primer sets were generated for quantification of nascent DNA strands by real-time PCR (see Materials and Methods). The primer set LB amplifies the lamin B2 replication initiation site (cf. 5,7). The two adjacent primer sets LBC1 and LBC2 amplify sequences 4 kb upstream and 3 kb downstream of the origin, respectively. (B) Initiation of DNA replication in vivo. Abundance of nascent DNA strands was quantified by real-time PCR using the LBC1, LB and LBC2 primer sets (see Materials and Methods). Nascent strands were prepared from HeLa cells arrested with mimosine in late G1 phase (grey bars) and from cells that were released for 3 h from a mimosine block into early S phase in vivo (black bars). Means ± SD from two independent experiments are shown. (C) Initiation of DNA replication in vitro. Abundance of nascent strands from nuclei of mimosine-arrested cells incubated in a cytosolic extract of proliferating cells was analysed as detailed for (B).
Then, we analysed nascent DNA prepared from nuclei of mimosine-arrested cells incubated in cytosolic extract in vitro. The lamin B2 origin sequences were amplified ∼10-fold over the neighbouring sequences (Fig. 3C). These data establish that DNA replication initiates at an early firing origin with sequence specificity in the human cell-free system.
rDNA repeats. We next investigated origins that fire at later stages of S phase. The human rRNA genes are amplified ∼400 times and are clustered in tandem repeats (Fig. 4A), forming the nucleolar organiser regions on chromosomes 13, 14, 15, 21 and 22 (9). The transcription units for the 45S precursor rRNA are separated from each other by non-transcribed 31 kb spacers. In human cells, DNA replication initiates at multiple sites within the non-transcribed spacers (9). The nucleoli are seen by confocal fluorescent microscopy to replicate in mid and late S phase (cf. Fig. 2; 22). Replication timing of the rDNA repeats was analysed at higher resolution taking into account the polymorphic nature of the amplified rDNA locus (9). A subpopulation of rDNA repeats lacking a polymorphic EcoRI site at the promoter (Fig. 4A, bracketed symbol) has been reported to replicate predominantly earlier than the subpopulation possessing this polymorphic EcoRI site (14). We investigated the generation of replication intermediates on both variant fragments during S phase. Since the replication of rDNA has previously been studied by 2D gel electrophoresis, we decided to use this approach also for the present study (Fig. 4B), rather than the nascent strand abundance assay, which has been used successfully in investigations of the lamin B2 origin.
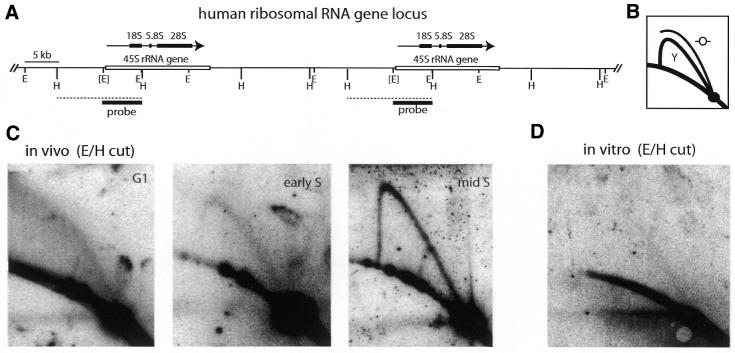
Figure 4.
Lack of initiation of DNA replication at the human rDNA locus in early S phase in vivo and in vitro. (A) Map of the human rDNA locus (cf. 9,14). Two of the repeated rDNA transcription units and the intergenic spacers are shown. The region coding for the 45S rRNA precursor is indicated by a white box, the 45S rRNA transcript as a black arrow with the 18S, 5.8S and 28S rRNA coding sequences highlighted as a series of black boxes. Restriction sites for EcoRI (E) and HindIII (H) are shown. The EcoRI site 5′ to the transcription start site is polymorphic and is indicated by brackets. The position of the hybridisation probe (EcoRI fragment B; 9) is indicated by a black box and the position of the analysed polymorphic E/H DNA fragment is indicated by a dashed line. (B) Principle of neutral/neutral 2D gel electrophoresis mapping of replication intermediates (46). DNA restriction fragments containing replication intermediates are first run from top to bottom and in the second dimension from left to right. Linear fragments migrate along the lower arc and unit length fragments accumulate at a discrete spot on this arc. Restriction fragments containing a single replication fork migrate on arc Y and fragments containg a centrally located defined origin of bidirectional replication migrate on arc O. (C) Neutral/neutral 2D gel analysis of rDNA replication in vivo. Total genomic DNA was purified from cells in G1 phase (arrested by mimosine), early S phase (released from mimosine for 3 h) and mid S phase (released from a double thymidine block for 3 h), enriched for replicative intermediates, cut with EcoRI and HindIII and subjected to 2D gel electrophoresis. Replicative intermediates were visualised by hybridisation to radioactively labelled EcoRI fragment B and autoradiography. (D) 2D gel analysis of rDNA replication in vitro. G1 phase nuclei from mimosine-arrested cells were incubated in cytosolic extract from proliferating cells for 3 h. Replicative intermediates were isolated and visualised as detailed for (C).
Replication intermediates were prepared from late G1 phase (mimosine-arrested), early S phase (released from mimosine for 3 h) and mid S phase cells (released from double thymidine block for 3 h), cut with EcoRI and HindIII and subjected to 2D gel electrophoresis (Fig. 4C). In G1 phase, no replication intermediates were seen. In very early S phase we could only detect a very faint simple Y arc in either the early or the late replicating variant fragments. In contrast, in mid S phase, we detected a prominent simple Y arc on both types of fragments. The results for the large (later replicating) variant covering the initiation zone are shown in Figure 4C. Although we did not detect a significant bubble arc on the late variant, a weak bubble arc in addition to a strong simple Y arc was detected when the blot was reprobed with probes CHB and CPE, which detect an early replicating HindIII–EcoRI variant restriction fragment that is centred on the region of highest frequency of initiation upstream of the gene promoter (9; data not shown). The coexistence of replication intermediates of both EcoRI variant fragments in the same time point is consistent with previous results (14). However, we have not investigated the degree to which the two variants replicate asynchronously under our conditions (see Discussion). Overall, these results suggest that efficient DNA replication of the amplified rDNA originates mainly in the non-transcribed spacer, consistent with earlier results (9,14), and does not occur at the beginning of S phase, but does so during later stages of S phase.
We therefore asked whether this replication timing in vivo is recapitulated in the human in vitro system. Strikingly, no DNA replication intermediates were formed in the rDNA cluster when nuclei from mimosine-arrested G1 phase cells were incubated in initiating cytosol (Fig. 4D). This result demonstrates that initiation of DNA replication in this system is restricted to early firing origins (such as the lamin B2 origin) and that later firing origins (such as in the non-transcribed spacer of the rDNA) are repressed from firing in an untimely manner.
DISCUSSION
In this paper we show for the first time that a homologous cell-free system entirely derived from human somatic cells (32,33) initiates DNA replication in isolated cell nuclei in an origin-specific manner. Furthermore, replication initiation timing is recapitulated in this system: the early firing lamin B2 origin initiates replication upon incubation of late G1 phase nuclei in cytosolic extract, whereas the later firing origins in the rDNA cluster do not initiate in vitro. Therefore, the results presented here establish the validity of the human cell-free system as a model system to study entry into S phase in a spatially and temporally controlled manner.
Initiation of DNA replication foci in the human cell-free system
Our previous work has established that human late G1 phase nuclei can be triggered to initiate semi-conservative DNA replication upon incubation in extracts from human cells that were synchronised in S phase (32) or, alternatively, in extracts from asynchronously proliferating cells (33). DNA replication is observed by confocal immunofluorescence microscopy on a per nucleus basis. In pilot control experiments we confirmed that late G1 phase nuclei initiate new DNA replication foci in the absence of pre-existing active or dormant replication forks (see Supplementary Material). New foci were established in vitro exclusively at intranuclear sites that had replicated when cells entered S phase of the previous cell cycle in vivo (Fig. 1). Amongst one of those sites initiated in vitro, initiation was specific for DNA sequences of the lamin B2 origin (Fig. 3). This very early firing origin was identified and precisely mapped before in intact human cells in a series of elegant experiments (5–8) and we were able to confirm this origin specificity in vivo for cells entering S phase upon release from a block in late G1 phase by mimosine (Fig. 3). From a technical viewpoint, our observation that the origin specificity is conserved between intact human cells and the cell-free initiation system should allow future exploitation of the cell-free system for the isolation and identification of earliest replicating DNA sequences from human cells.
Our data furthermore establish that in the human cell-free system the order of replication timing of individual replicons is not violated. We found that chromatin sites replicating in mid S phase, such as perinuclear and perinucleolar heterochromatin, do not initiate prematurely in this system (Fig. 2). Furthermore, domains replicating even later, such as nucleoli, are clearly excluded from initiating DNA replication in vitro and upon entry into S phase in vivo (Figs 1 and 2). With regard to sequence specificity in the nucleolar rDNA cluster, no significant replication intermediates are formed in vitro (Fig. 4), despite the presence of origins of replication or initiation zones in the intergenic spacer (9–11,14). This mirrors the situation in vivo, where these sequences are not initiated upon entry into S phase, but replicate at later stages of S phase (Fig. 4 and data not shown). The precise replication timing of all of the rDNA clusters, however, has not yet been established in detail in human cells. The origin mapping experiments were performed on asynchronously proliferating cells (9–11). However, one report shows differential timing of rDNA replication in S phase: one subpopulation is replicating late and another subpopulation is replicating apparently early in S phase (14). In the work of Larner et al., human cells were released for 80 min from block with a low concentration of mimosine (0.2 mM) and replication intermediates were observed in the rDNA locus (14). These observations are not inconsistent with ours, as 0.2 mM mimosine arrests human cells in early S phase and not in late G1 phase, as in the presence of 0.5 mM (35). A release from this early S phase block for 80 min therefore leaves the cells in mid S phase, similar to our synchronisation using release of thymidine-arrested early S phase cells (Fig. 4C). We are therefore tempted to argue that the earlier replicating cohort reported by Larner et al. (14) is not replicating at the onset of S phase, but at a later time after the G1 to S phase transition, and would therefore be fully consistent with our observations (Fig. 4).
Determinants of initiation competence, timing and sequence specificity
The molecular mechanisms that define and regulate the timing of origin firing during S phase are only beginning to be understood in metazoa (19). At discrete stages during G1 phase progression, replication timing and origin sequence specificity are established. The replication timing of chromosome domains is established in the nucleus at the TDP (17,18), which takes place 1–2 h post-metaphase in early G1, when chromosome domains reposition within the interphase nucleus following exit from mitosis (17). In the nuclei of mimosine-arrested human cells used in our study, proper replication timing is maintained in vitro and in vivo (Figs 1–4). These observations suggest that mimosine arrests human cells after execution of the TDP. Usage of specific DNA sites as replication origins is established at the ODP in the nucleus at 3–4 h after metaphase in mid G1 phase (15). Nuclei from mimosine-arrested cells used as templates in the human system in vitro can initiate DNA replication at a specific origin DNA sequence which is also used in vivo upon entry into S phase (Fig. 3), suggesting that mimosine arrests human cells after execution of the ODP. The experiments identifying the ODP and TDP made use of isolated hamster cell nuclei incubated in initiating extract from Xenopus eggs (15,17,18). Subsequent work confirmed that the ODP also operates in intact hamster cells in vivo (49). Therefore, constraints defining replication timing and origin specificity were associated with the hamster nuclei in cis and soluble initiation factors were supplied by the Xenopus extract. By analogy, we conclude that constraints specifying timing and site specificity of initiation in the homologous human system used in our studies are also associated with the late G1 phase template nuclei in cis and soluble initiation factors are supplied by the human cell extract.
A variant model of the differential timing of replication origin activation in our in vitro system could be built, postulating that mimosine blocks cells part way through a multistep initiation cascade, where early firing origins would be more advanced than later firing origins. Cytosolic initiation extract, in this model, would only support later steps of this cascade, with the result that early origins fire and late origins do not fire. We cannot rule out this scenario at present, and ongoing work in the laboratory of one of us (T. Krude) is aimed at dissecting the molecular steps of initiation in vitro by fractionation and reconstitution experiments of the initiating cell extract.
In any case, the competence of isolated human cell nuclei to initiate DNA replication in vitro is established in vivo during later stages of G1 phase progression. Nuclei prepared from early G1 phase human cells (32) or from rodent cells released from quiescence into G1 phase for <16 h (50) are relatively poor templates for initiation in human cell extracts. These nuclei lack factors that confer replication initiation competence (and hence preclude analysis of origin specificity and timing). In contrast, efficient initiation is observed in nuclei prepared either from dynamic populations of cells progressing through a narrow window of competence in late G1 phase, just before the onset of S phase in vivo (32,36,50), or from human cells arrested in late G1 phase with mimosine (33,35,36). These observations indicate that the initiating human cell extracts require a late G1 phase-specific structure within template nuclei to trigger initiation and cannot, unlike Xenopus egg extracts, induce replication on any DNA sequence or chromatin structure.
Taken together, we conclude for the human cell-free system used here that the constraints which dictate replication competence, replication timing and origin sequence specificity have all been assembled in vivo, before preparation of template nuclei, and are associated with the nuclei in cis.
Implications from a yeast cell-free initiation system and outlook
In the budding yeast Saccharomyces cerevisiae, DNA replication initiates in the chromosomal context at genetically defined short specific origin DNA sequences known as autonomous replicating sequences (2). The molecular mechanisms ensuring a controlled once per cell cycle firing of these origins have been characterised in detail (51). In a cell-free system derived from yeast cells, origin-specific initiation was observed in isolated G1 phase nuclei upon incubation in S phase nuclear extract (52). Initiation depended furthermore on the prior assembly and presence of the pre-replication complex including the origin recognition complex (ORC) and the protein kinase Cdc7-Dbf4 in the template nuclei (52,53). Amongst the soluble initiation factors required from the nuclear extract was the activity of yeast cyclin Clb/Cdc28 complexes (53).
In the human system, a strict requirement of S phase-promoting cyclin/Cdk activity for initiation has already been established (32,33,36), and ongoing research is focused on identifying and purifying additional initiation factors from the cytosolic extract. Our demonstration of origin specificity in the human system (this paper) adds another apparent layer of similarity of these two systems, despite the different organisation and sequence requirements for origin activity between yeast and man (2,3). The reason for this similarity is most likely associated with the requirement for an initiation-competent nuclear structure assembled in vivo as templates for initiation in cell extracts.
In human cells, the homologues of the yeast pre-replication complexes including the ORC, Cdc6 and MCM proteins are assembled following exit from mitosis and are bound to chromatin in late G1 phase (51). Preliminary western blot experiments confirm that this is also the case for the mimosine-arrested, late G1 phase cell nuclei used as templates in the human system (D. Szüts and L. Kitching, personal communication). Cross-linking and quantitative chromatin immunoprecipitation analysis demonstrated that the ORC is bound to the initiation site of the human lamin B2 origin (54). In addition, we recently identified two novel binding sites for the ORC in human chromatin that coincide with DNA sequences which function as origins of DNA replication, suggesting that the ORC may mark initiation sites for genome replication in general (54; C.Keller, E.Ladenburger, M.Kremer and R.Knippers, submitted for publication). Future experiments, which have now become possible in vitro through the cell-free system, should be aimed at studying in molecular detail the association of soluble initiation factors with pre-replication complexes bound to initiation-competent chromatin at human origin sequences.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at NAR Online.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Cristina Pelizon and Dávid Szüts for critically reading the manuscript and Ron Laskey and Tony Mills for discussions. We also thank Jim Sylvester (DuPont Hospital for Children, Wilmington, USA) for the generous gift of rDNA probes. This work was supported by Cancer Research UK (grant SP2546/0101 to T.K.), the Bundesministerium für Bildung und Forschung, BMBF (Förderkennzeichen 01 KW 9708 to R.K.) and the Association pour la Recherche sur le Cancer and the Ligue Nationale contre le Cancer, Comité de Paris (to O.H.).
REFERENCES
- 1.Hand R. (1978) Eukaryotic DNA: organization of the genome for replication. Cell, 15, 317–325. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert D.M. (2001) Making sense of eukaryotic DNA replication origins. Science, 294, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Todorovic V., Falaschi,A. and Giacca,M. (1999) Replication origins of mammalian chromosomes: the happy few. Front. Biosci., 4, D859–D868. [DOI] [PubMed] [Google Scholar]
- 4.DePamphilis M.L. (1999) Replication origins in metazoan chromosomes: fact or fiction? Bioessays, 21, 5–16. [DOI] [PubMed] [Google Scholar]
- 5.Giacca M., Zentilin,L., Norio,P., Diviacco,S., Dimitrova,D., Contreas,G., Biamonti,G., Perini,G., Weighardt,F., Riva,S. et al. (1994) Fine mapping of a replication origin of human DNA. Proc. Natl Acad. Sci. USA, 91, 7119–7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S., Giacca,M., Norio,P., Biamonti,G., Riva,S. and Falaschi,A. (1996) Utilization of the same DNA replication origin by human cells of different derivation. Nucleic Acids Res., 24, 3289–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdurashidova G., Deganuto,M., Klima,R., Riva,S., Biamonti,G., Giacca,M. and Falaschi,A. (2000) Start sites of bidirectional DNA synthesis at the human lamin B2 origin. Science, 287, 2023–2026. [DOI] [PubMed] [Google Scholar]
- 8.Biamonti G., Giacca,M., Perini,G., Contreas,G., Zentilin,L., Weighardt,F., Guerra,M., Della Valle,G., Saccone,S., Riva,S. et al. (1992) The gene for a novel human lamin maps at a highly transcribed locus of chromosome 19 which replicates at the onset of S-phase. Mol. Cell. Biol., 12, 3499–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Little R.D., Platt,T.H. and Schildkraut,C.L. (1993) Initiation and termination of DNA replication in human rRNA genes. Mol. Cell. Biol., 13, 6600–6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon Y., Sanchez,J.A., Brun,C. and Huberman,J.A. (1995) Mapping of replication initiation sites in human ribosomal DNA by nascent-strand abundance analysis. Mol. Cell. Biol., 15, 2482–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haaf T. (1997) Analysis of replication timing of ribosomal RNA genes by fluorescence in situ hybridization. DNA Cell Biol., 16, 341–345. [DOI] [PubMed] [Google Scholar]
- 12.Gencheva M., Anachkova,B. and Russev,G. (1996) Mapping the sites of initiation of DNA replication in rat and human rRNA genes. J. Biol. Chem., 271, 2608–2614. [DOI] [PubMed] [Google Scholar]
- 13.Scott R.S., Truong,K.Y. and Vos,J.M. (1997) Replication initiation and elongation fork rates within a differentially expressed human multicopy locus in early S phase. Nucleic Acids Res., 25, 4505–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larner J.M., Lee,H., Little,R.D., Dijkwel,P.A., Schildkraut,C.L. and Hamlin,J.L. (1999) Radiation down-regulates replication origin activity throughout the S phase in mammalian cells. Nucleic Acids Res., 27, 803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J.R. and Gilbert,D.M. (1996) A distinct G1 step required to specify the Chinese hamster DHFR replication origin. Science, 271, 1270–1272. [DOI] [PubMed] [Google Scholar]
- 16.Wu J.R. and Gilbert,D.M. (1997) The replication origin decision point is a mitogen-independent, 2-aminopurine-sensitive, G1-phase event that precedes restriction point control. Mol. Cell. Biol., 17, 4312–4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimitrova D.S. and Gilbert,D.M. (1999) The spatial position and replication timing of chromosomal domains are both established in early G1 phase. Mol. Cell, 4, 983–993. [DOI] [PubMed] [Google Scholar]
- 18.Li F., Chen,J., Izumi,M., Butler,M.C., Keezer,S.M. and Gilbert,D.M. (2001) The replication timing program of the Chinese hamster beta-globin locus is established coincident with its repositioning near peripheral heterochromatin in early G1 phase. J. Cell Biol., 154, 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert D.M. (2001) Nuclear position leaves its mark on replication timing. J. Cell Biol., 152, F11–F15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berezney R., Dubey,D.D. and Huberman,J.A. (2000) Heterogeneity of eukaryotic replicons, replicon clusters and replication foci. Chromosoma, 108, 471–484. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura H., Morita,T. and Sato,C. (1986) Structural organizations of replicon domains during DNA synthetic phase in the mammalian nucleus. Exp. Cell Res., 165, 291–297. [DOI] [PubMed] [Google Scholar]
- 22.Nakayasu H. and Berezney,R. (1989) Mapping replicational sites in the eucaryotic cell nucleus. J. Cell Biol., 108, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kill I.R., Bridger,J.M., Campbell,K.H., Maldonado-Codina,G. and Hutchison,C.J. (1991) The timing of the formation and usage of replicase clusters in S-phase nuclei of human diploid fibroblasts. J. Cell Sci., 100, 869–876. [DOI] [PubMed] [Google Scholar]
- 24.van Dierendonck J.H., Keyzer,R., van de Velde,C.J. and Cornelisse,C.J. (1989) Subdivision of S-phase by analysis of nuclear 5-bromodeoxyuridine staining patterns. Cytometry, 10, 143–150. [DOI] [PubMed] [Google Scholar]
- 25.O’Keefe R.T., Henderson,S.C. and Spector,D.L. (1992) Dynamic organization of DNA replication in mammalian cell nuclei: spatially and temporally defined replication of chromosome-specific alpha-satellite DNA sequences. J. Cell Biol., 116, 1095–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manders E.M., Stap,J., Brakenhoff,G.J., van Driel,R. and Aten,J.A. (1992) Dynamics of three-dimensional replication patterns during the S-phase, analysed by double labelling of DNA and confocal microscopy. J. Cell Sci., 103, 857–862. [DOI] [PubMed] [Google Scholar]
- 27.Manders E.M., Stap,J., Strackee,J., van Driel,R. and Aten,J.A. (1996) Dynamic behavior of DNA replication domains. Exp. Cell Res., 226, 328–335. [DOI] [PubMed] [Google Scholar]
- 28.Jackson D.A. (1995) S-phase progression in synchronized human cells. Exp. Cell Res., 220, 62–70. [DOI] [PubMed] [Google Scholar]
- 29.Jackson D.A. and Pombo,A. (1998) Replicon clusters are stable units of chromosome structure: evidence that nuclear organization contributes to the efficient activation and propagation of S phase in human cells. J. Cell Biol., 140, 1285–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma H., Samarabandu,J., Devdhar,R.S., Acharya,R., Cheng,P.C., Meng,C. and Berezney,R. (1998) Spatial and temporal dynamics of DNA replication sites in mammalian cells. J. Cell Biol., 143, 1415–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sparvoli E., Levi,M. and Rossi,E. (1994) Replicon clusters may form structurally stable complexes of chromatin and chromosomes. J. Cell Sci., 107, 3097–3103. [DOI] [PubMed] [Google Scholar]
- 32.Krude T., Jackman,M., Pines,J. and Laskey,R.A. (1997) Cyclin/Cdk-dependent initiation of DNA replication in a human cell-free system. Cell, 88, 109–119. [DOI] [PubMed] [Google Scholar]
- 33.Krude T. (2000) Initiation of human DNA replication in vitro using nuclei from cells arrested at an initiation-competent state. J. Biol. Chem., 275, 13699–13707. [DOI] [PubMed] [Google Scholar]
- 34.Lalande M. (1990) A reversible arrest point in the late G1 phase of the mammalian cell cycle. Exp. Cell Res., 186, 332–339. [DOI] [PubMed] [Google Scholar]
- 35.Krude T. (1999) Mimosine arrests proliferating human cells before onset of DNA replication in a dose-dependent manner. Exp. Cell Res., 247, 148–159. [DOI] [PubMed] [Google Scholar]
- 36.Laman H., Coverley,D., Krude,T., Laskey,R. and Jones,N. (2001) Viral cyclin-cyclin-dependent kinase 6 complexes initiate nuclear DNA replication. Mol. Cell. Biol., 21, 624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coverley D. and Laskey,R.A. (1994) Regulation of eukaryotic DNA replication. Annu. Rev. Biochem., 63, 745–776. [DOI] [PubMed] [Google Scholar]
- 38.Gilbert D.M., Miyazawa,H. and DePamphilis,M.L. (1995) Site-specific initiation of DNA replication in Xenopus egg extract requires nuclear structure. Mol. Cell. Biol., 15, 2942–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lucas I., Chevrier-Miller,M., Sogo,J.M. and Hyrien,O. (2000) Mechanisms ensuring rapid and complete DNA replication despite random initiation in Xenopus early embryos. J. Mol. Biol., 296, 769–786. [DOI] [PubMed] [Google Scholar]
- 40.Hyrien O. and Mechali,M. (1993) Chromosomal replication initiates and terminates at random sequences but at regular intervals in the ribosomal DNA of Xenopus early embryos. EMBO J., 12, 4511–4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hyrien O., Maric,C. and Mechali,M. (1995) Transition in specification of embryonic metazoan DNA replication origins. Science, 270, 994–997. [DOI] [PubMed] [Google Scholar]
- 42.Aten J.A., Bakker,P.J., Stap,J., Boschman,G.A. and Veenhof,C.H. (1992) DNA double labelling with IdUrd and CldUrd for spatial and temporal analysis of cell proliferation and DNA replication. Histochem. J., 24, 251–259. [DOI] [PubMed] [Google Scholar]
- 43.Dimitrova D.S., Todorov,I.T., Melendy,T. and Gilbert,D.M. (1999) Mcm2, but not RPA, is a component of the mammalian early G1-phase prereplication complex. J. Cell Biol., 146, 709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giacca M., Pelizon,C. and Falaschi,A. (1997) Mapping replication origins by quantifying relative abundance of nascent DNA strands using competitive polymerase chain reaction. Methods, 13, 301–312. [DOI] [PubMed] [Google Scholar]
- 45.Dijkwel P.A., Vaughn,J.P. and Hamlin,J.L. (1991) Mapping of replication initiation sites in mammalian genomes by two-dimensional gel analysis: stabilization and enrichment of replication intermediates by isolation on the nuclear matrix. Mol. Cell. Biol., 11, 3850–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brewer B.J. and Fangman,W.L. (1987) The localization of replication origins on ARS plasmids in S. cerevisiae. Cell, 51, 463–471. [DOI] [PubMed] [Google Scholar]
- 47.Hyrien O. and Mechali,M. (1992) Plasmid replication in Xenopus eggs and egg extracts: a 2D gel electrophoretic analysis. Nucleic Acids Res., 20, 1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biamonti G., Perini,G., Weighardt,F., Riva,S., Giacca,M., Norio,P., Zentilin,L., Diviacco,S., Dimitrova,D. and Falaschi,A. (1992) A human DNA replication origin: localization and transcriptional characterization. Chromosoma, 102, S24–S31. [DOI] [PubMed] [Google Scholar]
- 49.Wu J.R., Keezer,S.M. and Gilbert,D.M. (1998) Transformation abrogates an early G1-phase arrest point required for specification of the Chinese hamster DHFR replication origin. EMBO J., 17, 1810–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stoeber K., Mills A.D., Kubota,Y., Marheineke,K., Krude,T., Romanowski,P., Laskey,R.A. and Williams,G.H. (1998) Cdc6 causes premature entry into S phase in a mammalian cell-free system. EMBO J., 17, 7219–7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelly T.J. and Brown,G.W. (2000) Regulation of chromosome replication. Annu. Rev. Biochem., 69, 829–880. [DOI] [PubMed] [Google Scholar]
- 52.Pasero P., Braguglia,D. and Gasser,S.M. (1997) ORC-dependent and origin-specific initiation of DNA replication at defined foci in isolated yeast nuclei. Genes Dev., 11, 1504–1518. [DOI] [PubMed] [Google Scholar]
- 53.Pasero P., Duncker,B.P., Schwob,E. and Gasser,S.M. (1999) A role for the Cdc7 kinase regulatory subunit Dbf4p in the formation of initiation-competent origins of replication. Genes Dev., 13, 2159–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ladenburger E.M., Keller,C. and Knippers,R. (2002) Identification of a binding region for human origin recognition complex proteins 1 and 2 that coincides with an origin of DNA replication. Mol. Cell. Biol., 22, 1036–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.