Abstract
Background
Various types of crowns are used for full-coverage restoration of primary teeth affected by caries, developmental defects, or after pulp therapy. Prefabricated Stainless Steel and Zirconia crowns are commonly utilized. Bioflx crowns, which blend the properties of Stainless Steel and Zirconia, provide a flexible and aesthetically pleasing alternative.
Aim
This study aimed to evaluate the vertical marginal gap and fracture resistance of Bioflx pediatric crowns compared to Zirconia and Stainless Steel crowns following thermomechanical aging.
Methods
This in-vitro study was conducted using mandibular second primary crowns of three different materials (n = 30). Crowns were divided into three groups; Zirconia crowns group (n = 10, Nu Smile, USA), Bioflx crowns group (n = 10, Nu Smile, USA) and Stainless Steel crowns group (n = 10, Nu Smile, USA). The crowns were cemented onto standardized acrylic resin dies and subjected to thermomechanical aging. Vertical marginal gap measurements were obtained using a USB digital microscope with an integrated camera, while fracture resistance was assessed with a universal testing machine. Data were analyzed for outliers and tested for normality using the Shapiro-Wilk or Kolmogorov-Smirnov tests, with statistical significance set at 0.05.
Results
Significant differences were observed in the vertical marginal gaps among the groups after cementation and thermomechanical aging (P = 0.013 and P = 0.001, respectively). Zirconia crowns exhibited the largest average marginal gap, followed by Bioflx and Stainless Steel crowns. Stainless steel crowns demonstrated the highest fracture resistance, followed by Bioflx crowns, while Zirconia crowns showed the lowest.
Conclusions
Bioflx crowns exhibit the largest vertical marginal gap but show greater fracture resistance compared to Zirconia crowns, although they are still less resistant than Stainless Steel crowns after undergoing thermomechanical aging.
Keywords: Stainless steel crowns, Bioflx crowns, Zirconia crowns, Marginal gap, Fracture resistance
Background
The American Academy of Pediatric Dentistry recommends prefabricated full-coverage restorations for large or multisurface cavitated lesions in primary teeth [1, 2]. Stainless Steel crowns are considered the gold standard due to their cost-effectiveness, adaptability, minimal tooth reduction, and superior retention [3]. Moreover, their reliability and longevity are well-documented [4]. However, these crowns have limitations due to their metallic appearance, which is not aesthetically pleasing, and their potential for biological incompatibility. Such aesthetic concerns can adversely affect both the child’s and the parent’s perception, which cannot be disregarded [5, 6].
Consequently, aesthetic materials have been developed to replace Stainless Steel crowns, including open-faced crowns and pre-veneered stainless-steel crowns [7]. While these crowns offer an acceptable aesthetic, they present several drawbacks, such as poor gingival health, extended chair time, limited contouring or crimping ability, and the potential for veneer resin chipping or fracture [8, 9].
Zirconia crowns have been used in primary teeth as aesthetic treatment options, offering satisfactory mechanical and biological properties [10]. They are composed of polycrystalline ceramic without a glass component often referred to as “ceramic steel,” [11, 12]. The advantages of Zirconia crowns include high compressive strength, longevity, and biocompatibility [13, 14]. Their color, translucency, high polish and potential for subgingival extension minimize the risk of gingival irritation in primary teeth and ensure a natural appearance [15, 16]. However, Zirconia crowns require more extensive tooth preparation when compared to conventional Stainless Steel crowns [14, 17, 18]. Their greater hardness may cause wear on the opposing enamel [19], they can’t be easily modified or adjusted at the margins, relying solely on dental cement for retention [20]. Furthermore, their high cost also makes them more expensive compared to other aesthetic dental treatment options [21, 22].
Bioflx crowns represent a noteworthy innovation in pediatric dentistry, combining the desirable features of both Stainless Steel and Zirconia crowns. These crowns are made from a biocompatible, high-impact and high-strength hybrid resin polymer [23]. They provide an aesthetic benefit similar to Zirconia, providing a natural appearance. Bioflx crowns are highly flexibility, conforming to the anatomical cervical convexity of primary teeth and ensuring an active fit to the tooth [24]. Additionally, they require less tooth removal compared to Zirconia crowns [25, 26].
Marginal adaptation plays a crucial role in the success of full-coverage dental restorations. Achieving proper marginal adaptation ensures a tight fit between the restoration and the tooth [27]. Any discrepancies at the margins can lead to increased plaque accumulation and bacterial growth, potentially resulting in secondary caries, periodontal issues, and eventually tooth loss [1].
Another critical property that should be considered in pediatric crowns is fracture resistance which reflects both the resilience of the material and the ability to withstand the forces of chewing and other oral stresses [28]. Fracture resistance is measured by the material’s capacity to inhibit the progression of cracks that begin from inherent flaws, potentially leading to minor fractures at the restoration’s edges or extensive fractures within the filling itself [29].
Finding aesthetically pleasing and functional coverage solutions for primary molars is a key concern for pediatric dentists. Consequently, there is an immediate need to identify new materials that are cost-effective, repairable, and tailored to specific requirements. Ideally, such materials would support less invasive preparations, enhance fit and retention, shorten chair time, and simplify the placement process. Therefore, this study aims to compare the recently developed Bioflx crowns with two traditional pediatric crowns, Zirconia and Stainless Steel, in terms of vertical marginal gap and fracture resistance.
The null hypothesis of this study asserted that there were no notable differences in the vertical marginal gap and fracture resistance between Zirconia, Bioflx, and Stainless Steel crowns after undergoing thermomechanical aging.
Methods
This in-vitro experiment was carried out in the Dental Biomaterials Department at the Faculty of Dentistry, Suez Canal University, with the approval of the institution’s Research Ethical Committee (approval number 754/2023).
Sample size calculation was performed using G*Power version 3.1.9.2 [30], based on the effect size of 0.80 using an alpha (α) level of 0.05 and Beta (β) level of 0.20, corresponding to a power of 80%. Consequently, the calculated minimum number of samples required was thirty.
Grouping
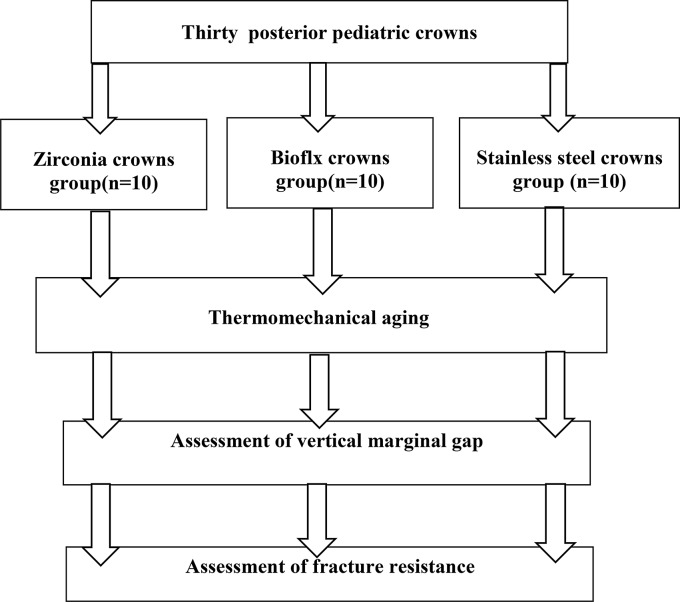
Thirty-three primary mandibular right second molar crowns size five were used in this study (n = 33) (3 crowns used for die fabrication were excluded from the laboratory tests). The crowns (n = 30) were equally divided into three groups; Zirconia crowns group (n = 10, Nu Smile, USA), Bioflx crowns group (n = 10, Nu Smile, USA) and Stainless Steel crowns group (n = 10, Nu Smile, USA). (Fig. 1)
Fig. 1.
Schematic representation of experimental study design
Die fabrication
Initially, three acrylic dies were produced by filling one crown of each type with acrylic resin (Cold cure acrylic resin, Acrostone, Egypt) and allowing them to harden for one hour. The dies were then evaluated for fit, and any visible undercuts in the dies were removed with a finishing bur. The dies were placed in a PVC (polyvinyl chloride) tube, filled with the same acrylic resin to form a die resin base and left to solidify for an additional hour. Impressions of each crown die were taken using silicone impression material (Zhermack S.p.A Tropicalgin, Italy), which hardened within an hour. This process yielded three negative master impressions, which were then used to precisely fabricate ten acrylic dies for each crown category, subsequently the dies cured for 24 h [15, 31].
Crowns cementation
Thirty crowns were cemented onto acrylic dies using glass ionomer cement (Riva self-cure, SDI, Australia), prepared according to the manufacturer’s instructions and applied to the inner surfaces of the crowns. The crowns were then accurately positioned on the relevant dies and allowed to harden for 10 min under static finger pressure, followed by axially loaded with a 3 kg weight using a custom-made holding device [32] (Fig. 2). Excess cement was carefully removed with a dental explorer. Finally, the crowns were left to be completely set for 24 h [33].
Fig. 2.
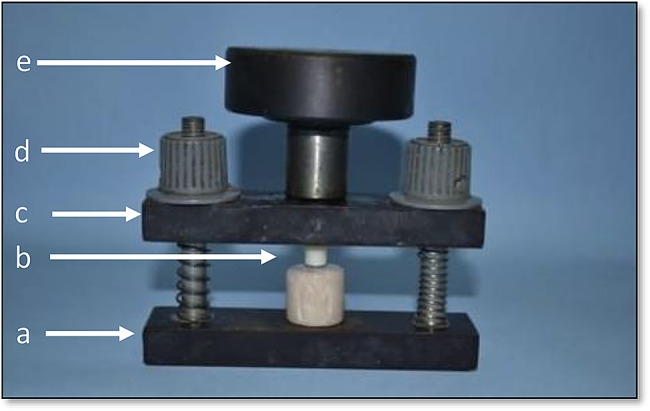
Cementation holding device. (a) Fixed base portion. (b) Position of a crown sample (c) Upper movable portion. (d) Tightening plastic cups. (e) T shape weight-bearing portion (3 kg)
Thermomechanical aging
To replicate the conditions of six months in the oral environment, the crowns underwent 5000 thermal cycles ranging from 5 to 55 °C, with a dwell time of 25 s and a lag time of 10 s using a Robota automated thermal cycler (BILGE, Turkey) [34]. Subsequently, a chewing simulator (Robota, model ACH-09075DCT, AD-Tech Technology CO., LTD., Germany) was employed to administer 75,000 cycles of a 50 N occlusal load at a frequency of 1.6 Hz [28, 35].
Assessment methods
The vertical marginal gap of the thirty crowns was assessed first, followed by an evaluation of their fracture resistance. The entire procedure was performed by a single operator. The validity and reliability were assessed as the intra-observer reliability for the measurements.
Assessment of vertical marginal gap
Marginal discrepancy was assessed by measuring the vertical distance between both the crown and corresponding die margins (parallel to the tooth axis). Each sample was documented using a USB digital microscope equipped with an integrated camera; the photographs were captured using the specified image acquisition system [27, 36–39]. A digital camera (U500x Digital Microscope, Guangdong, China) with a resolution of 3 megapixels were positioned vertically 2.5 cm away from the samples. The images were captured at the highest resolution and interfaced with a compatible personal computer set to a fixed magnification of 40X. Gap width measurements were conducted using a digital image analysis system (Image J 1.43U, National Institute of Health, USA). Calibration was made by comparing an object of known size (a ruler in this study) with a scale generated by the Image J software. Shots of the margins were taken for each specimen for all surfaces (buccal, mesial, lingual and distal). Finally, morphometric measurements were done for each shot (4 equidistant landmarks along the circumference for each surface) [40]. The average values, along with standard deviations, were expressed in micrometres (µm).
Assessment of fracture resistance
After thermomechanical aging, each sample was positioned on a computer-controlled materials testing apparatus (Model 3345; Instron Industrial Products, Norwood, MA, USA) that featured a 5 kN load cell following ISO specifications No ISO 7500-1. Data collection was conducted using Bluehill Lite Software (Instron®). The samples were firmly attached to the lower fixed part of the machine using screws. Fracture testing proceeded in compressive mode, with the force directed occlusal via a metallic rod with a rounded tip (5.6 mm diameter) connected to the upper movable part of the machine. The machine functioned at a crosshead speed of 1 mm/min, and a tin foil sheet was inserted between the specimen and the rod to facilitate uniform stress distribution and minimize localized force spikes. The occurrence of a fracture was signalled by an audible crack and a noticeable decline in the load-deflection curve which was recorded by Software. The force required to induce the fracture was quantified in Newtons (N) [14, 16, 28]. The Mode of failure of the tested crowns was assessed using a light microscope (Leica Microsystems GmbH, Germany) to detect and verify the surface deformation, perforations and fractures [41].
Statistical analysis
Data compilation, verification, and organization into tables were performed using Microsoft Excel 2016. The dataset underwent outlier detection and normality testing, using the Shapiro-Wilk and Kolmogorov-Smirnov tests at a 0.05 significance level. Parametric descriptive statistics were presented numerically in tables, with data represented as mean and standard deviation. For inferential statistics, a one-way Analysis of Variance (ANOVA) was performed, followed by Duncan’s Multiple Range Test (DMRT) at 0.05 level. Statistical analyses were performed using IBM-SPSS version 29.0 form Mac OS (IBM SPSS, release 2023, Armonk, NY: IBM Corp).
Results
The results in Table 1 showed a significant difference in the vertical marginal gap between groups (Zirconia, Bioflx, and Stainless Steel) across all surfaces before cementation, after cementation, and after aging, except at the distal surface after cementation and after aging.
Table 1.
Mean and standard deviation SD (±) values (µm) of the vertical marginal gap among different groups across all surfaces before cementation, after cementation and after thermomechanical aging
| Zirconia | Bioflx | Stainless Steel | F test | P value | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||||
| Buccal | Before cementation | 31.14 ± 1.64b | 26.86 ± 4.29c | 35.08 ± 5.28a | 5.178 | 0.024** |
| After cementation | 45.08 ± 2.51a | 31.62 ± 7.15c | 42.38 ± 3.26b | 11.173 | 0.002** | |
| After aging | 53.86 ± 2.45a | 40.38 ± 7.52c | 47.58 ± 4.87b | 7.918 | 0.006** | |
| Mesial | Before cementation | 35.70 ± 2.33a | 27.72 ± 4.38b | 27.66 ± 8.00b | 1.162 | 0.034** |
| After cementationt | 47.14 ± 1.55b | 50.38 ± 4.72a | 44.82 ± 7.48b | 0.426 | 0.0461** | |
| After aging | 74.64 ± 10.23a | 74.28 ± 7.44a | 49.12 ± 5.67b | 7.281 | 0.009** | |
| Lingual | Before cementation | 31.94 ± 1.87a | 27.52 ± 8.06b | 28.40 ± 4.49b | 0.927 | 0.0422** |
| After cementationt | 44.42 ± 4.13a | 31.86 ± 6.44b | 46.84 ± 5.52a | 10.901 | 0.002** | |
| After aging | 57.20 ± 6.49b | 63.32 ± 2.58a | 46.54 ± 5.19c | 14.272 | 0.001** | |
| Distal | Before cementation | 30.50 ± 2.76a | 26.28 ± 5.41b | 22.72 ± 2.30c | 5.387 | 0.021** |
| After cementationt | 43.54 ± 2.78b | 38.26 ± 7.10a | 47.12 ± 8.84a | 2.188 | 0.155 | |
| After aging | 64.60 ± 4.15a | 57.32 ± 7.96a | 54.74 ± 6.32a | 3.255 | 0.074 |
** Means significant at P <0.05
Different superscript letters indicate statistically significant differences among groups at P<0.05
Statistical analysis showed no significant differences in the average of vertical marginal gap between Zirconia, Bioflx, and Stainless Steel crowns before cementation (P = 0.063), while after cementation and after aging showed significant differences between groups (P = 0.013 and 0.001 respectively). The pairwise comparison revealed a statistically significant difference in the percentage change of vertical marginal gap among groups after cementation, with Zirconia showing a 39.3% change, Bioflex showing a 40.2% change, and Stainless Steel showing a higher change of 58.9%. Additionally, after aging, there was a significant difference in the percentage change where Zirconia exhibited a 93.8% change, Bioflex a 117.0% change, and Stainless Steel a lower change of 75.4%. (Table 2; Fig. 3).
Table 2.
Mean, standard deviation SD (±) values (µm) and percentage change of the vertical marginal gap among different groups before cementation, after cementation, and after thermomechanical aging
| Before cementation (A) | After cementation (B) | After aging (C) | % change (B-A) |
% change (A-C) |
F test | P value | |
|---|---|---|---|---|---|---|---|
| Zirconia crowns | 32.3 ± 1.0 c A | 45.0 ± 0.8 b A | 62.6 ± 2.6 a A | 39.3 | 93.8 | 424.4 | < 0.001** |
| Bioflx crowns | 27.1 ± 4.8 c A | 38.0 ± 5.3 b B | 58.8 ± 6.5 a A | 40.2 | 117.0 | 41.83 | < 0.001** |
| Stainless Steel crowns | 28.5 ± 2.7 c A | 45.3 ± 3.4 b A | 50.0 ± 1.6 a B | 58.9 | 75.4 | 90.79 | < 0.001** |
| F test | 3.51 | 6.36 | 12.19 | ||||
| P value | 0.063 | 0.013** | 0.001** |
**; Means significant differences P<0.05
Small superscript letters (Row) mean the significant difference between before cementation, after cementation and after aging at P<0.05
Capital superscript letters (Column) mean significant difference among groups at P<0.05
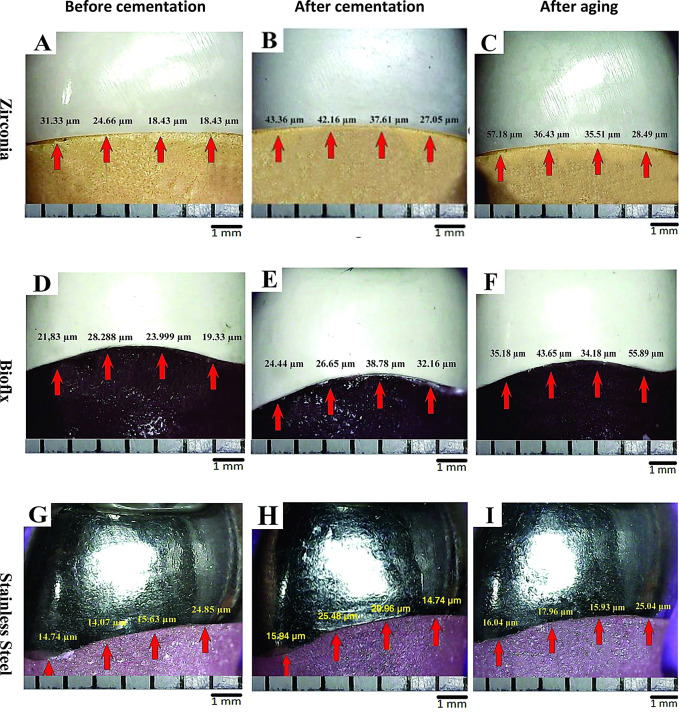
Fig. 3.
Digital light microscope images showing the vertical marginal gap of the tested crowns. Zirconia crowns (A; before cementation, B; after cementation and C; after thermomechanical aging), Bioflx crowns (D; before cementation, E; after cementation, and F; after thermomechanical aging) and Stainless Steel crowns (G; before cementation, H; after cementation, and I; after thermomechanical aging). Arrows refer to equidistant landmarks along the circumference for each surface
The fracture resistance test results after thermomechanical aging revealed a significant difference among the study groups (P < 0.001). Stainless steel crowns exhibited the highest mean fracture resistance (3062.14 ± 408.97) followed by Bioflx crowns (2403.44 ± 92.65), while Zirconia crowns demonstrated the lowest resistance (1286.30 ± 91.56) among different study groups, Table 3. The failure mode for Stainless Steel and Bioflx crowns was characterized by permanent deformation of the occlusal surface and micro perforations, whereas Zirconia crowns experienced fracture lines (Table 4; Fig. 4).
Table 3.
Mean and standard deviation SD (±) values (N) of fracture loads among groups after thermomechanical aging
| Mean ± SD | F test | P value | |
|---|---|---|---|
| Zirconia crowns | 1286.30 c ± 91.56 | 65.619 | < 0.001** |
| Bioflex crowns | 2403.44 b ± 92.65 | ||
| Stainless Steel crowns | 3062.14 a ± 408.97 |
**; Means significant differences
Small letters mean the significant difference among groups at P<0.05
Table 4.
Quantitative analysis of the failure mode among groups
| Crown type | Surface deformation | Micro perforations | Fractures and loss |
|---|---|---|---|
| Zirconia crowns | ---- | ----- | 10 |
| Bioflx crowns | 10 | 4 | ---- |
| Stainless Steel crowns | 10 | 6 | ---- |
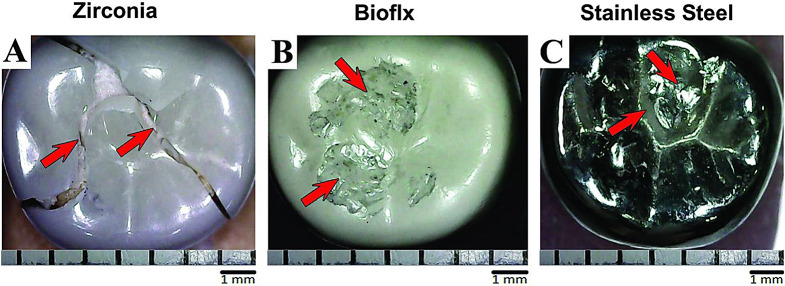
Fig. 4.
Light microscope images showing the mode of failure among tested crowns after thermomechanical aging. (A) Arrows refer to the fracture line in Zirconia crowns. (B) Arrows refer to the permanent surface deformation in Bioflx crowns. (C) Arrows refer to permanent surface deformation in Stainless Steel crowns
Discussion
With advancements in aesthetic dentistry, the demand for more visually appealing alternatives to traditional Stainless Steel crowns is increasing among both young patients and their guardians [24, 25].
While utilizing natural teeth in in-vitro studies can closely mimic actual clinical conditions, this study used resin dies to avoid the inherent variability of natural teeth. Differences in tooth anatomy, hard tissue thickness, age, storage conditions, shape, and size complicate the standardization of tooth preparation. Such variability could lead to inconsistent cement thickness across samples, increasing stress on the restorative material and raising the risk of fractures [42].
Thermal and mechanical cycling was used to simulate the aging of restorations and replicate the impact of masticatory forces on the crowns as if they had been in the oral cavity for six months [43]. Cyclic loading mimics the repetitive sub-threshold stresses from daily chewing activities, which can contribute to the failure of restorations. In this study, thermomechanical aging provides a more accurate assessment of the crowns’ performance and offers insights into their potential clinical lifespan [15, 44].
In this study, the assessment of marginal gaps was performed using a USB digital microscope with integrated illumination. This non-destructive method is preferred as it is external and does not require intermediate materials like impression materials. It is also less time-consuming than other techniques and minimizes potential errors from multiple steps, improving result accuracy. Furthermore, this method is cost-effective. The evaluation focused on the vertical cervical marginal gap, a commonly used indicator of restoration fit precision [27, 36–39].
The averaged vertical marginal gap values for the buccal, mesial, lingual and distal surfaces were reported for simplicity, clarity, and ease of comparison with other studies. The findings revealed no significant differences in the marginal gaps among the Zirconia, Bioflx, and Stainless-Steel crowns before cementation. However, significant differences were observed among groups after cementation and following aging.
It has been established that a maximum marginal discrepancy of 120 μm is deemed clinically acceptable for traditionally manufactured restorations [39, 45]. The average discrepancy values for all crowns examined at various stages in this study remained consistently below or within this acceptable range, indicating that the fit of all crowns is clinically suitable [46, 47].
This study’s findings align with those of several researchers [48–50] who noted a considerable increase in the marginal gap post-cementation compared to pre-cementation. Variations in cementation techniques, such as unregulated manual pressure (thus, a standardized pressure was applied using a specialized device in this study) or the over-application of cement, can lead to inconsistent cement distribution, resulting in one side of the axial wall having a thicker cement layer while the opposite side has a thinner one [51, 52].
In this study, there was a significant increase in marginal discrepancy values among groups following thermomechanical aging. These findings are consistent with those reported by Fouad, M. [36], who indicated that thermal aging could exacerbate marginal discrepancies between crowns and their corresponding dies. This phenomenon may arise from the cementation process, which is influenced by various factors, including the thermal mismatch between the dies and the crowns. Furthermore, Al-Haj Ali et al. [53], and Elsayed et al. [54] found that Zirconia crowns exhibited the most significant microleakage, potentially linked to artificial aging and thermocycling. These processes are crucial as they tend to diminish the adhesive strength between Zirconia crowns and luting cements. Additionally, thermocycling can cause repeated stresses from shrinkage and expansion, result from the mismatching of thermal coefficients of the materials used in the restoration [55].
In fracture resistance measurements, every tested specimen withstood a load greater than the typical occlusal forces observed in the posterior region of children’s mouths during the transition from early primary to permanent dentition. According to research by Talekar et al. [56], the average occlusal biting forces were recorded as 176 N in early primary dentition, 240 N in late primary dentition, 289 N in early mixed dentition, and 433 N in late mixed dentition.
In this study, the findings of fracture resistance among groups after thermomechanical aging noted a distinction among Zirconia, Bioflx, and Stainless Steel crowns. Stainless Steel crowns exhibited the greatest average fracture resistance, followed by Bioflx crowns, while Zirconia crowns displayed the lowest fracture resistance.
The findings on fracture resistance from this study align with the findings reported by Kist et al. [13] and Elsayed et al. [54] who investigated the fracture load and chewing simulation of Zirconia and Stainless-Steel crowns for primary molars. Their research documented the high fracture load values for Stainless-Steel crowns that might be a result of the higher ductility of the Stainless-Steel crowns compared with the more brittle Zirconia. Additionally, Townsend et al. [57], and Akila et al. [1], reported that Stainless Steel crowns exhibited better compressive strength compared to Zirconia crowns.
The primary types of damage observed in Stainless Steel crowns are fatigue and permanent occlusal surface deformation which is considered a functional failure. Fatigue damage occurs because metals, (Stainless Steel), are susceptible to weakening under repeated alternating loads. This susceptibility is compounded by potential thinning of the occlusal surface due to plastic deformation from chewing simulations. Over time, the repeated stresses can lead to noticeable deformations in the crowns [58]. Despite their durability as restorative materials for deciduous teeth, Stainless Steel crowns can suffer damage and deformation over long-term use if subjected to forces exceeding typical chewing pressures [59].
Zirconia crowns showed the lowest fracture resistance and presented with cracks and fracture lines on the occlusal surface after being subjected to compressive loading which is considered a brittle fracture. The vulnerability of these crowns to fracture can be attributed to their inability to absorb significant amounts of plastic strain energy [60]. When subjected to excessive force, they are likely to break, as is typical with ceramic materials, these crowns do not withstand flexure well and are prone to easy fracturing. Unlike other materials (Stainless Steel), Zirconia crowns don’t flex and can’t withstand permanent deformation and is susceptible to fracture under excessive compressive load [61, 62].
The average fracture resistance for Bioflx crowns ranks them slightly less than Stainless Steel crowns yet more than Zirconia crowns. The observed occlusal failures are characterized by fatigue and permanent occlusal surface deformation which may be attributed to the crowns’ composition of a biocompatible hybrid resin polymer, which possesses more flexibility and greater elasticity. This material is designed to improve ductility, adaptability, and durability [25, 26].
This finding aligns with Deolikar et al. [26], who concluded that the Bioflx crown can withstand perpendicular forces and has superior resistance to crown fracture compared to zirconia crowns.
The null hypothesis was rejected, as significant differences were observed in both marginal integrity and fracture resistance among the Zirconia, Bioflx, and Stainless Steel crowns following thermomechanical aging.
Bioflx crowns have emerged as a promising new flexible and aesthetic alternative to both Zirconia and Stainless Steel crowns in pediatric dentistry.
This study encounters several limitations, as this study considers pioneering for investigation of Bioflx crowns and calculating the percentage change of a marginal gap of the tested groups however, limited literature was available. In addition, this study assesses 2D measurements of the vertical cervical marginal gap rather than the 3D space of the margin (depth as well as height).
Conclusions
After thermomechanical aging, Bioflx crowns exhibit the largest vertical marginal gap when compared to Zirconia and Stainless Steel crowns. In terms of fracture resistance Bioflx crowns are greater than Zirconia crowns, while the Stainless Steel crowns offer the highest fracture resistance among the three groups.
The failure mode for Stainless Steel and Bioflx crowns is characterized by permanent deformation of the occlusal surface and micro perforations, whereas Zirconia crowns experience fracture lines.
Recommendations
Further studies are needed to evaluate the physical and mechanical properties of Bioflx crowns, both in vitro and in vivo studies. Furthermore, a 3D assessment provides a more comprehensive evaluation of the crown’s fit.
Acknowledgements
Not applicable.
Author contributions
N.A and E.M carried out the lab work. A.A conceived the idea, reviewed, revised the manuscript, designed the study, collected and analyzed data, drafted the manuscript. N.A, E.M and A.A read and approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding was received.
Data availability
Data cannot be shared openly but are available on request from authors.
Declarations
Ethical approval
The research ethics committee of the Faculty of Dentistry, Suez Canal University granted an approval for this research with reference number (754/2023).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.V A, et al. Evaluation of compressive strength, microleakage and amount of primary tooth reduction required for posterior zirconia and stainless steel crowns-an invitro study. Int J Sci Res Publ. 2021;11:544–7. [Google Scholar]
- 2.Dentistry AA. of P. Pediatric restorative dentistry. Ref. Man. Pediatr. Dent. 2021, 371–383 (2020).
- 3.Abushanan A, Sharanesha RB, Aljuaid B, Alfaifi T, Aldurayhim A. Fracture resistance of primary Zirconia crowns: an in Vitro Study. Children. 2022;9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.youness badr sherine, Rayyan M, elshiekh manal. Evaluation of fracture resistance and color stability of innovative esthetic crowns for primary posterior molars. Egypt Dent J. 2021;67:1879–86. [Google Scholar]
- 5.Holsinger DM, Wells MH, Scarbecz M, Donaldson M. Clinical evaluation and parental satisfaction with pediatric Zirconia anterior crowns. Pediatr Dent. 2016;38:192–7. [PubMed] [Google Scholar]
- 6.Pani SC et al. Esthetic concerns and acceptability of treatment modalities in primary teeth: A comparison between children and their parents. Int. J. Dent. 2016, (2016). [DOI] [PMC free article] [PubMed]
- 7.Agrawal R, Khanduja R, Singhal M, Gupta S, Kaushik M. Clinical Evaluation of Stainless Steel Crown versus Zirconia Crown in primary molars: an in vivo study. Int J Clin Pediatr Dent. 2022;15:15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champagne C, Waggoner W, Ditmyer M, Casamassimo PS. Parental satisfaction with preveneered stainless steel crowns for primary anterior teeth. Pediatr Dent. 2007;29:465–9. [PubMed] [Google Scholar]
- 9.Shah PV, Lee JY, Wright JT. Clinical success and parental satisfaction with anterior preveneered primary stainless steel crowns. Pediatr Dent. 2004;26:391–5. [PubMed] [Google Scholar]
- 10.Taran PK, Şahinbaş A, Bayraktar GA. Effect of different restorative materials on primary tooth wear: a quantitative evaluation using microcomputed tomography. Pediatr Dent. 2021;43:395–402. [PubMed] [Google Scholar]
- 11.Khatri A. Esthetic zirconia crown in pedodontics. Int J Pedod Rehabil. 2017;2:31–3. [Google Scholar]
- 12.Shi HY et al. Overview of Several Typical Ceramic Materials for Restorative Dentistry. Biomed Res. Int. 2022, 8451445 (2022). [DOI] [PMC free article] [PubMed]
- 13.Kist S, Stawarczyk B, Kollmuss M, Hickel R, Huth KC. Fracture load and chewing simulation of zirconia and stainless-steel crowns for primary molars. Eur J Oral Sci. 2019;127:369–75. [DOI] [PubMed] [Google Scholar]
- 14.Ali AAEM, Abo-ELsoud AAE, Helmy YS. The fracture resistance of pulpotomized primary molars restored with zirconia crowns, lithium disilicate or resin based ceramic endocrowns. BMC Oral Health. 2024;24:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.el habashy, laila m, Aboushelib OA. Fatigue and 3-D wear resistance of Fiberglass versus Stainless steel crowns for restoration of primary teeth. Egypt. Dent. J. 67, 1835–1841 (2021).
- 16.El Makawi Y, Khattab N. In Vitro Comparative Analysis of Fracture Resistance of Lithium Disilicate Endocrown and Prefabricated Zirconium Crown in Pulpotomized Primary molars. Open Access Maced J Med Sci. 2019;7:4094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark L, Wells MH, Harris EF, Lou J. Comparison of amount of primary tooth reduction required for anterior and Posterior Zirconia and Stainless Steel Crowns. Pediatr Dent. 2016;38:42–6. [PubMed] [Google Scholar]
- 18.Falahchai, M., Babaee Hemmati, Y., Neshandar Asli, H. & Neshandar Asli, M. Marginal adaptation of zirconia-reinforced lithium silicate overlays with different preparation designs. J. Esthet. Restor. Dent. Off. Publ. Am. Acad. Esthet. Dent. … et al.] 32, 823–830 (2020). [DOI] [PubMed]
- 19.Jitwirachot K, Rungsiyakull P, Holloway JA, Jia-Mahasap W. Wear Behavior of Different Generations of Zirconia: Present Literature. Int. J. Dent. 2022, 9341616 (2022). [DOI] [PMC free article] [PubMed]
- 20.Cohn C. Zirconia-Prefabricated crowns for Pediatric patients with primary dentition: technique and cementation for esthetic outcomes. Compend Contin Educ Dent. 2016;37:554–8. [PubMed] [Google Scholar]
- 21.Bica C, et al. Applicability of zirconia-prefabricated crowns in children with primary dentition. Rev Chim. 2017;68:1940–3. [Google Scholar]
- 22.Lee J-H. Guided tooth preparation for a pediatric zirconia crown. J Am Dent Assoc. 2018;149:202–e2082. [DOI] [PubMed] [Google Scholar]
- 23.Rahate I, Fulzele P, Thosar N. Comparative evaluation of clinical performance, child and parental satisfaction of Bioflx, zirconia and stainless steel crowns in pediatric patients. F1000Research 12, 756 (2023). [DOI] [PMC free article] [PubMed]
- 24.Https://nusmile.com/pages/bioflx), (. No Title.
- 25.Almajed OS. Shaping smiles: a narrative review of Crown Advancements in Pediatric Dentistry. Cureus. 2024;16:e52997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deolikar S, Rathi N, Mehta V. Comparative evaluation of stress generation in primary teeth restored with zirconia and BioFlx crowns: a finite element analysis. Dent J. 2024;57:80–6. [Google Scholar]
- 27.Hegazy M, Shakal M, Sharkawy E, S., Sultan S. Effect of Cement Space settings on the Vertical marginal gap of Cement retained - Implant supported crowns: an in vitro study. Adv Dent J. 2022;4:110–22. [Google Scholar]
- 28.El Shahawy OI, Azab MM. Fracture resistance of prefabricated versus custom-made zirconia crowns after thermo-mechanical aging: an in-vitro study. BMC Oral Health. 2022;22:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bianchi E, Silva AA, méli, et al. Influence of restorative techniques on fracture load of endodontically treated premolars. Stomatologija. 2013;15:123–8. [PubMed] [Google Scholar]
- 30.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. [DOI] [PubMed] [Google Scholar]
- 31.Al Shobber MZ, Alkhadra TA. Fracture resistance of different primary anterior esthetic crowns. Saudi Dent J. 2017;29:179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sultan S, Hegazy M, Shakal M, Magdy S. Effect of virtual cement gap settings on the marginal fit of cemented resin-ceramic crowns on implant abutments. J Prosthet Dent. 2021;125:e8041–6. [DOI] [PubMed] [Google Scholar]
- 33.Salman NR, Khattab NMA, Gomaa YF. Assessment of the external marginal adaptation of prefabricated zirconia crowns for restoring primary molars using a stereomicroscope. Ann Rom Soc Cell Biol. 2021;25:20218–26. [Google Scholar]
- 34.Morresi AL, et al. Thermal cycling for restorative materials: does a standardized protocol exist in laboratory testing? A literature review. J Mech Behav Biomed Mater. 2014;29:295–308. [DOI] [PubMed] [Google Scholar]
- 35.Nawafleh N, Hatamleh M, Elshiyab S, Mack F. Lithium disilicate restorations fatigue testing parameters: a systematic review. J Prosthodont off J Am Coll Prosthodont. 2016;25:116–26. [DOI] [PubMed] [Google Scholar]
- 36.Fouad M. Marginal adaptation related to margin design of CAD/CAM ceramic crowns. Egypt Dent J. 2023;69:447–55. [Google Scholar]
- 37.Farag E, Sabet A, Ebeid K. El Sergany, O. Build angle effect on 3D-printed dental crowns marginal fit using digital light-processing and stereo-lithography technology: an in vitro study. BMC Oral Health. 2024;24:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji M-K, et al. Evaluation of marginal fit of 2 CAD-CAM anatomic contour zirconia crown systems and lithium disilicate glass-ceramic crown. J Adv Prosthodont. 2015;7:271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emam M, Metwally MF. Effect of coping materials zirconia or polyetheretherketone with different techniques of fabrication on vertical marginal gap and fracture resistance of posterior crowns with composite veneering. BMC Oral Health. 2023;23:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Möhn M, Frankenberger R, Krämer N. Wear and marginal quality of aesthetic crowns for primary molars. Int J Paediatr Dent. 2022;32:273–83. [DOI] [PubMed] [Google Scholar]
- 41.Kessler A, et al. Two-body wear and fracture behaviour of an experimental paediatric composite crown in comparison to zirconia and stainless steel crowns dependent on the cementation mode. Dent Mater. 2021;37:264–71. [DOI] [PubMed] [Google Scholar]
- 42.Vinson LA. Fracture resistance of full ceramic primary crowns. Dent Oral Heal Cosmesis. 2016;1:1–4. [Google Scholar]
- 43.Bankoğlu Güngör M, Karakoca Nemli S. Fracture resistance of CAD-CAM monolithic ceramic and veneered zirconia molar crowns after aging in a mastication simulator. J Prosthet Dent. 2018;119:473–80. [DOI] [PubMed] [Google Scholar]
- 44.Anusavice KJ. Informatics systems to assess and apply clinical research on dental restorative materials. Adv Dent Res. 2003;17:43–8. [DOI] [PubMed] [Google Scholar]
- 45.Özal Ç, Ulusoy M. In-vitro evaluation of marginal and internal fit of 3-unit monolithic zirconia restorations fabricated using digital scanning technologies. J Adv Prosthodont. 2021;13:373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Fiore A, et al. Influence of ceramic firing on marginal gap accuracy and metal-ceramic bond strength of 3D-printed co-cr frameworks. J Prosthet Dent. 2020;124:75–80. [DOI] [PubMed] [Google Scholar]
- 47.Zhu H, Zhou Y, Jiang J, Wang Y, He F. Accuracy and margin quality of advanced 3D-printed monolithic zirconia crowns. J Prosthet Dent. 2023. 10.1016/J.PROSDENT.2023.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Stappert CFJ, Dai M, Chitmongkolsuk S, Gerds T, Strub JR. Marginal adaptation of three-unit fixed partial dentures constructed from pressed ceramic systems. Br Dent J. 2004;196:766–70. discussion 760, quiz 780. [DOI] [PubMed] [Google Scholar]
- 49.Okutan M, Heydecke G, Butz F, Strub JR. Fracture load and marginal fit of shrinkage-free ZrSiO4 all-ceramic crowns after chewing simulation. J Oral Rehabil. 2006;33:827–32. [DOI] [PubMed] [Google Scholar]
- 50.Jesús Suárez M, Lozano JFL, Paz Salido M, Martínez F. Marginal fit of titanium metal-ceramic crowns. Int J Prosthodont. 2005;18:390–1. [PubMed] [Google Scholar]
- 51.Pilo R, Cardash HS. In vivo retrospective study of cement thickness under crowns. J Prosthet Dent. 1998;79:621–5. [DOI] [PubMed] [Google Scholar]
- 52.Nawafleh NA, Mack F, Evans J, Mackay J, Hatamleh MM. Accuracy and reliability of methods to measure marginal adaptation of crowns and FDPs: a literature review. J Prosthodont off J Am Coll Prosthodont. 2013;22:419–28. [DOI] [PubMed] [Google Scholar]
- 53.Al-Haj Ali SN, Farah R. I. In vitro comparison of microleakge between preformed metal crowns and aesthetic crowns of primary molars using different adhesive luting cements. Eur Arch Paediatr Dent off J Eur Acad Paediatr Dent. 2018;19:387–92. [DOI] [PubMed] [Google Scholar]
- 54.Elsayed DH, Eldin MSS, Mohamed S, Omer M. Comparison between Stainless-Steel, Zirconia and Fiberglass crowns as full Coverage Restoration for deciduous molars of children. Afr J Bio Sc. 2024;6:2663–2187. [Google Scholar]
- 55.Euán R, Figueras-Álvarez O, Cabratosa-Termes J, Oliver-Parra R. Marginal adaptation of zirconium dioxide copings: influence of the CAD/CAM system and the finish line design. J Prosthet Dent. 2014;112:155–62. [DOI] [PubMed] [Google Scholar]
- 56.Talekar A et al. Ex Vivo Assessment of Natural Teeth Wear against Zirconia and Novel Glass-Fiber-Reinforced Composite Crowns in Primary Teeth by a Three-Dimensional Assessment Method. Int. J. Dent. 2021, 9670982 (2021). [DOI] [PMC free article] [PubMed]
- 57.Townsend JA, et al. In vitro fracture resistance of three commercially available zirconia crowns for primary molars. Pediatr Dent. 2014;36:125–9. [PubMed] [Google Scholar]
- 58.Bamdadian Z, Pasdar N, Alhavaz A, Ghasemi S, Bijani A. Comparative evaluation of Physical and Mechanical Properties of Different Brands of primary Molar Stainless-Steel crowns: an in Vitro Study. Open Access Maced J Med Sci. 2019;7:4120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maruyama N, Mori D, Hiromoto S, Kanazawa K, Nakamura M. Fatigue strength of 316L-type stainless steel in simulated body fluids. Corros Sci. 2011;53:2222–7. [Google Scholar]
- 60.Passi P, Girardello GB, Vesentini A. Resistance to fracture of ceramic jacket crowns. Quintessence Int. 1992;23:845–7. [PubMed] [Google Scholar]
- 61.Waggoner WF. Restoring primary anterior teeth: updated for 2014. Pediatr Dent. 2015;37:163–70. [PubMed] [Google Scholar]
- 62.Rocha MCM, et al. Zirconia crowns as an esthetic alternative for oral rehabilitation in pediatric dentistry: a review. Pediatr Dent J. 2021;31:224–34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data cannot be shared openly but are available on request from authors.